Abstract
Patients with Alzheimer’s disease have category-specific semantic memory difficulty for natural relative to manufactured objects. We assessed the basis for this deficit by asking healthy adults and patients to judge whether pairs of words share a feature (e.g. “banana:lemon – COLOR”). In an fMRI study, healthy adults showed gray matter (GM) activation of temporal-occipital cortex (TOC) where visual-perceptual features may be represented, and prefrontal cortex (PFC) which may contribute to feature selection. Tractography revealed dorsal and ventral stream white matter (WM) projections between PFC and TOC. Patients had greater difficulty with natural than manufactured objects. This was associated with greater overlap between diseased GM areas correlated with natural kinds in patients and fMRI activation in healthy adults for natural than manufactured artifacts, and the dorsal WM projection between PFC and TOC in patients correlated only with judgments of natural kinds. Patients thus remained dependent on the same neural network as controls during judgments of natural kinds, despite disease in these areas. For manufactured objects, patients’ judgments showed limited correlations with PFC and TOC GM areas activated by controls, and did not correlate with the PFC-TOC dorsal WM tract. Regions outside of the PFC–TOC network thus may help support patients’ judgments of manufactured objects. We conclude that a large-scale neural network for semantic memory implicates both feature knowledge representations in modality-specific association cortex and heteromodal regions important for accessing this knowledge, and that patients’ relative deficit for natural kinds is due in part to their dependence on this network despite disease in these areas.
Keywords: semantic, fMRI, DTI, Alzheimer’s, temporal, prefrontal
1. INTRODUCTION
Semantic memory is the long-term representation of knowledge about our world (Tulving, Tulving, & Donaldson, 1972). While impairment of episodic memory is the best known clinical characteristic of Alzheimer’s disease (AD), semantic memory deficits are also frequently present (Chertkow & Bub, 1990; Chertkow, Whatmough, Saumier, & Duong, 2008; Grossman & Koenig, 2001; Hodges, Salmon, & Butters, 1992; Martin & Fedio, 1983). Semantic memory difficulty in AD is characterized by a relative impairment for natural kinds compared to manufactured objects (Garrard, Lambon Ralph, Hodges, & Patterson, 2001; Garrard et al., 2001; Grossman, White-Devine, Robinson, Biassou, & D’Esposito, 1998; Mauri, Daum, Sartori, Riesch, & Birbaumer, 1994; Moss, De Mornay Davies, Jeppeson, McLellan, & Tyler, 1998; Silveri, Daniele, Giustolisi, & Gainotti, 1991). Questions nevertheless remain about the basis for this deficit. In this report, we use functional MRI (fMRI) and diffusion tensor imaging (DTI) in healthy adults to help identify the joint contribution of perceptual feature knowledge in modality-specific association cortex and regions of prefrontal cortex that help select feature knowledge for natural and manufactured object categories. We further use structural MRI and DTI in patients to examine the basis for their relative difficulty with natural kinds.
AD is a neurodegenerative condition that involves disease in both modality-specific association areas (such as temporal-occipital cortex; TOC) and heteromodal association regions (such as lateral prefrontal cortex; PFC) (Braak et al., 1997; Dickerson et al., 2009; Forman et al., 2006). Both of these areas are regularly recruited in fMRI studies of healthy adults during semantic memory tasks (Binder, Desai, Graves, & Conant, 2009). As noted above, patients with AD often have semantic memory difficulty that typically includes relative impairment for natural kinds compared to manufactured artifacts. Since this deficit has been shown in studies using well-matched category-specific materials administered in the identical manner, the impairment cannot be easily attributed to non-semantic deficits such as limited attention, impaired mental imagery or difficulty perceiving stimuli.
At least two hypotheses relate the anatomic distribution of disease in AD to their category-specific semantic memory deficit. One hypothesis attributes their semantic memory deficit primarily to degradation of the perceptual features that contribute disproportionately to the representations of natural kinds than manufactured objects (Chertkow, Bub, & Schwartz, 1990; Farah & McClelland, 1991; Fung et al., 2001; Grossman, et al., 1998; Whatmough, Chertkow, Murtha, & Hanratty, 2002). This hypothesis is associated with an anatomic model of semantic memory that proposes that object knowledge depends largely on the activation of brain regions that store the visual-perceptual features contributing to object concepts (Barsalou, 2008; Martin, 2007). We refer to this as the sensory-motor hypothesis. From this perspective, TOC plays a critical role in storing visual-perceptual features because it is near areas that are important for perceptual processing of this information. Disease in AD involving modality-specific association regions like TOC may therefore interfere with the representation of natural kinds because these object concepts are thought to be more dependent on visual-perceptual features than are manufactured objects (Saffran, Schwartz, Umilta, & Moscovitch, 1994).
To date, few studies of AD have examined imaging evidence relating disease in modality-specific regions like TOC to performance on semantic-memory tasks involving object knowledge. In a resting PET correlation study of AD, judgments of visual attributes of natural kinds were associated with TOC, while judgments of visual attributes of manufactured objects were associated with premotor and anterior temporal regions (Zahn et al., 2006). In a BOLD fMRI activation study, increased recruitment in left TOC was seen in AD relative to healthy seniors for natural kinds, and this increased recruitment was greater during judgments of natural kinds relative to manufactured objects, suggesting compensatory up-regulation of TOC during AD patients’ judgments of natural kinds as disease accumulates in areas critical for representing perceptual features contributing to natural object concepts (Grossman, 2003). Together, these findings suggest that patients continue to depend on TOC during judgments of natural kinds despite disease in this area, while other areas may be recruited — including parietal and premotor areas thought to be important for storing motion (Chao & Martin, 2000) and action (Hauk, Johnsrude, & Pulvermuller, 2004) features — to support the representation of manufactured objects. Features of manufactured objects may be more distributed than those of natural kinds, and thus may be less susceptible to disease in AD (Devlin, Gonnerman, Andersen, & Seidenberg, 1998; Gonnerman, Andersen, Devlin, Kempler, & Seidenberg, 1997; Koenig, Smith, & Grossman, 2010; Rogers, 2004).
A second semantic memory hypothesis builds on this sensory-motor approach by invoking a second component — top-down control and selection of sensory-motor features (Koenig & Grossman, 2007; Putnam, 1970; Thompson-Schill, 2003). This component may be critical for selecting target information in semantic representations, generalizing across specific instances of an object category, and making inferences about objects regardless of their sensory-motor features (Caramazza, Hillis, Rapp, & Romani, 1990). From this perspective, a large-scale neural network for semantic memory may depend not only on regions of modality-specific sensory cortex, but also on association cortices that are not modality-specific. We refer to this as the heteromodal hypothesis. The concept of a large-scale network underlying semantic memory is an important consideration because there are few lesion studies reporting semantic memory deficits following disease restricted to a single region. This raises the possibility that disruption of a network, rather than damage to a circumscribed region of cortex, compromises semantic memory.
Some support for this heteromodal hypothesis comes from the observation that PFC is frequently recruited in fMRI studies of word meaning in healthy controls, yet is not associated with a specific sensory-motor modality (Binder, et al., 2009; Martin, Haxby, Lalonde, Wiggs, & Ungerleider, 1995; Martin, Wiggs, Ungerleider, & Haxby, 1996; Thompson-Schill, 2003). In one study, for example, activation of dorsolateral portions of PFC was seen when subjects judged whether a list of attributes describes a target word, presumably helping to control and select features that contribute to an object concept (Peelle, Troiani, & Grossman, 2009). Likewise, PFC was recruited in a concept acquisition study when it was necessary to identify the specific visual-perceptual features required for membership of an object in the new category (Koenig et al., 2005). PFC activation was not seen in these studies for other judgments, such as the overall resemblance of an object to a prototypical member of the novel category. Recently, investigators found PFC areas that are sensitive to conceptual and perceptual differences between pictured natural kinds in a category membership judgment task (Gotts, Milleville, Bellgowan, & Martin, 2011). Thus, there is substantial evidence supporting a role for PFC in semantic memory.
Multi-component models of semantic memory such as this also necessarily implicate white matter projections between the gray matter (GM) regions contributing to this network. Indeed, anatomic studies of nonhuman primates have identified converging white matter (WM) projections between modality-specific association cortices such as TOC and heteromodal regions such as PFC (Mesulam, 2000; Mesulam, van Hoesen, Pandya, & Geschwind, 1977; Petrides & Pandya, 1999; Seltzer & Pandya, 1984), making PFC particularly suitable for a supramodal role in semantic memory. There is a long history of work underlining the critical role of the arcuate fasciculus in language processing (Geschwind, 1965). More recently, DTI studies have identified direct projections through the arcuate fasciculus that connect TOC and PFC (Catani, Jones, & Ffytche, 2005). In addition to this dorsal stream, a ventral stream involving the inferior frontal-occipital fasciculus coursing between PFC and TOC also may contribute to language processing (Turken & Dronkers, 2011). Hypotheses regarding the roles of these projections in language processing have been forwarded (Friederici, 2011; Hickok & Poeppel, 2004, 2007), but few studies have provided empirical evidence regarding the contribution of these projections to a semantic memory network. Here we use DTI to examine whether these projections are implicated in an fMRI study recruiting PFC and TOC components of a large-scale neural network of semantic memory. If semantic memory difficulty in AD depends in part on the breakdown of this network, moreover, then this deficit also may depend in part on reduced connectivity between the regions implicated in the semantic memory network
In sum, the present study tests the hypothesis that selective breakdown of a large-scale neural network for semantic memory — including disease in TOC and PFC as well as degraded projections between TOC and PFC — contributes to the category-specific deficit for natural kinds in AD. We first describe an fMRI study in healthy adults probing knowledge about natural kinds and manufactured objects. We then report data from this same task in patients with AD, along with correlated neuroimaging measures of GM and WM integrity.
2. METHODS
2.1 Subjects
Participants in the fMRI task were 18 healthy adults (9 males) aged 18–33 years (mean = 24.4, SD = 3.4) from the University of Pennsylvania community. All were right-handed, native English speakers, and had good general health and no history of neurological difficulty as established by a pre-scan screening form.
We also studied 33 patients with AD spectrum disease, including 15 with probable AD (7 males) and 18 with amnestic Mild Cognitive Impairment (aMCI) (13 males), diagnosed according to published criteria (Albert et al., 2011; McKhann et al., 2011). We extended our assessment to aMCI because of the identical underlying histopathology and because these patients also appear to show semantic memory deficits (Adlam, Bozeat, Arnold, Watson, & Hodges, 2006; Joubert et al., 2010; Woodard et al., 2009). Patients with evidence for other neurological disorders such as stroke or hydrocephalus, primary psychiatric disorders such as major depression or schizophrenia, or medical conditions that can interfere with cognitive functioning such as encephalopathy or metabolic disorders were excluded from participation. Patients may have been taking a clinically indicated dosage of a medication such as a cholinesterase inhibitor or a small dosage of an anti-depressant, but dosage was stable throughout the entire study and no patients were suffering from medication-related cognitive side effects. We also studied 14 healthy seniors (6 males) who served as controls for the AD patients in the behavioral study. Patients were older than age-matched controls, as indicated in Table 1, but there was no correlation between performance on this simple task and age (r= −0.05). All subjects participated in an informed consent procedure approved by the Institutional Review Board of the University of Pennsylvania.
TABLE 1.
MEAN (± SD) CLINICAL AND DEMOGRAPHIC FEATURES IN PATIENTS AND CONTROLS
| PATIENTS (n=33)1 | CONTROLS (n=14) | |
|---|---|---|
| AGE | 73.1 (9.0) | 61.4 (8.5) |
| MMSE (max=30) | 23.9 (4.6) | 29.6 (0.8) |
| SEMANTIC | ||
| Pyramid and Palm Tree-words (max=52) | 46.9 (5.6) | 52.0 (0.0) |
| Pyramid and Palm Tree-pictures (max=52) | 46.7 (5.8) | 51.5 (0.7) |
| EPISODIC MEMORY | ||
| Verbal word list (max=9) | 0.2 (0.3) | 7.6 (0.9) |
| Visual geometric figure (max=24) | 6.2 (5.2) | 19.8 (5.6) |
| EXECUTIVE | ||
| Trails B (max=25) | 19.2 (7.4) | 25.0 (0.0) |
| FAS (# words/3 min) | 29.3 (14.0) | 40.1 (12.2) |
| VISUAL | ||
| Dot location copy (mm displacement from target) | 103.89 (107.5) | 62.5 (30.4) |
| Rey figure copy (max=36) | 26.0 (9.0) | 35.5 (1.0) |
NOTE
Three AD cases did not have Pyramid and Palm Tree Pictures, seven cases did not have Pyramid and Palm Tree Words, two cases did not have Verbal Word List, one case did not have Visual Geometric Figure Recall or Copy, three cases (3 AD) did not have Trails B, and four cases (3 aMCI, 1 AD) did not have dots.
As expected, patients were mildly impaired according to the MMSE. We also examined patients on a brief neuropsychological battery. This included measures of: semantic memory (Pyramid and Palm Tree test, a measure of semantic associativity knowledge involving pictures or words) (Howard & Patterson, 1992); episodic memory (delayed recall from a word list; and delayed recall of the complex Rey figure) (Libon et al., 2007; Libon et al., 1996); executive functioning (Trails B, a measure of planning and organization; FAS, a category naming fluency measure requiring mental search and working memory) (Libon, et al., 2007); and visuospatial functioning (localization of a 1 cm dot in a 5″ × 8″ space similar to a model; and copy of the complex Rey figure) (Libon, et al., 2007). Not all patients were able to perform all tasks for a variety of reasons (e.g., intercurrent medical needs, scheduling, technical difficulties). Performance on these measures is summarized in Table 1.
2.2 Stimulus Materials
We created pairs of printed nouns, half of which were natural kinds (e.g. banana, lemon) and half manufactured objects (e.g. spoon, knife) (see Supplement Table 1). We used words rather than pictures to minimize the possibility that purely visual-perceptual deficits could explain patients’ difficulties. Natural kinds consisted of fruits, vegetables and animals, and manufactured objects consisted of implements, sports equipment and means of transportation. We created 200 pairs, where half were natural kinds and half manufactured objects, and half of each of these stimulus subsets probed shape and half color. According to norming studies on subjects who did not participate in this study, half of the shape and half the color pairs of each semantic category were judged “same” and half “different.” Word frequencies (Francis & Kucera, 1982) and familiarity ratings obtained from a different group of 20 young adults were used to match lists of stimuli, and no significant differences (p>0.10) were found between natural kinds and manufactured objects, or between shape and color stimuli. All stimulus words were highly imageable. Intermixed were 50 filler pairs (100 words) that queried a third perceptual feature (“size”) of natural and manufactured objects; performance on these fillers did not differ between groups (according to pretesting), and these items were not considered further.
2.3 Behavioral fMRI study in Healthy Controls
We administered to healthy controls a subset of these materials while we monitored activation with BOLD fMRI. Each trial began with a 500 ms crosshair followed by presentation of a pair of nouns. Pairs remained on the screen for 2.5 sec or until subjects responded using a keypad to indicate “same” or “different.” An event-related design was used, and 80 word pairs (40 pairs of natural kinds and 40 pairs of manufactured objects) were presented in a fixed pseudorandom order for the block probing each perceptual attribute. Between each trial, there was an interval of 0, 3, 6, 9 or 12 seconds, during which time a blank, white screen was displayed. Subjects were trained in advance on the experimental materials with several practice items, and all subjects appeared to understand the task and the procedure for indicating their judgments during the practice session prior to the experiment. Presentation was blocked by material and probe in order to minimize executive control demands associated with trial-by-trial switching between materials or between probes. Blocks began with a question for 3 sec indicating the attribute to be compared during the block (e.g. “Are these the same color?”), and the relevant property (e.g. “color”) was written below each word pair during presentation of the remainder of the stimuli for a block.
2.4 BOLD fMRI Imaging Methods in Healthy Controls
MRI data were acquired on a Siemens Trio scanner (Siemens Medical Systems, Erlangen, Germany) at 3T, beginning with acquisition of a T1-weighted structural volume using a MPRAGE sequence (repetition time [TR] = 1620 ms, echo time [TE] = 3 ms, flip angle = 15°, 1 mm slice thickness, 192 × 256 matrix, voxel size = 0.9766 × 0.9766 × 1 mm). Blood oxygenation level-dependent functional MRI images were acquired with 3 mm isotropic voxels, flip angle = 90°, TR = 3 s, TEeff = 30 ms, and a 64 × 64 matrix.
Analysis of the fMRI data was performed using SPM8 software (Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm). For each participant, images were realigned to the first image, coregistered to the structural image, and normalized to Montreal Neurological Institute (MNI) space using unified segmentation (Ashburner & Friston, 2005), including resampling to 2 × 2 × 2 mm voxels, and spatially smoothed with a 10 mm full-width at half maximum (FWHM) Gaussian kernel. Responses to events were modeled using a canonical hemodynamic response function, and movement parameters were included as covariates of no interest. Parameter estimates from single-subject analyses were brought to second-level random effects analyses for making group inferences. Statistical maps for the MRI analyses were rendered on 3D MNI-space templates from SPM8.
2.5 Behavioral Procedure for the Patient Study
Subjects were asked to compare pairs of written object nouns based on a perceptual feature using the same materials described above. We recruited mildly impaired patients and used a simple task involving a single judgment of familiar objects to minimize the risk that executive-resource limitations may contribute to the patients’ deficit. Stimulus presentation was blocked by perceptual probe (color or shape) in order to minimize task-related demands associated with trial-by-trial switching between probes. Blocks began with a question for 3 sec indicating the feature to be compared during the block (e.g. “Are these the same color?”), and the relevant property (e.g. “color”) was written below each word pair during presentation of the remainder of the stimuli for a block to minimize any difficulty due to impaired episodic memory. One hundred and fifty word pairs (50 of natural, 50 manufactured, and 50 filler) were presented in a fixed pseudorandom order for each block.
Each trial within a block began with a 500 ms crosshair on the computer screen followed by presentation of a pair of nouns. Pairs remained on the screen until subjects responded in order to minimize task-related working memory demands, and subjects used the computer keyboard to indicate “same” or “different.” Subjects were trained beforehand on the experimental method with several practice items, and all subjects appeared to understand the task during the practice session.
2.6 T1-weighted Gray Matter Atrophy Imaging Methods for the Patient Study
For 17 patients (8 with AD and 9 with aMCI), we had a volumetric T1-weighted brain MRI scan available on average within 4 months of the behavioral task. These patients did not differ statistically from the larger set of patients on any demographic or cognitive measure. T1-weighted MRI scans were also available for 40 age-matched controls (MMSE >27 for all healthy seniors) who did not participate in the behavioral testing. We used a SIEMENS Trio 3.0T scanner at 1 mm slice thickness and a 192 × 256 matrix using an MPRAGE protocol (TR=1620ms, TE=3ms, flip angle=15°, in-plane resolution=0.9766 × 0.9766). Images were preprocessed by deforming into a standard local template space with 1-mm3 resolution using PipeDream (https://sourceforge.net/projects/neuropipedream/) and Advanced Normalization Tools (ANTS, http://www.picsl.upenn.edu/ANTS/) in a validated pipeline for multivariate normalization (Avants, Epstein, Grossman, & Gee, 2008; Klein et al., 2009). PipeDream and ANTS were used to map T1-weighted structural MRI images to an optimal template space using diffeomorphic and symmetric registration methods (Avants & Gee, 2004; Avants et al., 2010). Each subject’s T1 image was corrected for inhomogeneity using N4 (Tustison et al., 2010) and segmented into GM probability maps using template-based priors, then registered to MNI space for statistical comparisons. During normalization, no modulation was performed (i.e., normalized images reflect gray matter probability, not volume). GM probability images were smoothed using an 8 mm FWHM Gaussian kernel.
We used a two-sample t-test contrasting normalized GM probability between patients and healthy controls in order to identify regions of significant GM atrophy. For this atrophy analysis, we used a whole-brain threshold of p<0.05 (false discovery rate [FDR]-corrected for multiple comparisons at the voxel level), 400 μl extent.
We then conducted whole-brain regression analyses to relate GM probability to the accuracy of color and shape judgments of natural and manufactured objects. For the regression analysis, we used a statistical height threshold of p<0.05 (uncorrected) and accepted clusters containing a peak with z-score >3.09 (p<0.001) and an extent of at least 50 μl. We used this liberal statistical threshold because we wished to establish whether any significant voxels relating performance to atrophy emerged in the regions of interest (ROIs) defined by fMRI activations in healthy adults for the same materials.
2.7 Tractography Analysis with Diffusion Tensor Imaging
We assessed the white matter projections that directly connect the PFC and TOC regions implicated in task performance. We focused on these two regions because they were activated for both features of both semantic categories in the fMRI BOLD study (see below). DTI images were available for 15 young controls and 10 patients (6 with AD and 4 with aMCI, mean MMSE = 21.8) where we also had T1 structural imaging. Technical difficulties, the use of different image acquisition parameters, or lengthy discrepancies between T1 and DTI acquisitions limited DTI use in the remaining patients who had T1 structural imaging. DTI acquisition parameters were: FOV=240mm; matrix size=128 × 128; number of slices=70; imaging resolution=1.9 × 1.9 × 2mm; TR=8000ms; TE=82ms; fat saturation. In total, 31 volumes were acquired per subject, one without diffusion weighting (b=0 s/mm2) and 30 with diffusion weighting (b=1000 s/mm2) along 30 non-collinear directions.
Diffusion-weighted images were pre-processed using ANTS and the Camino toolkit (Cook et al., 2006) using the PipeDream processing pipeline. Motion and distortion artifacts were removed by affine co-registration of each diffusion-weighted image to the unweighted (b=0) image. Diffusion tensors were computed using a linear least squares algorithm (Salvador et al., 2005) implemented in Camino.
Using the T1 template described above, DTI images from each subject were relocated to the T1 template space by PipeDream. Any distortion between the subject’s T1 and DTI image was corrected by registering the fractional anisotropy (FA) to the T1 image. The DTI image was then warped to template space by applying both the intra-subject (FA to subject T1) and inter-subject (subject T1 to template) warps. We also reoriented the tensors using the preservation of principal directions algorithm (Alexander, Pierpaoli, Basser, & Gee, 2001). To establish the PFC and TOC ROIs that constitute the GM component of the network for each semantic category studied in the task, we used the regions activated during performance of the identical task by healthy subjects. Use of these ROIs allowed us to determine whether deviations from normal connectivity between theses areas emerge in patients. A morphological dilation, radius 3 voxels, was then applied to the functionally-defined ROIs from healthy controls to extend them into WM.
We then identified the WM tracts linking the ROIs for each semantic category in both healthy adults and patients. A DTI template was computed by averaging each participant’s DTI image after normalization to the T1 template space. Streamline tractography was performed separately for healthy adults and patients using Camino. For each group, streamlines of the participants’ DTI were seeded in each voxel of the left hemisphere of the DTI template with an FA>0.25, and proceeded according to the FACT algorithm (Xue, van Zijl, Crain, Solaiyappan, & Mori, 1999). Tracking was terminated upon reaching a voxel with FA<0.25 or if the streamline trajectory changed >45° in successive steps. WM connectivity between PFC and TOC was visualized by searching the set of left-hemisphere streamlines and retaining only those that intersected both ROIs.
3. RESULTS
3.1 Behavioral Results
Behavioral results confirmed a relative deficit for natural kinds in patients. T-tests showed a robust statistical difference in judgment accuracy between controls (mean ± S.D. = 35.1 ± 2.5 correct) and patients (31.7 ± 4.6 correct) for natural kinds [t(45)=2.62; p=0.01], and a smaller difference between controls (31.8 ± 2.3 correct) and patients (29.9 ± 3.2 correct) for manufactured objects [t(45)=2.00; p=0.051]. Since controls found manufactured objects more difficult than natural kinds [t(13)=6.14; p<0.001], we evaluated patients’ between-category performance comparatively while accounting for the different levels of controls’ performance with these stimuli by converting individual patients’ performance to z-scores relative to controls’ performance within each category. An analysis of variance (ANOVA) examined judgment accuracy in patients using within-subjects effects for semantic category (2: natural, manufactured) and visual-perceptual feature (2: color, shape). This revealed a main effect for semantic category, with greater difficulty for natural kinds (z= −1.11 ± 1.7) than manufactured objects (z= −0.67 ± 1.1) [F(1,32)=8.03; p=0.008]. An evaluation of individual patient performance profiles using these z-scores revealed greater difficulty for natural kinds compared to manufactured objects in 21 (64%) of patients. While the relative deficit for natural kinds was more common among AD patients than aMCI patients, this percentage did not statistically between subgroups (p>0.15). We did not observe a main effect for perceptual feature [F(1,32)=1.71; p=0.49], but there was an interaction for semantic category × perceptual feature [F(1,32)=9.26; p=0.005], with shape features of natural kinds more difficult than shape features of manufactured objects [t(32)=3.95; p<0.001]. We also found a significant correlation between overall judgment accuracy on this task and performance on the Pyramid and Palm Tree measure of semantic memory [r(24)=0.66; p<0.001], consistent with the view that our behavioral measure is related in part to semantic memory.
3.2 fMRI Results in Healthy Adults
BOLD fMRI in healthy adults showed activation of PFC and TOC regions for both categories, although there also appeared to be some additional selective activation for natural and manufactured categories. We conducted whole-brain analyses to identify regions of significant activation relative to resting baseline while participants made judgments about natural kinds and manufactured objects, using a voxelwise threshold of p < 0.001, corrected for multiple comparisons across the whole brain (p < .05) based on cluster extent using random field theory. These results are shown in Figure 1a. As summarized in Table 2, we found that semantic judgments for both categories resulted in increased activation bilaterally in both TOC and PFC, as well as left parietal cortex. Figure 1b shows the direct comparison of activations for natural kinds and manufactured objects. As summarized in Table 3, this demonstrated clusters in temporal and frontal cortex in which there was significantly greater activation for manufactured objects. We also observed greater activation in the left angular gyrus for natural kinds compared to manufactured objects.
Figure 1.
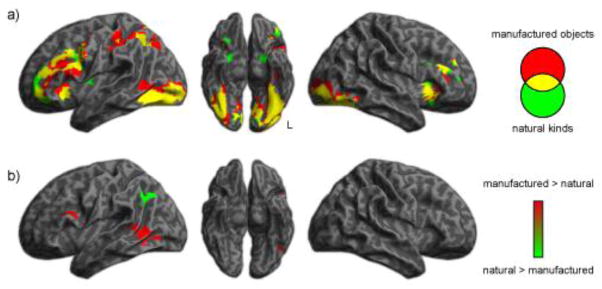
BOLD fMRI activations for natural kinds and manufactured objects in healthy adults. (a) Activity for manufactured objects and natural kinds relative to a resting baseline. (b) Direct comparisons of activations for natural kinds and manufactured objects.
TABLE 2.
fMRI ACTIVATIONS RESULTS IN YOUNG ADULTS FOR NATURAL KINDS AND MANUFACTURED OBJECTS RELATIVE TO RESTING BASELINE
| Region | # voxels | Coordinates
|
Z score | ||
|---|---|---|---|---|---|
| NATURAL KINDS > BASELINE | x | y | z | ||
| L fusiform gyrus (37) | 10863 | −38 | −60 | −20 | 5.60 |
| L fusiform gyrus (18) | −42 | −76 | −18 | 5.44 | |
| L fusiform gyrus (19) | −22 | −74 | −18 | 4.84 | |
| L lingual gyrus (18) | −6 | −82 | −18 | 5.01 | |
| L lingual gyrus (18) | −8 | −70 | 0 | 3.57 | |
| L inferior occipital gyrus (18) | −32 | −94 | −6 | 4.77 | |
| L inferior occipital gyrus (18) | −42 | −84 | −6 | 4.36 | |
| L cuneus (17) | −4 | −86 | 10 | 4.09 | |
| L cuneus (18) | −2 | −90 | 22 | 3.52 | |
| L cuneus (18) | −4 | −98 | 8 | 3.56 | |
| L cingulate gyrus (23) | −2 | −62 | 6 | 3.36 | |
| R fusiform gyrus (36) | 46 | −52 | −26 | 4.93 | |
| R fusiform gyrus (37) | 38 | −62 | −22 | 5.33 | |
| R fusiform gyrus (37) | 34 | −60 | −22 | 5.29 | |
| R fusiform gyrus (19) | 32 | −76 | −18 | 4.61 | |
| R fusiform gyrus (37) | 34 | −44 | −24 | 3.90 | |
| R lingual gyrus (18) | 12 | −84 | −20 | 4.74 | |
| B lingual gyrus (18) | 0 | −58 | 0 | 3.61 | |
| R inferior temporal gyrus (19) | 48 | −82 | −4 | 3.32 | |
| R inferior occipital gyrus (18) | 36 | −88 | −8 | 4.01 | |
| R cuneus (18) | 30 | −96 | −2 | 3.96 | |
| R middle occipital gyrus (19) | 36 | −94 | 4 | 3.92 | |
| R precuneus (19) | 2 | −84 | 40 | 3.24 | |
| B cuneus (18) | 0 | −88 | 34 | 4.03 | |
| R cuneus (18) | 16 | −76 | 14 | 3.66 | |
| R cuneus (17) | 10 | −82 | 6 | 3.59 | |
| B cerebellum | 2 | −62 | −32 | 3.64 | |
| B cerebellum | 4 | −80 | −30 | 4.73 | |
| B cerebellum | −6 | −56 | −32 | 3.54 | |
| B cerebellum | 0 | −60 | −22 | 3.22 | |
| L inferior frontal gyrus (44) | 3594 | −48 | 8 | 30 | 4.46 |
| L inferior frontal gyrus (47) | −48 | 50 | −8 | 3.88 | |
| L inferior frontal gyrus (47) | −32 | 30 | −16 | 3.64 | |
| L inferior frontal gyrus (47) | −32 | 28 | −12 | 3.63 | |
| L inferior frontal gyrus (47) | −34 | 20 | −6 | 3.57 | |
| L inferior frontal gyrus (47) | −32 | 24 | −8 | 3.57 | |
| L inferior frontal gyrus (47) | −42 | 44 | −4 | 4.03 | |
| L inferior frontal gyrus (44) | −52 | 22 | 28 | 3.85 | |
| L precentral gyrus (6) | −42 | 0 | 38 | 4.02 | |
| L precentral gyrus (6) | −42 | 0 | 14 | 3.39 | |
| L middle frontal gyrus (10) | −40 | 48 | 8 | 4.04 | |
| L middle frontal gyrus (46) | −44 | 42 | 14 | 3.99 | |
| L middle frontal gyrus (30) | −44 | 30 | 28 | 3.32 | |
| L middle frontal gyrus (9) | −44 | 40 | 28 | 3.21 | |
| L middle frontal gyrus (46) | −34 | 30 | 24 | 3.16 | |
| L middle frontal gyrus (45) | −30 | 26 | 22 | 3.16 | |
| L insula | −38 | −2 | 14 | 3.39 | |
| L fusiform gyrus (36) | −44 | 36 | −20 | 4.11 | |
| L fusiform gyrus (37) | −46 | 46 | −18 | 3.81 | |
| L hippocampus | −34 | −20 | −16 | 3.61 | |
| L hippocampus | −30 | −16 | −12 | 3.56 | |
| L hippocampus | −28 | −26 | −4 | 3.26 | |
| L putamen | −24 | 16 | 4 | 3.99 | |
| L putamen | −28 | −6 | 6 | 3.87 | |
| L putamen | −26 | 2 | −10 | 3.85 | |
| L putamen | −28 | −8 | 2 | 3.84 | |
| L putamen | −16 | 8 | 0 | 4.33 | |
| L putamen | −20 | 10 | 2 | 4.18 | |
| L putamen | −30 | −6 | −6 | 3.44 | |
| L putamen | −20 | 10 | 14 | 3.12 | |
| R middle frontal gyrus (46) | 576 | 50 | 36 | 24 | 4.56 |
| R middle frontal gyrus (46) | 48 | 40 | 24 | 4.50 | |
| R middle frontal gyrus (46) | 48 | 48 | 10 | 3.72 | |
| R middle frontal gyrus (10) | 40 | 56 | 14 | 3.31 | |
| R inferior frontal gyrus (44) | 42 | 4 | 26 | 3.55 | |
| R inferior frontal gyrus (44) | 50 | 22 | 30 | 3.47 | |
| L inferior parietal lobe (40) | 1136 | −30 | −54 | 42 | 4.73 |
| L inferior parietal lobe (40) | −44 | −38 | 46 | 3.50 | |
| L superior occipital gyrus (19) | −24 | −64 | 36 | 3.84 | |
| R putamen | 2043 | 20 | 2 | 14 | 5.13 |
| R putamen | 26 | 8 | −6 | 4.63 | |
| R putamen | 22 | 18 | −10 | 3.39 | |
| R insula | 26 | 18 | 12 | 4.12 | |
| R insula | 24 | 18 | 8 | 4.08 | |
| R insula | 26 | 16 | 2 | 3.92 | |
| R cingulate gyrus (32) | 10 | 24 | 36 | 3.81 | |
| R cingulate gyrus (24) | 18 | 12 | 30 | 3.48 | |
| R inferior frontal gyrus (47) | 30 | 26 | −16 | 3.80 | |
| R inferior frontal gyrus (47) | 34 | 26 | −8 | 3.67 | |
| R inferior frontal gyrus (47) | 30 | 28 | −12 | 3.66 | |
| R inferior frontal gyrus (47) | 36 | 24 | −4 | 3.56 | |
| L medial frontal gyrus (32) | −10 | 24 | 42 | 3.73 | |
| R ventral lateral nucleus of thalamus | 18 | −16 | 16 | 3.42 | |
| R amygdala | 28 | −2 | −12 | 4.15 | |
| MANUFACTURED OBJECTS > BASELINE
| |||||
| L inferior occipital gyrus (18) | 16922 | −28 | −94 | −8 | 6.09 |
| L inferior occipital gyrus (18) | −32 | −88 | −16 | 5.88 | |
| L fusiform gyrus (37) | −38 | −60 | −18 | 5.76 | |
| L fusiform gyrus (19) | −38 | −42 | −10 | 5.11 | |
| L fusiform gyrus (20) | −30 | −44 | −20 | 5.08 | |
| L lingual gyrus (19) | −40 | −50 | −6 | 5.20 | |
| L lingual gyrus (19) | −6 | −62 | −2 | 4.60 | |
| L lingual gyrus (18) | −12 | −64 | 0 | 4.46 | |
| L middle occipital gyrus (19) | −40 | −72 | −14 | 5.94 | |
| L middle occipital gyrus (19) | −42 | −88 | −2 | 5.47 | |
| L cuneus (34) | −20 | −66 | 4 | 4.68 | |
| R inferior occipital gyrus (18) | 36 | −86 | −8 | 5.45 | |
| R inferior occipital gyrus (19) | 36 | −82 | −10 | 5.40 | |
| R middle occipital gyrus (19) | 44 | −80 | −14 | 5.56 | |
| R lingual gyrus (18) | 12 | −76 | −16 | 5.56 | |
| L parahippocampal gyrus (36) | −30 | −30 | −22 | 4.44 | |
| R fusiform gyrus (37) | 36 | −62 | −20 | 6.01 | |
| R fusiform gyrus (20) | 28 | −44 | −24 | 5.15 | |
| R fusiform gyrus (20) | 38 | −44 | −34 | 4.84 | |
| R fusiform gyrus (20) | 38 | −46 | −30 | 4.73 | |
| R fusiform gyrus (19) | 30 | −76 | −18 | 4.57 | |
| R middle occipital gyrus (19) | 36 | −94 | 4 | 5.16 | |
| R cuneus (18) | 32 | −96 | 2 | 5.11 | |
| B lingual gyrus (19) | 0 | −64 | −2 | 4.57 | |
| L cerebellum | −2 | −82 | −28 | 5.52 | |
| R cerebellum | 2 | −80 | −30 | 5.50 | |
| B cerebellum | −2 | −74 | −24 | 5.05 | |
| B cerebellum | 0 | −60 | −32 | 4.91 | |
| B cerebellum | −6 | −58 | −32 | 4.71 | |
| B cerebellum | 0 | −58 | −24 | 4.49 | |
| B cerebellum | −4 | −56 | −20 | 4.40 | |
| L inferior frontal gyrus (44) | 6086 | −48 | 8 | 30 | 5.26 |
| L inferior frontal gyrus (44) | −52 | 12 | 16 | 4.73 | |
| L inferior frontal gyrus (47) | −32 | 28 | −2 | 4.47 | |
| L inferior frontal gyrus (47) | −50 | 46 | −12 | 3.76 | |
| L inferior frontal gyrus (47) | −48 | 50 | −6 | 3.81 | |
| L inferior frontal gyrus (47) | −42 | 42 | −20 | 3.62 | |
| L inferior frontal gyrus (11) | −44 | 46 | −18 | 3.83 | |
| L middle frontal gyrus (46) | −40 | 28 | 14 | 4.87 | |
| L middle frontal gyrus (45) | −32 | 20 | 22 | 4.45 | |
| L middle frontal gyrus (6) | −48 | 4 | 50 | 3.97 | |
| L middle frontal gyrus (6) | −40 | 2 | 58 | 3.65 | |
| L precentral gyrus | −42 | −2 | 40 | 4.46 | |
| L insula | −28 | 18 | 2 | 4.42 | |
| L insula | −30 | 22 | 0 | 4.35 | |
| R middle frontal gyrus (46) | 50 | 36 | 24 | 4.53 | |
| R inferior frontal gyrus (47) | 44 | 16 | −6 | 3.56 | |
| R inferior frontal gyrus (45) | 44 | 32 | 10 | 3.51 | |
| R inferior frontal gyrus (45) | 34 | 26 | 6 | 3.44 | |
| R insula | 34 | 26 | −6 | 4.31 | |
| R insula | 30 | 14 | −2 | 4.31 | |
| L putamen | −20 | 6 | 4 | 4.07 | |
| L putamen | −18 | 6 | −2 | 3.94 | |
| L globus pallidus | −18 | −6 | 10 | 4.29 | |
| L thalamus | −12 | −14 | 8 | 4.71 | |
| L thalamus | −10 | −18 | 8 | 4.64 | |
| R putamen | 18 | 6 | 6 | 4.14 | |
| R putamen | 24 | 0 | 6 | 3.63 | |
| R putamen | 30 | 4 | −2 | 3.37 | |
| R globus pallidus | 14 | −2 | −6 | 4.18 | |
| R globus pallidus | 16 | 2 | 6 | 4.07 | |
| R thalamus | 10 | −6 | −2 | 3.88 | |
| R thalamus | 16 | −12 | 10 | 4.83 | |
| L superior occipital gyrus (19) | 2317 | −24 | −66 | 34 | 4.97 |
| L superior occipital gyrus (19) | −28 | −72 | 26 | 4.32 | |
| L superior parietal lobe (7) | −30 | −54 | 50 | 4.94 | |
| L superior parietal lobe (7) | −18 | −64 | 54 | 4.43 | |
| L inferior parietal lobe (40) | −30 | −44 | 40 | 4.76 | |
| L inferior parietal lobe (40) | −48 | −32 | 48 | 4.32 | |
| L inferior parietal lobe (40) | −46 | −32 | 42 | 4.32 | |
| L inferior parietal lobe (40) | −38 | −44 | 54 | 3.94 | |
| L postcentral gyrus (3) | −56 | −22 | 40 | 3.17 | |
| L medial frontal gyrus (6) | 423 | −2 | 16 | 48 | 4.33 |
TABLE 3.
fMRI RESULTS IN YOUNG ADULTS DIRECTLY COMPARING ACTIVATIONS FOR NATURAL KINDS AND MANUFACTURED OBJECTS
| Region | # voxels | Coordinates
|
Z score | ||
|---|---|---|---|---|---|
| MANUFACTURED > NATURAL | x | y | z | ||
| L middle temporal gyrus (21) | 518 | −50 | minus;56 | 2 | 4.67 |
| L middle temporal gyrus (37) | −48 | −66 | 0 | 3.83 | |
| L inferior temporal gyrus (37) | −48 | −58 | −10 | 3.67 | |
| L inferior frontal gyrus (44) | 240 | −48 | 10 | 24 | 4.02 |
| L inferior frontal gyrus (45) | −44 | 16 | 20 | 3.60 | |
| NATURAL > MANUFACTURED
| |||||
| L inferior parietal lobule (40) | 275 | −44 | −68 | 44 | 5.21 |
| L superior temporal gyrus (39) | −52 | −56 | 28 | 3.17 | |
3.2 T1 Structural Atrophy Results in Patients
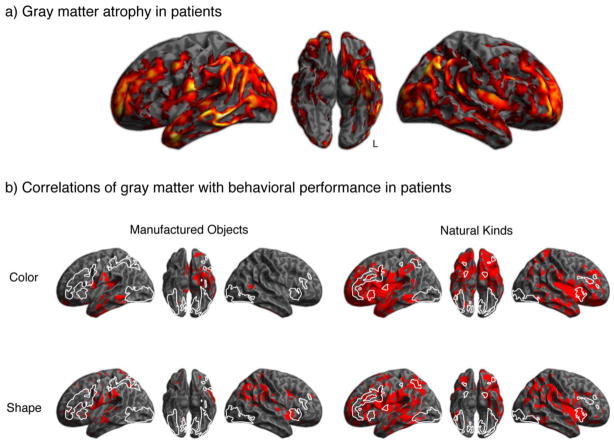
Gray matter atrophy in patients is illustrated in Figure 2a and summarized in Supplement Table 2. Patients had widespread gray matter atrophy throughout temporal, frontal, parietal and occipital lobes. Although this general atrophy was expected to impact upon patients’ overall level of performance, we were most interested in the relationship between GM atrophy and impaired behavioral performance.
Figure 2.
Gray matter atrophy in patients and regression analyses relating gray matter atrophy to behavioral performance for natural kinds and manufactured objects. (a) Areas of significantly reduced gray matter density in patients relative to healthy controls. (b) Regression analyses relating gray matter density to behavioral performance for the four experimental conditions in patients. Areas activated by healthy adults during the corresponding judgments of manufactured objects or natural kinds (from Figure 1) are outlined in white.
Regression analyses relating behavioral performance to structural atrophy in patients showed a differential deficit for natural kinds. Regression analyses relating regional GM density in patients to their performance in each of the experimental conditions are shown in Figure 2b, along with the outline of regions activated by young adults (in white). The results of the regression analyses are summarized in Table 4. Multiple areas of GM atrophy were implicated in judgments of both natural kinds and manufactured artifacts, including portions of temporal, frontal and parietal cortex.
TABLE 4.
REGESSION ANALYSES RELATING BEHAVIORAL PERFORMANCE TO GRAY MATTER ATROPHY IN PATIENTS
| Region | # voxels | Coordinates
|
Z score | ||
|---|---|---|---|---|---|
| NATURAL KINDS - SHAPE | x | y | z | ||
| L middle temporal gyrus (21) | 283701 | −70 | −7 | −4 | 4.22 |
| L uncus (20) | −29 | −15 | −35 | 4.26 | |
| L uncus (28) | −31 | 8 | −25 | 4.06 | |
| L precentral gyrus (40) | −47 | −5 | 20 | 4.21 | |
| L medial frontal gyrus (6) | −14 | −6 | 61 | 3.89 | |
| L cingulate gyrus (31) | −12 | −50 | 27 | 3.87 | |
| L cingulate gyrus (23) | −15 | −51 | 22 | 3.91 | |
| R middle temporal gyrus (39) | 28 | −69 | 23 | 3.78 | |
| R postcentral gyrus (2) | 50 | −20 | 28 | 4.35 | |
| R precentral gyrus (4) | 43 | −4 | 17 | 4.18 | |
| R inferior frontal gyrus (44) | 38 | −5 | 33 | 3.86 | |
| R fusiform gyrus (20) | 4347 | 62 | −54 | −26 | 3.32 |
| NATURAL KINDS - COLOR
| |||||
| R inferior frontal gyrus (45) | 382665 | 35 | 23 | 14 | 4.98 |
| R medial frontal gyrus (9) | 7 | 39 | 31 | 4.35 | |
| R insula | 29 | 28 | 13 | 4.29 | |
| R superior frontal gyrus (11) | 20 | 45 | −14 | 4.24 | |
| L superior frontal gyrus (11) | −19 | 51 | −24 | 4.81 | |
| L inferior frontal gyrus (44) | −38 | 19 | 15 | 4.63 | |
| L precentral gyrus (6) | −54 | 5 | 10 | 4.24 | |
| L cingulate gyrus (24) | −14 | 34 | 13 | 4.73 | |
| L parahippocampal gyrus (36) | −33 | −20 | −29 | 4.56 | |
| L postcentral gyrus (40) | −59 | −17 | 19 | 4.26 | |
| L postcentral gyrus (2) | −59 | −19 | 25 | 4.14 | |
| R superior parietal lobule (7) | 12190 | 31 | −63 | 51 | 3.59 |
| R superior parietal lobule (7) | 35 | −59 | 53 | 3.49 | |
| R superior parietal lobule (19) | 33 | −77 | 28 | 3.41 | |
| R occipital lobe (18) | 1279 | 30 | −72 | −3 | 3.32 |
| R supramarginal gyrus (40) | 769 | 49 | −43 | 33 | 3.23 |
| MANUFACTURED OBJECTS - SHAPE
| |||||
| R inferior frontal gyrus (44) | 103938 | 39 | 14 | 20 | 4.37 |
| R inferior frontal gyrus (44) | 29 | 7 | 28 | 3.66 | |
| R precentral gyrus (4) | 44 | −2 | 17 | 3.99 | |
| L medial frontal gyrus (6) | −14 | −6 | 61 | 4.05 | |
| L cingulate gyrus (32) | −19 | 41 | −1 | 3.76 | |
| L cingulate gyrus (24) | −13 | 4 | 32 | 3.43 | |
| L cingulate gyrus (24) | −12 | 1 | 29 | 3.43 | |
| L cingulate gyrus (33) | −14 | 20 | 25 | 3.42 | |
| L cingulate gyrus (24) | −26 | 6 | 39 | 3.41 | |
| R inferior parietal lobule (40) | 39 | −60 | 47 | 3.49 | |
| R inferior parietal lobule (40) | 26 | −60 | 38 | 3.46 | |
| L precuneus (31) | 2721 | −26 | −54 | 37 | 3.57 |
| MANUFACTURED OBJECTS - COLOR
| |||||
| L inferior temporal gyrus (20) | 41397 | −54 | −9 | −26 | 3.94 |
| L inferior temporal gyrus (20) | −47 | −19 | −37 | 3.79 | |
| L inferior temporal gyrus (20) | −55 | −12 | −33 | 3.71 | |
| L middle temporal gyrus (21) | −59 | −47 | −13 | 3.31 | |
| L superior temporal gyrus (38) | −45 | 9 | −9 | 3.23 | |
| L postcentral gyrus (40) | −56 | −15 | 18 | 3.40 | |
| L insula | −32 | −28 | 16 | 3.29 | |
| R cingulate gyrus (29) | 21148 | 5 | −37 | 11 | 3.61 |
| R cingulate gyrus (24) | 5 | 25 | −5 | 3.47 | |
| L cingulate gyrus (32) | −5 | 21 | −9 | 3.50 | |
| L thalamus | −2 | −2 | 8 | 3.31 | |
| L superior frontal gyrus (11) | 1603 | −18 | 51 | −20 | 3.28 |
We examined whether patients depend on the same regions as healthy controls for their decisions about natural and manufactured objects. To this end, we assessed overlap between the results of these GM regression analyses in patients and BOLD fMRI activations in healthy controls. The greatest overlap between the regions implicated by the regression analyses for patients and the fMRI BOLD activations for healthy controls was for shape judgments of natural kinds (3205 voxels overlapping); there was also considerable overlap between regression analyses in patients and BOLD fMRI activations in healthy controls for color judgments of natural kinds (2648 voxels overlapping). The overlapping regions for both color and shape judgments of natural kinds include both TOC and PFC, implicating difficulty in multiple regions of the neural network supporting normal semantic judgments about perceptual features. Patients thus appeared to be using the same regions that controls recruited when judging natural kinds. Since these areas are significantly diseased in patients, their difficulty judging natural kinds may be due in part to degradation of the GM substrate that normally supports judgments of this category of objects. The overlap for judgments of manufactured objects was still present, but quantitatively lower, for judgments of both shape (439 voxels) and color (107 voxels). The lack of correspondence between the areas that healthy adults recruited and those that patients relied on for judgments about manufactured objects thus suggested that patients’ relative success with manufactured objects may be due in part to their use of brain regions other than those recruited by healthy adults for manufactured objects.
Patients’ relative dependence on the same areas as healthy controls for natural kinds was confirmed by similar analyses examining the BOLD fMRI regions selectively activated by healthy controls in direct comparisons of natural and manufactured categories. We found overlap for the angular gyrus area selectively activated for the natural category in healthy controls and areas implicated by regression analyses in patients’ decisions about natural kinds (112 voxels for shape judgments, 11 voxels for color judgments). By contrast, there was no overlap between the temporal and frontal areas selectively activated for the manufactured category in healthy controls and the areas implicated by the regression analyses in patients’ decisions about manufactured objects. Moreover, the areas of the regression analyses in patients appeared to be specific for semantic memory because a similar regression using the verbal episodic memory measure provided in Table 1 did not overlap with the fMRI activations in controls for semantic judgments (see Supplement Figure 1).
What areas, then, do patients rely on for their judgments of manufactured objects? The fMRI study in healthy adults demonstrated activation of the motor region of the frontal lobe (Figure 1), but there was no significant GM atrophy in this region in patients. Thus, patients may have been able to use knowledge about manufactured objects represented in this area to help support their judgments of these objects. The accuracy of patients’ color and shape judgments for manufactured objects also correlated to some extent with parietal gray matter atrophy, and the area implicated by this analysis was not encompassed by the parietal area activated during the same judgments by healthy adults. In sum, patients may have been relatively successful in their judgments about manufactured objects in part because they do not depend on the identical regions that healthy subjects do. This is consistent with the possibility that other mechanisms are available to support patients’ representation of manufactured objects.
3.3 DTI Tractography Imaging Results
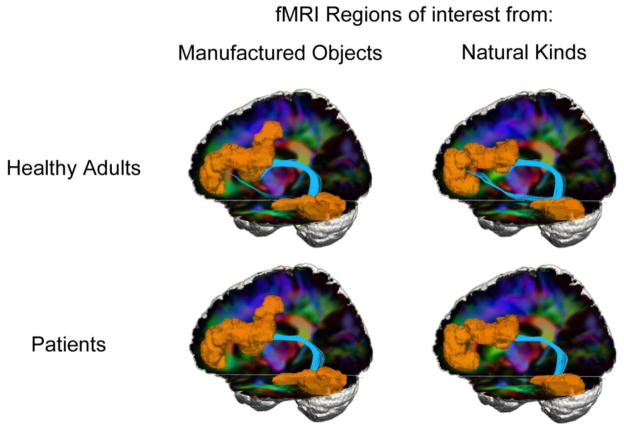
Another factor potentially contributing to the category-specific effects in patients is that there may be a difference in the WM projections supporting connectivity between PFC and TOC during judgments of natural kinds compared to manufactured objects. As illustrated in Figure 3, we observed two projections between PFC and TOC in controls in association with judgments of natural kinds and manufactured objects. One (mean FA = 0.51) coursed through the superior longitudinal fasciculus dorsal to the Sylvian fissure, and then proceeded between PFC and TOC regions via the descending arm of the arcuate fasciculus. We also observed a second, infra-Sylvian projection (mean FA = 0.53) between PFC and TOC through the inferior longitudinal fasciculus in the temporal lobe that also appeared to be denser for natural kinds than manufactured objects.
Figure 3.
White matter tractography in healthy adults and patients. Regions of interest (orange) were formed in left temporal-occipital cortex and prefrontal cortex based on fMRI results of healthy adults (Figure 1) showing common activations during judgments of manufactured objects or natural kinds. Streamline tractography between these regions is shown in light blue. RGB diffusion tensor imaging background shows water diffusion in tracts coursing in left-right (red), anterior-posterior (green), and superior-inferior (blue) orientations.
By comparison, patients’ judgments of natural kinds and manufactured objects were associated with a single, dorsal projection (mean FA = 0.45) between PFC and TOC that resembles the dorsal projection observed in controls. No ventral PFC-TOC projection was seen in patients. These findings suggested that patients may have limited connectivity within the large-scale neural network for object knowledge, and this may interact with limitations in GM portions of the semantic memory network to further interfere with judgments of natural kinds.
We also examined whether patients’ judgments correlated with the dorsal projection that they appear to have maintained. We found a significant correlation between FA in the portion of the dorsal tract associated with judgments of natural kinds [r(8)=0.58; p=0.04, one-tailed], but there was no correlation between FA and judgments of manufactured objects. Thus, resembling our analyses of GM, we found that the same dorsal tract implicated in the large-scale semantic memory network in controls is also related to judgments of natural kinds in patients. By comparison, for manufactured objects, patients appeared to be relying on other neuroanatomic mechanisms to help support connectivity within the semantic memory network.
4. DISCUSSION
Patients with AD have difficulty understanding the meanings of single words, and this is often manifested as a category-specific deficit for natural kinds. We examined the basis for this pattern of impaired semantic memory by assessing judgments of visual-perceptual features of natural and manufactured objects. We found a category-specific deficit, with patients having relatively greater difficulty with natural kinds. In healthy adults, we found activation of PFC and TOC during judgments of natural and manufactured objects, with both dorsal and ventral WM streams projecting between these regions. Our structural imaging analysis suggested that patients’ judgments of natural kinds depends on some of the same areas in TOC and PFC that are activated by healthy controls, and on the same, dorsal WM projection between PFC and TOC that appears to connect these regions. The damage to this network in patients thus may account in part for their relative difficulty with natural kinds. By comparison, patients were less dependent on the same network as controls for their judgments of manufactured objects, and this may account in part for their relative success judging these objects. We discuss the implications of these observations for the category-specific semantic memory deficit in patients and theories of semantic memory in greater detail below.
4.1 Sensory-motor Theories of Semantic Memory
Recent, anatomically-based theories of semantic memory suggest that the sensory-motor feature knowledge that is part of object meaning is represented in or near modality-specific association cortex that corresponds to the type of sensory-motor information in the object (Barsalou, 2008; Martin, 2007). For example, auditory association cortex is activated in lexicality judgments of words enriched with auditory features such as “thunder,” and disease in auditory association cortex interferes with lexicality judgments of these words (Bonner & Grossman, 2012).
In the fMRI results reported here, we found TOC recruitment during judgments about the shape and color of natural kinds in healthy controls. Since this area is important for visual-perceptual processing of shape and color, it is also thought to be involved in representing visual-perceptual features of objects. Patients have disease in these areas. Notably, the anatomic distribution of fMRI activation for these stimuli in healthy adults partially encompasses the areas implicated by our regression analysis relating judgments of shape and color of natural kinds to GM atrophy in patients. Previous imaging work in AD also has associated TOC with judgments of natural kinds (Grossman, 2003; Zahn, et al., 2006). Visual-perceptual processing deficits are reported in AD, including difficulty with shape and color (Kirby, Bandelow, & Hogervorst, 2010). Shape features and color features associated with object concepts may be degraded to some extent in AD as well since the perceptual knowledge associated with object concepts may be represented in or near the same brain areas responsible for perceptual processing. We used words as stimuli to minimize the likelihood that patients’ difficulty is due to visual-perceptual processing per se. The best example of overlapping findings in the present study comes from judgments of shape features of natural kinds, where we found substantial overlap between fMRI activation in healthy adults and the area of GM disease in TOC in patients that is related to their accuracy judging the shape of natural kinds. It was judgments of shapes of natural kinds that were the most difficult for patients. While we cannot rule out entirely that patients’ deficit is related in part to poor visual imagery, the relative effect for type of feature makes this argument less likely since we would otherwise have observed equal difficulty with shape and color attributes.
These findings appear to be consistent with the sensory-motor approach to the representation of object concepts, where visual-perceptual feature knowledge for objects is represented near visual association cortex where shape and color are processed. TOC is diseased in patients, they may have difficulty with judgments of the perceptual features of natural kinds in part because they are attempting to use the same TOC area that is activated by healthy controls to judge natural kinds. Moreover, patients’ greater difficulty with natural kinds has been related to the claim that natural kinds depend more heavily on shape and color features than do manufactured objects (Saffran, et al., 1994). Thus, disease in TOC that degrades visual-perceptual knowledge may disproportionately compromise natural object concepts in AD.
Another observation consistent with the sensory-motor approach concerns other brain regions that appear to support decisions about manufactured objects. The BOLD fMRI study in healthy adults demonstrated relatively greater activation in two additional regions during decisions about manufactured objects compared to natural kinds. One region with greater recruitment during the fMRI BOLD study – an area in premotor cortex – may be related to actions represented in or near the hand portion of the motor system that is associated with use of manufactured objects (Hauk, et al., 2004), and thus may be involved in representing action information about manufactured objects (although see an alternate account for activation of this area below). Patients with amyotrophic lateral sclerosis – a progressive disorder of the motor system – have disease in this same area that appears to be associated with their degraded action knowledge (Grossman et al., 2008). This area does not appear to have significant GM atrophy in the patients participating in the present study, and the fact that this area was relatively intact may have helped support patients’ knowledge of manufactured objects.
Findings for a second region – the lateral temporal lobe – also may be consistent with the sensory-motor approach. This area may related in part to the representation of visual-motion features associated more prominently with manufactured objects than natural kinds (Chao & Martin, 2000). This area showed some atrophy in patients but did not appear to be associated with manufactured objects. Other regions emerged in the regression analysis, even though the fMRI study of healthy adults did not implicate them, and we cannot rule out the possible contribution of these areas to patients’ relatively successful performance with manufactured objects.
4.2 Challenges to the Sensory-Motor Approach: Category-Specific Semantic Memory Deficit in Patients
Other findings in the present study argue against strong claims that disease in TOC can fully explain the pattern of semantic memory difficulty in patients. Specifically, we found that the association between visual-perceptual features in object knowledge and TOC in patients depends in part on the semantic category. While regression analyses in patients related judgments of color and shape features of natural kinds to TOC, regression analyses showed that shape and color features of manufactured objects have a minimal relationship to TOC. Yet, the fMRI BOLD study of healthy adults using the identical materials activated TOC fairly equally for both categories.
How can we account for this discrepancy, where patients’ performance is less affected by disease in TOC during their judgments of manufactured objects even though this area is recruited by healthy subjects? One speculation is related to differences in the way in which features from natural and manufactured categories may be represented in TOC. There may be biological constraints on shape and color features associated with natural kinds that limit the variability with which features of these objects may be represented (Caramazza & Shelton, 1998), while the features of manufactured artifacts may be comparatively less constrained. Likewise, distributed models of semantic memory posit greater sharing and redundancy of visual-perceptual features for natural than manufactured objects (Devlin, et al., 1998; Gonnerman, et al., 1997; Koenig, et al., 2010; Rogers, 2004). While we do not believe that features of object concepts are equally distributed across the entire cortical mantle, this category-specific distinction may be reflected in differences in the way in which features of these object categories are represented locally within TOC, where greater redundancy may result in a less flexible neuroanatomic representation. From these perspectives, it is possible that color and shape features of natural kinds are relatively fixed and stable in their representations within TOC, and thus may be more susceptible to degradation if disease is present in these critical areas. By comparison, limited biological constraints and reduced redundancy for color and shape features of manufactured artifacts may result in more variable and distributed representations of features associated with manufactured objects within TOC and possibly extending to other sensory-motor association cortices. Disease in TOC thus may be less likely to compromise judgments of manufactured objects.
4.3 A Second Challenge to the Sensory-Motor Account: Non-Sensory-Motor Regions and Semantic Memory Judgments
A second finding in the present study also weakens strong claims about the sensory-motor approach to semantic memory: Assessments of object knowledge appear to involve brain regions such as PFC that are not easily related to sensory and motor features (Koenig & Grossman, 2007; Patterson, Nestor, & Rogers, 2007; Thompson-Schill, 2003). Considerable work has associated PFC activation with the selection of visual-perceptual features during evaluations of object meaning in semantic memory (Koenig & Grossman, 2007; Thompson-Schill, 2003; Wagner, Pare-Blagoev, Clark, & Poldrack, 2001). PFC also is implicated in semantic memory in a large metaanalysis of well-conducted fMRI studies (Binder, et al., 2009), even though PFC is not directly involved in the representation of sensory-motor features. In a passive reading study assessing words that name shapes and colors, Pulvermuller and his co-workers observed activation of PFC only for shape words, and this was attributed to the relative motor component associated with outlining a shape with hands or eyes that is not available for colors (Pulvermuller & Hauk, 2006). However, this hypothesis would not explain the observation in this study and elsewhere that PFC is recruited in fMRI studies for both natural and manufactured objects, nor the finding of relatively greater activation for manufactured artifacts in an anatomically inaccurate area – in this study, for example, in an inferior frontal distribution that does not correspond well to the hand area (Bedny, Caramazza, Grossman, Pascual-Leone, & Saxe, 2008). Our view is that PFC is a region of heteromodal association cortex that has reciprocal projections with multiple modality-specific sensory and motor association cortices (Pandya & Yeterian, 1985, 1996; Petrides & Pandya, 1999); and based on the connectivity pattern of this region, PFC may play a role in the top-down organization and selection of features in object knowledge. A direct comparison of natural and manufactured objects revealed greater PFC activation for manufactured objects. From this perspective, PFC activation may be due in part to the greater variability of shape and color features of manufactured artifacts and thus increased effort required for their selection, while features of natural kinds may be more stable and thus may require less effort for selection.
In the present study, evidence relating the PFC region to semantic memory in patients comes from the findings that accuracy judging both color and shape of natural kinds was related to cortical atrophy in PFC. Notably, the same area overlapped with the fMRI activations seen for judgments of these same features of natural kinds in healthy controls. Since patients appear to depend on PFC during judgments of natural kinds, disease in this area likely contributes to their deficit when making judgments about natural kinds.
While we found a robust relationship between PFC atrophy and judgments of natural kinds in patients, regression analyses in patients provided only minimal support for a relation between PFC and judgments of manufactured objects. The observation of patients’ minimal dependence on a diseased brain area that is recruited to perform the identical task in healthy controls may explain in part why manufactured objects are relatively preserved in AD. The category-specific effect for PFC also suggests that involvement of this area cannot be attributed to non-specific resource demands during performance of this simple task. Likewise, we do not believe that non-specific difficulty with manufactured objects can entirely account for our findings. While raw judgments of manufactured objects were worse than natural kinds for controls and patients alike, the relative deficit for natural kinds was evident to a statistically significant extent even after differences between natural and manufactured categories in controls were factored into the category-specific comparisons in patients. There may have been somewhat more extensive recruitment for manufactured than natural objects in the fMRI study, but direct comparisons of fMRI recruitment patterns revealed that each category was associated with its own areas of significantly greater activation. Thus, we found more activation for manufactured objects in a mid-lateral temporal-occipital distribution and in inferior frontal cortex, possibly related to the motion and action features, respectively, that are associated with manufactured objects much more than natural kinds. Conversely, we found greater fMRI recruitment for natural kinds than manufactured objects in the angular gyrus, the area most frequently activated in studies of semantic memory (Binder, et al., 2009). Findings such as this suggest that the absolute extent of recruited regions is unlikely to explain fully the category-specific observations in our study, and that the specific anatomic distribution of recruited regions also contributes to the category-specific effects we found.
While our findings are consistent with a model of semantic memory that includes heteromodal regions like PFC, our observations are less consistent with specific involvement of the anterior temporal lobe as a critical component in a network of brain regions supporting semantic memory (Patterson, et al., 2007). We did not observe activation of the anterior temporal lobe during the fMRI study, for example, although this negative finding must be interpreted cautiously because of susceptibility artifact in anterior and ventral temporal regions (Visser, Embleton, Jefferies, Parker, & Ralph, 2010). Regression analyses in patients implicated the anterior temporal lobe to some extent in their difficulties judging natural and manufactured objects, but these regressions did not overlap with fMRI activations and thus are difficult to interpret. Additional work using these materials is needed to assess patients with semantic dementia who have anterior temporal disease.
4.4 Connectivity within the Large-Scale Neural Network for Semantic Memory
In our fMRI study, we demonstrated not only a role for TOC and PFC, but additional observations were consistent with the possibility that these two regions work together in considering word meaning. Thus, we found two WM projections between activated regions in PFC and TOC that connect these areas. There was a dorsal WM tract involving the superior longitudinal fasciculus and the arcuate fasciculus, and a ventral WM tract involving the inferior frontal-occipital fasciculus. According to one account focusing on the auditory system, the dorsal stream is important for auditory-motor integration, while the ventral stream plays a crucial role in mapping sound to meaning (Hickok & Poeppel, 2004, 2007). A linguistically-motivated hypothesis focuses on the role of the dorsal stream in long-distance syntactic dependencies, and the contribution of the ventral stream to lexical representations (Friederici, 2011). In AD, previous work has indicated that there are deficits in visual processing associated with both the ventral stream and the dorsal stream (Kirby, et al., 2010). However, deficits associated with impairment of the ventral stream appear to occur earlier (Binetti et al., 1998) and more commonly (Mendola, Cronin-Golomb, Corkin, & Growdon, 1995) than those associated with dorsal stream functions. Greater impairment in the ventral stream in AD also appears to be consistent with more severe imagery deficits (van Rhijn et al., 2004) and greater AD pathology (Arnold, Hyman, Flory, Damasio, & van Hoesen, 1991) than found in dorsal stream regions of the visual system.
We examined the integrity of dorsal and ventral WM projections connecting TOC and PFC in patients. The same supra-Sylvian WM projection through the superior longitudinal fasciculus and arcuate fasciculus that was implicated in controls’ judgments appears to be relatively intact in patients. However, the ventral projection between PFC and TOC appears to be compromised. This would be consistent with previous work showing greater impairment associated with the ventral stream than the dorsal stream in AD. If this compromised ventral WM tract plays a relatively crucial role in the semantic content of lexical representations, its degradation in the patients we studied may contribute to their deficits in semantic memory.
Regression analyses relating behavior to WM tractography also revealed evidence consistent with a greater deficit for natural kinds in patients. Specifically, FA in the superior longitudinal fasciculus and the arcuate fasciculus projecting between PFC and TOC correlated with patients’ judgments of natural kinds. Thus, paralleling the analysis of GM atrophy, it appears that the same dorsal WM projection found in healthy controls was implicated in patients’ judgments of natural kinds. By comparison, the dorsal stream did not correlate with patients’ judgments of manufactured objects. Paralleling our analysis of GM regressions, it is possible to speculate that other projections may be supporting their semantic judgments of manufactured objects, and therefore their performance may be better with manufactured objects than with natural kinds. Regardless of the specific explanation, our findings suggest that patients’ judgments of natural kinds may be compromised in part by their dependence on a particular WM projection in the PFC-TOC network, despite the presence of AD-related pathology. Additional work is needed to identify the specific network supporting AD patients’ judgments of manufactured objects.
5. CONCLUSION
Our observations suggest that a large-scale neural network involving multiple GM areas as well as WM projections between these areas contributes to semantic memory. Some of our findings are consistent with sensory-motor approaches to semantic memory (Barsalou, 2008; Martin, 2007). fMRI studies of healthy adults thus showed activation of TOC during judgments of word pairs for feature knowledge. However, several of our findings are less consistent with strong versions of sensory-motor approaches to semantic memory. First, we found a category-specific deficit in patients. Even though regression analyses showed that patients appear to rely to some extent on the same modality-specific region in TOC as is activated in healthy controls, judgments of visual-perceptual features of natural kinds were relatively more dependent on disease in this area than their judgments of manufactured objects. This may be due in part to the greater vulnerability of features of natural kinds represented in TOC than features of manufactured objects. Second, disease in PFC appeared to be related to object judgment difficulty, even though PFC is not thought to be involved in the representation of modality-specific sensory-motor knowledge. PFC instead may contribute to the top-down selection and organization of features for object concepts. Third, differences emerged in patients’ WM projections between PFC and TOC, and these differences appeared to be modulated by the semantic category as well. Thus, examination of both GM and WM components of this large-scale neural network for semantic memory showed that the same network is implicated for natural kinds in patients as is found in healthy adults, even though this network is diseased in patients. By comparison, patients appeared to be less dependent on the same GM-WM network as healthy controls in their judgments of manufactured objects.
HIGHLIGHTS.
Alzheimer’s patients were impaired for natural compared to manufactured objects
fMRI during the same judgments in healthy adults showed temporal and prefrontal activation
Tractography showed projections between temporal and prefrontal activations
Patients’ temporal, prefrontal and white matter disease selectively disrupted natural judgments
Acknowledgments
Funding: This work was supported in part by National Institutes of Health (AG15116, AG17586, NS44266, AG32953, NS53488, and AG38490) and The Wyncote Foundation. Dr. Grossman receives support for participating in clinical drug trials unrelated to this study from Forest Pharmaceuticals, Bristol Myers-Squibb and Allon Pharmaceuticals
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Adlam ALR, Bozeat S, Arnold R, Watson PC, Hodges JR. Semantic knowledge in mild cognitive impairment and mild Alzheimer’s disease. Cortex. 2006;42(5):675–684. doi: 10.1016/s0010-9452(08)70404-0. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Partial transformations of diffusion tensor magnetic resonance images. IEEE Transactions for Medical Imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, van Hoesen GW. The topographic and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cerebral Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Unfied segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Avants B, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape and intensity averaging. Neuroimage. 2004;23:S139–S150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Avants B, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J, et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 2010;49(3):2457–2466. doi: 10.1016/j.neuroimage.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annual Review of Psychology. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Bedny M, Caramazza A, Grossman E, Pascual-Leone A, Saxe R. Concepts Are More than Percepts: The Case of Action Verbs. J Neurosci. 2008;28(44):11347–11353. doi: 10.1523/JNEUROSCI.3039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binetti G, Cappa SF, Magni E, Padovani A, Bianchetti A, Trabucchi M. Visual and spatial perception in the early phase of Alzheimer’s disease. Neuropsychology. 1998;12:29–33. doi: 10.1037//0894-4105.12.1.29. [DOI] [PubMed] [Google Scholar]
- Bonner MF, Grossman M. Gray matter density of auditory association cortex relates to knowledge of sound concepts in orimary progressive aphasia. The Journal of Neuroscienc. 2012;32(23):7986–7991. doi: 10.1523/JNEUROSCI.6241-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, Iqbal K, Winblad B, Nishimura T, Takeda M, et al. Alzheimer’s Disease: Biology, Diagnosis, k and Therapeutics. New York: Wiley; 1997. Patterns of cortical lesions in Alzheimer’s disease; pp. 227–237. [Google Scholar]
- Caramazza A, Hillis AE, Rapp BC, Romani C. The multiple semantics hypothesis:multiple confusions? Cognitive Neuropsychology. 1990;7:161–189. [Google Scholar]
- Caramazza A, Shelton JR. Domain-specific knowledge systems in the brain: The animate-inanimate distinction. Journal of Cognitive Neuroscience. 1998;10:1–34. doi: 10.1162/089892998563752. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D, Schwartz MF. Modular deficits in Alzheimer-type dementia. Vol. 1. Cambridge: MIT Press; 1990. Semantic memory loss in Alzheimer-type dementia; pp. 207–244. [Google Scholar]
- Chertkow H, Bub DN. Semantic memory loss in dementia of the Alzheimer’s type: What do the various measures measure? Brain. 1990;113:397–417. doi: 10.1093/brain/113.2.397. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Whatmough C, Saumier D, Duong A. Cognitive neuroscience studies of semantic memory in Alzheimer’s disease. In: Sossin WS, Lacaille J-C, Castellucci VF, Bellevile S, editors. Progress in Brain Research. Vol. 169. Amsterdam: Elsevier; 2008. pp. 393–407. [DOI] [PubMed] [Google Scholar]
- Cook PA, Bai Y, Nadjati-Gilani S, Seunarine KK, Hall MG, Parker GJM, et al. Camino: Open-source diffusion-MRI reconstruction and processing. Paper presented at the International Society for Magnetic Resonance in Medicine.2006. [Google Scholar]
- Devlin JT, Gonnerman LM, Andersen ES, Seidenberg M. Category-specific semantic deficits in focal and widespread brain damage: A computational account. Journal of Cognitive Neuroscience. 1998;10:77–94. doi: 10.1162/089892998563798. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The Cortical Signature of Alzheimer’s Disease: Regionally Specific Cortical Thinning Relates to Symptom Severity in Very Mild to Mild AD Dementia and is Detectable in Asymptomatic Amyloid-Positive Individuals. Cereb Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, McClelland JL. A computational model of semantic memory impairment: modality specificity and emergent category specificity. J Exp Psychol Gen. 1991;120:339–357. [PubMed] [Google Scholar]
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal dementia: Clinicopathological correlations. Annals of Neurology. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis WN, Kucera H. The frequency analysis of English usage. Boston: Houghton-Mifflin Co; 1982. [Google Scholar]
- Friederici AD. The brain basis of language processing: From structure to function. Physiological Reviews. 2011;91(4):1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Fung TD, Chertkow H, Murtha S, Whatmough C, Peloquin L, Whitehead V, et al. The spectrum of category effects in object and action knowledge in dementia of the Alzheimer’s type. Neuropsychology. 2001;15:371–379. [PubMed] [Google Scholar]
- Garrard P, Lambon Ralph MA, Hodges JR, Patterson K. Prototypicality, distinctiveness and intercorrelation: analyses of the semantic attributes of living and nonliving items. Cogn Neuropsychol. 2001;18:125–174. doi: 10.1080/02643290125857. [DOI] [PubMed] [Google Scholar]
- Garrard P, Lambon Ralph MA, Watson PC, Powis J, Patterson K, Hodges JR. Longitudinal profiles of semantic impairment for living and nonliving concepts in dementia of the Alzheimer’s type. Journal of Cognitive Neuroscience. 2001;13:892–909. doi: 10.1162/089892901753165818. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnection syndromes in animals and man. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Gonnerman LM, Andersen ES, Devlin JT, Kempler D, Seidenberg MS. Double dissociation of semantic categories in Alzheimer’s disease. Brain and Language. 1997;57:254–279. doi: 10.1006/brln.1997.1752. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Milleville SC, Bellgowan PSF, Martin A. Broad and Narrow Conceptual Tuning in the Human Frontal Lobes. Cerebral Cortex. 2011;21:477–491. doi: 10.1093/cercor/bhq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. Neural basis for semantic memory difficulty in Alzheimer’s disease: an fMRI study. Brain. 2003;126:292–311. doi: 10.1093/brain/awg027. [DOI] [PubMed] [Google Scholar]
- Grossman M, Anderson C, Khan A, Avants B, Elman L, McCluskey L. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology. 2008;71(18):1396–1401. doi: 10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Koenig P. Encyclopedia of Cognitive Science. San Diego: Academic Press; 2001. Semantic memory. [Google Scholar]
- Grossman M, White-Devine T, Robinson KM, Biassou N, D’Esposito M. Semantic memory in Alzheimer’s disease: Ontologic category, representativeness, and material. Neuropsychology. 1998;12:34–42. doi: 10.1037//0894-4105.12.1.34. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermuller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer’s disease: Failure of access or degraded knowledge. Neuropsychologia. 1992;30:301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and Palm Trees: A Test of Semantic Access from Pictures and Words. Bury St. Edmonds, UK: Thames Valley Test Company; 1992. [Google Scholar]
- Joubert S, Brambati SM, Ansado J, Barbeau EJ, Felician O, Didic M, et al. The cognitive and neural expression of semantic memory impairment in mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia. 2010;48(4):978–988. doi: 10.1016/j.neuropsychologia.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Kirby E, Bandelow S, Hogervorst E. Visual impairment in Alzheimer’s disease: A critical review. Journal of Alzheimer’s Disease. 2010;21(1):15–34. doi: 10.3233/JAD-2010-080785. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig P, Grossman M. Neural Basis of Semantic Memory. Cambridge, UK: Cambridge University Press; 2007. Process and content in semantic memory; pp. 247–264. [Google Scholar]
- Koenig P, Smith EE, Glosser G, DeVita C, Moore P, McMillan C, et al. The neural basis for novel semantic categorization. Neuroimage. 2005;24(2):369–383. doi: 10.1016/j.neuroimage.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Grossman M. Categorization of novel tools by patients with Alzheimer’s disease: Category-specific content and process. Neuropsychologia. 2010;48(7):1877–1885. doi: 10.1016/j.neuropsychologia.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Massimo L, Moore P, Coslett HB, Chatterjee A, Aguirre GK, et al. Screening for frontotemporal dementias from Alzheimer’s disease with The Philadelphia Brief Assessment of Cognition: A preliminary analysis. Dementia and Geriatric Cognitive Disorders. 2007;24:441–447. doi: 10.1159/000110577. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Matson RE, Glosser G, Kaplan E, Malamut M, Sands LP, et al. A nine word dementia version of the California Verbal Learning Test. The Clinical Neuropsychologist. 1996;10:237–244. [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Fedio P. Word production and word comprehension in Alzheimer’s disease: The breakdown of semantic knowledge. Brain and Language. 1983;19:124–141. doi: 10.1016/0093-934x(83)90059-7. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Mauri A, Daum I, Sartori G, Riesch G, Birbaumer N. Category-specific semantic impairment in Alzheimer’s disease and temporal lobe dysfunction: A comparative study. Journal of Clinical and Experimental Neuropsychology. 1994;16:689–701. doi: 10.1080/01688639408402682. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendola JD, Cronin-Golomb A, Corkin S, Growdon JH. Prevalence of visual deficits in Alzheimer’s disease. Optometry and Vision Science. 1995;72:155–167. doi: 10.1097/00006324-199503000-00003. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Principles of Behavioral and Cognitive Neurology. Philadelphia: F.A. Davis Company; 2000. Behavioral neuroanatomy: Large-scale networks, association cortex, frontal syndromes, the limbic system, and hemispheric specializations; pp. 1–120. [Google Scholar]
- Mesulam MM, van Hoesen GW, Pandya DN, Geschwind N. Limbic and sensory connections of the inferior parietal lobule (Area PG) in the rhesus monkey: a study with a new method of horseradish peroxidase histochemistry. Brain Research. 1977;136:393–414. doi: 10.1016/0006-8993(77)90066-x. [DOI] [PubMed] [Google Scholar]
- Moss HE, De Mornay Davies P, Jeppeson C, McLellan S, Tyler LK. The relationship between knowledge of nouns and verbs in a category-specific deficit for living things. Brain and Language. 1998;1998:65–92. [Google Scholar]
- Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Peters A, Jones EG, editors. Cerebral Cortex. Volume 4: Association and Auditory Cortex. New York: Plenum Press; 1985. pp. 3–61. [Google Scholar]
- Pandya DN, Yeterian EH. Neurobiological and Neuropsychological Approaches to the Study of Cognitive and Executive Function Comparison of Prefrontal Architecture and Connections. Philosophical Transactions of the Royal Society of London B. 1996;351:1423–1432. doi: 10.1098/rstb.1996.0127. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Grossman M. Interaction between process and content in semantic memory: An fMRI study of noun feature knowledge. Neuropsychologia. 2009;47(4):995–1003. doi: 10.1016/j.neuropsychologia.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Hauk O. Category-specific conceptual processing of color and form in left fronto-temporal cortex. Cerebral Cortex. 2006;2006:1193–1201. doi: 10.1093/cercor/bhj060. [DOI] [PubMed] [Google Scholar]
- Putnam H. Is semantics possible? Metaphilosophy. 1970;1:187–201. [Google Scholar]
- Rogers TT. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Saffran E, Schwartz MF, Umilta C, Moscovitch M. Attention and performance XV: Conscious and nonconscious information processing. Cambridge: MIT Press; 1994. Of cabbages and things: Semantic memory from a neuropsychological perspective -- A tutorial review; pp. 507–536. [Google Scholar]
- Salvador R, Pena A, Menon DK, Carpenter TA, Pickard JD, Bullmore ET. Formal characterization and extension of the linearized diffusion tensor model. Human Brain Mapping. 2005;24:144–155. doi: 10.1002/hbm.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Parietal, temporal and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: A retrograde tracer study. Journal of Comparative Neurology. 1984;343:445–463. doi: 10.1002/cne.903430308. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Daniele A, Giustolisi L, Gainotti G. Dissociation between living and nonliving things in dementia of the Alzheimer type. Neurology. 1991;41:545–546. doi: 10.1212/wnl.41.4.545. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring “how” from “where”. Neuropsychologia. 2003;41:280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Tulving E, Tulving E, Donaldson W. Organization of memory. Vol. 1. New York: Academic Press; 1972. Episodic and semantic memory. [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011;5:1–20. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants B, Cook PA, Egan A, Zheng Y, Yushkevich PA, et al. N4ITK: Improved N3 bias correction. IEEE Transactions for Medical Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rhijn SJ, Glosser G, de Vries JJ, Clark CM, Newberg AB, Alavi A. Visual processing impairments and decrements in regional brain activity in Alzheimers disease. J Clin Exp Neuropsychol. 2004;26:11–23. doi: 10.1076/jcen.26.1.11.23931. [DOI] [PubMed] [Google Scholar]
- Visser M, Embleton KV, Jefferies E, Parker GJ, Ralph MAL. The inferior, anterior temporal lobes and semantic memory clarified: Novel evidence from distortion-corrected fMRI. Neuropsychologia. 2010;48(6):1689–1696. doi: 10.1016/j.neuropsychologia.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–336. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Whatmough C, Chertkow H, Murtha S, Hanratty K. Dissociable brain regions process object meaning and object structure during picture naming. Neuropsychologia. 2002;40:174–186. doi: 10.1016/s0028-3932(01)00083-5. [DOI] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Antuono P, Guidotti L, Durgerian S, et al. Semantic memory activation in amnestic mild cognitive impairment. Brain. 2009;132(8):2068–2078. doi: 10.1093/brain/awp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R, van Zijl PCM, Crain B, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magnetic Resonance in Medicine. 1999;42:1123–1127. doi: 10.1002/(sici)1522-2594(199912)42:6<1123::aid-mrm17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Zahn R, Garrard P, Talazko J, Gondan M, Bubrowski P, Juengling FD, et al. Patterns of regional brain metabolism associated with knowledge of semantic features and categories in Alzheimer’s disease. Journal of Cogitive Neuroscience. 2006;18:2138–2151. doi: 10.1162/jocn.2006.18.12.2138. [DOI] [PubMed] [Google Scholar]