Abstract
Children with fragile X syndrome (FXS) face high risk for anxiety disorders, yet no studies have explored FXS as a high-risk sample for investigating early manifestations of anxiety outcomes. Negative affect is one of the most salient predictors of problem behaviors and has been associated with both anxiety and autistic outcomes in clinical and non-clinical pediatric samples. In light of the high comorbidity between autism and anxiety within FXS, the present study investigates the relationship between longitudinal trajectories of negative affect (between 8 and 71 months) and severity of anxiety and autistic outcomes in young males with FXS (n= 25). Multilevel models indicated associations between elevated anxiety and higher fear and sadness, lower soothability, and steeper longitudinal increases in approach. Autistic outcomes were unrelated to negative affect. These findings suggest early negative affect differentially predicts anxiety, not autistic symptoms, within FXS. Future research is warranted to determine the specificity of the relationship between negative affect and anxiety, as well as to explore potential moderators. Characterizing the relationship between early negative affect and anxiety within FXS may inform etiology and treatment considerations specific to children with FXS, as well as lend insight into precursors of anxiety disorders in other clinical groups and community samples.
Keywords: Fragile X syndrome, Anxiety, Autism, Temperament, Longitudinal
Introduction
Anxiety disorders constitute the most commonly diagnosed psychological condition in the United States, affecting approximately 25 % of individuals during their lifetime (Kessler et al. 1994) and 2–13 % of children (Costello et al. 2003; Shaffer et al. 1996). Anxiety disorders are associated with both genetic and environmental vulnerabilities and have been attributed to disruptions in the emotion-regulating and executive regions of the brain (see Martin et al. 2010, for review). Several clinical subgroups face higher risk for anxiety outcomes, including children with neurodevelopmental disorders such as fragile X syndrome (FXS). Fragile X syndrome is the most common heritable form of intellectual disability, affecting approximately 1:4000 individuals (Crawford et al. 2001, 2002). The FXS phenotype is caused by a CGG triplet expansion on the X chromosome (>200 repeats), which reduces FMR1 protein (FMRP) production and negatively impacts brain function (Bassell and Warren 2008). Children with FXS demonstrate a constellation of symptoms including heightened emotional and physiological reactivity, sensory issues, aggression, social avoidance and withdrawal, stereotypical behavior, impulsivity, and hyperactivity (e.g. Hall et al. 2008; Hatton et al. 2009; Roberts et al. 2009b).
In light of this broad spectrum of FXS symptoms, it is unsurprising that many children with FXS are affected by multiple co-occurring conditions that impair learning opportunities and challenge daily functioning (Bailey et al. 2008a). Anxiety disorders and autism represent two of the most common and devastating comorbid conditions in individuals with FXS, with 70–83 % experiencing anxiety disorders (Bailey et al. 2008a; Cordeiro et al. 2011) and 25–60 % meeting diagnostic criteria for autism (Bailey et al. 2008c; Kaufmann et al. 2004). Although anxiety and autism are highly prevalent and problematic in FXS, their emergence and co-occurrence in children with FXS are unclear. Prospectively studying the precursors and manifestation of anxiety and autism in FXS offers valuable insight into precursors of these disorders in the general population, as well as informs etiology and treatment considerations specific to children with FXS. The present study longitudinally investigates the relationship between one salient indicator of psychopathology, early negative affect, and anxiety and autistic outcomes in young males with FXS.
Anxiety in FXS
Measuring anxiety in FXS is complicated by the cognitive and verbal challenges common to the phenotype (Boyle and Kaufmann 2010). As such, researchers often rely on parent report to assess anxiety symptoms. Parents have associated anxiety in children with FXS with increased arguing, distractibility, and sleeping problems (Sullivan et al. 2007; n= 43, ages 6–14 years). Data from a national parent survey indicate 70 % of males and 56 % of females with FXS have received anxiety diagnoses or treatments (Bailey et al. 2008a). The most common cluster of problem behaviors in males included anxiety in conjunction with developmental delay, attention problems, and hyperactivity. Notably, within the subset of males with FXS without developmental delay, 68 % reported high anxiety, indicating anxiety is not restricted to individuals with FXS and intellectual disability.
In the first study to examine prevalence of anxiety disorders in FXS using Diagnostic and Statistical Manual criteria (DSM-IV-TR; American Psychiatric Association 2000), Cordeiro et al. (2011) found 83 % of participants (n=97, ages 5.5–33.3 years) met criteria for any anxiety disorder, and 58 % exhibited multiple anxiety disorders. Symptoms were measured using the Anxiety Disorder Interview Schedule for the DSM-IV: Parent Report Version (ADIS-IV; Silverman and Albano 2004), a semi-structured parent interview. The examiners considered age and the presence of intellectual disabilities when determining diagnoses. Consistent with DSM-IV guidelines, adults functioning at the developmental level of a child were permitted to meet anxiety criteria based on symptoms typically exhibited by children. For example, these adults were not required to be described by their parents as recognizing fears as unreasonable or excessive, and they could express specific phobias by crying, tantruming, clinging or freezing. The authors validated the ADIS-IV by demonstrating correspondence with the anxiety subscales of the Anxiety Depression and Mood Scale (Esbensen et al. 2003), a psychiatric screening tool for individuals with intellectual disability. The presence of anxiety disorders in FXS was significantly higher than in comparison groups with nonspecific intellectual disabilities (11 %), Williams Syndrome (62 %) and the general population (10 %). The most common anxiety diagnoses within the FXS group included specific phobia (60 %), social phobia (58 %), selective mutism (25 %), generalized anxiety disorder (24 %) and obsessive compulsive disorder (24 %). Social phobia and post-traumatic stress disorder were more common in individuals over age 18, consistent with findings in the general population (Kessler et al. 2007). Gender and autism status were not associated with consistent patterns of anxiety diagnoses, although individuals with autism were somewhat more likely to exhibit selective mutism, specific phobias, and social phobia.
The presentation of anxiety in FXS is consistent with behavioral and neurobiological models of anxiety in non-FXS samples. Mood and anxiety disorders are most commonly attributed to dysregulation of limbic system in regions such as the hippocampus and amygdala, which function to modulate stress response and process emotional stimuli, respectively (see Martin et al. 2010, for review). Repetitive and poorly-modulated activation of stress response systems results in compromised capacity to adaptively respond to acute challenges (McEwen 1998), which may be particularly debilitating to children during key periods of early development (Duke 2008). These neurobiological processes relate to a variety of biobehavioral symptoms. For example, Barlow (1988, 2000) characterizes anxiety as an integrated cognitive-affective construct that involves complex, cyclical interactions among environmental cues, negative affect, attention, arousal modulation, hypervigilence, and dysfunctional behavior. Consistent with this theoretical framework, avoidant behaviors in FXS have been linked to physiological dysregulation and hyperarousal (Hall et al. 2009; Hessl et al. 2002; Roberts et al. 2001), and several studies have reported structural and functional limbic system differences in FXS (see Hessl et al. 2004, for review). Thus, although cognitive components of anxiety may present differently in FXS compared to non-FXS samples, examining the FXS phenotype in light of these neurobiological models may contribute to our understanding of the multifaceted underpinnings of anxiety.
Autism in FXS
In addition to the high proportion of anxiety disorders in FXS, up to 90 % of individuals with FXS display autistic symptoms, and 25–60 % meet full autism diagnostic criteria (Bailey et al. 2008c; Kaufmann et al. 2004). Autism spectrum disorder is a neurodevelopmental disability that affects approximately 1:54 males in the United States (Center for Disease Control 2012). Three primary domains of autism symptoms include social problems, repetitive behaviors or restricted interests, and impaired language; with the manifestation of these symptoms varying across individuals (American Psychiatric Association 2000). Similar to elevated rates of anxiety in FXS, approximately 25–49 % of children and adolescents with idiopathic autism (non-FXS) meet anxiety disorder criteria (see White et al. 2009, for review). Within FXS, comorbid autism is associated with increased behavior problems (Hatton et al. 2002), lower adaptive behavior (Hatton et al. 2003; Kau et al. 2004; Rogers et al., 2001), receptive language delays (Rogers et al. 2001), withdrawal (Kau et al., Kaufmann et al. 2004; Roberts et al. 2007), social indifference (Budimirovic et al. 2006), and more severely impaired cognitive profiles (Bailey et al. 2001).
Despite the high prevalence of autistic symptoms in FXS, researchers have debated the utility and appropriateness of differentially diagnosing children with FXS+autism given the broad spectrum of socio-communicative and repetitive behavior deficits associated with FXS without autism. For example, Hall et al. (2010) suggest diagnostic instruments often produce inconsistent results in FXS, perhaps due to the high prevalence and wide spectrum of autistic behavioral symptoms present in this population. The neurobiological underpinnings of autistic symptoms may also differ in FXS and non-FXS samples, as children with FXS+autism and idiopathic autism have shown differing patterns of brain volumetrics, particularly in areas related to social cognition (Hazlett et al. 2009: Hoeft et al. 2011). Despite these potential differences, other researchers have advocated for differential diagnosis of FXS+autism as an accurate and efficacious mechanism for describing symptoms and organizing treatment. Budimirovic and Kaufmann (2011) propose autism within FXS presents distinct behavioral, neural, and potential molecular profiles, justifying a dual diagnosis. Findings from this group suggest the co-occurrence of autism in young children with FXS can be distinguished by impaired peer relationships and adaptive socialization (Hernandez et al. 2009), whereas other researchers with older samples have emphasized atypicalities in communication and repetitive behavior domains (McDuffie et al. 2010). Clarifying the early presentation of autistic symptoms in FXS, particularly in relation to cognitive abilities, may facilitate stronger conceptualization of this growing and important area of research.
Distinguishing Anxiety and Autism in FXS
Symptoms of anxiety and autism overlap and interrelate within FXS, although the disorders have been distinguished through behavioral and physiological profiles. Kaufmann, Budimirovic and colleagues have published a series of papers characterizing anxiety and autism as distinct social interaction disorders in FXS (e.g. Budimirovic et al. 2006; Budimirovic and Kaufmann 2011). The authors propose that social anxiety emerges from a combination of lower non-verbal abilities and moderate social withdrawal, whereas autism (either alone or in conjunction with social anxiety) is characterized by a more complex constellation of severe social withdrawal and lower adaptive socialization or verbal skills. The authors attribute the emergence of autism in FXS to two neurobiological vulnerabilities: (1) abnormal cortical development resulting in delayed communicative and attention processes, and (2) limbic dysfunction resulting in avoidant behavior (Budimirovic et al. 2006). Although anxiety may contribute to the intensification of self-regulatory symptoms such as hand-flapping or social avoidance, the rates of anxiety disorders in FXS appear relatively similar across individuals with and without autism. In one of the few studies to specifically compare anxiety symptoms in FXS versus FXS+autism, Cordeiro et al. (2011) found that individuals with FXS+ autism did not demonstrate consistent patterns of anxiety diagnoses, although they were somewhat more likely to exhibit selective mutism, specific phobias, and social phobia than participants without autism. This inconsistent relationship between anxiety disorders and autism in FXS underscores the importance of considering the unique impact and manifestation of each disorder within FXS.
Behavioral and physiological studies have further clarified withdrawal profiles of children with FXS versus FXS+autism. Roberts et al. (2007) found that children with FXS and low levels of autistic symptoms showed improved social approach behaviors, particularly eye contact, as time spent with an unfamiliar person increased, whereas children with FXS and elevated autistic symptoms maintained social avoidance during the interaction. Roberts et al. (2009b) later replicated these results and found that elevated baseline levels and blunted change in cortisol across time predicted increased autistic symptoms in children with FXS+autism. Several additional studies have documented associations between atypical physiological arousal and autistic-like symptoms, such as eye contact, within FXS (Hall et al. 2008; Hessl et al. 2006). These findings suggest that although autism may present similarly to anxiety in initial approach behaviors, FXS+autism is associated with a more severe and consistent patterns of social withdrawal, which may be related to poor modulation of physiological arousal.
Negative Affect
The complicated co-occurrence of anxiety and autism in FXS warrants investigation into the early manifestation and predictors of differential psychopathology outcomes in this population. Negative affect is one of the most commonly studied predictors of problem behaviors in non-clinical (e.g., Calkins et al. 2007; Lemery et al. 2002) and clinical (e.g. Hutman et al. 2010) populations. Negative affect is composed of several specific dimensions of temperament; including fear, approach, soothability, sadness, anger, discomfort, and motor activity (Putnam et al. 2008). Within typical development, negative affect can represent an adaptive or appropriate response to environmental challenges. However, both under- or over-regulation of negative affect are associated with poor outcomes such as anxiety, depression, and externalizing disorders (Chaplin et al. 2005). Recent research suggests genetic underpinnings for negative affect in infancy, endorsing the endogenous nature of negative affect and the potential predictive power of this construct (Holmboe et al. 2010; Sheese et al. 2009).
Early negative affect has been linked to anxiety outcomes through several prospective, longitudinal studies. Gartstein et al. (2010) found that both initial levels of fear at 4 months, as well as increases in infant fearfulness over time, predicted anxiety symptoms in typically developing toddlers. Similarly, Putnam and Stifter (2005) identified a relationship between infant inhibition and later internalizing symptoms, as well as between infant extraversion and later externalizing symptoms. Using a large, nationally representative sample (n=768,600), Grant et al. (2009) found that fussiness at ages 2–3 years prospectively predicted anxiety at ages 6–7, and poor adaptability predicted anxiety at ages 8–9. However, neither fussiness nor adaptability predicted anxiety outcomes at ages 10–11, which the authors attribute to environmental factors that may moderate anxiety outcomes at older ages.
Negative Affect in FXS
Several studies have examined parent-reported negative affect in children with FXS compared to typical and clinical samples. Levels of temperament have been shown to remain relatively stable in FXS across early childhood (Hatton, et al.). Compared to typical controls, parents have indicated elevated activity and withdrawal; as well as lower intensity, approachability, adaptability, sadness, anger, and persistence in young boys with FXS (Bailey et al. 2000; Hatton et al. 1999; Roberts et al. 2006; Shanahan et al. 2008). Evidence suggests that temperament does not differ across participants FXS+autism and FXS-only (Bailey et al. 2000); however, dampened facial sadness has been associated with autistic symptoms in FXS (Shanahan et al.). Compared to children with developmental delay, young children with FXS (n=41, ages 3–6 years) exhibit higher avoidance behavior, lower withdrawal behavior, and higher positive mood (Kau et al. 2000). No studies have longitudinally examined whether these early patterns of temperament, negative affect in particular, relate to later anxiety or autistic outcomes. Furthermore, although levels of negative affect have been compared across disorders, it is unclear whether levels of negative affect within the population of children with FXS predict problem behaviors.
Characterizing the relationship between negative affect and psychopathology in FXS is particularly warranted in light of the growing literature examining similar patterns in high risk infant siblings of children with autism. Similar to infants with FXS, “infant siblings” face higher rates of autism diagnoses (10–61 %; Brian et al. 2008; Landa and Garrett-Mayer 2006; Ozonoff et al. 2011; Yoder et al. 2009) than the general population. Infant and toddler siblings later diagnosed with autism have shown increased negative affect, irritability, and object fixation; as well as decreased positive affect, soothability, social engagement, and cognitive abilities (Bryson et al. 2007; Garon et al. 2009; Zwaigenbaum et al. 2005). Despite individual differences, the broader infant sibling literature generally endorses negative affectivity as one of many early indicators associated with autism, warranting examination of whether similar trends are present in young children with FXS.
Predicting Anxiety and Autism in FXS
Understanding the emergence of anxiety and autism in children with FXS bears important implications for promoting early, targeted, and effective interventions for affected children and their families. However, as demonstrated by the high degree of overlap among symptoms associated with FXS, autism, and anxiety, understanding the specificity of early indicators to outcomes is an essential first step to informing the emergence of specific symptoms in FXS. No published studies have prospectively examined the emergence of anxiety in FXS, and few have prospectively surveyed the emergence of autistic symptoms in young children with FXS (see Roberts et al. 2011, for exception). This work is particularly important given the possibility that neurobiological underpinnings of autistic symptoms and anxiety may differ in FXS and non-FXS samples. As a first step to addressing this important question, the present study aims to prospectively examine the relationship between early negative affect and both (1) anxiety and (2) autistic outcomes in young males with FXS. Based on the documented relationship between negative affect and both anxiety (e.g. Gartstein et al. 2010; Putnam and Stifter 2005) and autism (e.g. Zwaigenbaum et al. 2005) in non-FXS samples, we hypothesized that elevated mean levels of negative affect, as well as accelerated negative affect over time, would predict both autism and anxiety outcomes within FXS.
Methods
Participants
Data were drawn from two consecutive longitudinal studies focused on the early development of FXS, which used similar methods and overlapping samples. The initial study focused on infants and included 13 males with FXS assessed at 9, 12, 18, and 24 months of age. The subsequent study focused on preschool development and included 45 males with FXS assessed at 18 month intervals, beginning between 12 and 40 months of age. Our current study includes participants from either study who had at least 2 assessments and complete data, resulting in 25 boys assessed on negative affect measures between 2 and 7 times (average 4.2). The average age of first assessment was 15.2 months (range 8–35; SD=6.88). Descriptive statistics are listed in Table 1.
Table 1.
Descriptive statistics
| M | SD | Min | Max | |
|---|---|---|---|---|
| Age of first assessment | 15.16 | 6.88 | 8 | 35 |
| Age across all assessments | 31.91 | 18.20 | 8 | 71 |
| Number of assessments | 4.16 | 1.60 | 2 | 7 |
| Time in study (in months) | 40.16 | 14.38 | 6 | 61 |
| CARS total score | 27.48 | 4.93 | 18 | 40 |
| CBCL DSM-Anxiety raw score | 3.80 | 2.66 | 0 | 10 |
| CBCL DSM-Anxiety t-score | 56.08 | 7.47 | 50 | 75 |
| Age at CARS and CBCL | 58.43 | 10.71 | 35 | 72 |
Age reported in months. CARS childhood autism rating scale. CBCL childhood behavior checklist. “Assessments” refer to sessions in which parent-reported temperament and mental age were measured. CARS and CBCL data were collected either concurrently or shortly following the final assessment of parent-reported negative affect
Measures
Negative Affect
We measured negative affect using the Rothbart Temperament scales, which include the Infant Behavior Questionnaire (IBQ, 3 to 12 months; Rothbart 1981), Toddler Behavior Assessment Questionnaire- Revised (TBAQ-R, 16 to 35 months; Goldsmith 1996; Rothbart et al. 2003), and Children’s Behavior Questionnaire (CBQ, 3 to 7 years; Rothbart et al. 2001). Mothers completed the scale corresponding to their child’s current age at each assessment (range 8–71 months). Each questionnaire includes multiple items asking parents to rate recent frequency of temperament characteristics using a seven-point scale.
The present study focuses on four subscales that consistently load on the Negative Affect domain of temperament and demonstrate continuity across each scale: Fear, Anger/ Frustration, Sadness, and Soothability (Putnam et al. 2008). We also examined participants’ scores on the Approach scale, which loads on the Surgency temperament domain, due to the relationship between atypical social approach and autistic symptoms in FXS (Roberts et al. 2009a, b). The Rothbart Scales demonstrate appropriate convergent validity, discriminant validity, and continuity of temperament dimensions over time (Putnam et al. 2008). Notably, Putnam and colleagues explicitly state that traits across scales “can be meaningfully combined… utilizing growth-curve analyses to examine influences maintaining and lawfully altering longitudinal stability” (p. 402).
Autism Symptom Severity
Autistic outcomes were measured using the Childhood Autism Rating Scale (CARS; Schopler et al. 1998), an examiner rating scale of autistic behavior. The CARS demonstrates internal consistency ratings of 0.94 and highly correlates with clinical ratings of autism (range= 0.80 to 0.88; Schopler et al. 1998) and standardized instruments including the Autism Diagnostic Interview-Revised (Rutter et al. 2003; Pilowsky et al. 1998). The CARS has been commonly used with children with FXS (e.g. Bailey et al. 2001; Hatton et al. 2002; Hatton et al. 2006; Shanahan et al. 2008). The measure includes 15 items (rated 1 to 4) that measure a range of autistic behaviors. Total scores above 30 indicate mild to severe autistic behavior. Consistent with administration guidelines, the CARS was completed after each research assessment using clinical consensus between at least two examiners. All examiners were trained in CARS administration by reading the manual, reviewing videotaped vignettes of behavior, and practicing CARS scoring. Training was overseen by a master rater who had a Ph.D. and extensive experience with early development in FXS. Each research assessment lasted approximately 5 hours and included substantial opportunities for both structured and unstructured interactions between examiners and the participant. Because examiners maintained unique roles working with the parent or child and were therefore likely to observe different behaviors throughout the assessment, both examiners provided input on each item prior to consensus scoring.
Although multiple CARS scores were available for the majority of participants, we selected each child’s earliest score obtained after 35 months of age (range 35–72 months) to use as an outcome in the present study, as current DSM-IV criteria require symptoms emerge prior to age three to consider an autism diagnosis (American Psychiatric Association, 2000). This selection was also based on Hatton et al. (2006) finding that children with FXS, including a portion of participants in our current sample, show subtle but stable increases in CARS scores over time (approximately .20 points per year). To account for the variability in outcome ages, chronological age at outcome was included as a covariate in all analyses. Outcome data were collected either concurrently or shortly following their final assessment of parent-reported temperament. We treated CARS scores as a continuous variable for several reasons. First, research supports measuring autism as a continuous array of symptoms (see Constantino 2011, for review). Second, we were interested in examining the full spectrum of autistic behavior in FXS rather than categorical distinctions. Finally, quantifying autism as continuous permits powerful statistical modeling and reveals more specific information about range of functioning (Constantino 2011; Yoder et al. 2009).
Anxiety
The Child Behavior Checklist (CBCL/1.5–5, 6–18; Achenbach 1991; Achenbach and Rescorla 2001) is a widely-used parent report scale of internalizing and externalizing symptoms. Two separate forms are used for children ages 1.5–5 and 6–18 years. The CBCL has been normed on a large sample and demonstrates high test-retest reliability (r=0.95). The measure is organized into syndrome-specific scales, broad composite scales, and DSM-based symptoms scales. Raw scores are converted into T-scores, which may fall in the average range (≤65), borderline clinical range (66–69), and clinically significant range (70+) for the syndrome-specific and DSM scales. The CBCL has been widely used with children with disabilities including autism (Bolte et al. 1999), FXS (Bargagna et al. 2002; Hatton et al. 2002; Kau et al. 2004), Prader Willi Syndrome (Dykens and Kasari 1997; van Lieshout et al. 1998), and Down syndrome (Bargagna et al. 2002; Dykens and Kasari 1997).
The focus of the present study is on the DSM-Anxiety subscale of the CBCL, which consists of six of the eight CBCL anxiety/depression syndrome scale items that are consistent with DSM anxiety disorder criteria (Achenbach et al. 2003). This subscale is present on both forms of the CBCL (ages 1.5–5, 6–18). The DSM subscales of the CBCL/6–18 have shown similar reliability, convergent validity, and discriminative validity as the broad symptom scales of the CBCL (Nakamura et al. 2009). Compared to the broader depression/anxiety scale of the CBCL, the DSM-anxiety subscale specifically targets anxiety symptoms and has demonstrated higher correspondence with anxiety disorder diagnoses made through structured clinical interviews (Ebesutani et al. 2010). Many of the studies using the CBCL in FXS were conducted or published prior to the addition of the DSM subscales of the CBCL in 2003. However, all of the items on the DSM-Anxiety subscale are from the same item pool used in the anxiety/depression syndrome scale, which has been extensively used in FXS (e.g. Hatton et al. 2002; Kau et al. 2004). We selected the earliest CBCL score after 35 months of age to parallel our selection of the autism outcome measure. The average T-score for our sample is 56.08 (average age of 58.43 months). Notably, we followed publisher recommendation to use raw scores in research, as the CBCL syndrome and DSM-scale T-scores are truncated at 50 (Achenbach 1991). The average CBCL raw score in our sample is 3.80 (SD 2.66, range 0–10).
Developmental Age
The Mullen Scales of Early Learning (MSEL; Mullen 1995) is an individually-administered standardized assessment of developmental abilities for children ages 0–68 months. The MSEL has been used to assess children a wide range of abilities, including children with FXS (e.g. Roberts et al. 2009a, b; Zingerevich et al. 2009) and autism (e.g. Akshoomoff 2006; Munson et al. 2008). The five MSEL domains include gross motor, fine motor, visual reception, receptive language, and expressive language; which are reported using T-scores and age equivalents. We calculated total age equivalents by averaging mental age across four MSEL domains, excluding gross motor. In light of the previous literature suggesting relationships between mental age and autistic symptoms (Kau et al. 2004; Roberts et al. 2009a, b; Rogers et al. 2001) and strong correlation between MSEL and CARS scores in our sample (Pearson r (89)=−0.79, p<0.001, controlling age), we covaried each participant’s total MSEL age equivalent at outcome. To ensure that correlations between chronological and mental age did not significantly affect results, we preliminarily ran each temperament model with one age variable (chronological or mental). Results were relatively consistent across models, thus we included both chronological and mental age in the final models.
Results
Data were analyzed using multilevel modeling (MLM) in SAS 9.2 PROC MIXED. MLM enables simultaneous analysis of within- and between-subject variance (Singer 1998), providing a powerful method for conceptualizing individualized change over time (Raudenbush and Bryk 2002). Unlike other longitudinal modeling procedures, MLM accommodates data in which the timing and frequency of assessments varies across participants. This feature is particularly important for our sample, as participants entered our studies at various ages and completed different numbers of assessments.
We characterized the level and change for each component of negative affect (fear, approach, soothability, sadness, anger) by estimating the unconditional means and growth for each temperament factor. Based on our relatively small sample and visual inspection of empirical growth plots, we only estimated linear growth in MLM. Next, we estimated fixed effects to test how (1) anxiety and (2) autistic outcomes related to the initial level and change in parent-reported temperament over time. To include outcome measures within the multilevel framework, we treated anxiety and autistic symptoms as time-invariant predictors of levels and change in negative affect.
Data were screened for non-normality, outliers, and heteroskedasticity. We examined empirical growth plots to confirm the general linear form of data. Q–Q plots indicated residuals were generally normally distributed both within-and between-subjects, although the variability at extreme levels of negative affect was slightly lower than at moderate levels. Patterns of residuals and estimates across individuals suggested outliers were not substantially affecting the data. Standardized plots suggested equal variances of residuals across ages. Covariance matrices between observations were set as unstructured, and age was centered at zero. Table 2 lists partial correlations between predictors and outcomes, collapsing across observations. Controlling for developmental age, autistic and anxiety symptoms did not significantly correlate, r(22)=−0.28, p=.19, supporting examination of these predictors in separate models, particularly given our small sample. We confirmed this decision by also running less powerful combined models (autism and anxiety in same model), which presented similar estimates and are therefore not reported.
Table 2.
Partial correlations among predictors and outcome variables, controlling mental age
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| Age | – | 0.15 | 0.06 | −0.10 | 0.18 | −0.46** | 0.28* | 0.40** |
| CARS | – | −0.28^ | −0.02 | 0.01 | 0.07 | −0.02 | −0.15 | |
| CBCL | – | 0.38* | 0.17 | −0.49** | 0.34* | 0.32* | ||
| Fear | – | −0.10 | −0.22 | 0.24* | −0.12 | |||
| Anger | – | −0.38* | 0.55** | 0.38* | ||||
| Soothability | – | −0.64** | −0.52** | |||||
| Sadness | – | 0.49** | ||||||
| Approach | – |
CARS childhood autism rating scale total score. CBCL childhood behavior checklist, DSM-Anxiety Raw Score
Partial correlation between CBCL and CARS conducted with one observation per participant
p<0.05
p<0.01
Unconditional means and growth models estimated level and change in negative affect over time
| (1) |
Yij refers to the dependent variable (e.g. fear) of observation i within individual j. β0j and β1j represent the true change trajectory intercept and slope, respectively, of individual j. eij represents random error for prediction of the unconditional model. Table 3 includes parameter estimates, variance components, and fit indices of unconditional means and growth models. Intercepts significantly differed from zero across the sample. Mean levels of approach, sadness, and anger increased over time; soothability decreased over time; and fear did not change over time. Notably, non-clinical samples have shown similar patterns of change in these temperament dimensions across ages 18–36 months, with the exception of fear increasing over time (Putnam et al. 2006).
Table 3.
Unconditional multilevel models
| Model | Est. | SE | df | t | p | Variance Component | −2 Res Log Likelihood | χ2 model fit improvement |
|---|---|---|---|---|---|---|---|---|
| Fear | ||||||||
| Intercept | 3.40 | 0.14 | 24 | 25.04 | <0.0001 | 0.22 | 270.7 | 0 |
| Slope | 0.004 | 0.005 | 68 | 0.88 | 0.38 | 0 | 270.7 | |
| Approach | ||||||||
| Intercept | 4.34 | .15 | 23 | 29.46 | <0.0001 | .35 | 189.3 | 2.1 |
| Slope | 0.02 | 0.006 | 50 | 3.25 | 0.002 | 0.0003 | 187.8 | |
| Soothability | ||||||||
| Intercept | 4.89 | .11 | 24 | 45.89 | <0.0001 | .17 | 228.0 | 1.6 |
| Slope | −0.02 | 0.004 | 73 | −3.61 | 0.0006 | 0.0001 | 226.4 | |
| Sadness | ||||||||
| Intercept | 3.08 | .14 | 23 | 22.79 | <0.0001 | .25 | 199.6 | 6.6* |
| Slope | 0.01 | 0.007 | 52 | 2.11 | 0.04 | 0.0005 | 193.0 | |
| Anger | ||||||||
| Intercept | 3.72 | .15 | 24 | 25.69 | <0.0001 | .36 | 258.4 | 2.4 |
| Slope | 0.02 | 0.005 | 71 | 3.50 | 0.0008 | 0.0002 | 256.0 | |
p<0.05
To test our hypothesis that problem behavior outcomes would be predicted by both mean level and increased negative affect over time, we fit separate MLM to test the effect of each outcome (level 2 time-invariant explanatory variables) on mean level and change in each component of parent-reported negative affect.
| (2) |
| (3) |
Equation 2 predicts overall level of negative affect (intercept coefficient β0j) from problem behavior outcomes (Zj). Equation 3 predicts the relationship between negative affect and age, expressed as the slope coefficient β1j, from problem behavior outcomes (Zj). g00 and u0j represent the fixed and random effects of time-invariant Level 2 predictors (e.g. autism, autism x age) and covariates (chronological and mental age at outcome) on the intercept of negative affect (β0j). g10 and u0i represent fixed and random effects of Level 2 predictors on the slope, (β1j).
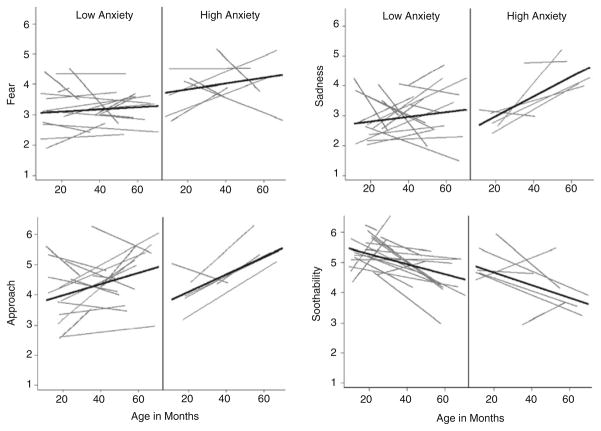
Contrary to our hypothesis, autism outcomes did not relate to the level or slope of any negative affect dimension over time (p’s>0.05). Fixed effects estimates indicated anxiety outcomes significantly relate to mean level of fear, g00=0.14 (0.05), p<0.009; soothability, g00=−0.10 (0.04), p=0.01; and sadness, g00=0.08 (0.04), p=0.055. Anxiety outcomes also significantly related to change in approach over time, g01= 0.004 (0.002), p=0.052. Change over time in sadness and mean levels of anger approached a relationship with anxiety outcomes (Fig. 1). Fixed effects and variance components are listed in Table 4.
Fig. 1.
Individual linear temperament trajectories across age, separated by level of anxiety at outcome. Participants are grouped by CBCL DSM-Anxiety t-score into Low (<60) or High (≥60) groups for display purposes only. Results indicate significant relationship between anxiety and level of fear, sadness, and soothability; as well as increasing approach over time
Table 4.
Fixed effects and variance components of multilevel models
| Model | Estimate | SE | df | t-value | p | Variance component | Pseudo R2 |
|---|---|---|---|---|---|---|---|
| CBCL DSM-Anxiety raw score | |||||||
| Fear | |||||||
| Intercept | 0.14 | 0.05 | 21 | 2.87 | <0.009 | 0.13 | 0.41 |
| Slope | 0.00006 | 0.002 | 67 | 0.03 | 0.98 | 0 | 0 |
| Approach | |||||||
| Intercept | 0.07 | 0.06 | 20 | 1.27 | 0.22 | 0.34 | 0.03 |
| Slope | 0.004 | 0.002 | 49 | 1.99 | 0.05^ | 0.0001 | 0.67 |
| Soothability | |||||||
| Intercept | −0.10 | 0.04 | 21 | −2.71 | 0.01 | 0.11 | 0.35 |
| Slope | −0.002 | 0.002 | 72 | −1.10 | 0.27 | 0.0001 | 0.00 |
| Sadness | |||||||
| Intercept | 0.08 | 0.04 | 20 | 2.04 | 0.06* | 0.07 | 0.72 |
| Slope | 0.004 | 0.002 | 51 | 1.72 | 0.09 | 0.0003 | 0.40 |
| Anger | |||||||
| Intercept | 0.09 | 0.05 | 21 | 1.76 | 0.09 | 0.23 | 0.36 |
| Slope | −0.0001 | 0.002 | 70 | −0.05 | 0.96 | 0.0003 | −0.50 |
| CARS total score | |||||||
| Fear | |||||||
| Intercept | 0.003 | 0.06 | 21 | 0.05 | 0.96 | 0.27 | 0.23 |
| Slope | −0.0009 | 0.001 | 67 | −0.77 | 0.45 | 0 | 0 |
| Approach | |||||||
| Intercept | −0.08 | 0.05 | 20 | −1.51 | 0.15 | 0.33 | 0.06 |
| Slope | −0.001 | 0.001 | 49 | −0.90 | 0.37 | 0.0002 | 0.33 |
| Soothability | |||||||
| Intercept | 0.05 | 0.04 | 21 | 1.21 | 0.24 | 0.18 | −0.06 |
| Slope | 0.002 | 0.001 | 72 | 0.21 | 0.83 | 0.0002 | −1.00 |
| Sadness | |||||||
| Intercept | −0.03 | 0.04 | 20 | −0.64 | 0.53 | 0.13 | 0.48 |
| Slope | −0.0003 | 0.001 | 51 | −0.20 | 0.85 | 0.0005 | 0.00 |
| Anger | |||||||
| Intercept | −0.01 | 0.05 | 21 | −0.20 | 0.85 | 0.28 | 0.22 |
| Slope | 0.0004 | 0.001 | 70 | 0.38 | 0.70 | 0.0002 | 0.00 |
CBCL childhood behavior checklist. CARS childhood autism rating scale. Pseudo R2 indicates proportion of variance in unconditional model explained by conditional model (Singer and Willett 2003). Models reaching p<0.05 significance are in boldface
p=0.052
p=0.055
To estimate the proportion of variance in the unconditional models explained by anxiety outcomes, we calculated pseudo R2 values using procedures outlined by Singer and Willett (2003, p. 103–4):
Within intercept models, anxiety accounted for approximately 41 % of the variability in fear, 35 % of variability in soothability, 72 % of variability in sadness, and 67 % of variability in approach change over time. Notably, although pseudo R2 provides a standard method for estimating relative proportions of variance explained by predictors within a common trajectory model, the statistic is influenced by both between and within-subject variability and thus should be interpreted with caution (Singer & Willett).
Power Analysis
To determine whether null effects of CARS outcomes on temperament trajectories related to underpowered models, we conducted a post hoc Monte Carlo power simulation using Mplus 6.11 software (Muthén and Muthén 2010). For each model, we used covariance and parameter estimates from our sample to calculate slope coefficients that could be significant at p=0.05 with 80 % power. For every 1 unit change in CARS scores, our sample was powered to detect annual corresponding change of 0.02 units of negative affect (range of Rothbart Scales is 1–7). In our sample, the standard deviation (SD) of negative affect scores ranges from 0.90 to 1.03, and the SD of CARS scores is 4.93. Thus, our sample is powered to detect annual rates of change of approximately .10 SD of negative affect per 1 SD of CARS. Given our sample is adequately powered to detect these modest effects, we can assume the null relationship between early negative affect and later autistic symptoms does not result from lack of power.
Discussion
The present study employed multilevel modeling to investigate whether trajectories of early negative affect predict anxiety and autistic outcomes in young males with FXS. Our results indicate that higher mean levels of fear and sadness, lower levels of soothability, and increasing levels of approach over time predicted anxiety, not autism. These data suggest that early negative affect is an important trait to increase our understanding of the emergence and prediction of problem behavior in FXS. However, our findings also confirm the complexity of these relationships, warranting future examination of the multifaceted factors related to negative affect and problem behaviors in FXS.
Anxiety
Our results replicate the well-documented relationships between behavioral inhibition, a construct closely linked to negative affect, and anxiety outcomes in typically developing populations (e.g. Kagan et al. 1987). With the exception of fear, the general patterns of age-related negative affect change in our sample were similar to trends reported in non-clinical samples (e.g. Putnam et al. 2006). Our results also mirror specific work implicating fear (Gartstein et al. 2010) and soothability (Grant et al. 2009) in anxiety and withdrawal outcomes in non-clinical samples. Although elevated mean levels of negative affect in our sample are consistent with findings linking negative affect and anxiety in typical populations (Gartstein et al. 2010), we did not replicate findings that increasing levels of negative affect over time also predict anxiety (Gartstein et al. 2010). This inconsistency may reflect the narrow and young age range of our sample; the median age of onset for most anxiety disorders in the general population is in middle childhood or adolescence (Kessler et al. 2007), and the average age of outcome assessment in our sample is 4.8 years (range 2.9–6.0). Thus, our findings supported our hypothesis that early negative affect relates to anxiety outcomes in FXS; however, this relationship is isolated to mean levels rather than change over time.
Our study also produced several unexpected findings, including a null relationship between early anger and anxiety outcomes. This finding lends support to theories that early anger is more closely related to externalizing traits (Cole et al. 1994; Eisenberg et al. 2001), with atypical physiological responses potentially moderating this relationship (Putnam et al. 2008). Dysregulated arousal is well-documented in children with FXS (Hall et al. 2006, 2009; Roberts et al. 2001, 2006, 2009a, b). In light of this physiological vulnerability within FXS, future research may investigate whether externalizing behaviors in FXS, rather than internalizing symptoms, link to early anger in young children with FXS.
Although we expected to find a relationship between anxiety outcomes and longitudinal increases in negative affect, we instead found a relationship between anxiety and increased approach, a construct sometimes classified under the extraversion domain of temperament (e.g. Putnam et al. 2008). This effect may relate to several facets of the FXS phenotype. Children with FXS show increases in approach behaviors as familiarity with a communicant increases (e.g. Hall et al. 2009; Roberts et al. 2007). More specifically, increased engagement in environmental activities may be paralleled by amplified sensitivity to social cues and influences, which may pose particular challenges in FXS due to difficulties physiologically modulating arousal in stressful situations (e.g., Roberts et al. 2001, 2006). In older children, deficits in cognitive flexibility and executive function inherent to the FXS phenotype (e.g. Hooper et al. 2008) may further aggravate this system. Thus, a child with FXS who struggles to self-regulate yet shows high levels of environmental engagement may actually be more vulnerable to anxiety than a child who displays lower approach. Further work is needed to test this speculation.
Autism
Although no published studies have prospectively examined the relationship between autism and temperament in FXS, our results are incongruent with previous research linking negative affect to autism in infant siblings of children with autism (Bryson et al. 2007; Garon et al. 2009; Watson et al. 2007; Zwaigenbaum et al. 2005), as well as emerging work suggesting early atypical self-modulation in FXS relates to later autistic symptoms (Roberts et al. 2012). This inconsistency may reflect overlapping phenotypic features of autism and FXS phenotype, such as withdrawal and social anxiety, which may be difficult to tease apart using non-diagnostic rating-scale measures. Our findings may also indicate that negative affect operates as an etiologically specific identifier in infant siblings that does not apply to children with FXS. This possibility is consistent with findings of distinct neural profiles in young children with idiopathic autism compared to children with FXS+autism (Hazlett et al. 2009). Understanding the unique predictors of autism across etiological groups is essential to accurate and early identification of the disorder.
In summary, our findings suggest a specific relationship between negative affect and anxiety, not autism, within FXS. To further understand this relationship, future research may explore outcomes specific to children with FXS+autism, such as persistent deficits in social approach (Roberts et al. 2007), to better elucidate autism precursors from global measures of autism and withdrawal symptoms. Similarly, investigating underlying physiological or neurocognitive processes associated with autism may be essential to distinguishing between comparable observable behaviors that may be rooted in distinct neurocognitive processes. For example, failure to improve gaze over time, a behavior specifically associated with FXS+autism (Roberts et al. 2007, 2009a, b), may reflect core socio-communicative deficits specific to autism, rather than withdrawal-related features common to FXS (see Volkmar 2011, for review of relevant autism symptoms). These possibilities warrant syndrome-sensitive examination of neurobiological components of anxiety and temperament, such as physiological arousal and attention (Barlow 2000), to more thoroughly elucidate the relationship among negative affect, anxiety, autism, and FXS.
Limitations and Future Directions
Despite our novel findings linking negative affect to anxiety in FXS, our study faces several limitations. Although the CARS is an empirically validated and reliable screening tool for autism, future work may use gold standard diagnostic tools such as the Autism Diagnostic Observation Scale (ADOS; Lord et al. 1989) to permit more fine-grained examination of these relationships. Similarly, we used a single anxiety subscale, which may provide limited information about the broad range of anxiety symptoms in our sample. Our reliance on parent-report measures of negative affect and anxiety may also reflect the subjective experience of parents and thus may be inconsistent with experimental assessments administered by clinicians. However, Shanahan et al. (2008) demonstrated general concordance between experimental and parent-report measures of childhood temperament in FXS, supporting the general validity of parent-reported temperament in this population. Supplementing parent-report measures in FXS may be particularly important in light of the high rates of depression and anxiety in mothers of children with FXS (Bailey et al. 2008a; Roberts et al. 2009a) and the association between maternal psycho-pathology and biased report of child behaviors (Weis and Lovejoy 2002). Furthermore, differential relationships between negative affect and our outcome measures may relate, in part, to rater variance, as parents and experimenters may report different perceptions of behavior. As such, future studies should investigate the relationship between negative affect and anxiety using multi-method, multi-informant measurement.
Future work may also explore the genetic and environmental vulnerabilities that contribute to anxiety outcomes in FXS. Maternal sensitivity and parenting style have been identified as moderators of the fear-anxiety relationship (Gartstein et al. 2010; Kiel and Buss 2009), which may be particularly important in FXS due to the high parenting stress associated with raising a child with FXS (e.g. Bailey et al. 2008b) and elevated rates of psychopathology in women with the FMR1 premutation (e.g. Bailey et al. 2008a). A substantial body of literature has linked parenting stress and depression to early negative affect and anxiety in children (e.g. Gartstein et al. 2010; Hudson and Rapee 2001, 2002), suggesting children with FXS may face particularly elevated risks due to both temperamental and environmental factors. Despite this dual vulnerability of child and family factors, no studies have specifically investigated how maternal symptoms relate to the negative affect in this sample. Increased attention to these early risk factors is essential to intervening on and minimizing the potential negative effects of chronic hyperarousal and anxiety, particularly during early developmental periods of neural plasticity (Duke 2008).
The current study presents the first prospective examination of negative affect as a predictor of anxiety and autistic outcomes in high-risk children with FXS. Understanding early predictors of psychopathology in FXS is particularly important given the extremely elevated rates of anxiety disorders and autism in this clinical population. In addition to endorsing temperament as a relevant predictor of anxiety outcomes in FXS, our work provides a foundation for future studies that may explore the early neurobiological and environmental processes related to anxiety and autistic outcomes in this population. This work is particularly important given the empirical void in early intervention FXS literature (see Hall 2009, for review). In addition to informing early characterization and treatment of anxiety in FXS, our findings may also inform theoretical models of anxiety disorders in general, thus potentially informing etiology and treatment in non-FXS samples.
Acknowledgments
This article is based on Bridgette Tonnsen’s thesis submitted to the University of South Carolina in partial fulfillment of the requirements for the master’s degree. This study was supported by the National Institute of Child Health and Human Development (P30-HD003110-35S1), the National Institute of Mental Health (R01-MH090194-01A1, F31-MH095318-01A1), and the office of Special Education Programs, U.S. Department of Education (H324C990042).
Contributor Information
Bridgette L. Tonnsen, Department of Psychology, University of South Carolina, 1512 Pendleton St., Columbia, SC 29208, USA
Patrick S. Malone, Department of Psychology, University of South Carolina, 1512 Pendleton St., Columbia, SC 29208, USA
Deborah D. Hatton, FPG Research Institute, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Jane E. Roberts, Email: Jane.Roberts@sc.edu, Department of Psychology, University of South Carolina, 1512 Pendleton St., Columbia, SC 29208, USA. FPG Research Institute, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
References
- Achenbach TM. Manual for the child behavior checklist/4–18 and 1991 profile. Burlington: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles. Burlington: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Achenbach TM, Dumenci L, Rescorla LA. DSM-oriented and empirically based approaches to constructing scales from the same item pools. Journal of Clinical Child and Adolescent Psychology. 2003;32:328–340. doi: 10.1207/S15374424JCCP3203_02. [DOI] [PubMed] [Google Scholar]
- Akshoomoff N. Use of the mullen scales of early learning for the assessment of young children with autism spectrum disorders. Child Neuropsychology. 2006;12:269–277. doi: 10.1080/09297040500473714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR. 4 2000. [Google Scholar]
- Bailey DB, Jr, Hatton DD, Mesibov G, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile X syndrome. Journal of Autism and Developmental Disorders. 2000;30:49–59. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism and Developmental Disorders. 2001;31:165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. American Journal of Medical Genetics. 2008a;146A(16):2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Sideris J, Roberts JE, Hatton DD. Child and genetic variables associated with maternal adaptation to fragile X syndrome: A multidimensional analysis. American Journal of Medical Genetics. 2008b;146A:720–729. doi: 10.1002/ajmg.a.32240. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Skinner D, Davis AM, Whitmarsh I, Powell C. Ethical, legal, and social concerns about expanded newborn screening: fragile X syndrome as a prototype for emerging issues. Pediatrics. 2008c;121:693–704. doi: 10.1542/peds.2007-0820. [DOI] [PubMed] [Google Scholar]
- Bargagna S, Canepa G, Tinelli F. Social adjustment in children with Down mental retardation (MRD) and Fragile-X mental retardation (MRX) Panminerva Medica. 2002;44:7–10. [PubMed] [Google Scholar]
- Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. New York: Guilford Press; 1988. [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Dickhut H, Poustka F. Patterns of parent-reported problems indicative in autism. Psychopathology. 1999;32:93–97. doi: 10.1159/000029072. [DOI] [PubMed] [Google Scholar]
- Boyle L, Kaufmann WE. The behavioral phenotype of FMR1 mutations. American Journal of Medical Genetics. 2010;154C:469–476. doi: 10.1002/ajmg.c.30277. [DOI] [PubMed] [Google Scholar]
- Brian J, Bryson SE, Garon N, Roberts W, Smith IM, Szatmari P, Rombough V, McDermott C. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12:433–456. doi: 10.1177/1362361308094500. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, et al. A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders. 2007;37:12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Budimirovic DB, Kaufmann WE. What can we learn about autism from studying fragile X syndrome? Developmental Neuroscience. 2011;33(5):379–394. doi: 10.1159/000330213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budimirovic DB, Bukelis I, Cox C, Gray RM, Tierney E, Kaufmann WE. Autism spectrum disorder in Fragile X syndrome: Differential contribution of adaptive socialization and social withdrawal. American Journal of Medical Genetics. 2006;140A(17):1814–1826. doi: 10.1002/ajmg.a.31405. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Blandon AY, Williford AP, Keane SP. Biological, behavioral, and relational levels of resilience in the context of risk for early childhood behavior problems. Development and Psychopathology. 2007;19:675–700. doi: 10.1017/S095457940700034X. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control. Prevalence of autism spectrum disorders: Autism and developmental disabilities monitoring network. Morbidity and Mortality Weekly Report. 2012;61(SS03):1–19. [PubMed] [Google Scholar]
- Chaplin TM, Cole PM, Zahn-Waxler C. Parental socialization of emotion expression: Gender differences and relations to child adjustment. Emotion. 2005;5:80–88. doi: 10.1037/1528-3542.5.1.80. [DOI] [PubMed] [Google Scholar]
- Cole PM, Zahn Waxler C, Smith KD. Expressive control during a disappointment - variations related to preschoolers behavior problems. Developmental Psychology. 1994;30:835–846. [Google Scholar]
- Constantino JN. The quantitative nature of autistic social impairment. Pediatric Research. 2011;69(5 Pt 2):55R–62R. doi: 10.1203/PDR.0b013e318212ec6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, Hessl D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. Journal of Neurodevelopmental Disorders. 2011;3:57–67. doi: 10.1007/s11689-010-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genetics in Medicine. 2001;3:359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, et al. Prevalence of the fragile X syndrome in African-Americans. American Journal of Medical Genetics. 2002;110:226–233. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- Duke BJ. Pathogenic effects of central nervous system hyperarousal. Medical Hypotheses. 2008;71:212–217. doi: 10.1016/j.mehy.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Kasari C. Maladaptive behavior in children with Prader-Willi syndrome, Down syndrome, and nonspecific mental retardation. American Journal of Mental Retardation. 1997;102:228–237. doi: 10.1352/0895-8017(1997)102<0228:MBICWP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ebesutani C, Bernstein A, Nakamura BJ, Chorpita BF, Higa-McMillan CK, Weisz JR. Concurrent validity of the child behavior checklist DSM-oriented scales: correspondence with dsm diagnoses and comparison to syndrome scales. Journal of Psychopathology and Behavioral Assessment. 2010;32:373–384. doi: 10.1007/s10862-009-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, Shepard SA. The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Development. 2001;72:1112–1134. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MG, Ruedrich S. Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. Journal of Autism and Developmental Disorders. 2003;33:617–629. doi: 10.1023/b:jadd.0000005999.27178.55. [DOI] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Zwaigenbaum L, Smith IM, Brian J, Roberts W, Szatmari P. Temperament and its relationship to autistic symptoms in a high-risk infant sib cohort. Journal of Abnormal Child Psychology. 2009;37(1):59–78. doi: 10.1007/s10802-008-9258-0. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Bridgett DJ, Rothbart MK, Robertson C, Iddins E, Ramsay K, Schlect S. A latent growth examination of fear development in infancy: contributions of maternal depression and the risk for toddler anxiety. Developmental Psychology. 2010;46:651–668. doi: 10.1037/a0018898. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. Studying temperament via construction of the toddler behavior assessment questionnaire. Child Development. 1996;67:218–235. [PubMed] [Google Scholar]
- Grant VV, Bagnell AL, Chambers CT, Stewart SH. Early temperament prospectively predicts anxiety in later childhood. Canadian Journal of Psychiatry. 2009;54:320–330. doi: 10.1177/070674370905400506. [DOI] [PubMed] [Google Scholar]
- Hall S. Treatments for fragile X syndrome: a closer look at the data. Developmental Disability Research Review. 2009;15:353–360. doi: 10.1002/ddrr.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, DeBernardis M, Reiss A. Social escape behaviors in children with fragile X syndrome. Journal of Autism and Developmental Disorders. 2006;36:935–947. doi: 10.1007/s10803-006-0132-z. [DOI] [PubMed] [Google Scholar]
- Hall SS, Burns DD, Lightbody AA, Reiss AL. Longitudinal changes in intellectual development in children with Fragile X syndrome. Journal of Abnormal Child Psychology. 2008;36:927–939. doi: 10.1007/s10802-008-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, Reiss AL. Physiological correlates of social avoidance behavior in children and adolescents with fragile x syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:320–329. doi: 10.1097/CHI.0b013e318195bd15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Hirt M, Rezvani A, Reiss AL. Autism in fragile X syndrome: a category mistake? Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:921–933. doi: 10.1016/j.jaac.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Bailey DB, Hargett-Beck MQ, Skinner M, Clark RD. Behavioral style of young boys with fragile X syndrome. Developmental Medicine and Child Neurology. 1999;41:625–632. doi: 10.1017/s0012162299001280. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Hooper SR, Bailey DB, Skinner ML, Sullivan KM, Wheeler A. Problem behavior in boys with fragile X syndrome. American Journal of Medical Genetics. 2002;108:105–116. doi: 10.1002/ajmg.10216. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Wheeler AC, Skinner ML, Bailey DB, Sullivan KM, Roberts JE, Clark RD. Adaptive behavior in children with fragile X syndrome. American Journal on Mental Retardation. 2003;108:373–390. doi: 10.1352/0895-8017(2003)108<373:ABICWF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics. 2006;140A:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Wheeler A, Sideris J, Sullivan K, Reichardt A, Roberts J, Bailey DB. Developmental trajectories of young girls with fragile x syndrome. American Journal of Intellectual and Developmental Disabilities. 2009;114:161–171. doi: 10.1352/1944-7558-114.3.161. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Lightbody AA, Gerig G, MacFall JR, Piven J. Teasing apart the heterogeneity of autism: Same behavior, different brains in toddlers with fragile X syndrome and autism. Journal of Neurodevelopmental Disorders. 2009;1:81–90. doi: 10.1007/s11689-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RN, Feinberg RL, Vaurio R, Passante NM, Thompson RE, Kaufmann WE. Autism spectrum disorder in fragile X syndrome: a longitudinal evaluation. American Journal of Medical Genetics Part A. 2009;149A(6):1125–1137. doi: 10.1002/ajmg.a.32848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Glasser B, Dyer-Friedman J, Blassey C, Hastie T, Gunnar M, Reiss AL. Cortisol and behavior in fragile X syndrome. Psychoneuroendocrinology. 2002;27(7):855–872. doi: 10.1016/s0306-4530(01)00087-7. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendrocrinology of fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(1):17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- Hessl D, Glasser B, Dyer-Friedman J, Reiss AL. Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of Child Psychology and Psychiatry. 2006;47(6):602–610. doi: 10.1111/j.1469-7610.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Walter E, Lighbody AA, Hazlett HC, Chang C, Piven J, Reiss AL. Neuroanatomical differences in toddler boys with fragile X syndrome and idiopathic autism. Archives of General Psychiatry. 2011;68(3):295–305. doi: 10.1001/archgenpsychiatry.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmboe K, Nemoda Z, Fearon RM, Sasvari-Szekely M, Johnson MH. Dopamine D4 receptor and serotonin transporter gene effects on the longitudinal development of infant temperament. Genes, Brain, and Behavior. 2010:10. doi: 10.1111/j.1601-183X.2010.00669.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SR, Hatton D, Sideris J, Sullivan K, Hammer J, Schaaf, Bailey DP. Executive functions in young males with fragile X syndrome in comparison to mental age-matched controls: baseline findings from a longitudinal study. Neuropsychology. 2008;22:36–57. doi: 10.1037/0894-4105.22.1.36. [DOI] [PubMed] [Google Scholar]
- Hudson JL, Rapee RM. Parent-child interactions and anxiety disorders: an observational study. Behaviour Research and Therapy. 2001;39:1411–1427. doi: 10.1016/s0005-7967(00)00107-8. [DOI] [PubMed] [Google Scholar]
- Hudson JL, Rapee RM. Parent-child interactions in clinically anxious children and their siblings. Journal of Clinical Child and Adolescent Psychology. 2002;31:548–555. doi: 10.1207/S15374424JCCP3104_13. [DOI] [PubMed] [Google Scholar]
- Hutman T, Rozga A, DeLaurentis AD, Barnwell JM, Sugar CA, Sigman M. Response to distress in infants at risk for autism: a prospective longitudinal study. Journal of Child Psychology and Psychiatry. 2010;51:1010–1020. doi: 10.1111/j.1469-7610.2010.02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Kau AS, Reider EE, Payne L, Meyer WA, Freund L. Early behavior signs of psychiatric phenotypes in fragile X syndrome. American Journal of Mental Retardation. 2000;105:286–299. doi: 10.1352/0895-8017(2000)105<0286:EBSOPP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kau AS, Tierney E, Bukelis I, Stump MH, Kates WR, Trescher WH, Kaufmann WE. Social behavior profile in young males with fragile X syndrome: characteristics and specificity. American Journal of Medical Genetics. 2004;126A:9–17. doi: 10.1002/ajmg.a.20218. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, Stanard P. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. American Journal of Medical Genetics. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: a review of recent literature. Current Opinion in Psychiatry. 2007;20:359–364. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kiel EJ, Buss KA. Maternal accuracy and behavior in anticipating children’s responses to novelty: relations to fearful temperament and implications for anxiety development. Social Development. 2009;19:304–325. doi: 10.1111/j.1467-9507.2009.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psychology and Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Lemery KS, Essex MJ, Smider NA. Revealing the relation between temperament and behavior problem symptoms by eliminating measurement confounding: expert ratings and factor analyses. Child Development. 2002;73:867–882. doi: 10.1111/1467-8624.00444. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergenk J, Jordan H, Mawhood L, Schopler E. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- McDuffie A, Abbeduto L, Lewis P, Kover S, Kim JS, Weber A, Brown WT. Autism spectrum disorder in children and adolescents with fragile X syndrome: within-syndrome differences and age-related changes. American Journal on Intellectual and Developmental Disabilities. 2010;115(4):307–326. doi: 10.1352/1944-7558-115.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychneuroendocrinology. Clinics in Laboratory Medicine. 2010;30:865–891. doi: 10.1016/j.cll.2010.07.006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostaticload. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning: AGS edition. San Antonio: Pearson; 1995. [Google Scholar]
- Munson J, Dawson G, Sterling L, Beauchaine T, Zhou A, Elizabeth K, et al. Evidence for latent classes of IQ in young children with autism spectrum disorder. American Journal of Mental Retardation. 2008;113:439–452. doi: 10.1352/2008.113:439-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- Nakamura BJ, Ebesutani C, Bernstein A, Chorpita BF. A psychometric analysis of the child behavior checklist DSM-oriented scales. Journal of Psychopathology and Behavioral Assessment. 2009;31:178–189. doi: 10.1007/s10862-009-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Stone WL. Recurrence risk for autism psectrum disorders: A baby siblings researh consortium study. Pediatrics. 2011 doi: 10.1542/peds.2010-2825. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky T, Yirmiya N, Shulman C, Dover R. The autism diagnostic interview-revised and the childhood autism rating scale: differences between diagnostic systems and comparison between genders. Journal of Autism and Developmental Disorders. 1998;28:143–151. doi: 10.1023/a:1026092632466. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Stifter CA. Behavioral approach-inhibition in toddlers: Prediction from infancy, positive and negative affective components, and relations with behavior problems. Child Development. 2005;76:212–226. doi: 10.1111/j.1467-8624.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: the early childhood behavior questionnaire. Infant Behavior & Development. 2006;29:386–501. doi: 10.1016/j.infbeh.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam SP, Rothbart MK, Gartstein MA. Homotypic and heterotypic continuity of fine-grained temperament during infancy, toddlerhood, and early childhood. Infant and Child Development. 2008;17:387–405. [Google Scholar]
- Raudenbush S, Bryk A. Hierarchal linear models: Applications and data analysis methods. 2. Thousand Oaks: Sage; 2002. [Google Scholar]
- Roberts JE, Boccia ML, Bailey DB, Hatton DD, Skinner M. Cardiovascular indices of physiological arousal in boys with fragile X syndrome. Developmental Psychobiology. 2001;39:107–123. doi: 10.1002/dev.1035. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Boccia ML, Hatton DD, Skinner ML, Sideris J. Temperament and vagal tone in boys with fragile X syndrome. Journal of Developmental and Behavioral Pediatrics. 2006;27:193–201. doi: 10.1097/00004703-200606000-00003. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Weisenfeld LA, Hatton DD, Heath M, Kaufmann WE. Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders. 2007;37:1748–1760. doi: 10.1007/s10803-006-0305-9. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Bailey DB, Jr, Mankowski J, Ford A, Sideris J, Weisenfeld LA, Golden RN. Mood and anxiety disorders in females with the FMR1 premutation. American Journal of Medical Genetics. 2009a;150B:130–139. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Mankowski JB, Sideris J, Goldman BD, Hatton DD, Mirrett PL, Bailey DB. Trajectories and predictors of the development of very young boys with fragile X syndrome. Journal of Pediatric Psychology. 2009b;34:827–836. doi: 10.1093/jpepsy/jsn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Hatton DD, Long ACJ, Anello V, Colombo J. Visual attention and autistic behavior in infants with fragile X syndrome. Journal of Autism and Developmental Disorders. 2011 doi: 10.1007/s10803-011-1316-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen BL, Robinson AR, Shrinkareva S. Heart activity and autistic behavior in toddlers with fragile X syndrome. American Journal of Intellectual and Developmental Disabilities. 2012;117:90–102. doi: 10.1352/1944-7558-117.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Developmental and Behavioral Pediatrics. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Measurement of temperament in infancy. Child Development. 1981;52:569–578. [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at three to seven years: the children’s behavior questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ellis LK, Rueda MR, Posner MI. Developing mechanisms of temperamental effortful control. Journal of Personality. 2003;71:1113–1143. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism diagnostic interview-revised. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Schopler E, Reichler R, Renner B. The childhood autism rating scale (CARS) Los Angeles: Western Psychological Services; 1998. [Google Scholar]
- Shaffer D, Fisher P, Dulcan MK, Davies M, Piacentini J, Schwab-Stone ME, Regier DA. The NIMH diagnostic interview schedule for children version 2.3 (DISC-2.3): Description, acceptability, prevalence rates, and performance in the MECA Study. Methods for the Epidemiology of Child and Adolescent Mental Disorders Study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:865–877. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Shanahan M, Roberts J, Hatton D, Reznick J, Goldsmith H. Early temperament and negative reactivity in boys with fragile X syndrome. Journal of Intellectual Disability Research. 2008;52:842–854. doi: 10.1111/j.1365-2788.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- Sheese BE, Voelker P, Posner MI, Rothbart MK. Genetic variation influences on the early development of reactive emotions and their regulation by attention. Cognitive Neuropsychiatry. 2009;14:332–355. doi: 10.1080/13546800902844064. [DOI] [PubMed] [Google Scholar]
- Silverman WK, Albano AM. Anxiety disorders interview schedule (ADIS-IV): Child and parent versions. Albany: Graywind; 2004. [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;23:323–355. [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- Sullivan K, Hooper S, Hatton D. Behavioural equivalents of anxiety in children with fragile X syndrome: parent and teacher report. Journal of Intellectual Disability Research. 2007;51:54–65. doi: 10.1111/j.1365-2788.2006.00899.x. [DOI] [PubMed] [Google Scholar]
- van Lieshout CF, de Meyer RE, Curfs LM, Koot HM, Fryns JP. Problem behaviors and personality of children and adolescents with Prader-Willi syndrome. Journal of Pediatric Psychology. 1998;23:111–120. doi: 10.1093/jpepsy/23.2.111. [DOI] [PubMed] [Google Scholar]
- Volkmar FR. Understanding the social brain in autism. Developmental Psychobiology. 2011;53:428–434. doi: 10.1002/dev.20556. [DOI] [PubMed] [Google Scholar]
- Watson LR, Baranek GT, Crais ER, Steven Reznick J, Dykstra J, Perryman T. The first year inventory: retrospective parent responses to a questionnaire designed to identify one-year-olds at risk for autism. Journal of Autism and Developmental Disorders. 2007;37:49–61. doi: 10.1007/s10803-006-0334-4. [DOI] [PubMed] [Google Scholar]
- Weis R, Lovejoy MC. Information processing in everyday life: emotion-congruent bias in mothers’ reports of parent-child interactions. Journal of Personality and Social Psychology. 2002;83:216–230. [PubMed] [Google Scholar]
- White SW, Oswald D, Ollendick T, Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clinical Psychology Review. 2009;29:216–229. doi: 10.1016/j.cpr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder P, Stone WL, Walden T, Malesa E. Predicting social impairment and ASD diagnosis in younger siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;39:1381–1391. doi: 10.1007/s10803-009-0753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingerevich C, Greiss-Hess L, Lemons-Chitwood K, Harris SW, Hessl D, Cook K, Hagerman MD. Motor abilities of children diagnosed with fragile X syndrome with and without autism. J Intellect Disabil Res. 2009;53:11–18. doi: 10.1111/j.1365-2788.2008.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]