Abstract
A major liability of existing nicotine vaccine candidates is the wide variation in anti-nicotine immune responses among clinical trial participants. In order to address this liability, significant emphasis has been directed at evaluating adjuvants and delivery systems that confer more robust potentiation of the anti-nicotine immune response. Toward that end, we have initiated work that seeks to exploit the adjuvant effect of liposomes, with or without Toll-like receptor agonist(s). The results of the murine immunization study described herein support the hypothesis that a liposomal nicotine vaccine formulation may provide a means for addressing the immunogenicity challenge.
Keywords: Immunopharmacotherapy, Liposome, Nicotine, Vaccine, Adjuvant
Chronic tobacco use, reinforced by nicotine dependence, is a widespread problem with costly impact. Current therapies to promote smoking cessation and prevent relapse include psychosocial interventions, antidepressants, and partial agonists. Despite these treatment options, relapse rates are still unacceptably high.
An alternative therapeutic strategy that has recently received intense attention is known as immunopharmacotherapy.1,2 This involves eliciting antibodies that are highly specific for a particular drug of abuse. Antibodies bind the drug in the periphery, and the resulting antibody-drug complexes are unable to cross the blood-brain barrier (BBB). As a result, brain exposure to the drug is reduced. The extent of this exposure reduction is related to both the quality and quantity of anti-drug antibodies elicited by the vaccine. We have carried out a wide variety of immunopharmacotherapeutic campaigns against several drugs of abuse, including cocaine,3 heroin,4 methamphetamine,5 and nicotine.6
In the case of nicotine, various candidate vaccine formulations have been or are currently being evaluated in human clinical trials.7 The hitherto lackluster performance of nicotine vaccines in the clinic underscores the notion that a sufficiently high concentration of nicotine-specific antibodies must be generated in order to provide broad efficacy in studies aimed at promoting cessation and preventing relapse.8 We continue to investigate tactics for enhancing nicotine vaccine immunogenicity.
We have previously scrutinized other parameters, such as linker position and length,9 hapten conformational constraint,10 and variation of protein carrier.6,11,12 Recently, a distinctly different tactic was employed for the generation of nicotine-specific antibodies: immunization of mice with an adeno-associated virus (AAV) vector carrying the genetic information for expressing the Fab fragment of NIC9D9, an anti-nicotine monoclonal antibody.13,14 While highly promising, the existing regulatory and developmental hurdles of such a gene therapy approach mean that further work within the more traditional active vaccination manifold merits pursuit. In this latter context, the vital role of adjuvant(s) for promoting robust nicotine-specific antibody production remains to be fully explored. The purpose of the present study was to evaluate the impact of liposomal delivery of a typical hapten-protein conjugate (i.e. AM1-KLH) on the resulting anti-nicotine immune response.
A means by which any weakly immunogenic vaccine can be rendered more immunogenic is through the use of adjuvants.15 Since the 1970s, it has been known that liposomal presentation of antigens can confer greater immunogenicity compared to antigen alone.16,17 Liposomal vaccines carry many advantages, including recruitment of various components of the immune system,18 opportunity for dose-sparing of antigen, and “plasticity” (tremendous flexibility) with regard to lipid composition.19 In addition to serving as an adjuvant by virtue of its direct stimulation of the immune response (e.g. macrophages and dendritic cells), liposomes also provide a physical means for either delivering encapsulated antigens or presenting surface-associated antigens with the ability to modulate epitope density and homogeneity. Also, orthogonal adjuvants, such as Toll-like receptor (TLR) agonists, may be encapsulated within the vesicles, or incorporated into the lipid bilayers. In other words, the liposome platform is one that boasts a great diversity of manipulations.
Generally speaking, a wide variety of surface functionalized liposomes have been studied for coupling with immunoglobulins, proteins, and peptides.20 Molecular Express, Inc. have developed VesiVax® Conjugatable Adjuvant Lipid Vesicles (CALVs) that offer a practical means for liposomal delivery of antigens of interest, by virtue of direct membrane anchorage through reactive functional groups. For example, the VesiVax® TLR4 conjugatable liposome contains a TLR4-specific agonist, monophosphoryl lipid A, and maleimide groups on the outer surface that allow covalent conjugation with sulfhydryl-containing molecules (e.g., cysteine groups on peptides or proteins). The VesiVax® TLR4 platform allows the user to covalently conjugate the desired target antigen for an easy-to-use antigen delivery method.
As a matter of course, we first evaluate new formulations for their ability to generate antibodies in mice, using results obtained from murine immunization studies to inform subsequent (e.g. pharmacokinetic and behavioral) studies in rats. There are several reports of active immunization against nicotine in mice.9,10,21,22 This is justifiable based on the relatively low cost output (compared to rats) and rapid immune response (1–2 months) exhibited by mice used in these studies.
This study began with the preparation of immunogen via hapten-protein conjugation. AM1 nicotine hapten6,23 was activated using EDC and sulfo-NHS in DMF/H2O (10:1 v/v) at room temperature for 4–6 h. After DMF removal under reduced pressure, activated AM1 was mixed with either bovine serum albumin (BSA) or keyhole limpet hemocyanin (KLH) in 0.1 M MOPS saline pH 7.2 at 4 °C for 18–20 h. The resulting hapten-protein conjugates, AM1-BSA and AM1-KLH, were purified by dialysis using PBS pH 7.2 at 4 °C. Protein concentrations were determined by BCA assay,24 and copy number (23–27) for AM1-BSA was determined by MALDI-TOF mass spectrometry. AM1-KLH was employed for immunization, while AM1-BSA was used for enzyme-linked immunosorbent assay (ELISA).
Next, liposomes were prepared. 1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) was purchased from Lipoid GmbH (Ludwigshafen, Germany), 1,2-dimyristoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DMPG) was purchased from Nippon Fine Chemical (Osaka, Japan), cholesterol was purchased from NOF (Japan), lipid-maleimide linker was synthesized by Molecular Express (Rancho Dominguez, CA), monophosphoryl lipid A (MPLA) was purchased from Avanti Polar Lipids (Alabaster, AL), and S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-N-palmitoyl-(R)-cysteinyl-alanyl-glycine (Pam3CAG) was purchased from Bachem (Torrance, CA). Briefly, VesiVax® CALVs25 were prepared by mixing DMPC, DMPG, cholesterol, lipid-maleimide, with or without MPLA and/or Pam3CAG in chloroform/methanol (1:1 v/v). The organic solvents were removed under a stream of nitrogen gas at 65 °C, and residual solvents were removed in vacuo for >24 h. Unilamellar liposomes were formed by hydrating the lipid films with 100 mM sodium phosphate pH 7.026–28 and probe sonicating until translucent. The particle sizes of the liposomes were 40–60 nm, as measured by dynamic light scattering (UPA 150, Microtrac, Montgomeryville, PA). The ratio of lipids was optimized to afford a maleimide functional group density of approximately 700 per liposome. For each liposome formulation evaluated, the abbreviation LP# indicates which type of TLR agonist was incorporated during lipid hydration and vesicle formation. LP2 refers to liposomes with TLR2 agonist Pam3CAG.29,30 LP4 refers to liposomes with TLR4 agonist MPLA.31,32 LP24, designed for agonism of both TLR2 and TLR4, refers to liposomes with both Pam3CAG and MPLA. LP0 refers to liposomes without any TLR agonist.
With the AM1-KLH immunogen and the set of CALVs in hand, union of the former with the latter commenced. The hapten-protein conjugate AM1-KLH was pre-treated with 40 mM dithiothreitol (DTT, final concentration) in PBS pH 7.2 at room temperature for 2 h in order to generate reactive sulfhydryl groups. Excess DTT was removed with an Econo-Pac 10DG desalting column (Bio-Rad, Hercules, CA). Immediately following the desalting process, the reduced hapten-protein conjugate was mixed with each of the four CALVs described above. After incubation at room temperature for 1 h, conjugation efficiencies were confirmed by HPLC; however, aggregation was observed. Bicinchoninic acid (BCA) assay of the conjugated samples (post-filtration, 0.22 μm syringe filter) suggested that ~70% of the AM1-KLH was lost, while the concentration of lipid components remained unchanged (HPLC analysis). A second conjugation was performed to reach the desired AM1-KLH dosage, using reduced and sterile-filtered AM1-KLH. Aggregation was again observed. However, no secondary filtration was performed since the reduced AM1-KLH and liposome samples were sterilized pre-conjugation. It is known that KLH is unstable under certain conditions. This protein is sourced from an oceanic creature that lives in a high-salt environment, and has a propensity to precipitate under conditions of lower salinity.33 Also, each intact KLH didecamer (8 MDa) is a hollow cylinder approximately 35 nm in diameter by 40 nm in length.34 For comparison, liposomes fabricated for this study were 40–60 nm in diameter.
To evaluate these liposomal nicotine vaccine formulations, groups of n=5 BALB/c mice (9 weeks, 25–30 g) were immunized subcutaneously on days 0, 14, and 35 with 200 μL of one of the following: AM1-KLH + Sigma Adjuvant System® (SAS, Sigma-Aldrich, St. Louis, MO), AM1-KLH-LP2, AM1-KLH-LP24, AM1-KLH-LP4, or AM1-KLH-LP0. Sera were collected via retro-orbital bleed on days 21, 42, and 56 (see Figure 1).
Figure 1.
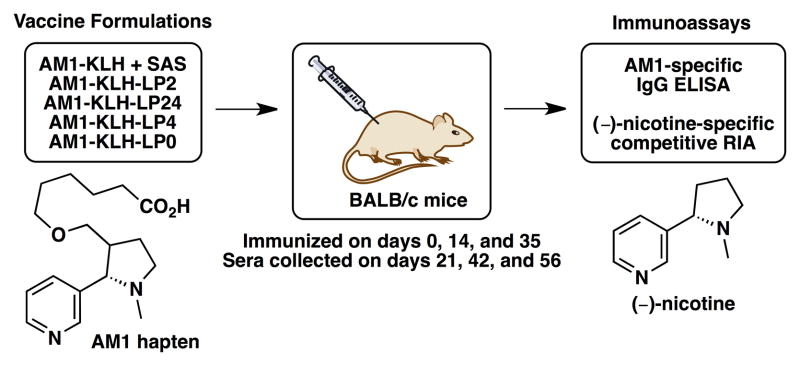
Summary of the five vaccine groups, immunization schedule, and immunoassays performed.
Serum anti-nicotine antibody titers were determined by ELISA.6 Mouse sera were serially diluted in 1% BSA across Costar 3690 plates pre-coated with AM1-BSA. After incubation at 37 °C in a moist chamber for 90 min, plates were washed with deionized H2O, treated with goat anti-mouse antibody horseradish peroxidase conjugate (Southern Biotech, Birmingham, AL) for 30 min, and washed again with deionized H2O. Plates were developed using 3,3′,5,5′-tetramethylbenzidine and H2O2 (TMB Substrate Kit, Thermo Pierce, Rockford, IL). Color development was halted by the addition of 2 M H2SO4, and plates were read on a plate reader (SpectraMax M2e, Molecular Devices, Sunnyvale, CA). By plotting absorbance versus log dilution, mid-point titers – dilutions affording an absorbance reading 50% of the maximum value – were obtained.
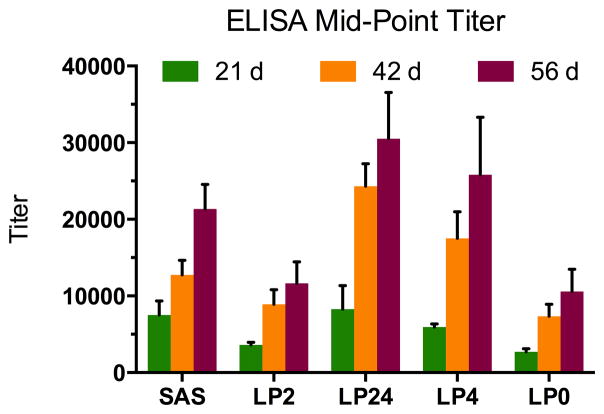
A summary of titer measurements is provided in Figure 2. Importantly, the data suggest superior performance of liposome formulations that were fortified with TLR4 agonist MPLA (with or without the help of TLR2 agonist Pam3CAG), compared to the conventional hapten-protein immunoconjugate approach. This is consistent with numerous studies underscoring the advantage of using liposomal lipid A.35–40 Notably, the non-liposomal group received SAS adjuvant, a component of which is, in fact, MPLA. For this group of mice that received AM1-KLH + SAS, each injection furnished 50 μg MPLA per animal, whereas, for the groups that received either AM1-KLH-LP24 or AM1-KLH-LP4, each injection furnished ~40 μg MPLA per animal. Despite containing a lesser amount of potent adjuvant MPLA, these two liposomal vaccine formulations outperformed the non-liposomal formulation. Finally, the vaccine formulations possessing LP2 and LP0 were comparable, suggesting little advantage in using the TLR2 agonist Pam3CAG alone.
Figure 2.
Mid-point titers determined by ELISA using AM1-BSA as coating antigen. Each vertical bar reflects a group average (n=5 mice per group). Error bars represent standard error of the mean (SEM).
Nicotine-specific serum antibody binding affinities and antibody concentrations were determined by competitive radioimmunoassay (RIA) using 3H-labeled nicotine.6,21,22 The protocol described herein is an adaptation of the procedure described by Müller.41 First, the serum dilution that binds ~50% of 3H-labeled nicotine is determined. Then, the affinity constant is calculated by competition with unlabeled nicotine. Since the sera were pooled for each vaccine group, the measured affinity constants are average affinities for each group.
Competitive RIA was carried out in a 5kDa MWCO Equilibrium Dialyzer-96 (Harvard Apparatus, Holliston, MA) to allow easy separation of bound and free L-[N-methyl-3H]-nicotine tracer; SA = 81.7 Ci/mmol (PerkinElmer, Boston, MA). Mouse sera were pooled and diluted in 2% BSA to a concentration that bound ~50% of ~30,000 dpm of 3H-nicotine tracer. Each sample chamber was loaded with 75 μL of diluted sera and 75 μL of radiolabelled tracer (~30,000 dpm), and each buffer chamber was loaded with 150 μL of unlabeled (−)-nicotine at varying concentrations in 1% BSA. The chamber contents were equilibrated on a plate rotator (Harvard Apparatus, Holliston, MA) at room temperature for at least 22 h. A 75 μL aliquot was removed from each sample/buffer chamber and added to 5 mL scintillation fluid (Ecolite(+)™, MP Biomedicals, Santa Ana, CA). Radioactivity (dpm) of each aliquot was measured in a Beckman LS 6500 Scintillation Counter.
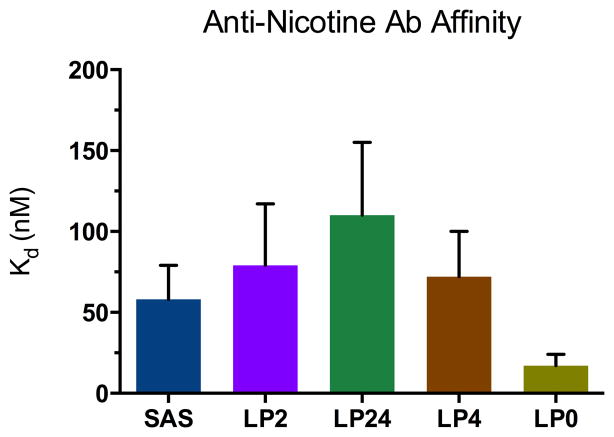
Figures 3 and 4 show results from competitive RIA of pooled sera from each of the five vaccine groups. Due to the limited volume of sera available, each measurement was performed twice. Additional replicates would further galvanize the Kd values and permit a more nuanced interpretation of the results. Nevertheless, one broad conclusion can be made. The CALV platform can indeed be exploited in the nicotine vaccine arena for production of murine polyclonal sera exhibiting nicotine-specific Kd values that are on par with others that have been reported. For instance, immunization of BALB/c mice with 3′-EstNic-rCTB + Alum resulted in anti-nicotine antisera with Kd ~56 nM,21 and immunization of BALB/c mice with 3′-EstNic-rQbVLP + Alum resulted in anti-nicotine antisera with Kd ~46 nM.22
Figure 3.
Serum anti-nicotine antibody affinities measured by competitive RIA. Each vertical bar reflects an average of two measurements using pooled sera (day 56) for each vaccine group (n=5 mice per group). Error bars represent standard error of the mean (SEM).
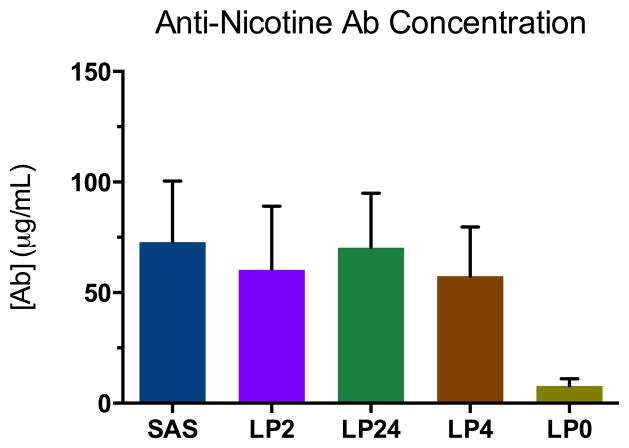
Figure 4.
Serum anti-nicotine antibody concentrations measured by competitive RIA. Each vertical bar reflects an average of two measurements using pooled sera (day 56) for each vaccine group (n=5 mice per group). Error bars represent standard error of the mean (SEM).
Serum anti-nicotine antibody concentrations are also on par with reports of a comparable nature. For instance, immunization of rats with AM1-KLH + AS-03 elicited antisera with anti-nicotine [Ab] ~40 μg/mL,6 and immunization of rats with 3′-AmNic-rEPA + complete/incomplete Freund’s adjuvant elicited antisera with anti-nicotine [Ab] ~184 μg/mL.42 It has been stated that an efficacious nicotine vaccine should be able to elicit a serum anti-nicotine antibody concentration of at least 200 μg/mL in animals. However, Freund’s adjuvant, despite being highly potent, is not approved for human use. The results herein demonstrate that, without the aid of TLR2 and/or TLR4 agonism, the anti-nicotine antibody response is minimal.
Based on these encouraging initial results, we will continue to evaluate VesiVax® CALVs for the rapid generation of additional nicotine vaccine formulations. In this vein, efforts to integrate a carrier protein (or peptide) other than KLH are underway, and results will be reported in due course. Additionally, adjustment of antigen and TLR agonist dosages and screening of other TLR agonists may lead to further increases in immunogenicity.
Acknowledgments
This work was supported by Tobacco-Related Disease Research Program (TRDRP) grant 20XT-0156 (to K.D.J.) and from National Institutes of Health/National Institute on Drug Abuse (NIH/NIDA) grant R41-DA032454 (to G.F.). This is manuscript # 22011 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Moreno AY, Janda KD. Pharmacol, Biochem Behav. 2009;92:199. doi: 10.1016/j.pbb.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meijler MM, Matsushita M, Wirsching P, Janda KD. Curr Drug Discovery Technol. 2004;1:77. doi: 10.2174/1570163043484851. [DOI] [PubMed] [Google Scholar]
- 3.Treweek JB, Janda KD. Mol Pharmaceutics. 2012;9:969. doi: 10.1021/mp200588v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, Janda KD. J Med Chem. 2011;54:5195. doi: 10.1021/jm200461m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno AY, Mayorov AV, Janda KD. J Am Chem Soc. 2011;133:6587. doi: 10.1021/ja108807j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno AY, Azar MR, Warren NA, Dickerson TJ, Koob GF, Janda KD. Mol Pharmaceutics. 2010;7:431. doi: 10.1021/mp900213u. [DOI] [PubMed] [Google Scholar]
- 7.Gorelick DA. Future Med Chem. 2012;4:227. doi: 10.4155/fmc.11.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann-Boyce J, Cahill K, Hatsukami D, Cornuz J. Cochrane Database Syst Rev. 2012;8:CD007072. doi: 10.1002/14651858.CD007072.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isomura S, Wirsching P, Janda KD. J Org Chem. 2001;66:4115. doi: 10.1021/jo001442w. [DOI] [PubMed] [Google Scholar]
- 10.Meijler MM, Matsushita M, Altobell LJ, 3rd, Wirsching P, Janda KD. J Am Chem Soc. 2003;125:7164. doi: 10.1021/ja034805t. [DOI] [PubMed] [Google Scholar]
- 11.De BP, Boyer JL, Alsaied O, Moreno AY, Janda K, Worgall S, Crystal RG. Mol Ther. 2009;17:S281. [Google Scholar]
- 12.Rosenberg JB, De BP, Hicks MJ, Moreno AY, Janda KD, Kaminsky SM, Crystal RG. Mol Ther. 2012;20:S127. [Google Scholar]
- 13.Hicks MJ, Rosenberg JB, De BP, Pagovich O, Young CN, Qiu JP, Kaminsky SM, Hackett NR, Worgall S, Janda KD, Davisson RL, Crystal RG. Mol Ther. 2012;20:S43. doi: 10.1126/scitranslmed.3003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hicks MJ, Rosenberg JB, De BP, Pagovich OE, Young CN, Qiu J-P, Kaminsky SM, Hackett NR, Worgall S, Janda KD, Davisson RL, Crystal RG. Science Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schijns VE, Lavelle EC. Expert Rev Vaccines. 2011;10:539. doi: 10.1586/erv.11.21. [DOI] [PubMed] [Google Scholar]
- 16.Allison AG, Gregoriadis G. Nature. 1974;252:252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- 17.Uemura K, Nicolotti RA, Six HR, Kinsky SC. Biochemistry (Mosc) 1974;13:1572. doi: 10.1021/bi00705a003. [DOI] [PubMed] [Google Scholar]
- 18.Garçon NM, Six HR. J Immunol. 1991;146:3697. [PubMed] [Google Scholar]
- 19.Watson DS, Endsley AN, Huang L. Vaccine. 2012;30:2256. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jølck RI, Feldborg LN, Andersen S, Moghimi SM, Andresen TL. Adv Biochem Eng/Biotechnol. 2011;125:251. doi: 10.1007/10_2010_92. [DOI] [PubMed] [Google Scholar]
- 21.Cerny EH, Lévy R, Mauel J, Mpandi M, Mutter M, Henzelin-Nkubana C, Patiny L, Tuchscherer G, Cerny T. Onkologie. 2002;25:406. doi: 10.1159/000067433. [DOI] [PubMed] [Google Scholar]
- 22.Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, Renner WA, Muller P, Bachmann MF. Eur J Immunol. 2005;35:2031. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- 23.Janda KD. Nicotine haptens, immunoconjugates and their uses. US 2012/0225087 A1. 2012 Sep 6;
- 24.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Anal Biochem. 1985;150:76. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 25.Fujii G, Szoka FC, Jr, Watson DS. Methods and compositions for liposomal formulations of antigens and uses thereof. US 2010/0226973 A1. 2010 Sep 9;
- 26.Ernst WA, Kim HJ, Tumpey TM, Jansen AD, Tai W, Cramer DV, Adler-Moore JP, Fujii G. Vaccine. 2006;24:5158. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Olson K, Macias P, Hutton S, Ernst WA, Fujii G, Adler-Moore JP. Vaccine. 2009;28:548. doi: 10.1016/j.vaccine.2009.09.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adler-Moore J, Munoz M, Kim H, Romero J, Tumpey T, Zeng H, Petro C, Ernst W, Kosina S, Jimenez G, Fujii G. Vaccine. 2011;29:4460. doi: 10.1016/j.vaccine.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes I, Frisch B, Muller S, Schuber F. Mol Immunol. 1997;34:569. doi: 10.1016/s0161-5890(97)00090-4. [DOI] [PubMed] [Google Scholar]
- 30.Espuelas S, Roth A, Thumann C, Frisch B, Schuber F. Mol Immunol. 2005;42:721. doi: 10.1016/j.molimm.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Alving CR, Rao M, Steers NJ, Matyas GR, Mayorov AV. Expert Rev Vacc. 2012;11:733. doi: 10.1586/erv.12.35. [DOI] [PubMed] [Google Scholar]
- 32.Alving CR, Rao M. Vaccine. 2008;26:3036. doi: 10.1016/j.vaccine.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Hermanson GT. Bioconjugate Techniques. 2. Academic Press; San Diego: 2008. p. 748. [Google Scholar]
- 34.Jaenicke E, Büchler K, Decker H, Markl J, Schröder GF. IUBMB Life. 2011;63:183. doi: 10.1002/iub.435. [DOI] [PubMed] [Google Scholar]
- 35.Richards RL, Rao M, Wassef NM, Glenn GM, Rothwell SW, Alving CR. Infect Immun. 1998;66:2859. doi: 10.2307/1366431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards RL, Alving CR, Wassef NM. J Pharm Sci. 1996;85:1286. doi: 10.1021/js9601593. [DOI] [PubMed] [Google Scholar]
- 37.Alving CR. Immunobiology. 1993;187:430. doi: 10.1016/S0171-2985(11)80355-4. [DOI] [PubMed] [Google Scholar]
- 38.Verma JN, Rao M, Amselem S, Krzych U, Alving CR, Green SJ, Wassef NM. Infect Immun. 1992;60:2438. doi: 10.1128/iai.60.6.2438-2444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alving CR, Verma JN, Rao M, Krzych U, Amselem S, Green SM, Wassef NM. Res Immunol. 1992;143:197. doi: 10.1016/s0923-2494(92)80165-h. [DOI] [PubMed] [Google Scholar]
- 40.Alving CR. J Immunol Methods. 1991;140:1. doi: 10.1016/0022-1759(91)90120-5. [DOI] [PubMed] [Google Scholar]
- 41.Müller R. Methods Enzymol. 1983;92:589. doi: 10.1016/0076-6879(83)92046-3. [DOI] [PubMed] [Google Scholar]
- 42.Keyler DE, Roiko SA, Earley CA, Murtaugh MP, Pentel PR. Int Immunopharmacol. 2008;8:1589. doi: 10.1016/j.intimp.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]