Abstract
Pure myoepithelioma of breast is an extremely rare tumor. Only a few cases have been reported in the literature so far. A 30-year old female presented with a large fungating mass arising from the areolar region of her right breast of six months duration. A clinical diagnosis of breast carcinoma was made and a mastectomy was performed. The specimen measured 23×22×9 cm with attached skin, and showed a large white ulcerated growth with areas of necrosis and hemorrhage. No normal breast tissue, nipple or areolar region was seen. Histopathological examination showed oval to spindle cells arranged in fascicles and bundles with whorling pattern in places showing mild pleomorphism with oval to spindle-shaped vesicular nuclei, prominent eosinophilic nucleoli, eosinophilic cytoplasm and clear cell changes in places, along with perivascular hyalinization and collagenization. Differential diagnosis of pleomorphic hyalinizing angiectatic tumor, solitary fibrous tumor, perivascular epithelioid cell tumor, mammary type myofibroblastic tumor and myoepithelioma were all considered. Immunohistochemistry for vimentin, smooth muscle actin, calponin, caldesmon, p63, epithelial membrane antigen, S-100, CD-31, CD-34, muscle specific antigen, myogenin, desmin, and pancytokeratin was carried out. On the basis of positive staining for vimentin, actin, p63 (nuclear), calponin and caldesmon (focal), a final diagnosis of myoepithelioma was considered; however, cytokeratin negativity was an unusual finding. This case was considered worthy of documentation because of its rarity, and because it highlights the importance of proper clinical examination and radiological examination to prevent misdiagnosis.
Key words: breast, myoepithelioma, p63, cytokeratin.
Introduction
Pure myoepithelial neoplasm of the breast is a rare condition and often presents a diagnostic challenge.1 The biological behavior of myoepitheliomas of the breast varies. They include a variety of lesions ranging from benign to locally invasive and recurring, to involvement of regional lymph nodes.2 These lesions are either derived from, or composed of, a dominant to pure population of myoepithelial cells which appear either spindle-shaped or as large ovoid cells, sometimes with clear cytoplasm.3 Myoid transformation is most frequently encountered around terminal ducts and lobules in the absence of appreciable epithelial proliferation.4 The diagnosis of malignancy is based on the presence of marked cellularity, frequent mitosis, necrosis, or local recurrence of the tumor.
Tumor cells express smooth muscle actin, calponin, caldesmon, S100 protein, cytokeratin and nuclear immunoreactivity with p63. This immunohistochemical phenotype allows a differential diagnosis from other breast tumors. Treatment varies from simple excision for myoepitheliosis to mastectomy, with axillary node dissection for malignant myoepitheliomas. A large excision is necessary because of the risk of recurrence and, more rarely, the proclivity to metastasis.5
Case Report
A 30-year female presented with a large fun-gating mass arising from the areolar region of her right breast of six months duration. A clinical diagnosis of breast carcinoma was made, a mastectomy was performed, and the specimen was sent for histopathological examination. There was no documentation of previous radiological or other investigations, or of any treatment having been given.
Gross
The specimen measured 23×22×9 cm with attached skin on the inferior surface, and showed a large white ulcerated growth (Figure 1A, B). The cut surface revealed solid, firm grayish-white areas. Focal areas of necrosis and hemorrhage were also seen. No normal breast tissue, nipple or areolar region was seen.
Figure 1.

A) Specimen showing a large white ulcerated growth; B) inferior surface of specimen shows attached skin; C) spindle-shaped cells showing whorling pattern around blood vessels (Haematoxylin and Eosin 100×); D) spindle-shaped cells arranged in fascicles around blood vessels (Haematoxylin and Eosin 100×).
Histopathology
Microscopic examination showed a pseudoencapsulated mass with areas of ulceration showing oval to spindle cells arranged in fascicles and bundles with herring bone and whorling patterns in places (Figure 1C, D). These cells showed mild pleomorphism with oval to spindle-shaped vesicular nuclei, prominent eosinophilic nucleoli, eosinophilic cytoplasm and clear cell changes in places (Figure 2A, B). No breast tissue or acini were seen. Numerous dilated blood vessels of varying sizes with focal areas of perivascular hyalinization were also noted (Figure 2C). Areas of collagenization, mild mixed inflammatory infiltrate and mitosis (1–2/10 hpf) were also seen. There was no evidence of invasion. On the basis of histological features, a differential diagnosis of pleomorphic hyalinizing angiectatic tumor, solitary fibrous tumor, perivascular epithelioid cell tumor, mammary type myofibroblastic tumor and myoepithelioma was considered.
Figure 2.
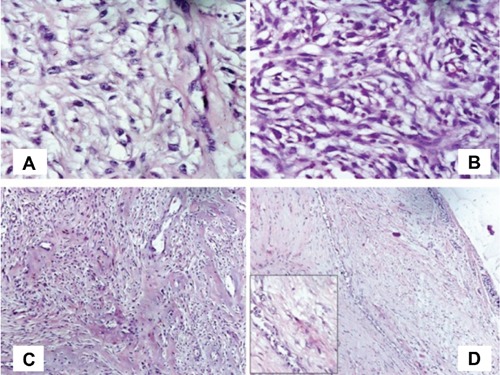
A) Clear cell changes in spindle-shaped cells with prominent nucleoli (Haematoxylin and Eosin 400×); B) spindle-shaped cells showing eosinophilic cytoplasm and prominent nucleoli with clear cell changes at places (Haematoxylin and Eosin 400×); C) focal areas of hyalinization and collagenization (Haematoxylin and Eosin 100×); D) few compressed glands at the margin of tumor (Haematoxylin and Eosin 100×). Inset shows myoepithelial proliferation around a duct (400×).
Immunohistochemistry
Immunohistochemistry for vimentin, smooth muscle actin (SMA), calponin, caldesmon, p63, epithelial membrane antigen (EMA), S-100, CD-31, CD-34, muscle specific antigen, myogenin, desmin, pancytokeratin was performed. Details of various antibodies used are given in Table 1.
Table 1. Antibodies used.
| N. | Name | Clone | Company | Dilution |
|---|---|---|---|---|
| 1 | Vimentin | V9 | Dako | Ready to use |
| 2 | Smooth muscle actin | 1A4 | Dako | Ready to use |
| 3 | Calponin | BSB5/20 | Biosb | Ready to use |
| 4 | Caldesmon | h-CD | Dako | Ready to use |
| 5 | Epithelial membrane antigen | E29 | Dako | Ready to use |
| 6 | S-100 protein | Polyclonal | Dako | Ready to use |
| 7 | CD31 | JC70A | Dako | Ready to use |
| 8 | CD34 | QBEnd | Dako | Ready to use |
| 9 | Muscle specific antigen | HHF35 | Dako | Ready to use |
| 10 | Myogenin | F50 | Dako | Ready to use |
| 11 | Desmin | D33 | Dako | Ready to use |
| 12 | Pan CK | AE1/AE3 | Dako | Ready to use |
| 13 | Pan CK | MNF116 | Biogenex | Ready to use |
| 14 | P63 | 7JUL | Lieca | Ready to use |
Vimentin, actin and p63 (nuclear immunopositivity) were positive (Figure 3A–C). Focal positivity for calponin and caldesmon was seen (Figure 3D, E). EMA, S-100, pancytokeratin (both AE1/AE3 and MNF116), CD-34 (Figure 3F) and others were negative. Later, after processing further sections, only a few scattered small ducts were seen at the margin of the tumor (Figure 2D). On the basis of immunopositivity, a final diagnosis of myoepithelioma was made (Table 2).
Figure 3.

A) Diffuse cytoplasmic positivity for vimentin (400×); B) cytoplasmic positivity for smooth muscle actin (400×); C) nuclear immunopositivity for p63 (400×); D) focal positivity for calponin (400×); E) focal positivity for caldesmon (400×); F) CD34 positivity in blood vessel lining but negative in tumor cells (400×).
Table 2. Immunoprofile of various tumors considered for differential diagnosis.
| Tumors | Vi | SMA | P63 | Cal | Cald | EMA | S100 | CD31 | CD34 | MSA | Myo | Des | Pan CK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHAT | + | - | - | NI | NI | - | - | - | + | NI | NI | - | - |
| SFT | + | - | - | + | NI | - | ± | - | + | NI | NI | NI | - |
| PEComa | + | + | - | NI | + | - | - | ± | ± | ± | ± | - | - |
| MFB | + | + | - | ± | ± | - | - | ± | ± | ± | ± | + | - |
| ME | ± | + | + | + | + | - | + | - | - | - | - | - | + |
NI, no information; PHAT, pleomorphic hyalinizing angiectatic tumor; SFT, Solitary fibrous tumor; PECOMA, Perivascular epithelioid cell tumor; MFB, myofibroblastoma; ME, myoepithelioma.
Discussion
Neoplasms composed entirely of myoepithelial cells represent one end of a spectrum of differentiation that includes tumors with adenomyoepithelial differentiation. Tavassori divided myoepithelial lesions of the breast into three categories: i) myoepitheliosis; ii) adenomyoepithelioma; and iii) malignant myoepithelioma.6 Pure myoepithelial neoplasms of the breast are extremely uncommon and reports are limited to case studies. A review of the subject by Hamperl in 1970 described lesions composed of epithelioid and spindle myoepithelial cells. Leiomyomatous proliferation in these neoplasms may be coordinated with glandular components, thus retaining adenomyoepitheliomatous features.4 Cameron et al. reported the case of a 40-year old woman with a 5–7 cm adenomyoepitheliomatous tumor in which a portion of the lesion was a highly cellular spindle cell neoplasm. One year after mastectomy, the patient developed a local recurrence involving fat and skeletal muscle, consisting entirely of spindle cells with no epithelial structures.4
Pure spindle cell myoepithelial tumors may be difficult to distinguish by light microscopy from other spindle cell mammary neoplasms. In most cases, the issue can be resolved by considering the patient's clinical history, as well as careful histological and immunohistochemical analysis, but electron microscopy is sometimes required. In the present case, which clinically masqueraded as breast carcinoma, histopathology indicated that the tumor was predominantly composed of spindle cells arranged in whorls and fascicles showing clear cell changes in places, vascularized stroma, perivascular collagenization and areas of hemorrhage. Myoepithelial origin was confirmed by immunoreactivity to SMA, P63 and focal positivity for calponin and caldesmon. Absence of ductal differentiation in the initial sections, and pan-cytokeratin (both AE1/AE3 and MNF116) negativity were unusual features. Although the biological behavior of the tumor remains to be ascertained, this tumor was considered to be of low grade due to mild nuclear pleomorphism, low mitotic count (1–2/10 hpf) and absence of invasion. The tumor described in this report is unusual for its rarity, its presentation, its large size and the fact that it mimicked a malignant tumor. The clinical significance of this entity lies primarily in its recognition as a distinctive neoplasm as these tumors can give rise to a wide range of clinical evolution. However, these breast tumors show a broad spectrum of histomorphological features that also overlap with some features of other tumors.7 The absence of staining for desmin and CD34 supported the exclusion of myofibroblastoma from other differential diagnoses. CD34 negativity excluded solitary fibrous tumor and pleomorphic hyalinizing angiectatic tumor. Smooth muscle actin and p63 positivity supported a myoepithelial origin for the tumor as ductal epithelium is negative for actin. Markers for glandular epithelial cells, such as epithelial membrane antigen and pancytokeratin were negative. Some of the earlier studies also showed cytokeratin negativity or weak positivity.8,9 However, this case presents diagnostic difficulties not only on paraffin embedded sections but also after immunohistochemistry.
Conclusions
In conclusion, although myoepithelioma of breast is a rare entity, awareness of this type of tumor is essential for patient diagnosis and optimal therapy.
Acknowledgments:
the authors appreciate the support received from the technical staff of the Department of Pathology, M.L.N. Medical College, Allahabad and Dr. Sanjay Navani, Lab Surgpath, Mumbai for help in immunohistochemistry.
References
- 1.Al Nafussi A. Spindle cell tumours of the breast: practical approach to diagnosis. Histopathology. 1999;35:1–13. doi: 10.1046/j.1365-2559.1999.00766.x. [DOI] [PubMed] [Google Scholar]
- 2.Irvanlou G, Mahjoub F. Malignant myoepithelioma of the breast. Acta Medica Iranica. 1997;35:90–4. [Google Scholar]
- 3.Abd El-All HS. Breast spindle cell tumours: about eight cases. Diagn Pathol. 2006;1:13–13. doi: 10.1186/1746-1596-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen PP. Rosen's breast pathology. 3rd Ed. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 5.Khattech R, Ben Othman M, Ben Romdhane K, et al. Breast myoepithelioma. Report of a case. Ann Pathol. 1995;15:138–41. [PubMed] [Google Scholar]
- 6.Miura Y, Kurose A, Kondo M, et al. Clear cell myoepithelial carcinoma of the breast. Tohoku J Exp Med. 2003;200:103–9. doi: 10.1620/tjem.200.103. [DOI] [PubMed] [Google Scholar]
- 7.Hikino H, Nagaoka S, Miura H, Kurosumi M. Benign myoepithelioma of the breast: origin and development. Pathol Int. 2009;59:422–6. doi: 10.1111/j.1440-1827.2009.02388.x. [DOI] [PubMed] [Google Scholar]
- 8.Lakhani S R, O'Hare M J, Monaghan P, et al. Malignant myoepithelioma (myoepithelial carcinoma) of the breast: a detailed cytokeratin study. J Clin Pathol. 1995;48:164–7. doi: 10.1136/jcp.48.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terada T. Malignant myoepithelioma of the breast. Pathol Int. 2011;61:99–103. doi: 10.1111/j.1440-1827.2010.02638.x. [DOI] [PubMed] [Google Scholar]


