Abstract
An 81-year old woman with a history of chronic lymphocytic leukemia (CLL) was admitted with night sweats and abdominal distension. A complete blood count showed hemoglobin 5 g/dL, white blood cell (WBC) count 28.5×109/L and platelets 38.4×109/L. Peripheral blood smear examination showed a large number of smudge cells and lymphocytosis composed of mature-looking lymphocytes with clumped nuclear chromatin. Computed tomography scan demonstrated enlarged cervical, axillary, paraaortic, retroperitoneal and mesenteric lymph nodes with concomitant omental thickening and ascites. Also, the liver and the spleen were enlarged in the presence of multiple ill-defined hypoechoic areas in the latter. Histopathological analysis of the cervical lymph node biopsy was consistent with CLL. Bone marrow examination showed diffuse infiltration of the marrow with small lymphocytes. Analysis of the ascitic fluid revealed an exudate with WBC 1220 cells/mL. Cytocentrifuge preparation of the ascitic fluid showed small mature lymphoid cells containing hyperchromatic nuclei with coarsely granular chromatin. On flow cytometric analysis of the ascitic fluid, expression of CD5, CD19, CD20, CD22, CD23, CD45 and HLA-DR was compatible with a diagnosis of CLL, in accordance with the results of the peripheral blood analysis. The patient was treated with chemotherapy consisting of cyclophosphamide, vincristine and prednisolone but died within one month after development of non-chylous ascites.
Key words: ascites, chronic lymphocytic leukemia, hematologic malignancies, lymphomas.
Introduction
Ascites is not an uncommon manifestation of certain recurrent and/or advanced solid tumors. Among hematologic malignancies, lymphomas tend to cause lymph node obstruction and subsequently result in accumulation of chylous ascites.1 Ascites is a rare entity in the course of chronic lymphocytic leukemia (CLL). CLL is a chronic lymphoproliferative disorder considered mainly to be a disease of the elderly, with a median age at diagnosis of 70 years and a male to female ratio of approximately 1.7:1.2,3 The most common presenting finding is painless swelling of lymph nodes, often in the cervical area.4 Approximately 25% of patients are asymptomatic at admission and the diagnosis is made in the presence of an absolute lymphocyte count of over 5×109/L and confirmation by flow cytometry of the clonality of the circulating B lymphocytes.5 Less commonly, patients may present with constitutional B symptoms, acquired immunodeficiency syndrome-associated symptoms, or autoimmune complications. Lymphadenopathy, splenomegaly, and hepatomegaly are most commonly encountered on initial physical examination.4 CLL cells may invade and cause malfunction of virtually any organ.4 Previous reports described ascites to be not common in CLL in the absence of prolymphocytic leukemia or transformation to a highly aggressive lymphoma (Richter's syndrome).6
Case Report
An 81-year old woman had been referred to our institute 20 months ago for the evaluation of multiple peripheral lymphadenopathies involving bilateral cervical, axillary and inguinal lymph areas, multiple intrathoracic and intraabdominal lymphadenopathies, and hepatosplenomegaly in the presence of B symptoms. On admission, her complete blood count showed a white blood cell (WBC) count of 145×109/L with 80% mature-looking lymphocytes, hemoglobin (Hb) 9 g/dL and platelet count 185×109/L. Flow cytometric analysis of the peripheral blood showed expression of CD5, CD19, CD20, CD23 and FMC7. The patient was diagnosed as CLL-Rai stage IV.7 Because of advanced age, the patient was treated with oral chlorambucil 0.08 mg/kg daily. Three months later, WBC returned to normal and Hb rose to 11 g/dL, accompanied by the disapperance of B symptoms. WBC counts obtained at regular 3-month intervals remained within normal limits.
At the 21st month after initial diagnosis, the patient was admitted with night sweats and abdominal distension. On physical examination, there was bilateral cervical and axillary lymphadenopathies and a protuberant abdomen with bulging flanks. The liver and spleen were palpated 7 cm and 5 cm below the costal margins, respectively. A complete blood count showed Hb 5 g/dL, WBC count 28.5×109/L and platelet count 38.4×109/L. On biochemical tests, lactate dehydrogenase (LDH) was elevated (660 U/L, normal range 240–480 U/L); alkaline phosphatase, serum aspartate amino-transferase, and serum glutamic pyruvic transaminase were normal. Peripheral blood smear examination showed a large number of smudge cells and lymphocytosis composed of mature-looking lymphocytes with clumped nuclear chromatin (Figure 1). The histopathological analysis of the cervical lymph node biopsy was consistent with CLL. Bone marrow examination showed diffuse infiltration of the marrow with small lymphocytes (Figure 2). Computed tomography scan demonstrated enlarged cervical, axillary, paraaortic, retroperitoneal and mesenteric lymph nodes with omental thickening and ascites. Also, the liver and the spleen were enlarged in the presence of multiple ill-defined hypoechoic areas in the latter. Serological tests for hepatitis B and C viruses were negative. Paracentesis was performed to provide symptomatic relief and perform diagnostic tests. Analysis of the ascitic analysis revealed an exudate with WBC 1220 cells/mL and the following results on biochemical analysis: ascitic total protein 2.4 g/dL (serum 4.9 g/dL), glucose 110 mg/dL (serum 98 mg/dL), LDH 638 U/L (serum 660 U/L), triglyceride 43 mg/dL (serum 149 mg/dL) and albumin 1.9 g/dL (serum 3.6 g/dL). Gram and acid fast bacilli stains of the ascitic fluid were negative. Also, ascitic fluid cultures remained negative both by the conventional method and by Bactec. Serum α-fetoprotein and carcinoembryonic antigen were within normal limits. On cytocentrifuge preparation of the ascitic fluid, there were small mature lymphoid cells containing hyperchromatic nuclei with coarsely granular chromatin (Figure 3). Flow cytometric analysis of the ascitic fluid showed the expression of CD5, CD19, CD20, CD22, CD23, CD45 and HLA-DR, compatible with a diagnosis of CLL, in accordance with the results of the peripheral blood analysis (Figure 4). Endoscopic examinations of the upper and lower gastrointestinal tract were normal, ruling out a gastrointestinal malignancy as the cause of ascites. Mammogram showed no abnormal findings. Transthoracic echocardiogram examination showed normal left ventricular function and normal heart valves. The patient was treated with chemotherapy consisting of cyclophosphamide, vincristine and prednisolone (CVP). One month after development of non-chylous ascites on the 10th day of therapy, the patient succumbed to her disease because of fungal esophagitis and septicemia.
Figure 1.
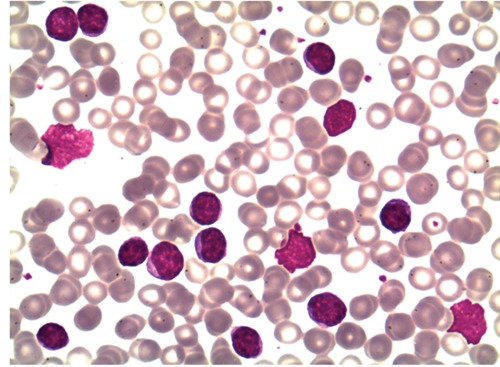
Peripheral blood smear examination showed a large number of smudge cells and lymphocytosis composed of mature-looking lymphocytes with clumped nuclear chromatin.
Figure 2.
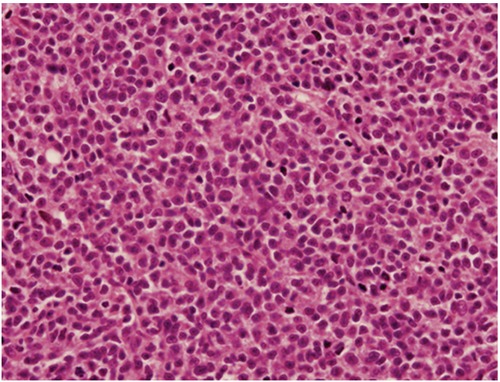
Bone marrow examination showed diffuse infiltration of the marrow with small lymphoid cells (Haematoxylin and Eosin 400×).
Figure 3.
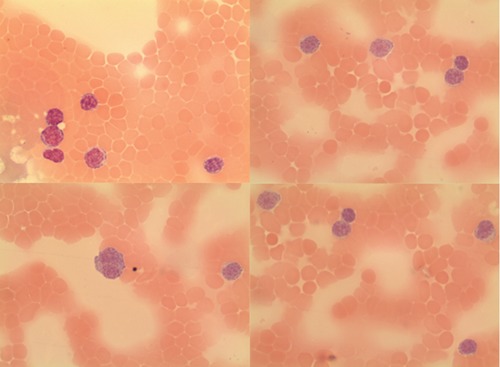
Cytocentrifuge preparation of the ascitic fluid showed small mature lymphoid cells containing hyperchromatic nuclei with coarsely granular chromatin.
Figure 4.
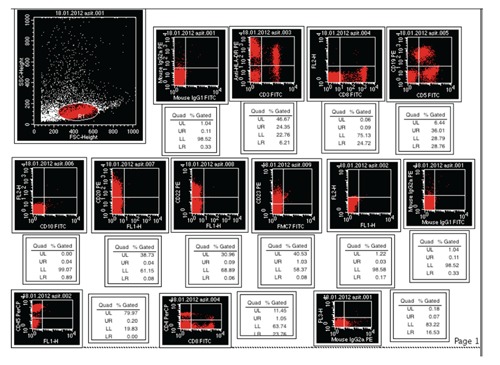
Flow cytometric analysis of the ascitic fluid showed the expression of CD5, CD19, CD20, CD22, CD23, CD45 and HLA-DR compatible with the diagnosis of chronic lymphocytic leukemia in accordance with the results of the peripheral blood analysis.
Discussion
CLL, a common hematologic malignancy in the elderly, is characterized by progressive accumulation of functionally incompetent monoclonal lymphocytes.8–10 Although CLL is generally regarded as an indolent disease with a prolonged clinical course, today we know that this observation is only true in less than 30% of all CLL patients. The natural history of CLL is extremely variable. Some patients die quickly, within 2–3 years of diagnosis due to complications or to causes directly associated with CLL, while others live well for 20 years. Ascites is a common manifestation of certain solid tumors, such as gastrointestinal malignancies, breast cancer and ovarian cancer. However, it is an unusual entity in lymphoproliferative malignancies.11 About 10% cases of CLL may transform to prolymphocytic leukemia and another 3% to aggressive or highly aggressive lymphoma (Richter's syndrome). In this limited population of CLL patients, development of ascites at the time of transformation has been observed.12–15 Yet, ascites as an initial manifestation of CLL has been rarely reported.6,16 In previously decsribed cases of ascites developing in the course of relapsed CLL, the ascitic fluid was reported to have transudative characteristics.11,17,18 Exudative ascites was reported only once as the initial manifestation of CLL and once in the course of relapsed CLL.16,19 Our patient demonstrates once again the rare presentation of ascites in a CLL patient, yet in the presence of the very unusual finding of exudative ascites. To date, development of ascites in CLL patients has mostly been reported during transformation to prolymphocytic leukemia and to aggressive lymphoma (Richter's syndrome).12–15 The ascitic fluid in those patients with Richter's transformation showed large blast-like lymphoma cells.13,14 In our patient, bone marrow and cervical lymph node biopsies showed the infiltration with small lymphocytes. It is well acknowledged that some lymph node and bone marrow areas can show infiltration with small lymphocytic lymphoma cells in concomitant presence of large cell transformation in other areas. Therefore, it may be argued that Richter's transformation was not completely ruled out in our case. However, both the morphological appeareance of the cells observed in the ascitic fluid and the flow cytometric analysis of the ascitic fluid were consistent with CLL, as observed in peripheral blood and bone marrow. Furthermore, our patient had no prolymphocytes in the ascitic fluid and had a very small population of prolymphocytes; therefore, transformation to prolymphocytic leukemia was not considered.
The pathogenesis of ascites in the course of lymphomas is obscure. It can be associated with a pressure effect of a tumor mass or an enlarged lymph node leading to an increase in hydrostatic pressure in the portal system.20 In vitro studies demonstrated that ascitic fluid contains increased levels of vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF) produced by lymphoma cells,21 thus suggesting VEGF/VPF stimulation of vascular leakage may be relevant to the pathogenesis of effusions in the course of lymphoproliferative diseases.
Possible causes of ascites during the course of CLL including subacute bacterial peritonitis, tuberculosis and portal hypertension have been excluded in our patient. Also, secondary solid tumors such as of the gastrointestinal tract and the lung, increasingly being reported in CLL,22 have been ruled out by endoscopies and CT scans, respectively. Ovarian carcinoma and breast carcinoma, important causes of exudative ascites in women, have been excluded by computed tomography (CT) scans and mammogram, respectively. Our patient had an enlarged liver but no hepatic lesion on CT scan and serum -fetoprotein was normal, making hepatocellular carcinoma an unlikely cause of ascites. Other probable etiologies of ascites, such as liver cirrhosis, cardiac ascites, severe hypoalbuminemia, hepatic and portal vein thrombosis, and renal failure, were also tested and ruled out. In our patient, radiological examination showed the presence of multiple lymph nodes with mesenteric thickening. It can, therefore, be speculated that either malignant cells of lymphoma involving the peritoneal surface or the portal system lymphatic obstruction due to extensive lymphatic infiltration lead to development of symptomatic ascites. A mesenteric biopsy in this case would have been helpful to confirm the infiltration of the peritoneal surface by lymphoma cells but this was not carried out because the patient's clinical condition was not good enough for her to undergo the procedure at that time.
Many treatment approaches are available for CLL patients. Presence of symptomatic disease, bulky lymphadenopathy and/or splenomegaly, risk of local compressive disease, marrow compromise, or rapid disease progression are the indications to initiate therapy. Performance status, age, circulating tumor load and presence of significant co-morbidities are important determinants in the choice of appropriate chemotherapy. Siddiqui et al. reported a 75-year old patient with CLL manifesting as ascites, in whom a good response to 3 cycles of CVP chemotherapy was obtained.16 On the contrary, our patient succumbed to her disease because of fungal esophagitis and septicemia on the 10th day of CVP chemotherapy. Our patient was frail, had low performance status and high WBC counts, reflecting a high circulating tumor load. After the administration of rituximab, particularly in patients with high circulating tumor load, tumor lysis syndrome and deaths have been reported as the likely result of high levels of cytokines released.23 Therefore, we did not add rituximab to CVP chemotherapy. Our patient had a short response to initial therapy lasting less than two years. There are reports of use of reduced dose of fludarabine, cyclophosphamide and rituximab, bendamustine-based chemotherapy, alemtuzumab-based chemotherapy, or ofatumumab to elderly short-term responders.24,25 Our patient was not able to tolerate purine analogs and the other aforementioned therapies because of her frail condition.
In a few cases, administration of intraperitoneal rituximab in cases of recurrent abdominal ascites due to non-Hodgkin's lymphoma has been reported as an alternative means of control of recurrant ascites.26,27 There are no published large series of peritoneal involvement with CLL and current knowledge is based on a few rare reported cases. Further studies are needed to standardize the treatment of this subgroup of patients.
Conclusions
In conclusion, we have reported an unusual progression of CLL, rapidly complicated with ascites. The ascitic fluid consisted of mature B-cell type lymphocytes, morphologically and immunophenotypically similar to the peripheral blood and bone marrow cells. Our patient survived less than one month after development of ascites. Therefore, it can be hypothesized that ascites is probably one of the poor prognostic manifestations of the disease. Recognizing development of ascites as a sign of progressive disease in CLL may help improve risk stratification and treatment.
References
- 1.Press OW, Press NO, Kaufman SD. Evaluation and management of chylous ascites. Ann Intern Med. 1982;96:358–358. doi: 10.7326/0003-4819-96-3-358. [DOI] [PubMed] [Google Scholar]
- 2.Hernández JA, Land KJ, McKenna RW. Leukemias, myeloma, and other lymphoreticular neoplasms. Cancer. 1995;75:381–381. doi: 10.1002/1097-0142(19950101)75:1+<381::aid-cncr2820751320>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105:1684–1684. doi: 10.1038/bjc.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–198. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5446. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis MN, Alloy AM, Chiesa JC, Pecora AA. Chronic lymphocytic leukemia presenting with massive chylous ascites. Am J Gastroenterol. 1990;85:593–6. [PubMed] [Google Scholar]
- 7.Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–34. [PubMed] [Google Scholar]
- 8.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3835. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 9.Swerdlow SH, Campo E, Harris NL, et al. World Health Organization classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 10.Tsimberidou AM, Wen S, O'Brien S, et al. Assessment of chronic lymphocytic leukemia and small lymphocytic lymphoma by absolute lymphocyte counts in 2,126 patients: 20 years of experience at the University of Texas M.D. Anderson Cancer Center. J Clin Oncol. 2007;25:4648–4648. doi: 10.1200/JCO.2006.09.4508. [DOI] [PubMed] [Google Scholar]
- 11.May JT, Costanzi JJ. Ascites in chronic leukemia: a case report and review of the literature. Oncology. 1982;39:55–8. doi: 10.1159/000225605. [DOI] [PubMed] [Google Scholar]
- 12.Shimoni A, Shvidel L, Shtalrid M, et al. Prolymphocytic transformation of B-chronic lymphocytic leukemia presenting as malignant ascites and pleural effusion. Am J Hematol. 1998;59:316–8. doi: 10.1002/(sici)1096-8652(199812)59:4<316::aid-ajh9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 13.Cuneo A, de Angeli C, Roberti MG, et al. Richter's syndrome in a case of atypical chronic lymphocytic leukaemia with the t(11;14)(q13;q32): role for a p53 exon 7 gene mutation. Br J Haematol. 1996;92:375–81. doi: 10.1046/j.1365-2141.1996.d01-1505.x. [DOI] [PubMed] [Google Scholar]
- 14.Desablens B, Gineston JL, Joly JP, et al. Immunoblastic lymphoma of the ileocecal region in chronic lymphoid leukemia. Richter's syndrome localized in the intestine and disclosed by ascites. Gastroenterol Clin Biol. 1987;11:901–3. [PubMed] [Google Scholar]
- 15.Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 31–1983. Chronic lymphocytic leukemia with the recent development of hepatosplenomegaly and ascites. N Engl J Med. 1983;309:297–305. doi: 10.1056/NEJM198308043090508. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui N, Al-Amoudi S, Aleem A, et al. World J Gastroenterol. Massive ascites as a presenting manifestation of chronic lymphocytic leukemia. 2008;14:3594–7. doi: 10.3748/wjg.14.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mouly S, Cochand-Priollet B, Halimi C, Bergmann JF. Portal hypertension caused by intra-hepatic block in chronic lymphoid leukemia. Presse Med. 1996;25:497–8. [PubMed] [Google Scholar]
- 18.Pauwels M, Pauwels S, Capron JP, et al. Portal hypertension caused by intra-hepatic block during chronic lymphoid leukemia. Gastroenterol Clin Biol. 2000;24:221–4. [PubMed] [Google Scholar]
- 19.Mamode C, Beauregard P, Langevin S, Mongeau CJ. Chronic lymphoid leukemia complicated with ascites. Can J Gastroenterol. 2000;14(SupplD):181D–184D. doi: 10.1155/2000/759693. [DOI] [PubMed] [Google Scholar]
- 20.Das DK. Serous effusions in malignant lymphomas: a review. Diagn Cytopathol. 2006;34:335–47. doi: 10.1002/dc.20432. [DOI] [PubMed] [Google Scholar]
- 21.Aoki Y, Tosato G. Vascular endothelial growth factor/vascular permeability factor in the pathogenesis of primary effusion lymphomas. Leuk Lymphoma. 2001;41:229–37. doi: 10.3109/10428190109057978. [DOI] [PubMed] [Google Scholar]
- 22.Hisada M, Biggar RJ, Greene MH, et al. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98:1979–81. doi: 10.1182/blood.v98.6.1979. [DOI] [PubMed] [Google Scholar]
- 23.Huhn D, von Schilling C, Wilhelm M, et al. Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood. 2001;98:1326–31. doi: 10.1182/blood.v98.5.1326. [DOI] [PubMed] [Google Scholar]
- 24.Smolej L. How I treat elderly or comorbid patients with chronic lymphocytic leukemia. Acta Medica (Hradec Kralove) 2010;53:213–20. doi: 10.14712/18059694.2016.79. [DOI] [PubMed] [Google Scholar]
- 25.Brown JR. The treatment of relapsed refractory chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:110–8. doi: 10.1182/asheducation-2011.1.110. [DOI] [PubMed] [Google Scholar]
- 26.Ng T, Pagliuca A, Mufti GJ. Intraperitoneal rituximab: an effective measure to control recurrent abdominal ascites due to non-Hodgkin's lymphoma. Ann Hematol. 2002l;81:405–6. doi: 10.1007/s00277-002-0479-y. [DOI] [PubMed] [Google Scholar]
- 27.Crysandt M, Neumann B, Das M, et al. Intraperitoneal application of rituximab in refractory mantle cell lymphoma with massive ascites resulting in local and systemic response. Eur J Haematol. 2007;79:546–9. doi: 10.1111/j.1600-0609.2007.00955.x. [DOI] [PubMed] [Google Scholar]


