Abstract
We report a very rare case of extensive ductal carcinoma in situ (DCIS) of the breast with secretory features in a 30-year old Japanese woman. The patient presented with a nodule in the lower inner quadrant of the left breast measuring approximately 2–3 cm, accompanied by an irregular tumor shadow with segmental microcalcification on mammography. These findings suggested malignancy, and excisional biopsy was performed following core needle biopsy. Pathological diagnosis was that of DCIS with secretory features. A treatment plan of simple mastectomy and sentinel lymph node biopsy was chosen. Most previous reports have only described invasive secretory carcinoma of the breast. We have only been able to find 2 case reports of non-invasive secretory lesion in the English literature to date. Because the characteristics of this lesion are not widely known, we thought it important to share our findings.
Key words: secretory carcinoma, breast cancer, ductal carcinoma in situ.
Introduction
Secretory carcinoma (SC) of the breast is a rare type of breast malignancy first described by McDivitt and Stewart in 1966.1 This type of carcinoma usually occurs in children and adolescent females. Many of the SC which had been previously reported were invasive ductal carcinoma. Non-invasive secretory carcinoma is extremely rare, and to the best of our knowledge, only 2 cases have been reported in the English literature to date.2,3 We report a rare case of ductal carcinoma in situ (DCIS) of the breast with secretory features in an adult female.
Case Report
A 30-year old unmarried female patient came to our hospital with a 6-month history of a nodule in the lower inner quadrant of her left breast. Her family history and past medical history were without significance. She had no family history of breast cancer and no past history of benign breast disease. She had no obstetric history. There was no history of previous use of oral contraceptives or hormonal replacement therapy. On physical examination, an irregular nodule with ill-defined borders was noted in the lower inner quadrant of the left breast measuring approximately 2–3 cm. Mammography showed an irregular tumor shadow with segmental microcalficication in the area (Figure 1). Sonographic examination revealed a heterogeneously hypoechoic lesion with ill-defined borders measuring approximately 20×16×15 mm (Figure 2). These findings suggested malignancy, and excisional biopsy was performed following core needle biopsy (CNB). Pathological diagnosis was that of DCIS with secretory features. The surgical margins were positive for malignancy and, therefore, a treatment plan of mastectomy with sentinel lymph node biopsy (SLNB) was programmed. Two sentinel lymph nodes were removed, and both were negative for metastases in the frozen and permanent section. The mastectomy specimen was confirmed to contain residual cancer. The microscopic findings were an extensive DCIS with secretory features measuring 10 cm at its largest diameter. The tumor cells showed a papillary growth pattern, forming focal sheets or cribriform pattern in the dilated mammary ducts (Figure 3A). The tumor nests showed a microcystic structure with round spaces containing an eosinophilic secretion and mimicked the appearance of pregnancy-like change (Figure 3B). The tumor contained abundant secretory material which showed a positive reaction for periodic acid-Schiff (PAS) (Figure 3C). The peripheral rim of myoepithelial cells were positive for HHF-35 immunoreactivity almost throughout (Figure 3D). The individual tumor cells had abundant pink-to-clear cytoplasm which vacuolated with abundant eosinophilic intracellular secretory material (Figure 3E). The oval-shaped nuclei varied in size and had moderate atypism. Immunohistochemically, the secretory material and cystoplasms of the tumor cells were negative for α-lactalbumin. Expression of estrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor (HER)-2 was evaluated by immunohistochemistry (IHC). The tumor was ER-positive, PgR-negative, and HER2-negative. Neither radiation nor adjuvant therapy were administered. The patient followed an uncomplicated postoperative course and is still followed on an outpatient basis. Five years after mastectomy, the patient has shown no evidence of recurrence.
Figure 1.
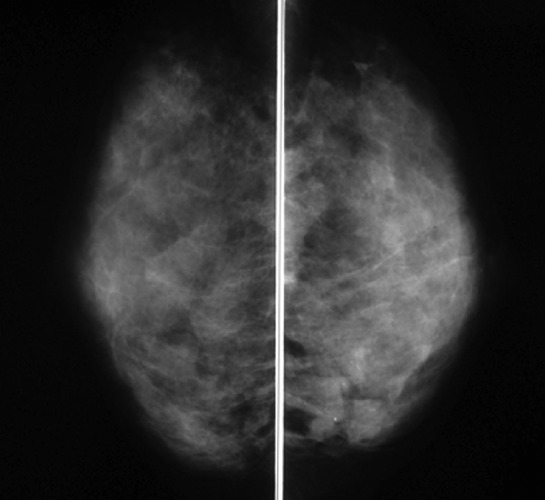
Mediolateral oblique mammography reveals an irregular tumor shadow with segmental microcalficication in the lower area of left breast.
Figure 2.
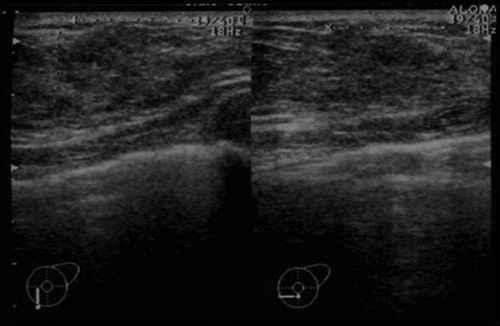
Ultrasonography shows a heterogeneously hypoechoic mass with ill-defined borders.
Figure 3.
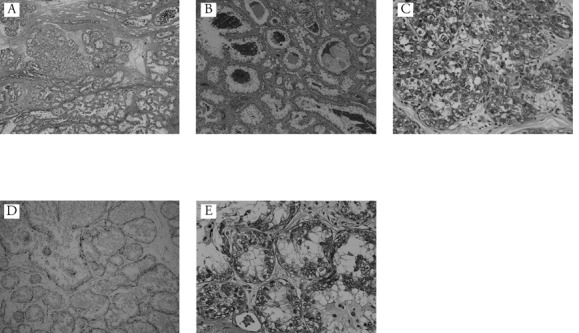
A) The tumor consisted of papillary and cribriform pattern in the dilated mammary ducts (Haematoxylin and Eosin 50×); B) the tumor nests show microcystic structure with round spaces containing an eosinophilic secretion. Histological features mimic those of pregnant or lactational changes (Haematoxylin and Eosin 100×); C) immunohistochemical staining for periodic acid-Shiff (immunohistochemical analysis of periodic acid-Shiff, 400×); D) immunohistochemical staining for HHF35 (immunohistochemical analysis of HHF35, 100×); E) most tumor cells have vacuolated cytoplasm, and eosinophilic materials can be seen in the vacuoles (Haematoxylin and Eosin 400×).
Discussion
Secretory carcinoma (SC) is one of the rarest types of breast cancer and accounts for less than 0.15% of all breast cancers.4 SC was initially reported in children and young girls, and was named juvenile carcinoma.1 Subsequently, cases in adult females have increasingly been reported. Tavassoli et al.4,5 reported that secretory carcinoma has been found in patients aged from 3 to 87 years of age (median 25 years). Ozguroglu et al.6 reported that most (approx. 70%) of the patients were over 20 years old and described the entity as a disease affecting adults. Therefore, the name secretory carcinoma is now preferred.7
SC can show several histological patterns including solid, microcystic, and tubular, and many tumors contain all three pattern.4,8 The tumor consists of polygonal cells with a vacuolated and granular eosinophilic cytoplasm.4,7,8 Atypia is mild or moderate and mitotic activity is usually low.3,6,7 A typical finding is the presence of secretory material both in the cytoplasm and in the glandular spaces formed by these cells.7 These tumor cells contain PAS or α-lactalbumin-positive secretory material.9,10
The histological features of SC mimic those of lactational changes and lactating adenoma.7,11 Lactating adenoma is actually diagnosed and excised during pregnancy, and does not produce lactational secretion.12 A clinical history of pregnancy is important in helping to distinguish it from SC.
We did not attempt a pregnancy test to determine whether the patient was pregnant, but her records confirmed the possibility of pregnancy to be unlikely.
The principal differential diagnosis of SC that have a prominent secretory or cystic component could be cystic hypersecretory carcinoma (CHC). In comparison with CHC, SC have only focal areas of cyst formation.13 SC contains vaculated cytoplasm and more bubbly secretions, which are not typical features of CHC.10 The histological features of our case were interpreted as consistent with SC. Myoepithelial cells in our case reacted strongly with HHF35. This finding meant that the tumor was not infiltrating the surrounding stroma. However, our case did not prove positive for α-lactalbumin. Therefore, we thought it was appropriate to describe this case as DCIS with secretory features. Secretory carcinoma of the breast is typically described as triple negative.3 Our case was ER-postive, PgR-negative, and HER2-negative. A previous study has reported that 4 and 2 out of 13 cases expressed ER and PgR, respectively, and only 2 were HER2 postive.14
Tognon et al.15 recently reported that SC of the breast are related to a characteristic balanced translocation, t(12;15), that causes an ETV6-NTRK3 gene fusion. The ETV6-NTRK3 is a chimeric tyrosine kinase protein with a potent transforming activity in multiple cell lineages.16 This chimeric protein seems to relate to a primary genetic lesion in SC.16 In addition, the ETV6-NTRK3 activates RAS-MAP kinases and PI3K-Akt pathways which are important for cancer cell proliferation.16 Lae et al.17 emphasize their genetic similarities from concordant ETV6 gene alterations in both in situ and invasive forms of SC. Detection of the t(12,15) translocation by fluorescence in situ hybridization (FISH) is the most reliable element for diagnosis of SC;15,16,18 however, we did not perform such a test.
SC belongs to the category of rare basal-like carcinomas that has a good prognosis.19 The metastases of axillary lymph node are uncommon, while a few patients have developed distant metastases.11,20 Several authors, therefore, propose that surgical treatment should be as conservative and minimally invasive as possible.
Many of the previously reported SC were invasive and, as far as we could find, our case is only the third reported case of non-invasive secretory carcinoma in the English literature to date. Because of the limited number of cases reported, the characteristics of non-invasive secretory carcinoma are difficult to grasp, and surgical treatment is a subject of debate.
DCIS is defined as non-invasive breast cancer and is, therefore, not expected to metastasize. No other invasive foci with a risk for metastasis could be found in the specimen apart from the DCIS. It has been previously reported that the rate of SLN positivity ranged from 0% to 13% in patients with pure DCIS, and from 10% to 30% in those with DCIS with micro-invasion (DCIS-MI).21 Previous reports showed that neither pre-operative CNB nor excisional biopsy are completely foolproof methods by which to identify pure DCIS, because there can be an invasive component in the specimen besides the DCIS.22–24 Because our case was an extensive DCIS tumor with indication for mastectomy, and since SLNB is impossible after mastectomy, we chose to treat with mastectomy with SLNB.
We describe a very rare case of DCIS of the breast with secretory features in a 30-year old Japanese woman who was treated with mastectomy and SLNB. To date, there has been no confirmation of local recurrence nor metastases.
Conclusions
SC is one of the rarest types of breast cancer, and many of the previously reported SC is invasive cancer. A typical finding is the presence of secretory material both in the cytoplasm and in the glandular spaces. The histological features of SC mimic those of lactational changes and lactating adenoma.
The principal differential diagnosis of SC that have a prominent secretory or cystic component is CHC. We reported a very rare case of extensive ductal carcinoma in situ (DCIS) of the breast with secretory features.
References
- 1.McDivitt RW, Stewart FW. Breast carcinoma in children. JAMA. 1966;195:388–90. [PubMed] [Google Scholar]
- 2.Kameyama K, Mukai M, Iri H, et al. Secretory carcinoma of the breast in a 51-year- old male. Pathol Int. 1998;48:994–7. doi: 10.1111/j.1440-1827.1998.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 3.Din NU, Idrees R, Fatima S, et al. Secretory carcinoma of breast: clinicopathologic study of 8 cases. Ann Diagn Pathol. 2012 doi: 10.1016/j.anndiagpath.2012.06.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Tavassoli FA, Devilee P. World Health Organization classification of tumors pathology and genetics of tumors of the breast and female genital organs. Lyon: IARC Press; 2003. [Google Scholar]
- 5.Tavassoli FA, Norris HJ. Secretory carcinoma of the breast. Cancer. 1980;45:2404–13. doi: 10.1002/1097-0142(19800501)45:9<2404::aid-cncr2820450928>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Ozguroglu M, Tascilar K, llvan S, et al. Secretory carcinoma of the breast. Case report and review of the literature. Oncology. 2005;68:263–8. doi: 10.1159/000086782. [DOI] [PubMed] [Google Scholar]
- 7.Rosen PP, Cranor ML. Secretory carcinoma of the breast. Arch Pathol Lab Med. 1991;115:141–4. [PubMed] [Google Scholar]
- 8.Richard G, Hawk JC, 3rd, Baker AS, Jr, et al. Multicentric adult secretory breast carcinoma: DNA flow cytometric findings, prognostic features and review of the world literature. J Surg Oncol. 1990;44:238–44. doi: 10.1002/jso.2930440410. [DOI] [PubMed] [Google Scholar]
- 9.Rosen PP. Rosen's breast pathology. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 10.Lamovec J, Bracko M. Secretory carcinoma of the breast: light microscopical, immunohistochemical and flow cytometric study. Mod Pathol. 1994;7:475–9. [PubMed] [Google Scholar]
- 11.Krausz T, Jenkins D, Grontoft O, et al. Secretory carcinoma of the breast in adults: emphasis on late recurrence and metastasis. Histopathology. 1989;14:25–36. doi: 10.1111/j.1365-2559.1989.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 12.Vesoulis Z, Kashkari S. Fine needle aspiration of secretory breast carcinoma resemling lactational changes. A case report. Acta Cytol. 1998;42:1032–6. doi: 10.1159/000331954. [DOI] [PubMed] [Google Scholar]
- 13.Tavassoli FA, Norris HJ. Secretory carcinoma of the breast. Cancer. 1980;45:2404–13. doi: 10.1002/1097-0142(19800501)45:9<2404::aid-cncr2820450928>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Diallo R, Schaefer KL, Bankfalvi A, et al. Secretory carcinoma of the breast: a distinct variant of invasive ductal carcinonma assessed by comparative genomic hybridization and immunohistochemistry. Hum Pathol. 2003;34:1299–305. doi: 10.1016/s0046-8177(03)00423-4. [DOI] [PubMed] [Google Scholar]
- 15.Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–76. doi: 10.1016/s1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 16.Lannon CL, Sorensen PH. ETV6-NTRK3: a chimeric protein tyrosine kinase with transformation activity in multiple cell lineages. Semin Cancer Biol. 2005;15:215–23. doi: 10.1016/j.semcancer.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Lae M, Freneaux P, Sastre-Garau X, et al. Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the basallike carcinoma. Mod Pathol. 2009;22:291–8. doi: 10.1038/modpathol.2008.184. [DOI] [PubMed] [Google Scholar]
- 18.Makretsov N, He M, Hayes M, et al. A fluorescence in situ hybridization study of ETV6-NTRK3 fusion gene in secretory breast carcinoma. Genes Chromosomes Cancer. 2004;40:152–7. doi: 10.1002/gcc.20028. [DOI] [PubMed] [Google Scholar]
- 19.Fadare O, Tavassoli FA. Clinical and pathologic aspects of basal-like breast cancers. Nat Clin Pract Oncol. 2008;5:149–59. doi: 10.1038/ncponc1038. [DOI] [PubMed] [Google Scholar]
- 20.Herz H, Cooke B, Goldstein D. Metastatic secretory breast cancer. Non-responsiveness to chemotherapy: case report and review of literature. Ann Oncol. 2000;11:1343–7. doi: 10.1023/a:1008387800525. [DOI] [PubMed] [Google Scholar]
- 21.Takács T, Paszt A, Szentpáli K, et al. Importance of sentinel lymph node biopsy in surgical therapy of in situ breast cancer. Pathol Oncol Res. 2009;15:329–33. doi: 10.1007/s12253-008-9123-z. [DOI] [PubMed] [Google Scholar]
- 22.Wilkie C, White L, Dupont E, et al. An update of sentinel lymph node mapping in patients with ductal carcinoma in situ. Am J Surg. 2005;190:563–6. doi: 10.1016/j.amjsurg.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Camp R, Feezor R, Kasraeian A, et al. Sentinel lymph node biopsy for ductal carcinoma in situ: an evolving approach at the University of Florida. Breast J. 2005;11:394–7. doi: 10.1111/j.1075-122X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 24.Zavagno G, Belardinelli V, Marconato R, et al. Sentinel lymph node metastasis from mammary ductal carcinoma in situ with microinvasion. Breast. 2007;16:146–51. doi: 10.1016/j.breast.2006.08.002. [DOI] [PubMed] [Google Scholar]


