Abstract
Polymorphonuclear leukocytes or neutrophils are the first immune cells to the site of injury and microbial infection. Neutrophils are crucial players in controlling bacterial and fungal infections, and in particular secondary infections, by phagocytosis, degranulation and neutrophil extracellular traps (NETs). While neutrophils have been shown to play important roles in viral pathogenesis, there is a lack of detailed investigation. In this article, we will review recent progresses toward understanding the role of neutrophils in viral pathogenesis.
Background
Neutrophils comprise the largest number of immune cells in the human body, approximately 80% at any given time, mainly during infections (Bai et al., 2010; Le Goffic et al., 2011; McNamara et al., 2003). While the majority of these cells remain housed in the bone marrow or immune centers (e.g. spleen and lymph nodes), about 2% of the total neutrophil population circulates in the blood (Navarini et al., 2009). These are short-lived cells, usually for 6–20 hours in circulation under healthy conditions (Croker et al., 2012; Navarini et al., 2009; Savill et al., 2002). During infections, however, their life-span is prolonged as neutrophils migrate from circulation (Colotta et al., 1992; Elbim et al., 2009) with the belief that this gives the cells the advantage to recruit more immune cells and prevent a pathogen from spreading. Several cytokines such as interferon-γ(IFN-γ), tumor necrosis factor (TNF) and granulocyte colony-stimulating factor (G-CSF) have been shown to prolong neutrophil survival during recruitment to the site of infection (Colotta et al., 1992). Respiratory syncytial virus (RSV) is able to prolong the survival of neutrophils, presumably through a PI3K- and NF-κB-dependent pathway (Lindemans et al., 2006). In addition, the life-span of neutrophils has also been reported to become significantly prolonged up to several days even when the body is under a homeostatic state and not under duress due to infection or inflammation (Pillay et al., 2010).
Being the first immune cells to the site of injury and infection, neutrophils act as the first line of defense against harmful microorganisms. Many studies have investigated the role of neutrophils in the pathogenesis of bacteria and fungi (Antachopoulos and Roilides, 2005; Choi and Dumler, 2003; Crameri and Blaser, 2002; Croker et al., 2012; Waldorf, 1989). Depletion of neutrophils or mutations in their key antimicrobial pathways increases susceptibility of the host to numerous bacterial and fungal pathogens, indicating that neutrophils play a vital protective role (Lekstrom-Himes and Gallin, 2000; Navarini et al., 2009). It has been demonstrated that neutrophils combat invading microorganisms by producing reactive oxygen species and releasing antimicrobial peptides and protein-decorated chromatin known as neutrophil extracellular traps (NETs) (Fuchs et al., 2007; Hemmers et al., 2011; Hoshino et al., 2008; Papayannopoulos and Zychlinsky, 2009; Segal, 2005). Conflicting to a protective role for the host, neutrophils have been found as reservoirs for microorganisms such as Staphylococcus aureus (Gresham et al., 2000; Voyich et al., 2005). In sand fly-mediated infections with the protozoan Leishmania, neutrophils initially capture the parasites and contribute to establishment of infection (Peters et al., 2008). Neutrophils also contribute to inflammation in a number of disease processes through a complex program of apoptosis (Kobayashi et al., 2003; Saez-Lopez et al., 2005; Savill et al., 1989) and an abundant zinc-chelating bacteriostatic and antifungal cytoplasmic protein, calprotectin (S100A8/A9) (Lusitani et al., 2003; Sohnle et al., 2000; Urban et al., 2009). Recently, there have been more and more studies concerning the role of neutrophils in viral pathogenesis. Here we will review the recent progresses toward understanding these important roles of neutrophils.
Protective roles
Neutrophils comprise a major element of the innate immune system and demolish invading microbes primarily by phagocytosing bacteria, fungi and virions (Choi and Dumler, 2003; Chtanova et al., 2008; Crameri and Blaser, 2002; Ferrante, 1989; Hachicha et al., 1998; Nathan, 2006; Soehnlein, 2009a; Tate et al., 2009; Wang et al., 2008). Viral infections will usually weaken and impair the host's immune system opening the way for opportunistic pathogens, such as bacteria or fungi, to take advantage of the debilitated host. Neutrophils play essential roles in protecting against such opportunistic infection including pathogens like Candida albicans (Djeu et al., 1993; Ruthe et al., 1978; Urban et al., 2006), S. aureus, Pseudomonas aeruginosa (Boucher, 2004; Ulrich et al., 2010; Worlitzsch et al., 2002), Aspergillus fumigatus (Tkalcevic et al., 2000) and Fusarium spp. (Gaviria et al., 1999; Roilides et al., 1993). If neutrophils are not efficiently recruited to the site of infection, there is a high chance of secondary infection occurring, leading to tissue damage, possible multi-organ failure and/or mortality. Clearance of neutrophils post-influenza infection impairs bacterial clearance (Elbim et al., 2009), but this appears not to have an effect on the pathology due to the actual viral affliction (Tumpey et al., 2005); while depletion prior to infection increases host mortality in the mouse model (Hemmers et al., 2011). However, others have found increased survival in mice when blocking with specific antibodies the recruitment chemokines CXCR2 and IRAK-M (IL-1 receptor-associated kinase-M) (Seki et al., 2010), which results in a lower neutrophil influx.
Activated neutrophils produce large amounts of reactive oxygen species (ROS) that can be devastating to invading microorganisms. One pathway to activate neutrophils is through the actions of NADPH oxidases in conjunction with the release of proteolytic enzymes (Fuchs et al., 2007; Nathan, 2006). Respiratory burst is another means by which neutrophils become activated and induce ROS production and potentially release various latent proteases that remain in specific granules (Colamussi et al., 1999; Engelich et al., 2002; Tkalcevic et al., 2000). ROS activate the innate immune system, not only during bacterial infections (Rada and Leto, 2008), but viral infections as well (Gonzalez-Dosal et al., 2011). Herpes simplex virus (Gonzalez-Dosal et al., 2011; Hu et al., 2011) and human cytomegalovirus (CMV) (Speir et al., 1996) have been shown to induce ROS production in phagocytic cells (Gonzalez-Dosal et al., 2011). Cells infected with respiratory syncytial virus (RSV) activate efflux of potassium ions and intracellular ROS as a signal for the stimulation of caspase-1 and IL-1β release (Segovia et al., 2012).
The extracellular formation of NETs is another intriguing and effective means of neutrophils to control and eliminate infections via containment and accentuates the synergistic effect of antimicrobials and elevating their concentrations. Mature neutrophils are able to release NETs following activation via a soluble agent such as LPS, IL-8 or phorbolmyristate acetate (PMA) (Brinkmann et al., 2004; Fuchs et al., 2007; Urban et al., 2006) or Fc receptors, chemokine and cytokine receptors, Toll-like receptor ligations (Wartha et al., 2007) and platelet-induced activation (Clark et al., 2007). NET formation has been shown to be independent of caspase activity (Fuchs et al., 2007). Evidence points to ROS generation as a major factor in the formation and release of NETs (Fuchs et al., 2007; Papayannopoulos and Zychlinsky, 2009). ROS-dependentNETs formation is associated with intra-phagosomal killing of neutrophils, which process is distinct from apoptosis and necrosis (Fuchs et al., 2007). NET formation is more recently referred to as NETosis, involving a unique form of cell death, but this process is more generally called ETosis based on other immune cells (e.g. eosinophils, mast cells) ability to form these antimicrobial constructs (Brinkmann and Zychlinsky, 2012; Fuchs et al., 2007; Guimarães-Costa et al., 2012). NETs are made up of chromatin, either nuclear (from dead or dying cells) or mitochondrial (from living cells) (Soehnlein, 2009b; Yousefi et al., 2008; Yousefi et al., 2009), embellished with proteins (e.g. myeloperoxidase and elastase) (Brinkmann et al., 2004; Urban et al., 2006; Wartha et al., 2007) and released from granules, which are effective at binding both Gram-positive and Gram-negative bacteria, as well as fungi; for instance calprotectin is a highly effective antifungal agent against C. albicans (Soehnlein, 2009a; Urban et al., 2009; Urban et al., 2006; Wartha et al., 2007). Accounting for approximately 70%, the most abundant component of NETs are histones (Urban et al., 2009), which are more effective against bacteria than fungi (Guimarães-Costa et al., 2012). These NET constructs are one of the ways to halt damage caused by fungal hyphae (e.g. C. albicans and Rhizopus) since these structures are too large to be phagocytosed by neutrophils (Crameri and Blaser, 2002; Urban et al., 2006). NETs can increase the local concentration of antimicrobial molecules that kill microbes effectively. Figure 1 shows neutrophil NETs formed by stimulation of S. aureus, PMA and glucose oxidase (Fuchs et al., 2007). In cases of viral infection, for instance, when cats are infected with feline leukemia virus (FeLV), NET formation becomes compromised (Wardini et al., 2010). A recent report showed that neutrophils recognize HIV-1 by TLR-7 and -8. Engagement of TLR7 and 8 induces ROS that triggers NET formation, which captures HIV and promote HIV-1 elimination through myeloperoxidase and α-defensin (Saitoh et al., 2012). However, HIV-1 induced IL-10 production may suppress ROS expression, thus inhibiting the ROS-dependentNET formation (Saitoh et al., 2012).
Figure 1. Immunostaining of NETs.
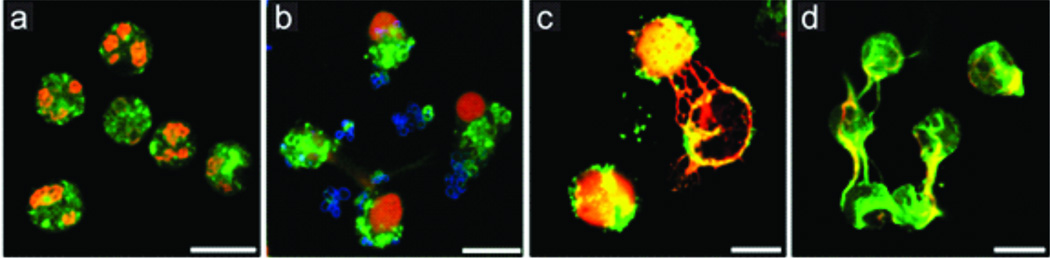
(green, neutrophil elastase; red, histone-DNA complex), (a) Unstimulated neutrophils (no NETs). NETs formed after neutrophils stimulated with S. aureus (b, blue), PMA (c) and glucose oxidase (d). Bars, 10 urn. © 2007 Rockefeller University Press. Originally published in Journal of Cell Biology. Vol 176. doi: 10.1083/jcb.200606027
The role of neutrophils during influenza infection ranges from protective to pathology-promoting. Depletion of neutrophils prior to infection with influenza A leads to increased mortality in mice, suggesting a protective role for neutrophils (Tumpey et al., 2005). Massive infiltration of neutrophils into the lungs during influenza virus infection can result in acute lung inflammation (Seki et al., 2010; Tate et al., 2008). In addition, a study has reported that increased amounts of NETs are detrimental during chronic lung inflammation (Marcos et al., 2010). Histone H3 and H4 deimination mediated by peptidylarginine deiminase 4 (PAD4) has been suggested to associate with NET formation (Wang et al., 2009), but PAD4-mediated NET formation seems to not be required for immunity against influenza infection in the mouse model. Mortality rates were equivalent in PAD4-deficient mice, which fail to form NETs, with wild-type which can form NETs (Hemmers et al., 2011). Beyond the most recent studies on FeLV, HIV-1 and influenza A, there is a lack of data on the capability for viruses to induce NET formation.
Increasing evidence also points to a direct influence of neutrophils on the adaptive immune response to viral infections (Elbim et al., 2009; Pillay et al., 2010). This act of direct influence could be accomplished through antigen presentation, transferring pathogens to draining lymph nodes and modulation of T helper cell responses (Pillay et al., 2010). A recent study reported that neutrophils served as antigen-presenting cells to anti-viral effector CD8+ T cells within the influenza A virus infected lung in mouse model (Hufford et al., 2012). Activation of dendritic cells via granule proteins either through granular release or as components of NETs activates TLR-9 in dendritic cells. Translation from innate immunity to the adaptive immune phase thus begins via dendritic cells with the production of interferons (Garcia-Romo et al., 2011; Lande et al., 2011; Yang et al., 2009). IFNs, which can modulate neutrophil responses to TLR ligands or neutrophil chemoattractant production of KC (CXCL1) and CXCL-2, during viral infections appear to have an effect on the adaptive immune system (Munir et al., 2011).
Activated neutrophils can release granular components and chemokines including IL-8, which recruits more neutrophils (Hachicha et al., 1998) and also prolongs their life-span via TNF and Fas-mediated receptors (Colotta et al., 1992). The major component, found in azurophilic granules of the neutrophil, is myeloperoxidase (MPO) (Segal, 2005; Seo et al., 2011). MPO is a pro-inflammatory heme enzyme comprising approximately 5% of total neutrophil protein, but 25% of granular protein (Segal, 2005). MPO, cathepsin G, and HNPs (human neutrophil peptides also known as α-defensin) have been shown to activate adaptive immune cells and ultimately shape an immune response during contagion (Soehnlein, 2009b; Soehnlein et al., 2008). HNPs are active against enveloped viruses and induce dendritic cell maturation (Yang et al., 2009). More work needs to be done to elucidate the exact mechanism(s) by which HNPs directly or indirectly drive chemotaxis and maturation of antigen-presenting cells, induce cytokine production, and increase lymphocyte activation and antigen presentation (Yang et al., 2009).
MPO-ANCAs (anti-neutrophil cytoplasmic autoantibodies) induce IL-17 production, playing a role in the progression of various diseases and allows activated neutrophils to alter the environment to differentiate Thl7 cells through the cytokines IL-6, IL-17 and IL-23 production. Cytokines IL-23 and IL-17 are involved in neutrophil recruitment during viral infections (Ferretti et al., 2003; Stark et al., 2005). Both cytokines have also been shown to improve resistance to bacterial and viral infections (e.g. vaccinia virus) (Kohyama et al., 2007). During the acute inflammatory response, MPO has also been determined to be a vital component in the initiation and execution against microbial infection (Hoshino et al., 2008). Investigation is lacking however on exactly how MPO-ANCAs stimulate cytokine production via neutrophils and in particular any potential role in viral infections.
Double-edged sword role
Neutrophils must be tightly regulated otherwise tissue damage is inevitable due to increased infiltration of these cells, which may lead to vascular leakage and high release of catalytic proteins and NETs. During the resolution phase of immune responses, clearance of neutrophils and other recruited immune cells occurs. Any defects in this process will cause inflammatory diseases (Croker et al., 2012) or susceptibility to secondary infections. Too many NETs and high amounts of microbial material can prove difficult for the host to clear during and after infection (Brinkmann et al., 2004). In addition, damage to host tissues from antimicrobial material released by neutrophils can occur, for instance elastase cleaves not only bacterial wall components, but also host proteins at the site of inflammation (Fujie et al., 1999). NETs have been shown to prevent such cases by minimizing diffusion of the granular components (Papayannopoulos and Zychlinsky, 2009).
Naturally dying neutrophils go through the apoptosis process. Apoptotic neutrophils are engulfed by macrophages to be cleared from the body's circulation (Savill et al., 1989). This engulfment has been shown to inhibit pro-inflammatory mediator production through release of TGF-β by macrophages (Elbim et al., 2009). Alteration in this apoptosis pathway impairs regulation causing neutrophil numbers to either increase or decrease, with either course potentially increasing the pathology of the affected tissue.
Neutrophil infiltration correlates with the level of inflammation, tissue damage and feed-back of the affected tissue (Seo et al., 2011). Inflammation is helpful in controlling viral infection, but it very so often becomes damaging to the host. A balance between beneficial and detrimental effects of the neutrophils initiated inflammatory responses must be maintained (Rosenthal et al., 2009; Rosenthal et al., 2011). Hyper-inflammation has been noted in chronic cases of hepatitis B virus infection in which a large influx of neutrophils causes liver damage (Zhang et al., 2011). Unregulated neutrophils into an infection site have also been observed with rift valley fever virus infections in mice (Gray et al., 2011), which can lead to permanent neurological disorders and the function of the liver can be significantly lost. Aggressive inflammatory cytokine production ("cytokine storm") is a key component of acute lung injury observed during influenza infections (e.g. 1918 avian strain; H5N1 strain) (Seo et al., 2011). Persistent infection by influenza virus may cause neutrophils to become over-active and produce excessive cytokines such as TNF and IL-1 (Fang et al., 2011). This considerable neutrophil influx ends up damaging the tissue, adding to the immunopathology associated with highly pathogenic viral strains such as the H5N1 strain and the 1918 H1N1 strain (Hemmers et al., 2011; Kobasa et al., 2007). Nagarkar et al. (2009) showed that the rhinovirus causes an increase in neutrophilic response and inflammation in the airways, which with regards to asthmatic patients, causes severe damage to the respiratory system (Nagarkar et al., 2009). Blockage or depletion of MIP-2 (CXCL2), a neutrophil-recruiting chemokine, has been shown to be associated with a reduced neutrophil recruitment into the lung of A/PR/8/34 virus (PR8, H1N1 strain) infected mice resulting in a reduced lung pathology (Tate et al., 2009). Interestingly, the pertussin toxin purified from Bordetellapertussis has been reported to enhance viral titers of the influenza A virus during concomitant infection by targeting an early immune response, reducing recruitment of neutrophils into infected tissue. However, another report shows the toxin, once inactivated, diminishes the cytokine storm, thus reducing neutrophil numbers into the infected lung tissue and subsequently reducing damage to the lung (Li et al., 2010). Hence, more work is needed to recognize which agent during concomitant infections between viral pathogens and those such as B. pertussis can cause one or the other to either protect or damage the host.
Herpes simplex virus (HSV-1) infection can cause stromal keratitis lesions, a chronic inflammation of the eye. During treatment using a variety of antiviral and anti-inflammatory agents, reactivation of the lesions commonly occur by the hosts' inflammatory response (Deshpande et al., 2004; Knickelbein et al., 2009) or a risk of intraocular pressure elevations and formation of cataracts. The disease can be partially controlled with the administration of a resolvin agent, RvEl, which are mediators derived from omega-3 fatty acids eicosapentaenoic and docosahexaenoic acid (Serhan et al., 2008). RvEl was shown to inhibit the production of IFN-γ, IL-6 and IL-17, which was correlated with a reduced influx of neutrophils in the neovascular system where lesions occurred (Rajasagi et al., 2012). IL-17 stimulates production of TNF and IL-1β from macrophages with implications that it stimulates granulopoiesis and recruitment of neutrophils into selective tissues (Ferretti et al., 2003). Depletion of neutrophils or neutralization of IL-17A during HSV-2 (Tkalcevic et al., 2000) and mouse CMV infections increases survival of mice and reduces damage to the liver (Stout-Delgado et al., 2009).
In the case of West Nile virus (WNV) infection, a rapid recruitment of neutrophils is observed to the site of infection and is caused by elevated chemokine levels, CXCL1 and CXCL2, produced by macrophages (Bai et al., 2010). An antiviral program can be initiated within 24 h post-infection by immune cells and entails the innate immune system utilizing pattern recognition receptors, such as Toll-like receptors, to recognize components of WNV to begin the antiviral signaling of the host with subsequent production of chemokines and cytokines for recruiting neutrophils (Bai et al., 2009; Daffis et al., 2007; Kobayashi et al., 2005; Town et al., 2009; Wang et al., 2004). Depletion of neutrophils prior to WNV infection, in addition to a deficiency in the chemokine receptor gene Cxcr2, results in reduced viremia and enhanced survival of the host (Bai et al., 2010). However, depletion of neutrophils after WNV infections results in higher viremia (Bai et al., 2010) with an earlier onset of mortality, suggesting that neutrophils have complicated roles during WNV infections.
Defective NET formation is common among people with chronic granulomatous disease (Brinkmann and Zychlinksy, 2007; Fuchs et al., 2007). The genes that encode the NADPH oxidase have mutations resulting in disruption of the complex which generates ROS (Papayannopoulos and Zychlinsky, 2009). It is possible that formation of NETs can contribute to autoimmune diseases through the initiation and/or propagation phase of disease (Brinkmann and Zychlinsky, 2012; Papayannopoulos and Zychlinsky, 2009). Autoimmune disorders are being increasingly studied with respect to the actions of neutrophils and their products (e.g. NETs, MPO) during viral infections; however, this area of research is still incredibly young and is without enough evidence to pin down the effects neutrophils have on the host while defending against viruses.
Neutrophils as viral transport vessels
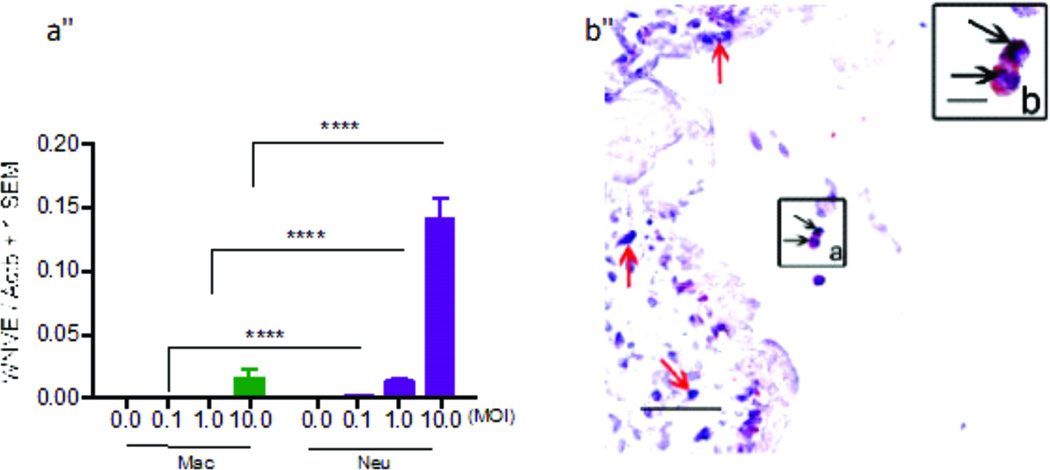
Neutrophils are the most abundant cells circulating in the human blood stream. Interestingly, neutrophils have shown to be productively infected by West Nile virus (WNV), implying that neutrophils may be responsible for WNV dissemination in the peripheral and central nervous system (CNS). Higher titers of WNV were detected in neutrophils compared to macrophages supporting the notion of viral replication in neutrophils (Bai et al., 2010) (Figure 2a). In addition, both positive- and negative-sense viral RNA of WNV have been detected in human and mouse neutrophils demonstrating that WNV is able to replicate within them (Bai et al., 2010). Neutrophils have been reported to be rapidly recruited into the CNS once WNV penetrates the brain (Bréhin et al., 2008; Rawal et al., 2006; Tyler et al., 2006), suggesting that infected neutrophils may bring more viruses into the CNS and make the infection more severe. Nevertheless, more investigation needs to be done to address whether this is the case.
Figure 2. Neutrophils may be a reservoir for H5N1 and WNV.
(a) Q-PCR results of WNV-infected human monocyte-derived macrophages and neutrophils at 8 h, suggesting neutrophils may act as a reservoir for WNV, **** denotes p < 0.0001, adapted from Bai et al, 2010. (b) H5N1 antigen (purple-blue; black arrows) is detected in CD15-positive cells (a neutrophil marker, red-brown; black arrows) in placental villi (inset a). Inset b shows enlargement of inset a, which shows that neutrophils contain H5N1 nucleotide sequences (bar, 10 urn), adapted from Zhao, et al., 2008. © Oxford Journals Oxford University Press.
Most of H5N1 virus replicate in the respiratory tract following initial infection. However, H5N1 particles have also been found in other places of the body such as the trachea and intestine (Gu et al., 2007; Zhao et al., 2008). Furthermore, viral antigens have been found in both the cytoplasm and nucleus of neutrophils located in the spleen and placental tissues (Zhao et al., 2008). Nuclear localization of the virus points to the possibility that H5N1 may be able to replicate in neutrophils after entering the cell through receptor-mediated endocytosis, implying that neutrophils transport the virus to other tissues from the respiratory tract (Gu et al., 2007; Zhao et al., 2008) (Figure 2b). Neutrophils may also be responsible for CMV transmission. It has been hypothesized that CMV stimulates endothelial cells to produce IL-8 and C-X-C chemokines, recruiting neutrophils to the site of infection and uses these cells for viral transmission (Grundy et al., 1998). When endothelial cells infected with CMV, they secrete chemokines recruiting neutrophils while at the same time release viral particles (Grundy et al., 1998). During transendothelial migration, neutrophil-endothelial cell contact allows the virus to spread to the neutrophils. Neutrophils coming into contact with other cells, in particular fibroblasts, can then spread CMV to those cells (Grundy et al., 1998).
Besides WNV and H5N1, Epstein-Barr virus (EBV) has also been reported to replicate in neutrophils (Savard and Gosselin, 2006). EBV viral genome has been detected in neutrophils, but neutrophils typically initiate a pronounced antiviral program leading to programmed cell death, which leads up to neutropenia (Douglas Jr. et al., 1966; Gosselin et al., 2001; Savard and Gosselin, 2006), thus not allowing the virus to reside and replicate in the cells. Further investigation is required for elucidation, but recent work points to EBV-encoded proteins that may be able to prevent phagocyte apoptosis, thus being able to circumvent the immune system (Savard and Gosselin, 2006).
Concluding remarks
Neutrophils, as a major component in the mammalian innate immune system, have essential roles in the battle with invading bacteria, fungi as well as viruses. More and more attentions have been drawn to dissecting the roles of neutrophils in viral pathogenesis; however, we still know very little about the true nature of neutrophils during the course of viral infections. Neutrophils appear to contribute to controlling viral infections, but unbalanced recruitment may cause severe damage to the targeting tissues or organs during influenza and other viral infections. WNV, H5N1 and EBV have been found to productively infect neutrophils, thus these viruses may utilize the infected neutrophils as transport vehicles for dissemination in the body. Clinical manipulation of neutrophils to control viral infection should be cautious for which may cause opportunistic infections or immunopathology. In conclusion, more investigations should be made to understand the complicated roles of neutrophils in viral pathogenesis and the immune system. Further investigations may aim at the following directions: the mechanisms by which neutrophils control virus infections, such as induction of ROS and NETs; the role neutrophils in induction of adaptive immunity; and mechanisms that neutrophils as transmission vectors for WNV and other viruses.
Acknowledgement
This work was supported in part by the Mississippi INBRE funded by grants from the National Center for Research Resources (5P20RR016476-11) and the National Institute of General Medical Sciences (8 P20 GM103476-11) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antachopoulos C, Roilides E. Cytokines and fungal infections. Br. J. Haematol. 2005;129:583–596. doi: 10.1111/j.1365-2141.2005.05498.x. [DOI] [PubMed] [Google Scholar]
- Bai F, Kong K-F, Dai J, Qian F, Zhang L, Brown C, Fikrig E, Montgomery R. A paradoxical role for neutrophils in the pathogenesis of west nile virus. J. Infect. Dis. 2010;202:1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Town T, Qian F, Wang P, Kamanaka M, Connolly T, Gate D, Montgomery R, Flavell R, Fikrig E. IL-10 signaling blockade controls murine West Nile virus infection. PLoS Pathog. 2009;5:e1000610. doi: 10.1371/journal.ppat.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher R. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Bréhin A, Mouriés J, Frenkiel M, Dadaglio G, Després P, Lafon M, Couderc T. Dynamics of immune cell recruitment during West Nile encephalitis and identificaiton of a new CD19+B220- BST-2+ leukocyte population. J. Immunol. 2008;180:6760–6767. doi: 10.4049/jimmunol.180.10.6760. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss D, Weinrauch Y, Zychlinksy A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Zychlinksy A. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J. Cell Biol. 2012;198:773–783. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K-S, Dumler J. Early induction and late abrogation of respiratory burst in A. phagocytophilum-infected neutrophils. Ann N.Y Acad. Sci. 2003;990:488–493. doi: 10.1111/j.1749-6632.2003.tb07415.x. [DOI] [PubMed] [Google Scholar]
- Chtanova T, Schaeffer M, Han S-J, van Dooren G, Nollmann M, Herzmark P, Chan S, Satija H, Camfield K, Aaron H, Striepen B, Robey E. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 2008;29:487–496. doi: 10.1016/j.immuni.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S, Ma A, Tavener S, McDonald B, Goodarzi Z, Kelly M, Patel K, Chakrabarti S, McAvoy E, Sinclair G, Keys E, Allen-Vercor E, Devinney R, Doig C, Green F, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- Colamussi M, White M, Crouch E, Hartshorn K. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood. 1999;93:2395–2403. [PubMed] [Google Scholar]
- Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- Crameri R, Blaser K. Allergy and immunity to fungal infections and colonization. Eur. Respir J. 2002;19:151–157. doi: 10.1183/09031936.02.00229102. [DOI] [PubMed] [Google Scholar]
- Croker B, Roberts A, Nicola N. Towards a four-dimensional view of neutrophils. In: Ashmann R, editor. Leukocytes: Methods and Protocols, Methods in Molecular Biology. Vol. 844. Springer; 2012. pp. 87–99. [DOI] [PubMed] [Google Scholar]
- Daffis S, Samuel M, Keller B, Gale M, Diamond M. Cell-specific IRF-3 responses protect against West Nile virus infection by interferon-depenednt and -independent mechanisms. PLoS Pathog. 2007;3:e106. doi: 10.1371/journal.ppat.0030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande S, Banerjee K, Biswas P, Rouse B. Herpetic eye disease: immunopathogenesis and therapeutic measures. Expert Rev Mol Med. 2004;6:1–14. doi: 10.1017/S1462399404007604. [DOI] [PubMed] [Google Scholar]
- Djeu J, Liu J, Wei S, Rui H, Pearson C, Leonard W, Blanchard D. Function associated with IL-2 receptor-β on human neutrophils. J. Immunol. 1993;150:960–970. [PubMed] [Google Scholar]
- Douglas R, Jr, Alford R, Cate T, Couch R. The leukocyte response during viral respiratory illness in man. Ann. Intern. Med. 1966;64:521–530. doi: 10.7326/0003-4819-64-3-521. [DOI] [PubMed] [Google Scholar]
- Elbim C, Katsikis P, Estaquier J. Neutrophil apoptosis during viral infections. Open Virol. J. 2009;3:52–59. doi: 10.2174/1874357900903010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelich G, White M, Hartshorn K. Role of the respiratory burst in co-operative reduction in neutrophil survival by influenza A virus and Escherichia coli. J. Med. Microbiol. 2002;51:484–490. doi: 10.1099/0022-1317-51-6-484. [DOI] [PubMed] [Google Scholar]
- Fang M, Wan M, Guo S, Sun R, Yang M, Zhao T, Yan Y, Zhang Y, Huang W, Wu X, Yu Y, Wang L, Hua S. An oligodeoxynucleotide capable of lessening acute lung inflammatory injury in mice infected by influenza virus. Biochem. Biophys. Res. Commun. 2011;415:342–347. doi: 10.1016/j.bbrc.2011.10.062. [DOI] [PubMed] [Google Scholar]
- Ferrante A. Tumor necrosis factor alpha potentiates neutrophil antimicrobial activity: increased fungicidal activity against Torulopsis glabrata and Candida albicans and associated increases in oxygen radical production and lysosomal enzyme release. Infect. Immun. 1989;57:2115–2122. doi: 10.1128/iai.57.7.2115-2122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti S, Bonneau O, Dubois G, Jones C, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- Fuchs T, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinksy A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujie K, Shinguh T, Inamura N, Yasumitsu R, Okamoto M, Okuhara M. Release of neutrophil elastase and its role in tissue injury in acute inflammation: effect of the elastase inhibitor, FR 134043. Eur J Pharmacol. 1999;374:117–125. doi: 10.1016/s0014-2999(99)00268-x. [DOI] [PubMed] [Google Scholar]
- Garcia-Romo G, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman R, Barrat F, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaviria J, van Burik J, Dale D, Root R, Liles W. Comparison of interferon-gamma, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor for priming leukocyte-mediated hyphal damage of opportunistic fungal pathogens. J. Infect. Dis. 1999;179:1038–1041. doi: 10.1086/314679. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Dosal R, Horan K, Rahbek S, Ichijo H, Chen Z, Mieyal J, Hartmann R, Paludan S. HSV infection induces production of ROS, which potentiates signaling from pattern recognition receptors: role for S-glutathione of TRAF3 and 6. PLoS Pathog. 2011;7:e1002250. doi: 10.1371/journal.ppat.1002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin J, Savard M, Tardif M, Flamand L, Borgeat P. Epstein-Barr virus primes human polymorphonuclear leucocytes for the biosynthesis of leukotriene B4. Clin. Exp. Immunol. 2001;126:494–502. doi: 10.1046/j.1365-2249.2001.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K, Worthy M, Juelich T, Agar S, Poussard A, Ragland D, Freiberg A, Holbrook M. Chemotactic and inflammatory responses in the liver and brain are associated with pathogenesis of rift valley fever virus infection in the mouse. PLoS Negl. Trop. Dis. 2011;6:e1529. doi: 10.1371/journal.pntd.0001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham H, Lowrance J, Caver T, Wilson B, Cheung A, Lindberg F. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 2000;164:3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- Grundy J, Lawson K, MacCormac L, Fletcher J, Young K. Cytomegalovirus-infected endothelial cells recruit neutrophils by the secretion of C-X-C chemokines and transmit virus by direct neutrophil-endothelial cell contact and during neutrophils transendothelial migration. J. Infect. Dis. 1998;177:1465–1474. doi: 10.1086/515300. [DOI] [PubMed] [Google Scholar]
- Gu J, Xie Z, Gao Z, Liu J, Korteweg C, Ye J, Lau L, Lu J, Gao Z, Zhang B, McNutt M, Lu M, Anderson V, Gong E, Yu A, Lipkin W. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet. 2007;370:1137–1145. doi: 10.1016/S0140-6736(07)61515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães-Costa A, Nascimento M, Wardini A, Pinto-da-Silva L, Saraiva E. ETosis: a microbicidal mechanism beyond cell death. J Parasitol Res 2012. 2012 doi: 10.1155/2012/929743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachicha M, Rathanaswami P, Naccache P, McColl S. Regulation of chemokine gene expression in human peripheral blood neutrophils phagocytosing microbial pathogens. J. Immunol. 1998;160:449–454. [PubMed] [Google Scholar]
- Hemmers S, Teijaro J, Arandjelovic S, Mowen K. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS ONE. 2011;6:e22043. doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Nagao T, Nagi-Miura N, Ohno N, Yasuhara M, Yamamoto K, Nakayama T, Suzuki K. MPO-ANCA induces IL-17 production by activated neutrophils in vitro via classical complement pathway-dependent manner. J. Autoimmun. 2008;31:79–89. doi: 10.1016/j.jaut.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng W, Schachtele S, Lokensgard J. Reactive oxygen species drive herpes simplex virus (HSV)-1-induced proinflammatory cytokine production by murine microglia. J. Neuroinflammation. 2011;8 doi: 10.1186/1742-2094-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufford M, Richardson G, Zhou H, Manicassamy B, CGarcia-Sastre A, Enelow R, Braciale T. Influenza-infected neutrophils within the infected lungs act as antigen presenting cells for anti- viral CD8(+) T cells. PLoS ONE. 2012;7:e46581. doi: 10.1371/journal.pone.0046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickelbein J, Hendricks R, Charukamnoetkanok P. Management of herpest simplex virus stromal keratitis: and evidence-based review. Surv Ophthalmol. 2009;54:226–234. doi: 10.1016/j.survophthal.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Kobasa D, Jones S, Shinya K, Kash J, Copps J, Ebihara H, Hatta Y, Kim J, Halfmann P, Hatta M, Feldmann F, Alimonti J, Fernando L, Li Y, Katze M, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Voyich J, Burlak C, DeLeo F. Neutrophils in the innate immune response. Arch. Immunol. Ther. Exp. (Warsz) 2005;53:505–517. [PubMed] [Google Scholar]
- Kobayashi S, Voyich J, Somerville G, Braughton K, Malech H, Musser J, DeLeo F. An apoptosis-differentiation program in human polymorphonuclear leukocytes facilitates resolution of inflammation. J. Leukoc. Biol. 2003;73:315–322. doi: 10.1189/jlb.1002481. [DOI] [PubMed] [Google Scholar]
- Kohyama S, Ohno S, Isoda A, Osamu M, Belladonna M, Hayashi H, Iwakura Y, Yoshimoto T, Akatsuka T, Matsui M. IL-23 enhances host defense against vaccinia virus infection via a mechanism partly involving IL-17. J. immunol. 2007;179:3917–3925. doi: 10.4049/jimmunol.179.6.3917. [DOI] [PubMed] [Google Scholar]
- Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu Y, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic R, Leymarie O, Chevalier C, Rebours E, Da Costa B, Vidic J, Descamps D, Sallenave J-M, Rauch M, Samson M, Delmas B. Transcriptomic analysis of host immune and cell death responses associated with the influenza A virus PB1-F2 protein. PLoS ONE. 2011;7:e1002202. doi: 10.1371/journal.ppat.1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekstrom-Himes J, Gallin J. Immunodeficiency diseases caused by defects in phagocytes. N. Engl. J. Med. 2000;343:1703–1714. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]
- Li R, Lim A, Phoon M, Narasaraju T, Ng J, Poh W, Sim M, Chow V, Locht C, Alonso S. Attenuated Bordetella pertussis protects against highly pathogenic influenza A viruses by dampening the cytokine storm. J. Virol. 2010;84:7105–7113. doi: 10.1128/JVI.02542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemans C, Coffer P, Shcellens I, de Graaff P, Kimpen J, Koenderman L. Respiratory syncytial virus inhibits granulocyte apoptosis through a phosphatidylinositol 3-kinase and NF-κB- dependent mechanism. J. Immunol. 2006;176:5529–5537. doi: 10.4049/jimmunol.176.9.5529. [DOI] [PubMed] [Google Scholar]
- Lusitani D, Malawista S, Montgomery R. Calprotectin, an abundant cytosolic protein from human polymorphonuclear leukocytes, inhibits the growth of Borrelia burgdorferi. Infect. Immun. 2003;71:4711–4716. doi: 10.1128/IAI.71.8.4711-4716.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos V, Zhou Z, Yildirim A, Bohla A, Hector A, Vitkov L, Wiedenbauer E, Krautgartner W, Stoiber W, Belohradsky B, Rieber N, Kormann M, Koller B, Roscher A, Roos D, Griese M, Eickelberg O, Döring G, Mall M, Hartl D. CXCR2 mediates NADPH oxidase- independent neutrophil extracellular trap formaiton in cystic fibrosis airway inflammation. Nat Med. 2010;16:1018–1023. doi: 10.1038/nm.2209. [DOI] [PubMed] [Google Scholar]
- McNamara P, Selby A, Hart C, Smyth R. Bronchoalveolar cellularity in infants with severe RSV bronchiolitis. Arch. Dis. Child. 2003;99:922–926. doi: 10.1136/adc.88.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir S, Hillyer P, Le Nouën C, Buchholz U, Rabin R, Collins P, Bukreyev A. Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response. PLoS ONE. 2011;7:e29386. doi: 10.1371/journal.ppat.1001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkar D, Wang Q, Shim J, ZHao Y, Tsai W, Lukacs N, Sajjan U, Hershenson M. CXCR2 is required for neutrophilic airway inflammation and hyperresponsiveness in a mouse model of human rhinovirus infection. J. Immunol. 2009;183:6698–6707. doi: 10.4049/jimmunol.0900298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Navarini A, Lang K, Verschoor A, Recher M, Zinkernagel A, Nizet V, Odermatt B, Hengartner H, Zinkernagel R. Innate immune-induce depletion of bone marrow neutrophils aggravates systemic bacterial infections. PNAS. 2009;106:7107–7112. doi: 10.1073/pnas.0901162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Peters N, Egen J, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay M, Germain R, Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay J, den Braber I, Vrisekoop N, Kwast L, de Boer R, Borghans J, Tesselaar K, Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- Rada B, Leto T. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib. Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasagi N, Reddy P, Suryawanshi A, Mulik S, Gjorstrup P, Rouse B. Controlling herpes simple virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J. Immunol. 2012;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal A, Gavin P, Sturgis C. Cerebrospinal fluid cytology in seasonal epidemic West Nile virus meningo-encephalitis. Diagn. Cytopathol. 2006;34:127–129. doi: 10.1002/dc.20410. [DOI] [PubMed] [Google Scholar]
- Roilides E, Uhlig K, Venzon D, Pizzo P, Walsh T. Enhancement of oxidative response and damage caused by human neutrophils to Asperigullus fumigatus hyphae by granulocyte colony- stimulating factor and gamma interferon. Infect. Immun. 1993;61:1185–1193. doi: 10.1128/iai.61.4.1185-1193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L, Amineva S, Szakaly R, Lemanske R, Gern J, Sorkness R. A rat model of picornavirus-induced airway infection and inflammation. Virol .J. 2009;6 doi: 10.1186/1743-422X-6-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L, Szakaly R, Amineva S, Xing Y, Hill M, Palmenberg A, Gern J, Sorkness R. Lower respiratory tract infection induced by a genetically modified picornavirus in its natural murine host. PLoS ONE. 2011;7:e32061. doi: 10.1371/journal.pone.0032061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthe R, Andersen B, Cunningham B, Epstein R. Effect of granulocyte transfusion in the control of systemic candidiasis in the leukopenic host. Blood. 1978;52:493. [PubMed] [Google Scholar]
- Saez-Lopez C, Ngambe-Tourere E, Rosenzwajg M, Petit J-C, Nicolas J-C, Gozlan J. Immediate-early antigen expression and modulation of apoptosis after in vitro infection of polymorphonuclear leukocytes by human cytomegalovirus. Microbes Infect. 2005;7:1139–1149. doi: 10.1016/j.micinf.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, Uehata T, Iwasaki H, Omori H, Yamaoka S, Yamamoto K, Akira S. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Savard M, Gosselin J. Epstein-Barr virus immunossuppression of innate immunity mediated by phagocytosis. Virus Res. 2006;119:134–145. doi: 10.1016/j.virusres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Savill J, Wyllie A, Henson J, Walport M, Henson P, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. J. Clin. Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. How neutrophils kill microbes. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia J, Sabbah A, Mgbemena V, Tsai S-Y, Chang T-H, Berton M, Morris I, Allen I, Ting J-Y, Bose S. TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS ONE. 2012;7:e29695. doi: 10.1371/journal.pone.0029695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Kohno S, Newstead M, Zeng X, Bhan U, Lukacs N, Kunkel S, Standiford T. Critical role of IL-1 receptor-associated kinase-M in regulating chemokine-dependent deleterious inflammation in murine influenza pneumonia. J. Immunol. 2010;184:1410–1418. doi: 10.4049/jimmunol.0901709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S-U, Kwon H-J, Ko H-J, Byun Y-H, Seong B, Uematsu S, Akira S, Kweon M-N. Type I interferon signaling regulates Ly6Chi monocytes and neutrophils during acute viral pneumonia in mice. PLoS Pathog. 2011;7:e1001304. doi: 10.1371/journal.ppat.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C, Chiang N, Van Dyke T. Resolving inflammation: dual anti-inflammatory and pro- resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehnlein O. Direct and alternative antimicrobial mechanisms of neutrophil-derive granule proteins. J. Mol. Med. 2009a;87:1157–1164. doi: 10.1007/s00109-009-0508-6. [DOI] [PubMed] [Google Scholar]
- Soehnlein O. An elegant defense: how neutrophils shape the immune response. Trends Immunol. 2009b;30:511–512. doi: 10.1016/j.it.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Soehnlein O, Zernecke A, Eriksson E, Rothfucks A, Pham C, Herwald H, Bidzhekov K, Rottenberg M, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory response. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohnle P, Hunter M, Hahn B, Chazin W. Zinc-reversible antimicrobial activity of recombinant calprotectin (Migration inhibitory factor-related proteins 8 and 14) J. Infect. Dis. 2000;182:1272–1275. doi: 10.1086/315810. [DOI] [PubMed] [Google Scholar]
- Speir E, Shibutani T, Yu Z, Ferrans V, Epstein S. Role of reactive oxygen intermediates in cytomegalovirus gene expression and in the response of human smooth muscle cells to viral infection. Circ. Res. 1996;79:1143–1152. doi: 10.1161/01.res.79.6.1143. [DOI] [PubMed] [Google Scholar]
- Stark M, Huo Y, Burcin T, Morris M, Olson T, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Stout-Delgado H, Du W, Shirali A, Booth C, Goldstein D. Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell Host Microbe. 2009;19:446–456. doi: 10.1016/j.chom.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate M, Brooks A, Reading P. The role of neutrophils in the upper and lower respiratory tract during influenza virus infection of mice. Respir. Res. 2008;9:57. doi: 10.1186/1465-9921-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate M, Deng Y-M, Jones J, Anderson G, Brooks A, Reading P. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J. Immunol. 2009;183:7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- Tkalcevic J, Novelli M, Phylactides M, Iredale J, Segal A, Roes J. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 2000;12:201–210. doi: 10.1016/s1074-7613(00)80173-9. [DOI] [PubMed] [Google Scholar]
- Town T, Bai F, Wang T, Kaplan A, Qian F, Montgomery R, Anderson J, Flavell R, Fikrig E. TLR7 mitigates lethal West Nile encephalitis by affecting interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009;30:242–253. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey T, Garcia-Sastre A, Taubenberger J, Palese P, Swayne D, Pantin-Jackwood M, Schultz- Cherry S, Solorzano A, van Rooijen N, Katz J, Basler C. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J. Virol. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler K, Pape J, Goody R, Corkill M, Kleinschmidt-DeMasters B. CSF findings in 250 patients with serologically confirmed West Nile virus meningitis and encephalitis. Neurology. 2006;66:361–365. doi: 10.1212/01.wnl.0000195890.70898.1f. [DOI] [PubMed] [Google Scholar]
- Ulrich M, Worlitzsch D, Viglio S, Siegmann N, Iadarola P, Shute J, Geiser M, Pier G, Friedel G, Barr M, Schuster A, Meyer K, Ratjen F, Bjarnsholt T, Gulbins E, Doring G. Alveolar inflammation in cystic fibrosis. J. Cyst. Fibros. 2010;9:217–227. doi: 10.1016/j.jcf.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban C, Ermert D, Schmid M, Abu-Adeb U, Goosmann C, Nacken W, Brinkmann V, Jungblut P, Zychlinksy A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PloS Patho. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban C, Reichard U, Brinkmann V, Zychlinksy A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- Voyich J, Braughton K, Sturdevant D, Whitney A, Said-Salim B, Porcella S, Long R, Dorward D, Gardner D, Kreiswirth B, Musser J, DeLeo F. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- Waldorf A. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol. Ser. 1989;47:243–271. [PubMed] [Google Scholar]
- Wang J, Bowen G, Padden C, Cemy A, Finberg R, Newburger P, Kurt-Jones E. Toll-like receptor-mediated activation of neutrophils by influenza A virus. Blood. 2008;112:2028–2034. doi: 10.1182/blood-2008-01-132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulon L, Anderson J, Fikrig E, Flavell R. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li M-R, Stadler S, Correll S, Li P, Danchen W, Hayama R, Lauriebeth L, Han H, Grigoryev S, Allis C, Coonrod S. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardini A, Guimarães-Costa A, Nascimento M, Nadaes N, Danelli M, Mazur C, Benjamin C, Saraiva E, Pinto-da-Silva L. Characterization of neutrophil extracellular traps in cats naturally infected with feline leukemia virus. J. Gen. Virol. 2010;91:259–264. doi: 10.1099/vir.0.014613-0. [DOI] [PubMed] [Google Scholar]
- Wartha F, Beiter K, Normark S, Henriques-Normark B. Neutrophil extracellular traps: casting the NET over pathogenesis. Curr. Opin. Microbiol. 2007;10:52–56. doi: 10.1016/j.mib.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer K, Birrer P, Bellon G, Berger J, Weiβ T, Botzenhart K, Yankaskas J, Randell S, Boucher R, Döring G. Reduced oxygen concentrations in airway mucus contribute to the early and late pathogenesis of Pseudomonas aeruginosa cystic fibrosis airways infection. J. Clin. Invest. 2002;109:295–309. [Google Scholar]
- Yang D, de la Rosa G, Tewary P, Oppenheim J. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30:531–537. doi: 10.1016/j.it.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi S, Gold J, Andina N, Lee J, Kelly A, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich G, Simon H. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- Yousefi S, Mihalache C, Kozlowski E, Simon H. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- Zhang J-Y, Zou Z-S, Huang A, Zhang Z, Fu J-L, Xu X-S, Chen L-M, Li B-S, Wang F-S. Hyper-activated pro-inflammatory CD16+ monocytes correlate with the severity of liver injury and fibrosis in patients with chronic hepatitis B. PLoS One. 2011;6:e17484. doi: 10.1371/journal.pone.0017484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Lu M, Lau L, Lu J, Gao Z, Liu J, Yu A, Cao Q, Ye J, McNutt M, Gu J. Neutrophils may be a vehicle for viral replication and dissemination in human H5N1 avian influenza. Clin. Infect. Dis. 2008;47:1575–1578. doi: 10.1086/593196. [DOI] [PMC free article] [PubMed] [Google Scholar]