Summary
CD8+ T cells confer host protection through T cell receptor (TCR)-mediated recognition of foreign antigens presented by infected cells. Thus, generation of CD8+ T cell populations with high antigen sensitivity is critical for efficient pathogen clearance. Besides selection of high affinity TCR, the molecular mechanisms regulating the antigen sensitivity of CD8+ T cells remain poorly defined. Herein, we have demonstrated that the antigen sensitivity of effector and memory CD8+ T cells is dynamically regulated and can be tuned by the pathogen-induced inflammatory milieu independently of selection of cells with higher affinity TCR. Mechanistically, we have demonstrated that the signal transduction capacity of key TCR proximal molecules was enhanced by inflammatory cytokines, which reduced the antigen density required to trigger antimicrobial functions. Dynamic tuning of CD8+ T cell antigen sensitivity by inflammatory cytokines likely optimizes immunity to specific pathogens while minimizing the risk of immunopathology at steady state.
Introduction
The capacity to clear infection relies on both the quantity and the quality of responding immune cells (Haring et al., 2006; Walker et al., 2010; Zhang and Bevan, 2011). Protection by CD8+T cells against intracellular pathogens strongly correlates with their ability to respond to low density of antigen (high antigen sensitivity) (Alexander-Miller, 2005). Conversely, highly sensitive T cells could be detrimental for the host, resulting in immunopathology or autoimmunity (Amrani et al., 2000; Han et al., 2005). As such, the antigen sensitivity of CD8+ T cells must be tightly regulated.
While B cells enhance antigen sensitivity through somatic hypermutation of the B cell receptor, individual T cells are unable to directly change the binding affinity of their T cell receptor (TCR). However, increases in antigen sensitivity (also termed functional avidity (Slifka and Whitton, 2001)) occur during T cell responses (Busch and Pamer, 1999; Malherbe et al., 2004; Zehn et al., 2009). During infection or immunization, higher avidity CD8+ T cell clones sustain proliferation compared to lower avidity clones, thus enhancing antigen sensitivity of the population (Zehn et al., 2009). Interestingly, monoclonal TCR transgenic (tg) CD8+ T cells also increase their antigen sensitivity as they progress from early to late effector stages after infection (Slifka and Whitton, 2001). Thus, T cell intrinsic properties, unrelated to repertoire selection, may also control the antigen sensitivity of CD8+ T cells. Together, these studies underscore the possibility that antigen sensitivity of individual CD8+ T cells may be dynamically regulated independently of changes in TCR utilization. However, the molecular mechanisms regulating antigen sensitivity of effector CD8+ T cells remain poorly understood. Additionally, it remains unknown whether functional avidity maturation is hardwired in cells with a specific TCR (Slifka and Whitton, 2001) or if antigen sensitivity can be modulated by pathogen-specific signals.
Inflammatory cytokines provide an additional signal that, along with TCR ligation and costimulation, is required for maximal effector CD8+ T cell accumulation (Curtsinger and Mescher, 2010; Curtsinger et al., 1999; Curtsinger et al., 2005). The inflammatory cytokines permitting optimal CD8+ T cell responses depend on the inflammatory milieu induced by the invading pathogen (Aichele et al., 2006; Kolumam et al., 2005; Pearce and Shen, 2007). The inflammatory environments induced by infection also sustain effector differentiation and delay formation of functional memory CD8+ T cells (Badovinac et al., 2005; Haring et al., 2006; Joshi et al., 2007; Joshi and Kaech, 2008; Pham et al., 2009). In contrast, immunization with peptide coated mature dendritic cells (DC) in the absence of systemic inflammation induces CD8+ T cells that rapidly acquire memory characteristics (Badovinac et al., 2005; Pham et al., 2009). Given that the inflammatory milieu plays multiple roles in regulating the T cell response, we tested the hypothesis that inflammatory cytokines directly regulate antigen sensitivity of CD8+T cells independently of clonal selection.
Results
Infection-induced inflammatory milieux regulate antigen sensitivity of endogenous effector CD8+ T cells
To determine if the inflammatory milieu regulates the antigen sensitivity of effector CD8+ T cells, mice were immunized with lipopolysacharide (LPS)-matured DC coated with ovalbumin257–264 (OVA) peptide (DC-OVA) in the presence or absence of infection with attenuated Listeria monocytogenes (LM), or lymphocytic choriomeningitis virus strain Armstrong (LCMV). Infection with LM or LCMV, which do not express OVA, induces multiple inflammatory cytokines (Biron, 1999; Pamer, 2004). This system allowed us to study the effect of the pathogen-specific inflammatory milieu in a setting where antigen presentation remains constant (Badovinac et al., 2005; Pham et al., 2009).
Antigen sensitivity of endogenous OVA-specific CD8+ T cells was measured on day 5 post-DC immunization by ex vivo stimulation with titrated doses of OVA peptide followed by intracellular cytokine staining for effector cytokines. We evaluated antigen sensitivity on day 5 as pathogen clearance occurs before peak CD8+ T cell numbers, thus effector function of CD8+ T cells at early time points are critical to an effective immune response (Badovinac et al., 2002; Pope et al., 2001). We observed that infection with either LM or LCMV significantly (p<0.01 and p<0.001, respectively) increased the antigen sensitivity of the responding OVA-specific CD8+ T cells as determined by the percentage of cells producing interferon-γ (IFN-γ) (Figure 1A, B). The antigen sensitivity of OVA-specific CD8+ T cells was increased 2.9 fold with LM infection and 10.7 fold with LCMV infection (Figure 1B) as measured by the peptide concentration required to obtain 50% of maximum IFN-γ production (effective concentration 50, EC50) (Figure 1B) compared to OVA-specific CD8+ T cells from mice immunized with only DC-OVA. In addition, exposure to inflammation significantly (p<0.01 and p<0.001, for LM and LCMV respectively) increased per cell IFN-γ production (Figure 1C and S1A). Thus, the inflammatory milieu induced by infection enhanced antigen sensitivity of OVA-specific effector CD8+ T cells. The inflammatory milieu associated with LCMV infection resulted in higher sensitivity to antigen than LM infection. Thus, the antigen sensitivity of early effector CD8+ T cells was tuned in a pathogen-specific manner.
Figure 1. Pathogen-specific inflammation increases antigen sensitivity of effector CD8+ T cells.
(A) % IFN-γ+ endogenous CD8+ T cells at day 5 after immunization with DC-OVA alone (gray squares) or DC-OVA with co-infection with LM (open circles) or LCMV (black triangles) determined following ex vivo stimulation with titrated concentrations of OVA peptide. Data (mean ± SEM) are normalized to % IFN-γ+ cells at peptide saturation (200nM). (B) Summary (mean ± SEM) of EC50 for stimulation of IFN-γ production by endogenous OVA-specific CD8+ T cells. (C) gMFI (mean ± SEM) of IFN-γ by endogenous OVA-specific CD8+ T cells at peptide saturation (200nM). (D, E, F) same as (A, B, C) for OT-I CD8+ T cells (saturating peptide=10nM). (G, H) Same as (A, B) for OT-I CD8+ T cells following infection with LCMV on day 3 post DC-OVA immunization. Data in (A, C, D, F, G) are from 3 mice per group and representative of at least 2 independent experiments. Data in (B, E, H) are cumulative from 2–3 independent experiments with at least 6 mice per group. Data in (B, C, E, F) were analyzed by One-way ANOVA with Tukey’s post-test of multiple comparisons. Data in (H) were analyzed by two-tailed, unpaired student’s t-test. (see also Figure S1)
Antigen sensitivity can be regulated independently of clonal selection
The preceding analyses of endogenous effector CD8+ T cells did not distinguish if enhanced antigen sensitivity depended on selection of high affinity T cells. To address this, we determined if infection-associated inflammation increased antigen sensitivity of monoclonal TCR-tg CD8+ T cells. Physiologic numbers of Thy1.1-1.1 or Thy1.1–1.2 OVA-specific OT-I TCR-tg CD8+ T cells were adoptively transferred into Thy1.2-1.2 mice. Recipients were immunized 24 hours later with DC-OVA in the presence or absence of LM or LCMV infection to induce pathogen-specific inflammation. To control for potential effects of infection on antigen presenting cell (APC) function during ex vivo stimulation, congenically marked OT-I cells from the DC-OVA only group were stimulated in the same well as OT-I cells from mice immunized with DC-OVA in the presence of LM or LCMV infection. Due to the fixed TCR and common APC pool in this scenario, any observed differences in antigen sensitivity between groups must be T cell intrinsic. We observed that infection with LM or LCMV also significantly (p<0.05 and p<0.001, respectively) increased the antigen sensitivity of monoclonal OT-I CD8+ T cells on day 5 following immunization (Figure 1D, E). The antigen sensitivity of OT-I CD8+T cell IFN-γ production was increased 2.4 fold with LM infection and 7.6 fold with LCMV infection compared to OT-I CD8+ T cells generated with DC-OVA alone as measured by the EC50 of peptide required for IFN-γ production (Figure 1E). Similarly, LM and LCMV infection enhanced the antigen sensitivity for OT-I TNF-α production (Figure S1C, D). This increase in antigen sensitivity permitted robust cytokine production at antigen concentrations that were insufficient to induce a cytokine response from OT-I CD8+T cells generated by DC-OVA immunization alone. As observed with endogenous responses, LCMV exerted a greater effect than LM on OT-I CD8+ T cell antigen sensitivity and the per cell production of IFN-γ (Figures 1E, F and S1B). These data show that the pathogen-specific inflammatory milieu regulates antigen sensitivity of CD8+T cells for effector cytokines production independently of clonal selection or APC function.
Inflammatory cytokines are rapidly produced after infection and could exert their effects by altering priming of naive CD8+ T cells. To address this, we infected mice with LCMV at day 3 after DC-OVA immunization, a time point when priming of new T cells by DC immunization is not detectable (Badovinac et al., 2005; Prlic et al., 2006). Infection with LCMV on day 3 post-immunization still significantly enhanced (p=0.015) the antigen sensitivity of OT-I CD8+ T cells for IFN-γ production at day 5 by 5.6 fold as measured by EC50 (Figure 1G, H). Thus, the infection induced inflammatory milieu rapidly influences antigen sensitivity of CD8+ T cells independently of effects on priming.
We next determined if inflammation simply accelerated acquisition of peak antigen sensitivity by CD8+ T cells or also enhanced antigen sensitivity for prolonged periods. Infection with LM or LCMV resulted in significantly higher antigen sensitivity (p=0.0003 and p= 0.004, respectively) and per cell IFN-γ production (p=0.0027 and p=0.0019, respectively) by OT-I CD8+ T cells on day 8 following immunization compared to OT-I CD8+ T cells generated by DC-OVA alone (Figure 2 and S2). Comparison of the EC50 values for IFN-γ production by OT-Is in the presence of LCMV infection revealed decreased antigen sensitivity at day 8 (EC50=44 pM) (Figure 2E) compared to day 5 (EC50=21 pM) (Figure 1F). Thus, the antigen sensitivity of CD8+T cells exposed to a pathogen-specific inflammatory milieu can be dynamically regulated to peak early following infection and then decrease as the infection is resolved.
Figure 2. Infection-induced inflammation enhances antigen sensitivity of CD8+ T cells for several days.
(A) % IFN-γ OT-I CD8+ T cells at day 8 after immunization with DC-OVA alone (gray square) or DC-OVA and LM (open circles) determined after ex vivo stimulation with titrated concentrations of OVA peptide. Data are normalized as in Fig. 1 except that peptide saturation was 10nM. (B) Summary (mean ± SEM) of EC50 for stimulation of IFN-γ production by OT-I CD8+ T cells. (C) gMFI (mean ± SEM) of IFN-γ expressed by OT-I CD8+ T cells at peptide saturation (10nM). (D, E, F) same as (A, B, C) for OT-I CD8+ T cells on day 8 following immunization with DC-OVA (gray squares) or DC-OVA + LCMV (black triangles). Data in (A, C, D, F) are from 3 mice per group and representative of 2 independent experiments. Data in (B, E) are cumulative from 2 independent experiments with at least 6 mice per group. Data were analyzed by two-tailed, unpaired student’s t-test. (see also Figure S2)
Infection-induced inflammatory milieu enhances cytolysis by CD8+ T cells
In addition to effector cytokines, CD8+ T cells are cytolytic through the perforin and granzyme B granule exocytosis pathways. OT-I CD8+ T cells from mice immunized with DC-OVA and LM or LCMV expressed more granzyme B (Figure 3A) and also exhibited a decreased EC50 of OVA peptide required for degranulation (as measured by surface CD107a expression, Figure 3B, C). Consistent with these data, OT-I T cells primed in mice receiving LCMV infection exhibited significantly (p=0.026 and p=0.023) enhanced per cell in vivo killing of splenocytes coated with two doses of OVA peptide (Figure 3D, E). Thus, the inflammatory milieu regulates the in vivo capacity of effector CD8+T cells to kill targets displaying low amounts of antigen.
Figure 3. Infection-induced inflammation enhances cytolysis byCD8 + T cells.
(A) Representative plots of granzyme B expression at day 5 following DC-OVA immunization with or without infection with LM or LCMV. Shaded histograms are isotype controls. Numbers show gMFI of granzyme B staining. (B) % CD107a+OT -I CD8+ T cells at day 5 following immunization with DC-OVA alone (gray square) or DC-OVA with co-infection with LM (open circles) or LCMV (black triangles) measured following ex vivo stimulation with titrated concentrations of OVA peptide. Data (mean ± SEM) are normalized as in Fig. 1 except that peptide saturation was 10nM. (C) Summary (mean ± SEM) of EC50 for degranulation (CD107a staining) of OT -I CD8+ T cells. (D) % in vivo specific killing of splenocytes coated with 0.05nM OVA peptide. Data are % killing for 106 OT -I CD8+ T cells in recipient mice. (E) Same as D for splenocytes coated with 0.025nM OVA peptide. Data in (A) are representative of at least 3 mice per group and 2 independent experiments. Data in (B) are from 3 mice per group and representative of 2 independent experiments. Data in (C) are cumulative from 2 independent experiments with a total of 6 mice per group and were analyzed by One-way ANOVA with Tukey’s post-test of multiple comparisons. Data in (D, E) are from at least 4 mice per group, representative of 2 independent experiments and analyzed by two-tailed, unpaired student’s t-test.
Inflammation enhances TCR signaling
To address underlying mechanisms of inflammation-induced T cell intrinsic increases in antigen sensitivity, we initially ruled out changes in surface expression of the alpha (Vα2) and beta (Vβ5) chains of the TCR or CD3ε, the costimulatory molecule CD28 or CD11a (α chain of LFA-1, a critical integrin that facilitates conjugation between T cells and DCs (Dustin et al., 1989)) (Figure 4A). However, we did observe that LCMV-induced inflammation enhanced the sensitivity of IFN-γ production by DC-OVA primed OT-I to plate bound CD3 antibody 5.3 fold compared to OT-Is immunized with DC-OVA alone (Figure 4B). These data suggest that inflammation regulates antigen sensitivity by increasing the capacity to translate TCR signals rather than by enhancing APC or costimulatory interactions.
Figure 4. Inflammation enhances TCR proximal signaling of CD8+ T cells.
(A) Representative plots of surface expression of the indicated marker at day 5 after immunization with DC-OVA alone (gray filled histograms) or DC-OVA and LCMV (black lines). (B) Summary (mean ± SEM) of EC50 for IFN-γ production by OT-I CD8+T cells following ex vivo stimulation with titrated concentrations of plate bound CD3 antibody. (C–G) Immunoblot analysis of cell lysates from OT-I CD8+ T cells for expression of the protein or phosphorylated protein indicated, on day 5 following DC-OVA immunization with or without co-infection with LCMV. Cells were stimulated by anti-CD3 crosslinking as indicated. Equivalent amounts of total protein were loaded in each lane. (H) Same as (C) for cells stimulated with PMA. (I) Summary fold increase in gMFI of IFN-γ expression following OVA peptide (10nM) or PMA and Ionomycin stimulation. Data are presented as fold increase compared to the mean gMFI of IFN-γ production by OT-I CD8+ transgenic T cells from mice immunized with DC-OVA only. Data in (A) are representative of 2 independent experiments. Data in (B) are cumulative from 2 independent experiments with 6 mice per group and were analyzed by two-tailed, unpaired student’s t-test. Samples in (C–H) were pooled from at least 5–10 mice. Data in (I) are cumulative of 2 independent experiments with at least 6 mice per group. (see also Figure S3)
Ligation of the TCR results in the activation of a signaling cascade initiated by the tyrosine kinases LCK and ZAP-70. Studies have suggested that enhanced antigen sensitivity following infection may be regulated by increased expression of these key signaling molecules (Amoah et al., 2012; Slifka and Whitton, 2001). However, exposure to inflammation did not increase the protein expression of either of these kinases at day 5 post-immunization (as measured by the relative signal intensity compared to the β-actin loading control) (Figure 4C, D and Fig S3A, B). Therefore, we assessed whether inflammation increased antigen sensitivity of CD8+ T cells by enhancing their capacity to translate TCR signals. Anti-CD3 stimulation of CD8+ T cells exposed to inflammation resulted in enhanced and more rapid phosphorylation of ZAP-70 compared to OT-I CD8+ T cells from mice immunized with DC-OVA alone (Figure 4D and S3C). Thus, CD8+T cells exposed to inflammation exhibit enhanced activation of ZAP-70 following TCR stimulation, which occurs independently of changes in total LCK or ZAP-70 protein.
Phosphorylation of ZAP-70 leads to the activation of phospholipase C (PLC)-γ, which cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into the secondary messengers inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). These secondary messengers initiate intracellular calcium release, activation of protein kinase C (PKC)’s and the mitogen activated protein kinase (MAPK) pathway, resulting in the transcription of target genes including IFN-γ (Abraham and Weiss, 2004; Smith-Garvin et al., 2009). We observed an increase in the phosphorylation of PLC-γ on activating tyrosine 783 in CD8+ T cells exposed to inflammation (Figure 4E and S3D), demonstrating that exposure to inflammation enhanced the activation of signaling pathways downstream of TCR ligation. This led to dramatic increases in the activation of PLC-γ dependent MAPK pathways (Nolz et al., 2006), as shown by the marked enhancement in the phosphorylation of extracellular-signal regulated kinases (ERK) 1 and 2 and c-Jun N-terminal kinases (JNK) 1 and 2 in CD3-stimulated CD8+ T cells exposed to inflammation (Figure 4F, G and S3E, F). We did not observe enhanced phosphorylation of another MAPK, p38 (Figure S3G, H) that appears to be activated in a non-canonical, linker of activated T cell (LAT) independent-manner, in T cells (Salvador et al., 2005). These data suggest that exposure to inflammation specifically enhances the canonical MAPK activation pathway. In contrast, we saw no differences in ERK1 and 2 activation when cells were stimulated with phorbol myristate acetate (PMA), a DAG analog that activates the MAPK pathway and phosphorylation of ERK1 and 2 while bypassing the requirement for TCR ligation and proximal signaling events (Figure 4H). Thus, while CD8+ T cells stimulated with DC-OVA in a non-inflammatory milieu were capable of robust MAPK activation, exposure of these cells to pathogen-induced inflammation enhanced TCR proximal signaling, amplified MAPK activation and increased antigen sensitivity. To determine whether the enhanced TCR signaling controlled antigen sensitivity in cells exposed to inflammation, we evaluated IFN-γ production from cells stimulated with either OVA peptide or PMA and ionomycin. We observed a larger fold increase in per cell IFN-γ production by OT-I T cells exposed to inflammation after peptide stimulation compared to stimulation with PMA and ionomycin (Figure 4I). These data confirmed that exposure to inflammation increased antigen sensitivity by enhancing proximal TCR signaling.
Direct regulation of antigen sensitivity by inflammatory cytokines
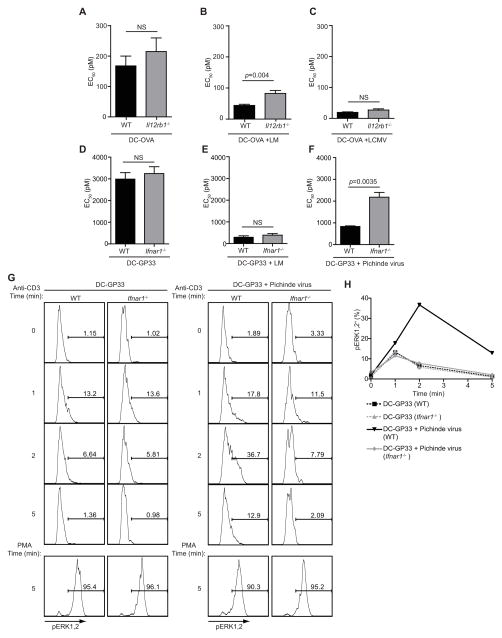
Pathogens elicit distinct and complex inflammatory cytokine responses. For example, LM infection induces several cytokines including IL-12 and type 1 interferon while LCMV elicits a dominant type 1 interferon response (Biron, 1999; Pamer, 2004). As such we determined if regulation of antigen sensitivity by exposure to inflammation required direct signaling to CD8+ T cells by specific cytokines. Congenically marked WT and IL-12 Receptor β1-deficient (Il12rb1−/−) OT-I CD8+ T cells were co-transferred into WT mice, which were immunized with DC-OVA in the presence or absence of infection with LM or LCMV. Il12rb1−/− OT -I CD8+ T cells responded to immunization with DC-OVA alone and did not exhibit changes in antigen sensitivity compared WT OT-I CD8+ T cells (Figure 5A). However, antigen sensitivity of Il12rb1−/− OT -I CD8+ T cells in mice receiving LM infection was significantly (p=0.004) reduced compared to WT OT-I CD8+ T cells (Figure 5B). Thus, IL-12 signaling directly regulates the antigen sensitivity of CD8+ T cells. Despite this, the LM-induced inflammatory milieu still partially increased the antigen sensitivity of Il12rb1−/− OT -I CD8+ T cells (Figure 5A, B) indicating that IL-12 is not the only cytokine involved in modulating the antigen sensitivity of responding CD8+ T cells following LM infection. Il12rb1−/− and WT OT-I cells exhibited the same enhancement of antigen sensitivity after DC-OVA immunization in the presence of LCMV infection (which primarily drives type 1 interferon and not IL-12) (Figure 4C).
Figure 5. Inflammatory cytokines regulate the antigen sensitivity of CD8+ T cells.
(A) Summary (mean ± SEM) of EC50 for IFN-γ production by congenically marked WT or Il12rb1−/− OT -I CD8+ T cells adoptively transferred into the same WT hosts at day 5 after immunization with DC-OVA alone. (B, C) same as (A) except for mice immunized with DC-OVA and co-infected with (B) LM or (C) LCMV respectively. (D, E, F) Same as (A, B, C) for WT or Ifnar1−/− P14 CD8+ T cells adoptively transferred into WT hosts immunized with (D) DC-GP33 alone or infected on day 3 post-immunization with (E) LM or (F) PV. (G) Representative flow cytometry plots of pERK1 and 2 from WT or Ifnar1−/− P14 CD8+ T cells on day 5 following immunization with DC-GP33 with or without infection with PV on day 3. Cells were stimulated with either CD3-crosslinking or PMA for the indicated times. (H) Summary data of percentage of pERK1 and 2+ WT or Ifnar1−/− P14 CD8+ T cells following activation by CD3-crosslinking. Data in (A, B, C) are cumulative (mean ± SEM) from 2 independent experiments with at least 6 mice per group.. Data in (D, E, F) are from 3 mice per group (mean ± SEM) and representative of 2 independent experiments. Data were analyzed by two-tailed, unpaired student’s t-test. Data in (G, H) are from 3 pooled mice per group and representative of 2 independent experiments.
To address a role for type 1 interferon signaling and extend our results to an additional TCR-tg CD8+ T cell, congenically marked WT and type 1 interferon receptor-deficient (Ifnar1−/−) P14 (specific for the GP33–41 epitope of LCMV) CD8+ T cells were co-transferred into WT mice, which were immunized with DC-GP33 followed by infection with LM or Pichinde virus (PV) on day 3 post-immunization. PV is an arenavirus that does not express the GP33–41 epitope of LCMV. Infection with either LM or PV enhanced the antigen sensitivity of WT P14 CD8+ T cells (Figure 5D–F), while the absence of the type 1 interferon receptor strongly abrogated the enhanced antigen sensitivity of OT-I CD8+ T cells in PV infected mice (Figure 5F). Thus, direct type 1 interferon signaling on CD8+ T cells plays a major role in the enhanced antigen sensitivity associated with the exposure to PV induced inflammation. Type 1 interferon receptor-deficiency did not affect the capacity of LM to enhance the antigen sensitivity of P14 CD8+ T cells suggesting that, while type 1 interferons are induced by LM infection (Pamer, 2004), these cytokines do not play a dominant role in regulating antigen sensitivity over the course of this bacterial infection. Collectively, these data demonstrate that inflammatory cytokines directly regulate the antigen sensitivity of effector CD8+ T cells in a pathogen-specific manner.
To determine if direct cytokine signaling on CD8+ T cells caused enhanced TCR signaling, congenically marked WT and Ifnar1−/− P14 CD8+ T cells were co-transferred to WT mice and immunized with DC-GP33 with or without infection with PV on day 3 following DC-GP33 immunization. Two days later, phosphorylation of ERK1 and 2 following CD3 stimulation was analyzed by flow cytometry, which allowed us to compare WT and Ifnar1−/− T cells immunized in the same mice. As described for WT OT-I CD8+ T cells (Figure 4), exposure to inflammation enhanced ERK1 and 2 phosphorylation following stimulation of WT P14 CD8+ T cells (Figure 5G, H). Enhanced ERK1 and 2 activation was abrogated in Ifnar1−/− P14 (Figure 5G, H). ERK1 and 2 phosphorylation in Ifnar1−/− P14 was similar to WT P14 when proximal TCR signaling was bypassed by PMA stimulation, demonstrating that Ifnar1−/− cells retain the capacity to signal normally (Figure 5H). These data demonstrate that inflammatory cytokines acted directly on effector CD8+ T cells to enhance proximal TCR signaling and increase antigen sensitivity.
Inflammatory cytokines enhance antigen sensitivity of memory CD8+ T cells
Hosts will contain memory CD8+ T cells specific for multiple pathogens and these cells enhance immunity to pathogen re-exposure. To determine if inflammatory cytokines also enhanced antigen sensitivity of memory CD8+ T cells, mice containing memory OT-I or P14 CD8+ T cells were infected with an unrelated pathogen to induce inflammatory cytokines in the absence of antigen stimulation (LCMV or PV respectively). On day 4 following infection, we observed that exposure to inflammatory cytokines significantly increased the antigen sensitivity of memory OT-I (Figure 6A, B) and memory P14 CD8+ T cells (Fig. 6C, D). (p=0.0026 and p=0.0017, respectively) as well as increased per cell IFN-γ production (Figure S3). Thus, antigen sensitivity of memory CD8+ T cells was also regulated by inflammatory cytokines. This change does not require recent TCR stimulation, suggesting that inflammatory cytokines enhance antigen sensitivity of memory cells prior to their potential interaction with cells expressing cognate antigen.
Figure 6. Inflammatory cytokines enhance the antigen sensitivity of memory CD8+T cells.
(A) % IFN-γ memory OT-I CD8+ T cells at day 4 after infection with LCMV (black triangles) or mock-infection with saline (gray squares) determined following ex vivo stimulation with titrated concentrations of OVA peptide. Data are normalized as in Fig. 1 except that peptide saturation was 10nM. (B) Summary (mean ± SEM) of EC50 for IFN-γ production by memory OT-I CD8+ T cells. (C, D) Same as (A, B) for memory P14 CD8+T cells following ex vivo stimulation with GP33–41 peptide on day 4 following infection with PV (black triangles) or mock-infection with saline (gray squares). (E) Same as (B) for memory OT-I CD8+ T cells isolated from the lung at day 3 following intranasal infection with LCMV (black triangles) or mock-infection with saline (grey squares). Saturating peptide=10nM (F) Same as (B) for memory OT-I T cells on day 4 (black bars) or day 14 (white bars) following infection with LCMV or mock-infection with saline (gray bars) Data in (A, C, D, E) are from 3 mice per group and representative of at least 2 independent experiments. Data in (B, F) are cumulative from at least 2 independent experiments with at least 6 mice per group. Data in (B, D, E) were analyzed by two-tailed, unpaired student’s t-test. Data in (F) were analyzed by One-way ANOVA with Tukey’s post-test of multiple comparisons. (see also Figure S4)
Chemokines induced by tissue-specific inflammation mediate rapid, antigen-independent, recruitment of memory CD8+ T cells (Ely et al., 2003; Kohlmeier et al., 2008). To determine if tissue specific inflammation enhanced antigen sensitivity, mice with memory OT-I CD8+ T cells were infected intranasally with LCMV to induce inflammatory cytokines and recruitment of memory cells to the lung. On day 3 following infection lung OT-I cells exposed to inflammation exhibited significantly (p=0.0097) increased antigen sensitivity (Figure 6E), indicating that exposure to inflammatory cytokines regulated the antigen sensitivity of memory cells that were localized within an inflamed tissue.
Since inflammatory cytokines tuned the antigen sensitivity of memory CD8+T cells independently of antigen re-encounter, we next determined if tuning is dynamic and antigen sensitivity returns to steady-state following clearance of infection. Mice containing memory OT-I CD8+ cells were mock-infected or infected with LCMV on day -14 or day -4 relative to analysis, such that antigen sensitivity in all groups could be measured on the same day. While OT-I antigen sensitivity was significantly (p<0.05) enhanced on day 4 post-infection, this increase was transient as antigen sensitivity was not significantly different from mock-infected mice on day 14 following LCMV infection (Figure 6F). Thus, tuning of memory CD8+ T cell antigen sensitivity is dynamic and in the absence of antigen re-encounter, antigen sensitivity of bystander memory CD8+T cells returns to steady-state.
Inflammatory cytokines enhance TCR signaling of memory CD8+ T cells
To determine if inflammatory cytokines increased antigen sensitivity by enhancing TCR signaling, memory OT-I TCR-tg CD8+ T cells were purified on day 4 following infection with LCMV or mock-infection and stimulated by CD3 cross-linking. The relatively low frequency of memory CD8+ T cells generated from physiologic naïve cell input numbers and low protein yield precluded analysis of ZAP-70 phosphorylation. However, as seen with effector CD8+ T cells, exposure to inflammation resulted in dramatically increased phosphorylation of ERK1 and 2 following TCR ligation (Figure 7A, B). This difference in ERK1 and 2 phosphorylation was no longer observed when TCR proximal signaling was bypassed with PMA (Figure 7C) demonstrating that exposure to inflammatory cytokines also enhanced proximal TCR signaling in memory CD8+ T cells. Collectively, these data demonstrate that TCR signaling of both effector and memory CD8+ T cells can be dynamically regulated by inflammatory cytokines in order to tune antigen sensitivity.
Figure 7. Inflammatory cytokines enhance TCR signaling of memory CD8+ T cells.
(A) Immunoblot analysis of cell lysates from memory OT-I CD8+ T cells on day 4 following infection with LCMV or mock infection with saline. Cells were stimulated by anti-CD3 crosslinking as indicated. Equivalent amounts of protein were loaded in each lane. (B) Relative signal intensity of pERK1 and 2 divided by relative signal intensity of total ERK1 and 2. Data are normalized to the maximal activation observed in memory CD8+ T cells from mock-infected mice. (C) Same as (A) for cells stimulated with PMA. Samples in (A) were pooled from 5–10 mice and are representative of 2 independent experiments. Samples in (B) were pooled from 5–10 mice.
Discussion
Generation of highly sensitive CD8+ T cell clones is important for the clearance of pathogen-infected cells, which may express low amounts of antigen (Alexander-Miller, 2005; Walker et al., 2010). Herein, we have demonstrated that inflammatory cytokines directly regulate the antigen sensitivity of CD8+ T cells independently of clonal selection. Our data have highlighted that the antigen sensitivity of CD8+ T cells is not hardwired, but rather is dynamically regulated in a pathogen-specific manner. Further, we have demonstrated that exposure to inflammatory cytokines enhanced TCR signaling of early effector and pre-existing memory CD8+ T cells to control antigen sensitivity. Dynamic tuning of TCR signaling by pathogen-specific inflammatory cytokines likely represents an important regulatory mechanism for the induction of optimal CD8+ T cell immunity.
Inflammatory cytokines provide a third signal that is required for optimal accumulation of effector CD8+ T cells (Curtsinger and Mescher, 2010; Curtsinger et al., 1999; Curtsinger et al., 2005) and to sustain effector differentiation (Badovinac et al., 2005; Joshi et al., 2007; Joshi and Kaech, 2008; Pham et al., 2009). Here, we have extended the role of these inflammatory cytokines by demonstrating their capacity to modulate the antigen sensitivity of individual CD8+ T cell clones. Of particular interest, we have demonstrated that the magnitude of increase in antigen sensitivity of the same CD8+ T cell clone varies depending on the inflammatory milieu induced by the pathogen used for infection. Thus, the precise pattern of inflammatory cytokines induced by infection can help tune antigen sensitivity in order to achieve optimal CD8+ T cell responses.
The rapid induction of peak antigen sensitivity by inflammatory cytokines may be of critical importance for efficient elimination of pathogens. CD8+ T cells with high antigen sensitivity are of particular importance for the clearance of viral pathogens. For example, patients that clear Hepatitis C virus infection contain CD8+ T cells with higher antigen sensitivity than CD8+ T cells harvested from chronically infected patients (Yerly et al., 2008). Here we have demonstrated that induction of inflammation with a viral pathogen, LCMV, results in a nearly 10-fold increase in the antigen sensitivity of OT-I CD8+ T cells and allows for more efficient in vivo killing of targets coated with a low density of antigen. Such increases in antigen sensitivity could allow antigen-specific CD8+ T cells to carry out effector function more efficiently during the course of infection by targeting cells when they are expressing lower amounts of antigen. This may be of particular importance as many viral pathogens are known to interfere with MHC class I trafficking and expression which would lead to lower amounts of cognate antigen expressed on infected cells (Donaldson and Williams, 2009; Griffin et al., 2010; Jackson et al., 2011). It should be noted that, while exposure of responding CD8+ T cell to inflammation enhanced antigen sensitivity, this is balanced by a delay in the acquisition of memory characteristics, including the capacity for robust secondary expansion, compared to cells primed in a low-inflammatory environment (Badovinac et al., 2005; Haring et al., 2006). As such, while enhanced antigen sensitivity may represent a significant advantage for short-term protection, the consequences of these changes for long-term protection and memory will require further study and must be considered when designing therapies or developing vaccine strategies.
It has previously been established that tissue specific infection results in non-specific recruitment of memory T cells to inflamed sites (Ely et al., 2003; Kohlmeier et al., 2008). We have demonstrated that exposure to inflammatory cytokines also dynamically enhances the antigen sensitivity of memory CD8+ T cells in the absence of antigen re-encounter in both the spleen and a peripheral tissue. This suggests that infection enhances the antigen sensitivity of memory CD8+ T cells regardless of their antigen specificity, thus preparing these cells for rapid deployment of effector functions should they encounter cells expressing low amounts of cognate antigen. Rapid enhancement in antigen sensitivity may in part explain why memory T cells respond to an invading pathogen by producing effector cytokines within hours of infection (Whitmire et al., 2008). Bystander activation of memory CD8+ T cells also occurs during acute infection in humans, suggesting that this represents a conserved regulatory mechanism for optimal memory CD8+ T cell function (Odumade et al., 2012).
A previous study suggested that antigen exposure results in “hardwired” changes in TCR signaling pathways that increased antigen sensitivity in a temporal fashion (Slifka and Whitton, 2001). This conclusion was drawn in evaluating the CD8+ T cell response to a single pathogen and while some changes in TCR signaling are likely to occur solely as a result of antigen exposure, our results show that TCR signaling capacity can be dynamically tuned by exposure to pathogen-specific inflammatory cytokines in both effector and memory CD8+ T cells. Indeed, we observed that inflammatory cytokines rapidly and transiently enhance the antigen sensitivity of effector CD8+ T cells. Similarly, we have demonstrated that the antigen sensitivity of a monoclonal population of memory CD8+ T cells can be transiently increased following exposure to inflammation. This demonstrated that the antigen sensitivity of circulating memory T cells that do not re-encounter their cognate antigen is blunted as inflammatory stimuli wane. As such, the modulation of the antigen sensitivity of memory cells likely represents an important regulatory mechanism aimed at minimizing the risks of immunopathology or autoimmunity.
One major question that remains is how inflammatory cytokines modulate the signaling capacity of responding CD8+ T cells. Inflammatory cytokines induce the transcription of a variety of genes including transcription factors and could lead to changes in the expression of key signaling components (Agarwal et al., 2009; Joshi et al., 2007; Pipkin et al., 2010). Slifka and colleagues reported a correlation between the amount of total LCK protein expression and the amount of IFN-γ per CD8+ T cells (Slifka and Whitton, 2001). They suggested that the total expression of this signaling molecule might regulate antigen sensitivity of CD8+ T cells. However, we have demonstrated that exposure to inflammatory cytokines directly enhanced the signaling capacity of early effector CD8+ T cells without increasing the total expression of either LCK or ZAP-70. This suggests that cytokine signaling to CD8+ T cells enhances the activity of signal transducing molecules involved in the TCR signaling pathway. To this effect, a recent study has demonstrated that antigen-stimulation of CD8+ T cells results in an increase in oligomeric T cell receptor complexes (Kumar et al., 2011). A contribution of inflammatory cytokines to reorganization of TCR complexes could explain the increased sensitivity of memory T cells to antigen stimulation. Alternatively, exposure to inflammatory cytokines may reduce the expression or activity of negative regulators of TCR signaling such as phosphatases. Further studies will be required to resolve these issues.
In conclusion, TCR signaling capacity can be directly regulated by inflammatory cytokines resulting in T cell intrinsic increases in antigen sensitivity and in vivo cytolytic capacity. Our study demonstrates that the antigen sensitivity of individual CD8+ T cell clones is not hardwired following infection but rather is a dynamic process dictated by the specific inflammatory milieu induced by an invading pathogen. This likely allows the rapid generation of optimal effector and memory CD8+ T cell responses while minimizing the risk of immunopathology.
Experimental procedures
Mice, pathogens and dendritic cells
C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD). TCR-tgOT -I and P14 mice were previously described (Hogquist et al., 1994; Pircher et al., 1987), Il12rb1−/− OT -I cells were provided by Dr. Mescher (U. Minnesota), Ifnar1−/− P14 cells were provided by Dr. Murali-Krishna Kaja (Emory University). Infected mice were housed at the appropriate biosafety level. The University of Iowa Animal Care and Use Committee approved animal experiments. An attenuated act-A deficient strain of L. monocytogenes (DPL1942) (Brundage et al., 1993) was grown and injected i.v. (1×106 CFU) as described (Harty and Bevan, 1995). LCMV Armstrong was propagated as described (Slifka and Whitton, 2001) and injected i.p. (2×105 PFU). PV was propagated as described (Varga et al., 2001) and injected i.p. (1×106 PFU). LPS matured OVA peptide coated DCs were prepared as previously described (Schmidt et al., 2008) and injected i.v. (5 × 105).
Adoptive transfers and generation of memory cells
Naïve TCR-tg CD8+ T cells were injected i.v. (OT-Is Thy1.1-1.1 or 1.1–1.2 (1×103) into naïve Thy1.2 recipients. Mice were immunized 24 hours later with DC-OVA with or without infection with LM or LCMV. Il12rb1−/− Thy1.2-1.2 OT-I were mixed 1:1 with WT Thy 1.1-1.1 OT-I (1×103 each) and injected i.v. into naïve CD45.1 recipients and immunized as indicated. Thy1.1-1.1 Ifnar1−/− P14 were mixed 1:1 with WT Thy1.1–1.2 P14s (5×103 each) and injected i.v. into naïve Thy1.2–1.2 recipients and immunized with DC-GP33 on day 0 followed by infection with LM or PV on day 3 post-immunization. For generation of primary memory cells, naïve Thy1.1 OT-I (5×102) or P14 CD8+T cells (1×104) were injected i.v. into naive Thy1.2 WT recipients that were infected with either actA−/− LM-OVA (5×106cfu) or LCMV (2×105 pfu). Mice were rested at least 50 days before use.
Ex vivo cytokine and degranulation analyses
Ex vivo cytokine detection and degranulation assays were performed as previously described and as detailed in supplemental methods (Butler et al., 2011; Nolz and Harty, 2011). Where indicated, congenically marked cells from DC-OVA only and co-infected groups were mixed and stimulated in the same well. Titration curves were fitted by nonlinear regression.
In vivo cytolytic assay
Assay was performed as previously described (Schmidt et al., 2011). Briefly, splenocytes were left untreated or coated with OVA peptide (0.05 or 0.025nM) for 1 hour at 37°C. Splenocytes were labeled with 0.8 μM CFSE (no peptide), 0.02 μM CFSE (0.05nM OVA), 2.5 μM Cell tracker violet (no peptide) or 0.25 μM (0.025nM OVA). Labeled cells (5 × 106 each, 2 ×107 total cells) were transferred i.v. into recipient mice. Killing was measured in the spleen at 4 hours following transfer. Percentage killing was calculated as: and is presented as the normalized percentage of killing per 1 ×106OT -I CD8+ T cells in recipient mice.
TCR signaling and immunoblot analysis
Naive Thy1.1 OT-Is were injected i.v. (1×104) into naïve Thy1.2 recipients, which were immunized 24 hours later as indicated. On day 5, spleens were harvested, stained with PE-anti-Thy1.1 antibody (Clone OX-7, BD Pharmingen) and purified with anti-PE magnetic beads using standard AutoMacs protocols. Memory OT-I CD8+ T cell were purified on day 4 following infection with LCMV or mock-infection with saline. 1–5 × 106 cells were incubated on ice for 30 minutes with 10 μg/ml biotinylated anti-CD3 (clone 145-2C11, eBioscience) antibody and cross-linked with streptavidin or stimulated with 200ng/ml of PMA for the indicated times at 37°C. Cells were washed with cold PBS and lysed in NP40 buffer (20 mM HEPES (pH 7.9), 100 mM NaCl, 5 mM EDTA, 0.5 mM CaCl, 1% Nonidet P-40, 1 mM PMSF, 10 μg/ml leupeptin, 5 μg/ml aprotinin, 1 mM Na3VO4). 5–10 μg of protein was resolved by SDS-PAGE, transferred to nitrocellulose and probed with antibodies as indicated. Antibodies were detected with goat anti-rabbit conjugated to HRP (Santa Cruz) and Supersignal (Thermo Scientific). Images were quantified with ImageJ software. Total protein quantification is presented as the ratio of the signal intensity of the protein of interest/signal intensity of β-actin and normalized to the average ratio for DC-OVA samples. Phosphorylation quantification is presented as the ratio of the signal intensity of the phosphorylated protein of interest/signal intensity of total protein and normalized to the maximal phosphorylation in DC-OVA samples. Alternatively, ERK1 and 2 phosphorylation was measured by flow cytometry. Briefly, congenically marked WT or Ifnar1−/− P14 CD8+ T cells (2.5×104) were adoptively transferred to naïve recipients. Mice were immunized with DC-GP33 with or without infection with PV on day 3 post-immunization. Splenocytes were isolated on day 5 post-immunization and stimulated with 10 μg/ml biotinylated anti-CD3 (clone 145-2C11, eBioscience) antibody and cross-linked with streptavidin for the indicated times at 37°C. Cells were fixed with BD cytofix (BD Bioscience) and permeabilized with BD Phosflow Perm Buffer III and stained with an antibody specific for pERK1 and 2 (T202 and Y204). Samples were analyzed with a BDLSRFortessa flow cytometer (BD Bioscience) and flowjo software (Tree Star).
Statistical Analyses
Data were analyzed with GraphPad Prism4 software. Specific tests to determine statistical significance are indicated in figure legends.
Supplementary Material
Acknowledgments
We thank M. Mescher for Il12rb1−/− OT -I, Murali-Krishna Kaja for Ifnar1−/− P14, S. Varga for PV and S. Bhaumik, L. Epping and L. Hancox for assistance. We thank members of the Harty Lab, V. Badovinac, S. Condotta and J. Houtman for comments and discussion. MJR is supported by a Canadian Institutes of Health Research fellowship, JCN is supported by a Leukemia and Lymphoma Society career development award. The JTH lab is supported by NIH grants (AI42767, AI50073, AI46653, AI085515).
Footnotes
Author contributions
MJR and JCN designed the experiments, performed the work, analyzed the data and wrote the manuscript. JTH designed the experiments, analyzed the data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nature reviews. Immunology. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- Agarwal P, Raghavan A, Nandiwada SL, Curtsinger JM, Bohjanen PR, Mueller DL, Mescher MF. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183:1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aichele P, Unsoeld H, Koschella M, Schweier O, Kalinke U, Vucikuja S. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- Alexander-Miller MA. High-avidity CD8+ T cells: optimal soldiers in the war against viruses and tumors. Immunologic research. 2005;31:13–24. doi: 10.1385/IR:31:1:13. [DOI] [PubMed] [Google Scholar]
- Amoah S, Yammani RD, Grayson JM, Alexander-Miller MA. Changes in Functional but Not Structural Avidity during Differentiation of CD8+ Effector Cells In Vivo after Virus Infection. J Immunol. 2012;189:638–645. doi: 10.4049/jimmunol.1102579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature. 2000;406:739–742. doi: 10.1038/35021081. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature medicine. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nature immunology. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- Biron CA. Initial and innate responses to viral infections--pattern setting in immunity or disease. Current opinion in microbiology. 1999;2:374–381. doi: 10.1016/s1369-5274(99)80066-6. [DOI] [PubMed] [Google Scholar]
- Brundage RA, Smith GA, Camilli A, Theriot JA, Portnoy DA. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. The Journal of experimental medicine. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nature immunology. 2011 doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Current opinion in immunology. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Williams DB. Intracellular assembly and trafficking of MHC class I molecules. Traffic. 2009;10:1745–1752. doi: 10.1111/j.1600-0854.2009.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Garcia-Aguilar J, Hibbs ML, Larson RS, Stacker SA, Staunton DE, Wardlaw AJ, Springer TA. Structure and regulation of the leukocyte adhesion receptor LFA-1 and its counterreceptors, ICAM-1 and ICAM-2. Cold Spring Harbor symposia on quantitative biology. 1989;54(Pt 2):753–765. doi: 10.1101/sqb.1989.054.01.089. [DOI] [PubMed] [Google Scholar]
- Ely KH, Cauley LS, Roberts AD, Brennan JW, Cookenham T, Woodland DL. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J Immunol. 2003;170:1423–1429. doi: 10.4049/jimmunol.170.3.1423. [DOI] [PubMed] [Google Scholar]
- Griffin BD, Verweij MC, Wiertz EJ. Herpesviruses and immunity: the art of evasion. Veterinary microbiology. 2010;143:89–100. doi: 10.1016/j.vetmic.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Han B, Serra P, Yamanouchi J, Amrani A, Elliott JF, Dickie P, Dilorenzo TP, Santamaria P. Developmental control of CD8 T cell-avidity maturation in autoimmune diabetes. The Journal of clinical investigation. 2005;115:1879–1887. doi: 10.1172/JCI24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Jackson SE, Mason GM, Wills MR. Human cytomegalovirus immunity and immune evasion. Virus research. 2011;157:151–160. doi: 10.1016/j.virusres.2010.10.031. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- Kohlmeier JE, Miller SC, Smith J, Lu B, Gerard C, Cookenham T, Roberts AD, Woodland DL. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29:101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. The Journal of experimental medicine. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Ferez M, Swamy M, Arechaga I, Rejas MT, Valpuesta JM, Schamel WW, Alarcon B, van Santen HM. Increased sensitivity of antigen-experienced T cells through the enrichment of oligomeric T cell receptor complexes. Immunity. 2011;35:375–387. doi: 10.1016/j.immuni.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Malherbe L, Hausl C, Teyton L, McHeyzer-Williams MG. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity. 2004;21:669–679. doi: 10.1016/j.immuni.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, Burkhardt JK, Freedman BD, Billadeau DD. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Current biology: CB. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz JC, Harty JT. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity. 2011;34:781–793. doi: 10.1016/j.immuni.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odumade OA, Knight JA, Schmeling DO, Masopust D, Balfour HH, Jr, Hogquist KA. Primary Epstein-Barr virus infection does not erode preexisting CD8(+) T cell memory in humans. The Journal of experimental medicine. 2012;209:471–478. doi: 10.1084/jem.20112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer EG. Immune responses to Listeria monocytogenes. Nature reviews. Immunology. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J Immunol. 2009;183:2337–2348. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H, Baenziger J, Schilham M, Sado T, Kamisaku H, Hengartner H, Zinkernagel RM. Characterization of virus-specific cytotoxic T cell clones from allogeneic bone marrow chimeras. European journal of immunology. 1987;17:159–166. doi: 10.1002/eji.1830170202. [DOI] [PubMed] [Google Scholar]
- Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrancois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. The Journal of experimental medicine. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador JM, Mittelstadt PR, Guszczynski T, Copeland TD, Yamaguchi H, Appella E, Fornace AJ, Jr, Ashwell JD. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nature immunology. 2005;6:390–395. doi: 10.1038/ni1177. [DOI] [PubMed] [Google Scholar]
- Schmidt NW, Butler NS, Harty JT. Plasmodium-host interactions directly influence the threshold of memory CD8 T cells required for protective immunity. J Immunol. 2011;186:5873–5884. doi: 10.4049/jimmunol.1100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nature immunology. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annual review of immunology. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga SM, Selin LK, Welsh RM. Independent regulation of lymphocytic choriomeningitis virus-specific T cell memory pools: relative stability of CD4 memory under conditions of CD8 memory T cell loss. J Immunol. 2001;166:1554–1561. doi: 10.4049/jimmunol.166.3.1554. [DOI] [PubMed] [Google Scholar]
- Walker LJ, Sewell AK, Klenerman P. T cell sensitivity and the outcome of viral infection. Clinical and experimental immunology. 2010;159:245–255. doi: 10.1111/j.1365-2249.2009.04047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire JK, Eam B, Whitton JL. Tentative T cells: memory cells are quick to respond, but slow to divide. PLoS pathogens. 2008;4:e1000041. doi: 10.1371/journal.ppat.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerly D, Heckerman D, Allen TM, Chisholm JV, 3rd, Faircloth K, Linde CH, Frahm N, Timm J, Pichler WJ, Cerny A, Brander C. Increased cytotoxic T-lymphocyte epitope variant cross-recognition and functional avidity are associated with hepatitis C virus clearance. Journal of virology. 2008;82:3147–3153. doi: 10.1128/JVI.02252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.