Abstract
Background
Fear extinction is a laboratory model of fear inhibition and is the basis of exposure therapy for anxiety disorders. Emerging evidence from naturally cycling female rodents and women indicates that estrogens are necessary to the consolidation of fear extinction. Hormonal contraceptives (HCs) inhibit estrogen production, yet their effects on fear extinction are unknown.
Methods
We used a cross-species translational approach to investigate the impact of HCs and estradiol supplementation on fear extinction in healthy women (n=76) and female rats (n=140).
Results
Women using HCs exhibited significantly poorer extinction recall compared to naturally cycling women. The extinction impairment was also apparent in HC-treated female rats and was associated with reduced serum estradiol levels. The impairment could be rescued in HC-treated rats either by terminating HC treatment after fear learning or by systemic injection of estrogen-receptor agonists prior to fear extinction, all of which restored serum estradiol levels. Finally, a single administration of estradiol to naturally cycling women significantly enhanced their ability to recall extinction memories.
Conclusions
Together, these findings suggest that HCs may impact women’s ability to inhibit fear, but that this impairment is not permanent and could potentially be alleviated with estrogen treatment.
Keywords: Estradiol, fear conditioning, anxiety, menstrual cycle, estrous cycle, gonadal hormones
Pavlovian fear conditioning and extinction are frequently utilized in studies of emotion regulation in rodents and humans. In such procedures a subject is presented a neutral conditioned stimulus (CS, e.g., a tone) that is paired with an aversive unconditioned stimulus (US, e.g., a mild shock), until the CS elicits conditioned fear responses independently of the US. Fear can be extinguished by repeatedly presenting the CS in the absence of any reinforcement. When presented the extinguished CS at a later time, the subject typically exhibits long-term retention of the extinction memory, as indexed by low levels of fear responding. Fear conditioning and extinction have proved to be useful models with which to investigate the mechanisms underlying associative memory formation(1). In addition, exposure therapy, which is among the most commonly used psychological treatments for anxiety disorders, was based on extinction(2).
Due to its theoretical and practical relevance, considerable progress in understanding the mechanisms underlying fear extinction has been achieved in past decades(3). Despite this, we know little about fear extinction in women. Most extinction research in humans has not considered sex or hormonal status to be variables of interest and preclinical research has predominantly used males as subjects. This omission is surprising as research has demonstrated clear sex differences in the formation of associative and emotional memories, as well as in the influence of stress on later learning and memory(4,5). Moreover, anxiety disorders are twice as common in women than men(6). Sex differences in the prevalence of anxiety do not emerge until after puberty(7), and may be partly due to activational effects of gonadal hormones on fear extinction. In support of this, recent evidence in naturally cycling rodents and women suggests that extinction is optimal during the high estrogen phase of the estrous/menstrual cycle, but is reduced during the low estrogen phase(8,9,10,11). In addition, estrogen agonists enhance, whereas estrogen antagonists impair, fear extinction in rodents(8,9,11). Furthermore, naturally high levels of estradiol enhance the function of brain regions involved in fear inhibition, such as the ventromedial prefrontal cortex(11).
The role of gonadal hormones in extinction has thus far been examined in naturally cycling subjects, however around 80% of women use hormonal contraception at some point. Combined hormonal contraceptives (HCs), which contain estrogen and progestin, inhibit ovulation by decreasing ovarian production of estradiol and progesterone(12). As a result, women using combined HCs have stable reductions in circulating estradiol, comparable to or lower than levels in naturally cycling women during the follicular (low estradiol) phase of the menstrual cycle(13,14). Given that elevated estradiol is associated with enhanced fear extinction, we hypothesized that HCs may impair fear extinction memories in both women and female rats. We also hypothesized that increasing estradiol levels, via termination of HCs prior to extinction training or systemic estradiol administration at the time of extinction training, would rescue HC-associated extinction impairments. Finally as a proof of concept, we tested the effects of orally administered estradiol on fear extinction in naturally cycling women low in estradiol. Our prediction was that this treatment would significantly facilitate extinction memory consolidation.
Materials and Methods
Human Participants
In Experiment 1, we recruited a cohort of healthy women (N =13) using combined monophasic HCs (Table S1) and contrasted their data to a previously studied cohort of naturally cycling women who underwent the same experimental design while in a high (H-EST) or low estradiol state (L-EST), as determined by serum analyses(11;see supplemental section for details). In Experiment 5, we recruited a cohort of naturally cycling women (N = 31) to participate ~5 days after the onset of menstruation (early-follicular phase).
Participants (18–30 years old) were recruited through advertisement from the local community, and were without neurologic, endocrinologic, or other medical conditions. Participants were excluded for past or present Axis I mental disorders as determined by the Structured Clinical Interview for DSM-IV. No participant reported to be using psychoactive or other potentially confounding drugs or medications. Written informed consent was obtained from all participants in accordance with the Partners Healthcare Human Research Committee.
Participants were administered a battery of psychiatric measures to assess personality traits, mood, and anxiety (see Table S2 for outcomes). Where significant group differences arose, analyses were repeated including these factors as covariates, and all results remained the same.
Human Conditioning and Extinction Procedure
The fear conditioning and extinction procedures were identical to those previously described (11;see Fig S1 and supplemental information for details). CSs were pictures of lamps, and the US was a mild electric shock delivered to the right hand. On Day 1, subjects underwent habituation (CS-alone presentations). The conditioning phase immediately followed, in which the to-be extinguished light (CS+E) and the to-be unextinguished light (CS+U) were each presented eight times with 62.5% partial reinforcement, while the light that was never followed by shock (CS−) was intermixed in 16 trials. The extinction training phase consisted of 16 CS+E and 16 CS-−trials presented in a new context. The extinction recall phase included eight trials each of the CS+U and CS+E, intermixed with 16 CS− trials, with no US presented, in the extinction context. The context images were presented for a total of 9s: 3s with the CS off followed by 6s with the CS on. The mean intertrial interval was 15s. In Experiment 1, conditioning and extinction training took place on Day 1, and extinction recall on Day 2. In Experiment 5 the phases took place across three consecutive days, in order to isolate any potential effects of estradiol administration to the extinction training phase (see below).
Psychophysiologic Data Analysis
Skin conductance responses (SCRs) were measured as conditioned responses. Conditioning strength was indexed as the average differential SCRs across conditioning trials (average SCRs to the CS+ minus average SCRs to the CS−). Within-session extinction was assessed by comparing average differential SCRs during conditioning to average differential SCRs during the last two extinction trials on Day 1 (Exp 1), or by comparing average differential SCRs during the first two extinction trials to the last two extinction trials on Day 2 (Exp 5). Extinction recall was assessed by calculating the percent recovery of fear, controlling for conditioning strength: each subject’s average SCRs during extinction recall were divided by their largest SCR to the CS+ trials during conditioning. The product was multiplied by 100, yielding a percent recovery of fear.
Estradiol Administration and Serological Measures
In Experiment 5 one dose of Femtrace (1.8mg, estrogen acetate tablets, Warner Chilcott) or placebo was orally administered 30 min prior to extinction training on Day 2. Blood samples were drawn at three time points: immediately before taking estradiol or placebo on Day 2 (T1); immediately after extinction training on Day 2 (T2) and 15 min prior to extinction recall on Day 3 (T3; Fig 3A).
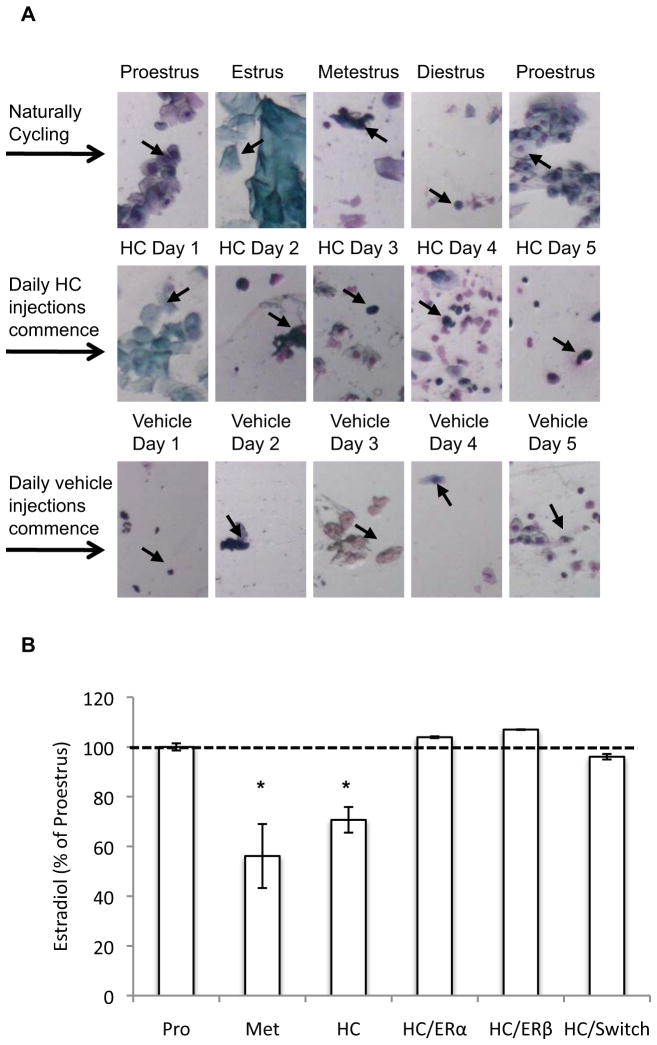
Fig. 3.
(A) Representative vaginal cytology of a naturally cycling rat that received HC treatment for 5 days, and was then switched to vehicle treatment for another 5 days, during Experiment 3 (arrows denote cell types referred to in the following descriptions). A naturally cycling rat progresses through different stages of the estrous cycle on a daily basis (row 1). Nucleated epithelial cells characterize proestrus; cornified cells characterize estrus, leukocytes characterize metestrus, and scant leukocytes along with some non-nucleated and nucleated cells characterize diestrus. HC treatment (row 2) causes persistent metestrus-like cytology (predominantly leukocytes, emerging on HC Days 2–5). Switching the rat from HC to vehicle treatment (row 3) results in a gradual return to normal cycling. Note the reduction in leukocytes by Day 3, and the emergence of cytology consistent with diestrus and then proestrus on Days 4 and 5, respectively. (B) Serum estradiol from naturally cycling rats (N = 5/group) sacrificed during proestrus (Pro) and metestrus (Met), and from HC-treated rats sacrificed after 5 days of HC treatment (HC), 5 days of HC treatment and 1 hr after s.c. injection of an ERα (HC/ERα) or ERβ (HC/ERβ) agonist, and 5 days of HC treatment followed by a switch to vehicle treatment for another 5 days (HC/switch). Estradiol levels are presented as a percentage of those obtained from rats during proestrus. * = p<0.05; HC and Met vs. Pro, HC/ERα, HC/ERβ, and HC/switch.
Animal Subjects
Female Sprague-Dawley rats (250–300g) were individually housed at the Massachusetts General Hospital Center for Comparative Medicine in Charlestown, Massachusetts. They were maintained on a 12-hour light/dark cycle, handled for 5 min/day for 3 days, and habituated to the conditioning chambers for 30 min/day. Vaginal smears were conducted as previously described(11). All procedures were approved by the Subcommittee on Research Animal Care of Massachusetts General Hospital in compliance with National Institutes of Health guidelines.
Animal Apparatus and Procedures
Apparatus were identical to those previously described(11). The CS was a 30s 80db, 4kHz tone. The US was a 0.5s 0.6mA footshock. Fear conditioning, extinction, and recall tests were identical in all rat experiments.
Fear Conditioning
Rats underwent habituation (5 CS trials alone) followed by conditioning (5 CS trials co-terminating with the US) on Day 5 in all experiments (after 4 days of HC or vehicle injections).
Extinction Training
24 hours after fear conditioning (or 4 days after fear conditioning in Exp 3), rats underwent extinction training consisting of 30 non-reinforced CS trials.
Recall Test
24 hours after extinction training all rats were tested for extinction recall, consisting of 3 non-reinforced CS trials.
Drug Administration
Hormonal Contraceptive
Rats were subcutaneously administered levonorgestrel, a progestin commonly used in HCs, or vehicle once daily for 4 days prior to and during conditioning, extinction, and recall. Levonorgestrel (Sigma) was reconstituted at a concentration of 0.25mg/ml in distilled H20 containing DMSO (2:1). In Experiment 2, rats were administered levonorgestrel in the doses of 100μg/kg of body weight and 500μg/kg of body weight. In all subsequent experiments rats were administered the 500μg/kg dose.
Estrogen-Receptor Agonists
In Experiment 4 two groups of rats received subcutaneous injections of the ERα agonist PPT (Tocris) or the ERβ agonist DPN (Sigma) 30 min prior to extinction. PPT and DPN were reconstituted at a concentration of 1.0mg/ml in a sesame oil vehicle. Rats were administered vehicle (sesame oil), PPT, or DPN at a dose of 1mg/kg of body weight. The doses and the timing of administration were based on our previous findings demonstrating that these doses enhance extinction recall when administered 30 mins prior to extinction training(11).
Serological Analysis
An experimentally-naïve group of rats were sacrificed and trunk blood was collected. Serum was analyzed for estradiol levels using a commercially available ELISA kit according to the manufacturer’s instructions (Calbiotech, Inc).
Behavioral Data Analysis
Freezing, defined as the absence of all movement other than that required for respiration, was the conditioned fear response. Each rat was scored every 3s as freezing or not freezing. A percentage score was calculated for each rat to determine the proportion of total observations during the 30s trial scored as freezing. The data for each trial are presented for conditioning and recall phases; the data for extinction training are presented as six blocks of trials, each representing the average of 5 trials. The scorer was unaware of the condition of the rats, and a sample of rats was cross-scored by a second scorer to determine inter-rater reliability. The correlation between the scorers exceeded 0.9.
Statistical Analysis
Analysis of variance with repeated measures was used to analyze data across experimental phases, and analysis of covariance was used to adjust for potentially confounding variables when performing between group analyses in human experiments. Post-hoc comparisons used Tukey’s Honestly Significant Difference test, or independent-samples t-tests when appropriate. Analyses used SPSS (Version 17.0, 2008; SPSS, Chicago, Illinois).
Results
Experiment 1: HCs impair fear extinction in healthy women
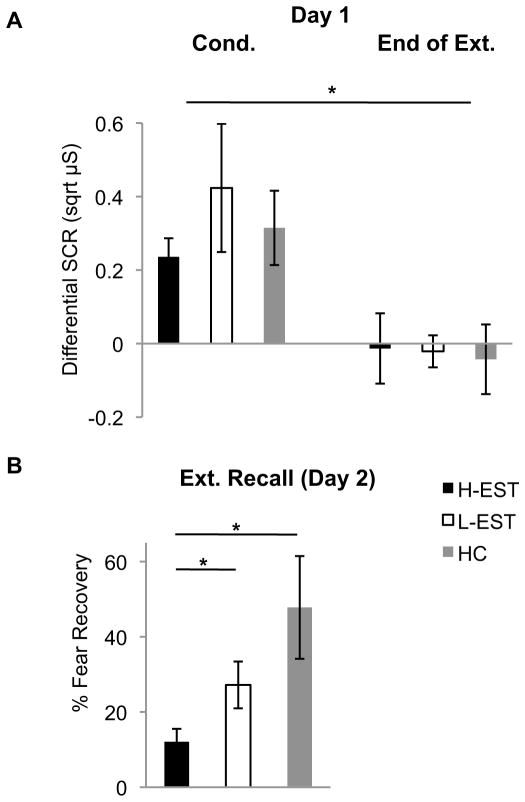
SCRs to conditioned and extinguished stimuli from women using HCs were compared to naturally cycling women who were high (H-EST) or low (L-EST) in estradiol. No group differences were observed across conditioning (F(2,44)=0.63,p=0.54). SCRs at the end of extinction training were significantly less than those during conditioning (F(1,42)=16.30,p<0.0001), with no effect of group (F(2,42)=0.79,p=0.47), nor phase-by-group interaction (F(2,42)=0.44,p=0.65), suggesting that all women attained comparable extinction on Day 1 (Fig 1A). The next day, however, there were group differences in the percent recovery of fear at extinction recall (F(2,44)=4.67,p=0.015). Post-hoc analyses confirmed that H-EST exhibited significantly less recovery of fear compared to both L-EST (t(30)=2.42,p=0.02) and HC (t(27)=2.63,p=0.02) groups (Fig 1B). These data indicate that HCs impair the consolidation or maintenance of extinction memories in healthy women, without altering conditioning or acquisition of extinction.
Fig. 1.
(A) Mean (±SEM) differential SCRs during conditioning (average across trials) and the end of extinction training (average of last two trials) on Day 1 in Experiment 1. * = p<0.05; cond. vs. end of ext. (B) Mean (±SEM) percent resovery of SCRs at extinction recall in Experiment 1. Ns = 16 per group for H-EST and L-EST, N = 13 for HC users. * = p<0.05; H-EST vs. L-EST, and HC.
Experiment 2: HCs impair fear extinction in female rats
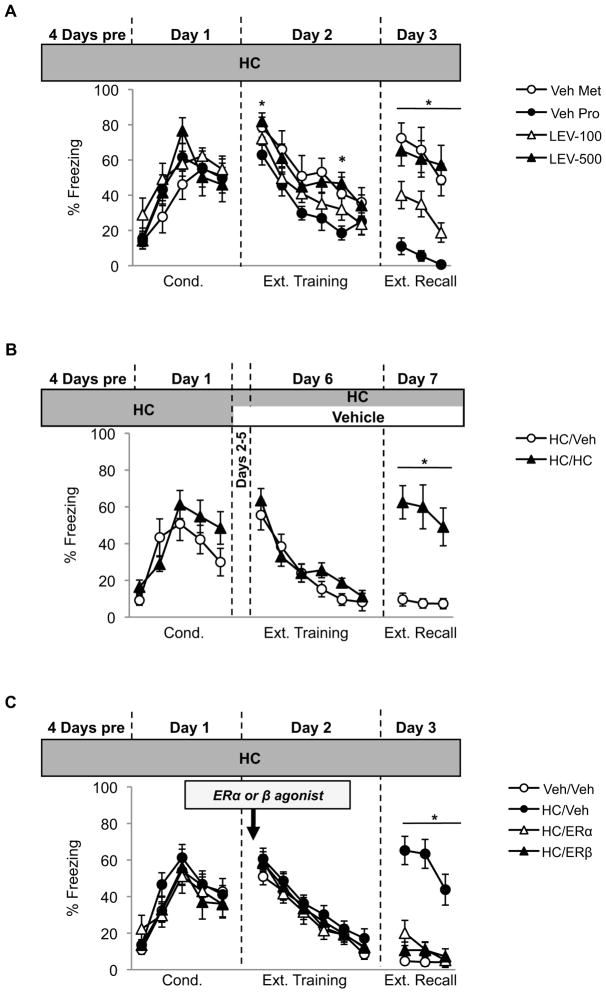
We next assessed whether the effects of HCs on extinction are consistent across species by treating adult female rats with a low (LEV-100) or high (LEV-500) dose of levonorgestrel or vehicle (Fig 2A). Vehicle-treated rats remained naturally cycling and were divided into proestrus (high estradiol; Veh-Pro) or metestrus (low estradiol; Veh-Met) groups depending on their estrous cycle phase during extinction training. Comparing freezing responses during conditioning, there was a significant effect of trial (F(4,124)=2.89,p<0.0001), and no significant effect of group (F(1,31)=0.75,p=0.53), nor a significant trial-by-group interaction (F(12,124)=1.01,p=0.44), suggesting that all groups acquired the fear comparably. Freezing significantly reduced across extinction training (F(5,185)=46.29,p<0.0001). There was a significant between-group effect (F(1,37)=3.30,p=0.03), due to LEV-500 rats freezing more than Veh-Pro rats during blocks 1 (p=0.04) and 5 (p=0.01), suggesting that the high dose HC may have caused enhanced consolidation of fear and/or resistance to extinction. Importantly, all groups obtained comparable extinction by the last block of extinction training (smallest p=0.645). During extinction recall, however, there were significant group differences (F(1,37)=14.63,p<0.0001). Veh-Pro rats froze significantly less than all other groups (largest p=0.045). HC treatment dose-dependently impaired extinction, as LEV-100 rats exhibited significantly less freezing at test compared to both LEV-500 and Veh-Met rats (largest p=0.03); whereas LEV-500 and Veh-Met groups exhibited comparable freezing (p=0.99).
Fig. 2.
(A) Mean (±SEM) freezing responses across experimental phases in Experiment 2. Ns = 11 (Veh-Pro), 7 (Veh-Met), 12 (LEV-100), 12 (LEV-500). Day 2 * = p<0.05; LEV-500 vs. Veh-Pro. Day 3 * = p<0.05; Veh-Pro vs. Veh-Met, LEV-100, and LEV-500; and LEV-100 vs. Veh-Met and LEV-500. (B) Mean (±SEM) freezing responses across experimental phases in Experiment 3. Ns = 8–9 per group. Day 3 * = p<0.05, HC/Veh vs. HC/HC. (C) Mean (±SEM) freezing responses across experimental phases in Experiment 4. Ns = 12–14/ group. Day 3 * = p<0.05; HC/Veh vs. Veh/Veh, HC/ERα, and HC/ERβ.
Experiment 3: Termination of HC treatment after fear conditioning prevents impairments in extinction recall in female rats
We next sought to determine on which phase of learning HCs exert their effects. Our previous rodent studies demonstrated that estrous cycle phase during extinction training, but not conditioning or recall, significantly impacts extinction recall(9). Thus, we hypothesized that HCs may also exert their effects during extinction training. Alternatively, it is possible that HCs alter the quality of the conditioning memory, rendering it resistant to extinction. To distinguish between these possibilities two groups of rats received HC-treatment for four days prior to and during fear conditioning. After fear conditioning, one group received vehicle injections (HC/Veh), while the other group continued to receive HCs (HC/HC). Four days later rats were extinguished and tested for extinction recall (Fig 2B).
There was a significant effect of conditioning trial (F(4,60)=9.65,p<0.0001), and of extinction block four days later (F(5,75)=36.45,p<0.0001). There were no overall effects of group and no significant trial- or block-by-group interactions during conditioning or extinction (ps>0.176), indicating that terminating HCs prior to extinction training had no effect on extinction acquisition. During extinction recall, however, rats that had been taken off HCs exhibited significantly less freezing compared to rats that remained on HCs (t(15)=4.12,p=0.004). Thus, it is necessary for rats to be on HCs during extinction training in order to induce a deficit in extinction recall. Furthermore, HC-treatment during fear conditioning is not sufficient to impair extinction recall, so long as treatment is terminated prior to extinction training.
Experiment 4: HC-induced extinction impairments can be rescued by systemic estrogen agonists in female rats
We next assessed whether the extinction deficit caused by HCs could be rescued without terminating HC treatment altogether. HCs reduce several hormones, including estradiol. If the impairment in extinction recall is partly due to a reduction in available estradiol at the time of extinction training, then increasing estradiol levels during extinction training should reduce the impairment in recall. To test this, we administered HC-treated rats an ERα agonist, an ERβ agonist, or vehicle, 30 min prior to extinction training, and compared these groups to a non-HC (vehicle) control (Fig 2C). There was a significant effect of conditioning trial (F(4,188)=26.115,p<0.0001), and extinction block (F(5,235)=75.47,p<0.0001). There were no effects of group or significant trial- or block-by-group interactions at either time point (ps>0.65), indicating that HC-treatment and estradiol supplementation had no effects on within-session extinction. However, there were group differences during extinction recall (F(1,47)=29.27,p<0.0001). HC-treated rats that received vehicle prior to extinction training froze significantly more than all other groups (ps<0.0001); whereas HC-treated rats that received the ERα or ER β agonist exhibited low and comparable levels of freezing to the non-HC control group (smallest p=0.65). These data suggest that the HC-induced impairments in extinction recall may be due to a reduction in estradiol binding to either the alpha or beta ER.
We used two approaches to confirm that our manipulations in rats altered the estrous cycle and estradiol levels. First, we examined vaginal cytology from rats in Exps 2–4. HC-treatment disrupted the daily variation in epithelial cells that is typical in naturally cycling rats, and led to a stable cytology comparable to that of rats in the metestrus stage, which persisted throughout the duration of HC-treatment. Normal cycling restored by 4 days after HC-termination (see Fig 3A caption for description of cell types). Second, we examined the effects of our manipulations in Exps 2–4 on serum estradiol. A new group of experimentally-naïve rats were sacrificed during the proestrus or metestrus phase of the estrous cycle, or after 5 days of HC treatment. Two other HC-treated groups were sacrificed 1hr after injection of an ERα (HC/ERα) or ERβ (HC/ERβ) agonist. A third HC-treated group was sacrificed after receiving vehicle treatment for 5 days (HC/switch). Thus, estradiol levels for each group were obtained at a time point corresponding to the occurrence of extinction training in Exps 2–4. Figure 3B presents estradiol levels as a percentage of proestrus rats. There was a significant effect of group (F(5,29)=13.01,p<0.0001), due to estradiol being significantly lower in HC and in metestrus groups compared to all other groups (ps<0.045), the latter of which did not differ (p=0.49). These data demonstrate that HCs reduce estradiol to a level comparable with metestrus, whereas removal from HCs or treatment with estrogen agonists restores serum estradiol to a level comparable with proestrus.
Experiment 5: Estradiol prevents extinction impairments in naturally cycling women
The previous experiments raised the possibility that estradiol may prevent extinction impairments in humans. In order to circumvent the difficulties of testing this in women on HCs (in whom the effects of additional estradiol further to those in their HC are unknown), and as a proof of concept, we addressed this question in a sample of healthy, naturally cycling women in the early-follicular (low estradiol) menstrual cycle stage. Women in this stage exhibit extinction impairments(11), similar to what we have observed in women on HCs, and so provide a useful cohort in which to test the effects of estradiol on fear extinction in humans.
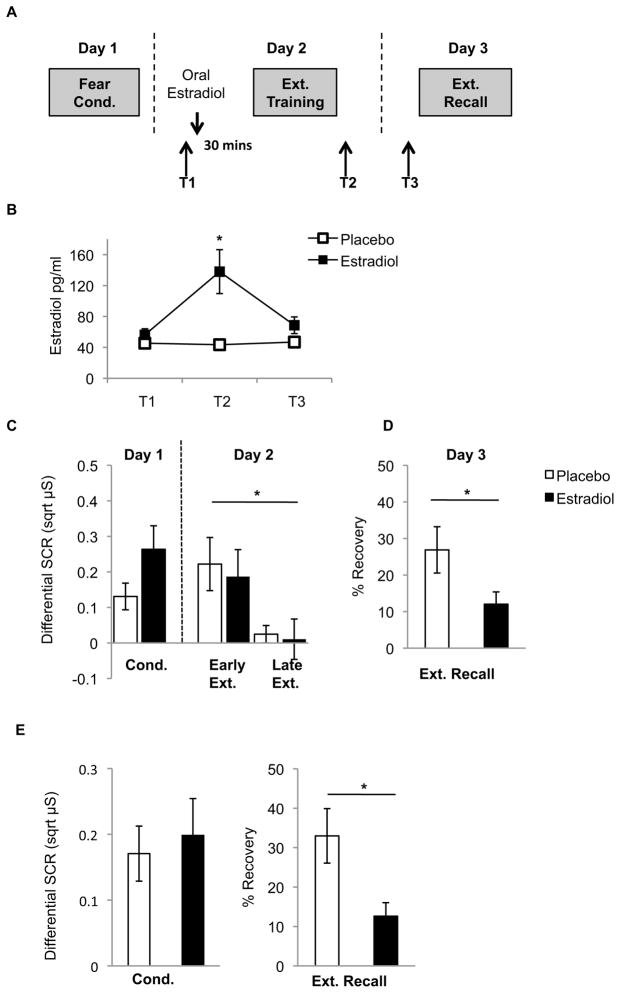
Femtrace selectively enhanced serum estradiol during extinction training, as estradiol was significantly greater in estradiol-treated women at T2 (immediately after extinction training; p=0.006), but not at any other point (Fig 4B; p>0.09). Estradiol-treated women also had significantly lower follicle-stimulating hormone than placebo-treated women at T3 prior to extinction recall (p=0.002), but did not differ on this, or other hormone assays (progesterone, luteinizing hormone, or thyroxin) at any other time (p>0.09; Table S3).
Fig. 4.
(A) Experimental protocol for Experiment 5. (B) Mean (±SEM) serum estradiol levels during the different phases of the experiment. T2 * = p< 0.05; Placebo vs. Estradiol. (C) Mean (±SEM) differential SCRs during conditioning, and differential SCRs during early and late extinction. * = p<0.05; Early vs. Late Ext. (D) Mean (±SEM) percent recovery of SCRs at extinction recall in Experiment 5 (Ns = 15–16/group). * = p<0.05; Placebo vs. Estradiol (E) A selection of estradiol (N = 13) and placebo-treated (N = 10) women were matched for conditioning strength. Estradiol-treated women exhibited significantly less percent recovery of fear at extinction recall than placebo-treated women when matching for conditioning strength. * = p< 0.05; Estradiol vs. Placebo.
No group differences were observed during conditioning (t(29)=1.72;p=0.097), and SCRs significantly declined across extinction training (F(1,29)=7.61,p=0.01), without any significant effects of group (F(1,29)=0.20,p=0.66), nor a time-by-group interaction (F(1,29)=0.025,p=0.88). This suggests that both groups recalled the fear memory and extinguished it comparably on Day 2 (Fig 4C). On Day 3, however, estradiol-treated women exhibited significantly less recovery of fear than placebo-treated women (t(29)=2.11,p=0.04) (Fig 4D), suggesting that, similar to HC-treated rats in the previous experiment, estradiol may have enhanced the consolidation of extinction.
Although this was a double-blind controlled study, to-be estradiol-treated women appeared to show greater conditioning strength than to-be placebo-treated women, albeit this was a non-significant trend (p=0.097). To ensure that the reduced recovery of fear observed in estradiol-treated women was not due to this difference, we selected women from each group to create two groups in which conditioning strength was identical (p=0.75). Using this method, estradiol-treated women still exhibited significantly reduced recovery of fear (p=0.02; Fig 4E).
Estradiol-treated women showed large variability in their response to Femtrace, as indexed by the percent change in serum levels between T1 (pre-extinction) and T2 (post-extinction) (range=23–1583%). We therefore divided estradiol-treated women into “responders” (>80% change) and “non-responders” (<80% change) on the basis of a median split in percent change in estradiol. There were no between-group differences in fear acquisition or within-session extinction (ps>0.05), however responders exhibited significantly less recovery of fear at recall than non-responders (t(13)=2.34,p=0.036) (responders=5.82%, non-responders=20.24%). Importantly, hormonal levels did not differ between groups at any point (p>0.05), suggesting that the magnitude of increase in estradiol may be more important in augmenting extinction ability than is the overall level of estradiol during extinction training.
Discussion
The present experiments demonstrated that although HCs had little or no effect on fear conditioning and acquisition of extinction, they significantly impaired extinction recall. The impairment was prevented in rats by terminating HCs or by administering ER agonists prior to extinction training, and pre-extinction estradiol administration also prevented extinction impairments in early-follicular women. HCs reduce ovarian hormones, and we found that manipulations that increased serum estradiol rescued HC-induced extinction deficits. It is therefore likely that the reduction in estradiol caused by HCs may underlie the deficit in extinction recall. Estradiol substantially impacts cell signaling, excitability, and morphology. For example, estradiol enhances dendritic spine density and synaptogenesis in the hippocampus(15), and the medial prefrontal cortex in rats and monkeys(16,17). Fear extinction is associated with dendritic spine density alterations in the prefrontal cortex(18). While the effects of HCs on spine density are unknown, ovariectomy, which immediately reduces estradiol levels, causes gradual reductions in spine density (15). As such, the extinction deficit observed in the present study may reflect a longer-term consequence of HCs on spine density following persistent reductions in estradiol.
We reported that a single dose of estrogen administered 30 minutes prior to extinction training rescued fear extinction in rats and women. This suggests that alterations in estrogen must also have more rapid, potentially nongenomic physiological consequences that may regulate the memory processes underlying fear extinction. Indeed, it has been determined that induction of spinogenesis and synaptogenesis by estradiol can occur in rodents within 30 minutes of systemic administration(19,20). Moreover, estrogen causes rapid increases in hippocampal NMDA receptor activation, brain-derived neurotrophic factor (BDNF), phosphorylation of CREB via MAPK/ERK signaling, and enhances long-term potentiation(21). Such nongenomic effects are most likely mediated by extranuclear ERs(22). Critically, there is much overlap between the rapid physiological consequences of estrogen and the molecular and cellular substrates of extinction(1). Therefore we speculate that the observed deficits in extinction caused by HCs may be mediated by a down-regulation of both the longer-term genomic and shorter-term nongenomic effects of estradiol. Supporting this idea are reports that HCs reduce peripheral BDNF(13) modify brain structure(14,23), and most notably, alter brain function during extinction learning(24). The rescue of extinction deficits by estradiol, on the other hand, may be due to the more rapid nongenomic consequences of estradiol on neuronal plasticity. Both of these possibilities will need to be addressed in future research. We also note that HC-induced changes in other hormones such as progesterone may contribute to the extinction deficit observed in our study, given progesterone’s role in other types of learning and memory(5), including fear extinction(9).
Some reports indicate that ERα and ERβ agonists have anxiogenic and anxiolytic effects on state anxiety, respectively(25). Consistent with our previous findings(9,11), we did not observe any effect of ER agonists on fear expression during extinction training, suggesting that estradiol may enhance extinction by modulating memory processes rather than fear expression. The non-selective facilitation of extinction by both agonists in HC-treated rats was unexpected, as we and others have previously shown that agonists of ERβ, but not ERα, facilitate extinction in naturally cycling and ovariectomized rats(8,9). There may be differences in ER expression in naturally cycling and ovariectomized versus HC-treated animals, which may in turn modulate their responsiveness to estradiol treatment. ER expression fluctuates across the estrous cycle and is modified by ovariectomy and administration of hormones(26). The effects of HCs on ERs remain uninvestigated. Nevertheless, there are reasons to suspect that both ERα and ERβ may modulate fear extinction as both receptors are involved in regulating neural plasticity underlying long-term memory(21,22), and both are expressed within the neural circuitry that mediates fear extinction(27,28,29).
Estrogen has been implicated in the pathophysiology of psychiatric disorders, including depression and schizophrenia(30), with several studies noting a worsening of symptoms during periods of low estradiol(27). Few studies have examined potential effects of estrogen on anxiety disorders, and the neurobiological reason for women’s greater vulnerability to anxiety remains unknown. It could be speculated that women may be less likely to naturally extinguish fearful memories, or further, may be less responsive to exposure therapy, when estradiol levels are low. Consistent with this premise is the recent finding that women with PTSD exhibited extinction deficits, along with greater symptom severity, only when estradiol levels were low(31). Future studies should examine the possibility that systemic estradiol might prevent extinction impairments, or enhance exposure-based therapies for anxiety, in women. Lastly, this study highlights the importance of taking into consideration HC use, and hormonal status in general, in women in clinical settings. In particular, our data suggest the need for further investigations into the impact of HCs on emotion regulation and therapeutic interventions for anxiety.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health grants K01MH080346, 1R01MH097880–01, and institutional funds from the Department of Psychiatry at MGH to MRM, and a Neurological Fellowship from the American Australian Association to BMG. The project was also supported by Harvard Clinical and Translational Science Center grant (1UL1 RR025758–01) from the National Center for Research Resources.
Footnotes
Financial Disclosures: MRM and BMG report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman SG. Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clin Psychol Rev. 2008;28:199–210. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2011;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeng LY, Waddell J, Shors TJ. The prefrontal cortex communicates with the amygdala to impair learning after acute stress in females but not in males. J Neurosci. 2010;30:16188–16196. doi: 10.1523/JNEUROSCI.2265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ertman N, Andreano JM, Cahill L. Progesterone at encoding predicts subsequent emotional memory. Learn Mem. 2011;18:759–763. doi: 10.1101/lm.023267.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 7.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu K. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor β. Hippocampus. 2009;19:1142–1150. doi: 10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- 9.Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 2011;70:920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. AJOG Reviews. 1999;181:1263–1269. doi: 10.1016/s0002-9378(99)70120-1. [DOI] [PubMed] [Google Scholar]
- 13.Pluchino N, Cubeddu A, Begliuomini S, Merlini S, Giannini A, Bucci F, Casarosa E, Luisi M, Cela V, Genazzani AR. Daily variation of brain-derived neurotrophic factor and cortisol in women with normal menstrual cycles, undergoing oral contraception and in postmenopause. Hum Reprod. 2009;24:2303–2309. doi: 10.1093/humrep/dep119. [DOI] [PubMed] [Google Scholar]
- 14.De Bondt T, Van Hecke W, Veraart J, Leemans A, Sijbers J, Sunaert S, Jacquemyn Y, Parizel PM. Does the use of hormonal contraceptives cause microstructural changes in cerebral white matter? Preliminary results of a DTI and tractography study. Eur Radiol. 2012 doi: 10.1007/s00330–012–2572–5. [DOI] [PubMed] [Google Scholar]
- 15.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 16.Chen JR, Yan YT, Wang TJ, Chen LJ, Wang YJ, Tseng GF. Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cerebral Cortex. 2009;19:2719–2727. doi: 10.1093/cercor/bhp048. [DOI] [PubMed] [Google Scholar]
- 17.Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WGM, Lou W, et al. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai CS, Franke TF, Gan W. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012 doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- 19.MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 20.Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor alpha and beta selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- 21.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, Kerschbaum HH. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Research. 2010;1348:55–62. doi: 10.1016/j.brainres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. Neuronal correlates of extinction learning are modulates by sex hormones. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nsr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lund TD, Rovis T, Chung WCJ, Handa RJ. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 26.Spary EJ, Maqbool A, Batten TFC. Changes in oestrogen receptor α expression in the nucleus of the solitary tract of the rat over the oestrous cycle and following ovariectomy. J Neuroendocrinol. 2010;22:492–502. doi: 10.1111/j.1365-2826.2010.01977.x. [DOI] [PubMed] [Google Scholar]
- 27.Östlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Science. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- 28.Wang ACJ, Hara Y, Janssen WGM, Rapp PR, Morrison JH. Synaptic estrogen receptor-α levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 2010;30:12770–12776. doi: 10.1523/JNEUROSCI.3192-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montague D, Weickert CS, Tomaskovic-Crook E, Rothmond DA, Kleinman JE, Rubinow DR. Oestrogen receptor α localisation in the prefrontal cortex of three mammalian species. J Neuroendocrinol. 2008;20:893–903. doi: 10.1111/j.1365-2826.2008.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perlman WR, Tomaskovic-Crook E, Montague DM, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. Alteration in estrogen receptor α mRNA levels in frontal cortex and hippocampus of patients with major mental illness. Biol Psychiatry. 2005;58:812–824. doi: 10.1016/j.biopsych.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 31.Glover EM, Jovanovich T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72:19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.