Abstract
A mouse model of heterosubtypic influenza A virus infections was used to determine the role of MyD88 signaling in CD4+ T-cell, CD8+ T-cell, and IgG immune responses. We found that MyD88 signaling played an important role in anti-influenza A virus heterosubtypic lung and spleen CD4+ T-cell, and spleen CD8+ T-cell, immune responses. MyD88 dependent signaling was important for T-helper 1 cytokine production in anti-influenza A virus lung and spleen heterosubtypic CD4+ T-cells, but not for their frequencies. Toll-like receptor 7 dependent signaling played a partial role in anti-influenza A virus lung heterosubtypic CD4+ T-helper 1 responses and anti-influenza A virus heterosubtypic IgG2c antibody levels. Our results have important implications for the generation of effective universal influenza vaccines.
Keywords: Influenza A virus, heterosubtypic, MyD88, TLR7, T-cell, antibody
INTRODUCTION
Influenza A virus (IAV) is a negative single stranded virus responsible for annual seasonal epidemics worldwide. Given seasonal viral antigenic drift, and occasional antigenic shift (Taubenberger and Kash, 2010), it is rare for an individual be serially infected with the same IAV subtype. In the quest for a universal influenza vaccine, lessons are often learned from heterosubtypic immune responses to serial IAV infections. Most often these heterosubtypic adaptive immune responses are directed against IAV internal proteins. These internal proteins tend to be conserved across the different subtypes of IAV (Thomas et al., 2006).
Several pattern recognition receptors (PRRs) mediate the initial recognition of IAV and shape the adaptive immune response (Jensen and Thomsen, 2012). Examples include the MyD88 signaling dependent toll-like receptor 7 (TLR7) (Diebold et al., 2004) and the MyD88 signaling independent receptors TLR3 (Guillot et al., 2005), retinoic-acid-inducible protein I (RIG-I) (Kato et al., 2006), nucleotide-binding domain and leucine-rich-repeat-containing protein 3 (NLRP3) (Kanneganti et al., 2006), and nucleotide-binding oligomerization domain-containing protein 2 (NOD2) (Sabbah et al., 2009). MyD88 signaling has been found to be essential for the optimal protection against various pathogens, including homologous challenge with IAV (Seo et al., 2010). However, the role of MyD88 signaling in heterosubtypic IAV infections is not well characterized.
We utilized a murine model of heterosubtypic IAV infections (Belz et al., 2002; Denton et al., 2007) to examine the role of MyD88 dependent signaling in heterosubtypic adaptive immune responses. Mice were first intranasally infected with a sublethal dose of H1N1 IAV A/Puerto Rico/8/34 (PR/8). 21 days later, the mice were then intranasally challenged with a sublethal dose of H3N2 IAV A/HK/X31 (HK/X31). The H3N2 HK/X31 virus contains all the H1N1 PR/8 internal proteins. Heterosubtypic adaptive immune responses to the internal IAV proteins, nucleoprotein (NP) and polymerase (PA), were examined 7 days after the second IAV infection. We found that MyD88 dependent signaling played an important role in anti-IAV heterosubtypic memory lung and spleen CD4+ T-cell, and spleen CD8+ T-cell, immune responses. MyD88 dependent signaling was important for T-helper 1 cytokine production in anti-influenza A virus lung and spleen heterosubtypic CD4+ T-cells, but not for their frequencies. TLR7 dependent signaling played a partial role in anti-IAV lung heterosubtypic CD4+ T-helper 1 (Th1) responses and anti-IAV heterosubtypic IgG2c antibody levels.
MATERIALS AND METHODS
Viruses, animals, and antibodies (Abs)
Purified influenza A/Puerto Rico/8/34 H1N1 (PR/8) and influenza A/HK/X31 H3N2 (HK/X31) were purchased from Charles River (Wilmington, MA). The HK/X31 virus is a laboratory recombinant that contains all the PR/8 internal proteins (Kilbourne, 1969). C57BL/6J (B6) mice were purchased from The Jackson Laboratory (Maine, USA). MyD88−/−, TLR7−/−, IL-1R−/−, and IL-18R−/− mice were provided by Drs. R. Finberg and K. Fitzgerald (University of Massachusetts Medical School, Worcester, MA) (Glaccum et al., 1997; Wang et al., 2007; Wang et al., 2010). IL-1α−/−, IL-1β−/− and IL-1αβ−/− mice were provided by Drs. K. Rock and H. Kono (University of Massachusetts Medical School) (Horai et al., 1998; Kono et al., 2010). The genetically modified knockout mice were backcrossed eight or more generations onto the B6 background and were then intercrossed to obtain the knockout genotypes. All animal procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health. The protocol was approved by the University of Massachusetts Medical School Animal Care and Use Committee (Protocol A-1884).
Purified PerCPCy5.5-anti-mouse CD4, PE-anti-mouse CD8, Alexa 700-anti-mouse TNFα and Allophycocyanin-anti-mouse IFN-γ were purchased from BD Biosciences (San Jose, CA). Purified Pacific Blue/V450-anti-mouse CD3 was purchased from eBiosciences (San Diego, CA).
Heterosubtypic IAV infection model
Primary IAV infections were carried out by intranasal infection of naïve mice with 10 plaque forming units (pfu) of the PR/8 virus (a sublethal dose). After 21 days, the mice were challenged with 104 pfu of the HK/X31 virus by intranasal infection (a sublethal dose). 7 days later, the mice were sacrificed and T-cell and IgG responses to immunodominant internal PR/8 proteins were examined.
Intracellular cytokine staining (ICS)
IFN-γ and TNFα-secreting CD4+ and CD8+ T-cells in mouse spleens and lungs were identified by ICS. Spleens were collected and homogenized by passing through 40 µm nylon strainers and lysing RBCs with ammonium chloride in Tris-HCl buffer. Lungs were treated with liberase enzyme solution (0.14 u/ml, Roche, Indianapolis, IN) and DNAse I (2,000 u/ml, New England BioLabs Inc., Ipswich, MA) for 45 min at 37°C prior to homogenization. The spleen & lung cell suspensions were washed with media, and then stimulated for 6 hours with immunodominant influenza A internal protein CD4+ or CD8+ T-cell peptides (CD4+ T-cell epitope-nucleoprotein (NP)311–325, AnaSpec, Inc., San Diego, CA) (CD8+ T-cell epitopes-NP366–374 and polymerase (PA)224–233, AnaSpec, Inc.) (Belz et al., 2002; Crowe et al., 2006; Townsend et al., 1986). The peptide stimulations were done in the presence of 1µl Brefeldin A (BD Biosciences). Cells were first stained with surface Abs, permeabilized with Cytofix/Cytoperm (BD Biosciences), stained with cytokine Abs, and fixed. Cells were analyzed using a FACSAria flow cytometer (BD, Franklin Lanes, NJ). LIVE/DEAD® Fixable Dead Cell Stain Kit (LDA) (Invitrogen, Carlsbad, CA) was used to exclude nonviable cells from analysis. IFN-γ or TNFα secreting CD4+ and CD8+ T-cells were identified as LDA−/CD3+/CD4+ or CD8+/ IFN-γ+ or TNFα+ cells (Supplementary Figure 1). Data was analyzed using FlowJo software (Treestar, Ashland, OR).
MHC Class I tetramer staining
MHC Class I peptide tetramers (PR/8 IAV nucleoprotein (NP)366–374/Db) were generated by the NIH Tetramer Facility (Atlanta, GA). Tetramer staining was performed on splenocytes for 30 min on ice, followed by staining for CD8+ T-cells. LDA was used to exclude nonviable cells from analysis.
Virus-specific antibody responses
Sera from IAV infected mice were obtained 21 days after primary infection and 7 days after secondary infection. Anti-NP specific antibodies were determined by ELISA. Briefly, 1 µg/ml of purified recombinant IAV PR/8 NP (Imgenex, San Diego, CA) was used as antigen on 96-well flat-bottomed microtiter plates (Thermo Scientific, Rochester, NY). Diluted sera were added to plates and incubated for 2 hours at room temperature. Then, horseradish peroxidase (HRP)-conjugated rat anti-mouse IgG, IgG1, or IgG2c were used as secondary antibodies (Southern Biotech, Birmingham, AL). The ELISA plates were developed using 3,3’,5,5’-tetramethylbenzidine (KPL, Gaithersburg, MD), and the reactions were stopped with 2N sulfuric acid. Relative antibody concentrations were determined using optical spectrophotometer readings at 450nm using a Spectra MAX microplate reader and analyzed with Softmax® Pro 5 Software (Molecular Devices, Sunnyvale, CA).
Statistics
Statistical analysis was done using GraphPad Prism Software version 5.04 (GraphPad, San Diego, CA). Comparisons between two normally distributed variables were performed using the unpaired Student’s t-test. Comparisons between two non-normally distributed variables were performed using the nonparametric Mann Whitney U test. A difference was considered significant if the p-value was < 0.05.
RESULTS
Murine model of heterosubtypic immunity to IAV
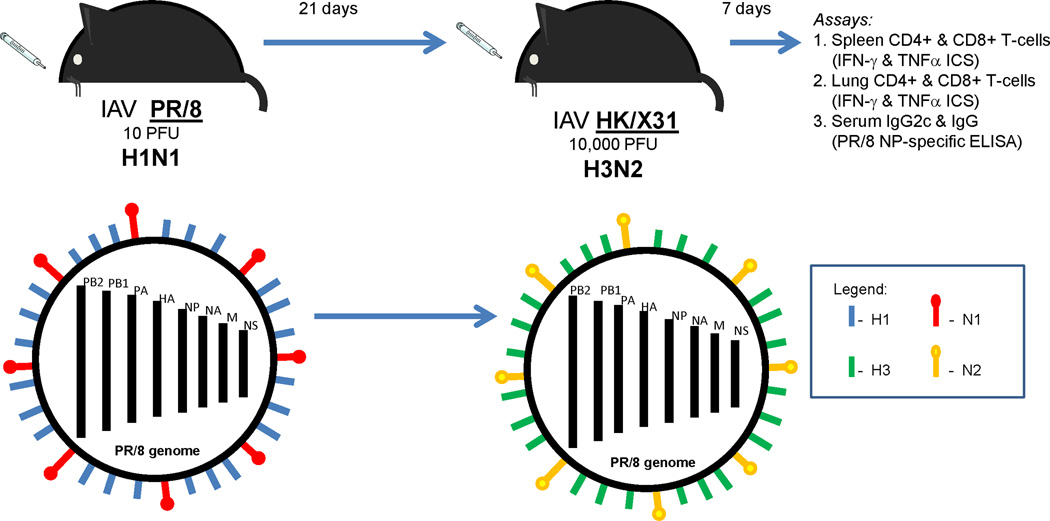
We utilized a murine model of heterosubtypic infections to investigate the role of PRR signaling pathways in memory adaptive immune responses to IAV. Mice were infected intranasally with a sublethal dose of influenza A PR/8 (H1N1) and later intranasally challenged with a sublethal dose of influenza A HK/X31 (H3N2), a strain that contains all the internal proteins of PR/8. All mice survived as both influenza A virus doses were sublethal. We examined heterosubtypic T-cell and IgG responses to the IAV internal proteins, NP and PA (Figure 1). This model allowed us to examine heterosubtypic immune responses to IAVs without the confounding effects of anti-hemagglutinin protective antibodies.
Figure 1. Heterosubtypic immunity to influenza A virus (IAV) internal proteins.
B6 mice were infected intranasally (i.n.) with 10 plaque forming units (PFU) of PR/8 IAV and challenged 21 days later with 10,000 PFU of HK/X31 IAV i.n. 7 days after HK/X31 infection, spleen, lung, and serum were collected for intracellular cytokine staining (ICS) and PR/8 nucleoprotein (NP)-specific IgG ELISA. PR/8 and HK/X31 IAVs have different hemagglutinin (HA) and neuraminidase (NA) surface proteins and are not recognized by any cross-reactive neutralizing antibodies. These IAV strains share 6 internal genes derived from PR/8 – NP, the polymerase complex heterotrimer (PB2, PB1 and PA), matrix protein (M) and nonstructural protein (NS).
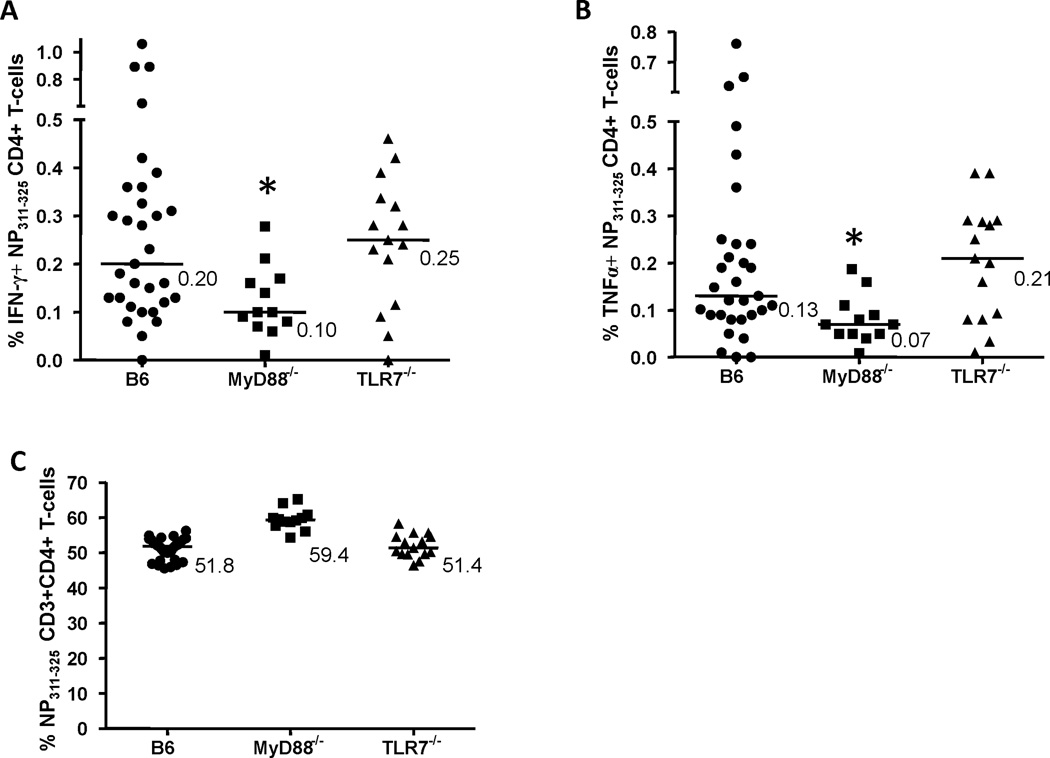
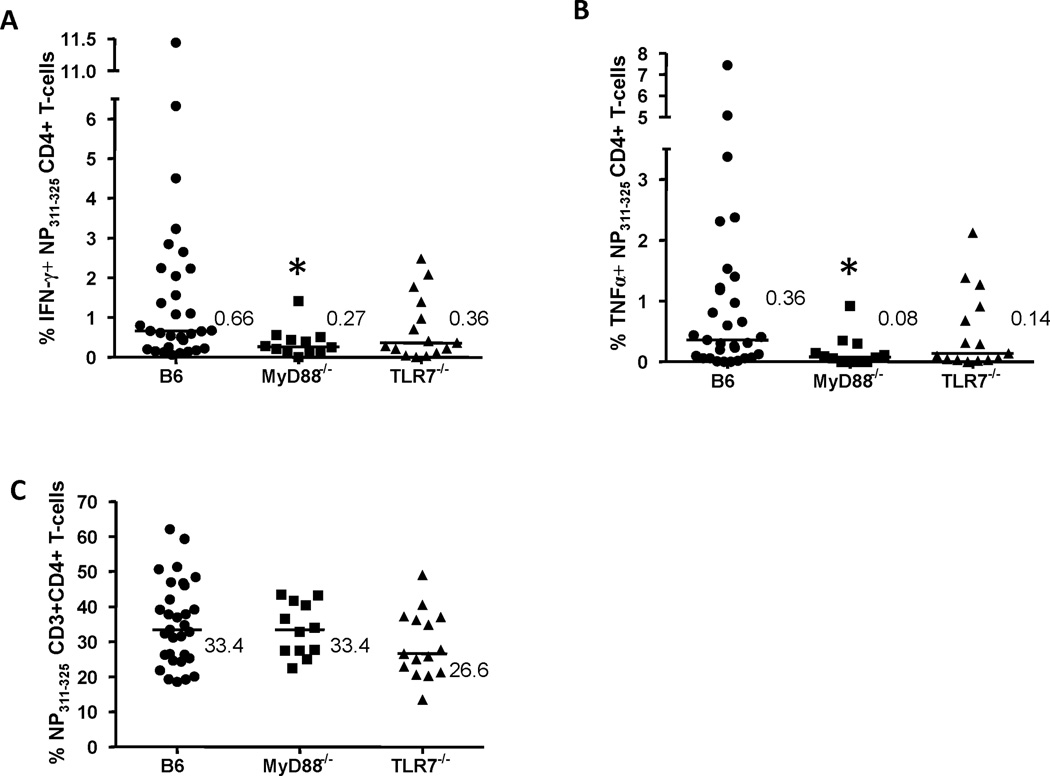
A MyD88-mediated signaling pathway is required for the induction of heterosubtypic CD4+ T-cell immune responses to IAV
In a murine homologous IAV challenge model, splenic CD4+ T-cell immune responses were previously reported to be dependent on TLR7/MyD88 signaling (Koyama et al., 2007). We found that anti-IAV NP311–325 CD4+ T-cell interferon-γ (IFN-γ) and tumor necrosis factor-α (TNFα) production in the lung and spleen were dependent on MyD88 signaling in a heterosubtypic IAV infection model (Figures 2&3). Nearly all NP311–325 specific CD4+ T-cells produced both cytokines (data not shown). Anti-IAV NP311–325 CD4+ Th1 cytokine production in the lung and spleen was MyD88 dependent, but the frequencies of anti-IAV NP311–325 CD4+ T-cells at these two sites were not (Figures 2c&3c). We further found that lung anti-IAV NP311–325 CD4+ Th1 cytokine production was partially dependent on TLR7 signaling, but spleen anti-IAV NP311–325 CD4+ Th1 cytokine production was TLR7 independent (Figures 2&3). Interleukin-1 receptor (IL-1R) and IL-18R signaling are also MyD88 dependent (Boraschi and Tagliabue, 2006). Lung and spleen anti-IAV NP311–325 CD4+ Th1 cytokine production were unchanged in IL-1α−/−, IL-1β−/−, IL-1αβ−/−, IL-1R−/−, or IL-18R−/− mice compared to wild type (data not shown).
Figure 2. Interferon-γ (IFN-γ) or tumor necrosis factor-α (TNFα) production by influenza A virus (IAV)-specific spleen CD4+ T-cells.
Splenocytes from mice infected with PR/8 and HK/X31 IAVs, as described in Figure 1, were stimulated for 6 hrs with nucleoprotein (NP)311–325 peptide, a MHC Class II restricted PR/8 IAV epitope. NP311–325-specific cytokine-producing CD4+ T-cells were identified as CD3+ CD4+ CD8− IFN-γ+ (A) or TNFα+ (B), and expressed as the percentage of CD4+ T-cells. NP311–325-specific cytokine-producing CD4+ T-cells were analyzed in B6, MyD88−/− and TLR7−/− mice. The frequencies of NP311–325-specific CD4+ T-cells in these mouse strains were also determined (expressed as the percentage of CD3+ T-cells) (C). Values shown (A & B) are the % of IFN-γ+ or TNFα+ NP311–325-specific splenic CD4+ T-cells minus unstimulated background. Bars are median values. *p<0.05 compared to NP311–325-specific B6 splenic CD4+ T-cells.
Figure 3. Interferon-γ (IFN-γ) or tumor necrosis factor-α (TNFα) production by influenza A virus (IAV)-specific lung CD4+ T-cells.
Lungs from mice infected with PR/8 and HK/X31 IAVs, as previously described in Figure 1, were homogenized and stimulated for 6 hrs with nucleoprotein (NP)311–325 peptide, a MHC Class II restricted PR/8 IAV epitope. The % of NP311–325-specific IFN-γ+ (A) or TNFα+ (B) producing lung CD4+ T-cells and the frequencies of NP311–325-specific lung CD4+ T-cells (C) were determined in B6, MyD88−/− and TLR7−/− mice (expressed as the percentage of CD3+ T-cells). Values shown (A & B) are the % of IFN-γ+ or TNFα+ NP311–325-specific CD4+ T cells minus unstimulated background (expressed as the percentage of CD4+ T-cells). Bars are median values. *p<0.05 compared to NP311–325-specific B6 lung CD4+ T-cells.
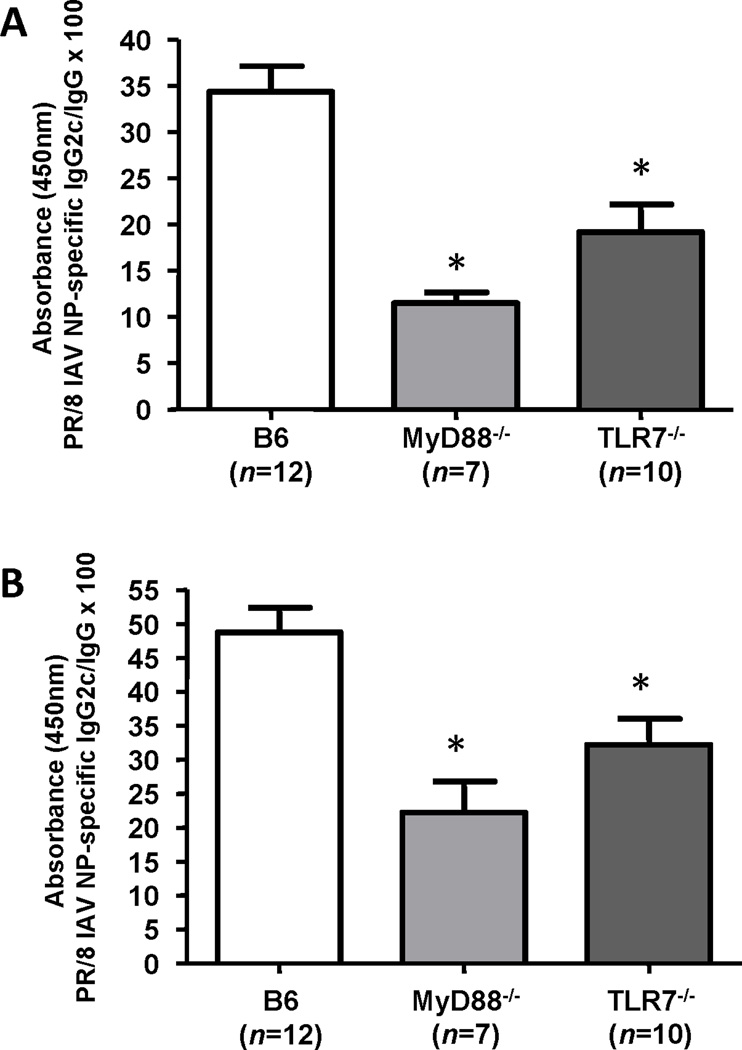
Anti-IAV NP Th1 antibody responses are partially dependent on TLR7 and MyD88 signaling in a heterosubtypic infection model
In C57BL/6 mice, CD4+ Th1 cells play a role in antibody isotype switching to IgG2c (Heer et al., 2007). In a murine homologous IAV challenge model, anti-IAV HA IgG2c levels were reported to be TLR7 and MyD88 dependent (Koyama et al., 2007). In our murine heterosubtypic IAV challenge model, we found that IgG2c directed against the IAV NP protein was partially TLR7 and MyD88 dependent. The partial TLR7 and MyD88 dependence for anti-NP IgG2c was seen after the primary IAV infection and after the second heterosubtypic IAV infection (Figure 4). Th2 dependent IgG1 levels directed against IAV NP were independent of TLR7 and MyD88 signaling (data not shown).
Figure 4. PR/8 influenza A virus (IAV) nucleoprotein (NP)-specific serum IgG2c/ IgG ratios.
B6, MyD88−/− and TLR7−/− mice were infected with PR/8 and HK/X31 IAVs, as described in Figure 1. Mouse sera were collected 20 days after primary PR/8 IAV infection (A) and 7 days after heterosubtypic HK/X31 IAV infection (B). Values shown are ELISA 450 nm absorbance (OD) values (×100) for anti-IAV NP IgG2c/ IgG. *p<0.05 compared to B6 sera.
A MyD88-mediated signaling pathway is required for the induction of some CD8+ T-cell immune responses to heterosubtypic IAV infections
In our heterosubtypic IAV challenge model, we examined CD8+ T-cell immune responses to peptides NP366–374 or PA224–233, two MHC Class I immunodominant epitopes in C57BL/6 mice (Belz et al., 2000; Deckhut et al., 1993). Anti-NP or PA spleen CD8+ T-cell type 1 cytokine production was dependent on MyD88 signaling, but largely independent of TLR7 signaling (Figures 5a&b). There were no differences in anti-NP or PA lung CD8+ T-cell cytokine production (IFN-γ or TNFα) and cell frequencies between wild type, MyD88−/−, or TLR7−/− mice (Figures 6a–c). There was also no difference in anti-NP or PA spleen CD8+ T-cell type 1 cytokine production between wild type and IL-1α−/−, IL-1β−/−, IL-1αβ−/−, IL-1R−/−, or IL18R−/− mice (data not shown). IL-33 signaling (also MyD88 dependent) was not examined (Bonilla et al., 2012). Unlike anti-NP311–325 splenic CD4+ T-cells, we found that the frequency of anti-NP or PA spleen CD8+ T-cells was MyD88 dependent (Figure 5c). This finding was confirmed by NP366–374 MHC Class I tetramer staining of splenic CD8+ T-cells (Supplementary Figure 2).
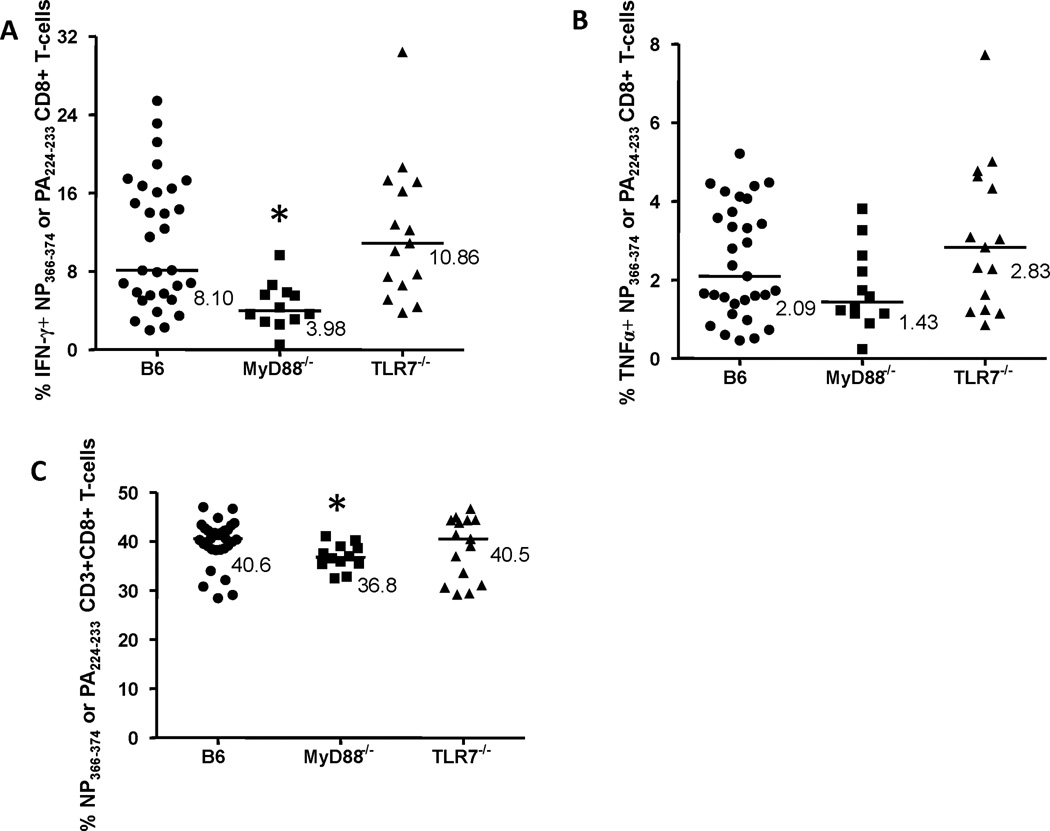
Figure 5. Interferon-γ (IFN-γ) or tumor necrosis factor-α (TNFα) production by influenza A virus (IAV)-specific spleen CD8+ T-cells.
Splenocytes from mice infected with PR/8 and HK/X31 IAVs, as described in Figure 1, were stimulated for 6 hrs with nucleoprotein (NP)366–374 and polymerase (PA)224–233 peptides, MHC Class I restricted PR/8 IAV epitopes. NP366–374 or PA224–233-specific cytokine-producing CD8+ T-cells were identified as CD3+ CD8+ CD4− IFN-γ+ (A) or TNFα+ (B), and expressed as the percentage of CD8+ T-cells. NP366–374 or PA224–233-specific cytokine-producing CD8+ T-cells were analyzed in B6, MyD88−/− and TLR7−/− mice. The frequencies of NP366–374 or PA224–233-specific CD8+ T-cells in these mouse strains were also determined (expressed as the percentage of CD3+ T-cells) (C). Values shown (A & B) are the % of IFN-γ+ or TNFα+ NP366–374 or PA224–233-specific splenic CD8+ T-cells minus unstimulated background. Bars are median values. *p<0.05 compared to NP366–374 or PA224–233-specific B6 splenic CD8+ T-cells.
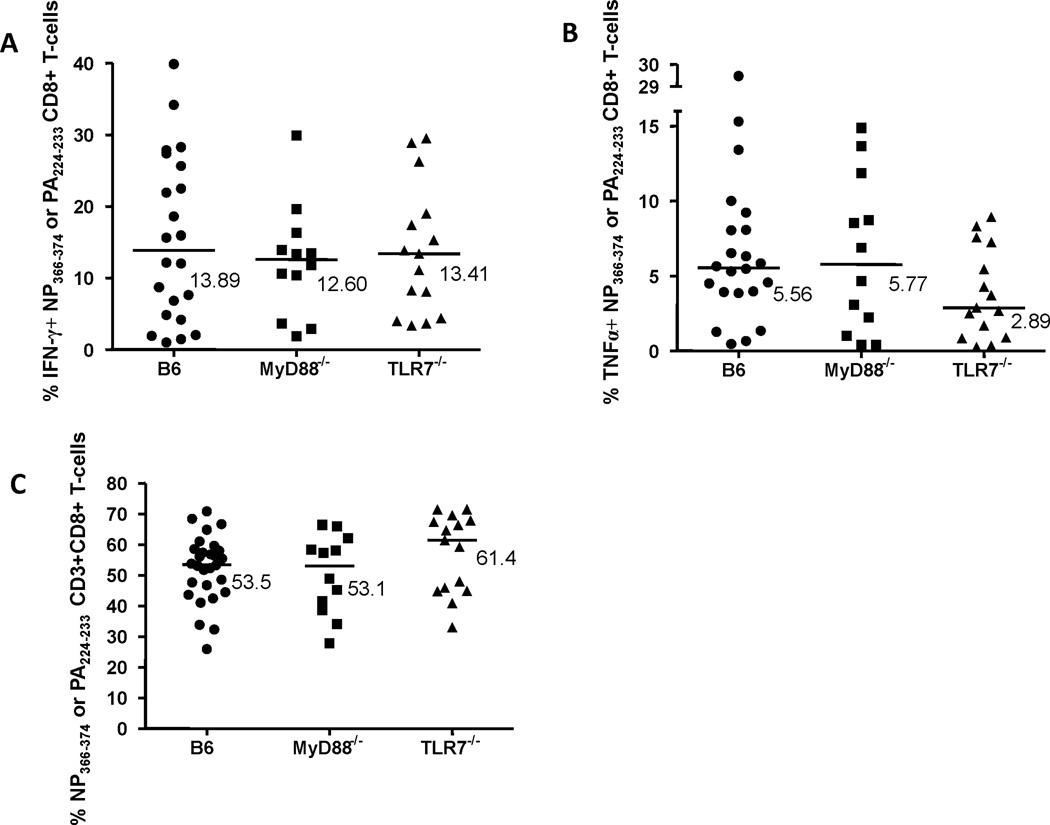
Figure 6. Interferon-γ (IFN-γ) or tumor necrosis factor-α (TNFα) production by influenza A virus (IAV)-specific lung CD8+ T-cells.
Lungs from mice infected with PR/8 and HK/X31 IAVs, as previously described in Figure 1, were homogenized and stimulated for 6 hrs with nucleoprotein (NP)366–374 and polymerase (PA)224–233 peptides, MHC Class I restricted PR/8 IAV epitopes. NP366–374 or PA224–233-specific cytokine-producing CD8+ T-cells were identified as CD3+ CD8+ CD4− IFN-γ+ (A) or TNFα+ (B), . The frequencies of NP366–374 or PA224–233-specific lung CD8+ T-cells (C) were also determined in B6, MyD88−/− and TLR7−/− mice (expressed as the percentage of CD3+ T-cells). Values shown (A & B) are the % of IFN-γ+ or TNFα+ NP366–374 or PA224–233-specific CD8+ T-cells minus unstimulated background (expressed as the percentage of CD8+ T-cells). Bars are median values. *p<0.05 compared to NP366–374 or PA224–233-specific B6 lung CD8+ T-cells.
DISCUSSION
The initial induction of influenza specific immune responses is mediated by PRR signaling pathways. PRR signaling pathways can be broadly categorized into MyD88 dependent and MyD88 independent ones (Ichinohe, 2010). We examined immune responses elicited during serial sublethal heterosubtypic IAV infections in a murine model. This model most closely reflects serial natural IAV infections in humans. Our findings point to an important role for MyD88 dependent signaling in anti-IAV heterosubtypic memory lung and spleen CD4+ T-cell, and spleen CD8+ T-cell, immune responses. MyD88 dependent signaling was important for Th1 cytokine production in anti-IAV heterosubtypic memory CD4+ T-cells, but not for their frequencies. TLR7 dependent signaling played a role only in anti-IAV heterosubtypic memory lung CD4+ Th1 and IgG2c antibody responses.
In our murine model, there was minimal proliferation of anti-IAV heterosubtypic memory lung and spleen CD4+ T-cells by in vivo EdU incorporation (data not shown). This implies that the majority of anti-IAV NP311–325 memory lung and spleen CD4+ T-cells were recruited to these sites, likely from regional lymph nodes. A similar finding has been reported for anti-IAV heterosubtypic memory CD8+ T-cells in a murine model (Ingulli et al., 2009; Kim et al., 2010). Our data demonstrated that MyD88 signaling was involved in generating anti-IAV heterosubtypic memory CD4+ Th1 cytokine responses, but not the number of CD4+ T-cells. Such a role has been reported in a LCMV murine infection model (Zhou et al., 2009), but the exact participation of MyD88 is unclear. Single dependence on TLR7 or IL-1R signaling was minimal. Other TLRs (except TLR3) singly or in combination could be involved either directly in CD4+ T-cells or in antigen-presenting cells.
For anti-IAV heterosubtypic memory CD8+ T-cells, the pulmonary type 1 cytokine response and cell frequency were MyD88 independent. This finding was consistent with other reports that the pulmonary anti-IAV memory CD8+ T-cells in our model were largely recruited from regional draining lymph nodes (Ingulli et al., 2009; Kim et al., 2010). The extrapulmonary anti-IAV heterosubtypic memory CD8+ T-cell type 1 cytokine response was MyD88 dependent, and this paralleled a MyD88 dependence for cell frequency.
The heterosubtypic anti-IAV NP IgG2c antibody response was partially dependent on TLR7 and MyD88 signaling. A similar finding has been reported for anti-hemagglutinin IgG2c antibody responses in a homologous IAV challenge model (Koyama et al., 2007). There are several places where TLR7/MyD88 signaling. could affect heterosubtypic anti-IAV NP IgG2c levels. Anti-IAV NP pulmonary CD4+ Th1 responses were also partially TLR7 dependent, and lung CD4+ T-cells may provide the B-cell help needed for isotype switching in heterosubtypic IAV infections. Alveolar epithelial cells are the primary site of replication in IAV pneumonia, and they express TLR7 (Jeisy-Scott et al., 2011). TLR7 stimulation might also occur directly in B-cells (Agrawal and Gupta, 2010), or plasmacytoid dendritic cells (Kaminski et al., 2012) might play a critical role in IgG isotype switching. It is notable that TLR7/MyD88 only played a partial role in isotype switching to IgG2c in heterosubtypic IAV infections. Anti-IAV NP IgG1 levels (a non-Th1 dependent isotype) were independent of TLR7 and MyD88 signaling.
CONCLUSION
MyD88 dependent signaling, not all of it TLR7 dependent, played important roles in a variety of T-cell and antibody memory immune responses in heterosubtypic IAV infections.
Supplementary Material
Interferon-γ (IFN-γ) or tumor necrosis factor-α (TNFα)-secreting CD4+ and CD8+ T-cells were gated as live/dead aqua (LDA)−/CD3+/CD4+ or CD8+ lymphocytes. Spleen and lung cell suspensions were stimulated for 6 hours with MHC class I or II-restricted IAV peptides, as described in previous figures, in the presence of Brefeldin A. One representative example of the gating strategy is shown.
MHC Class I peptide tetramers (PR/8 IAV nucleoprotein (NP)366–374/Db) were generated by the NIH Tetramer Facility (Atlanta, GA). Tetramer staining was performed on splenocytes for 30 min on ice, followed by staining for CD8+ T-cells. Live/Dead Aqua (LDA) was used to exclude nonviable cells from analysis. At least 200,000 events were collected for analysis. Data was analyzed using FlowJo software Treestar, Ashland, OR). Data shown are the relative frequencies of MHC class I tetramer positive splenic CD8+ T-cells following heterosubtypic IAV infections (see Figure 1).
Highlights.
Lung and spleen CD4+ Th1 responses to heterosubtypic IAV infection were MyD88 dependent
Lung and spleen CD4+ T-cell frequencies were MyD88 independent
Th1 dependent IgG2c levels were partially dependent on TLR7 and MyD88 signaling
Lung CD8+ Th1 responses to heterosubtypic IAV infection were MyD88 independent
Spleen CD8+ Th1 responses and T-cell frequencies were MyD88 dependent
ACKNOWLEDGEMENTS
This work was supported by a National Institutes of Health grant (NIH/NIAID U19 AI57319).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agrawal S, Gupta S. TLR1/2, TLR7, and TLR9 signals directly activate human peripheral blood naive and memory B cell subsets to produce cytokines, chemokines, and hematopoietic growth factors. J Clin Immunol. 2010;31(1):89–98. doi: 10.1007/s10875-010-9456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus-specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J Virol. 2002;76(23):12388–12393. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Xie W, Altman JD, Doherty PC. A previously unrecognized H-2D(b)-restricted peptide prominent in the primary influenza A virus-specific CD8(+) T-cell response is much less apparent following secondary challenge. J Virol. 2000;74(8):3486–3493. doi: 10.1128/jvi.74.8.3486-3493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, Kreutzfeldt M, Hegazy AN, Schrick C, Fallon PG, Klemenz R, Nakae S, Adler H, Merkler D, Lohning M, Pinschewer DD. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science. 2012;335(6071):984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- Boraschi D, Tagliabue A. The interleukin-1 receptor family. Vitam Horm. 2006;74:229–254. doi: 10.1016/S0083-6729(06)74009-2. [DOI] [PubMed] [Google Scholar]
- Crowe SR, Miller SC, Brown DM, Adams PS, Dutton RW, Harmsen AG, Lund FE, Randall TD, Swain SL, Woodland DL. Uneven distribution of MHC class II epitopes within the influenza virus. Vaccine. 2006;24(4):457–467. doi: 10.1016/j.vaccine.2005.07.096. [DOI] [PubMed] [Google Scholar]
- Deckhut AM, Allan W, McMickle A, Eichelberger M, Blackman MA, Doherty PC, Woodland DL. Prominent usage of V beta 8.3 T cells in the H-2Db-restricted response to an influenza A virus nucleoprotein epitope. J Immunol. 1993;151(5):2658–2666. [PubMed] [Google Scholar]
- Denton AE, Doherty PC, Turner SJ, La Gruta NL. IL-18, but not IL-12, is required for optimal cytokine production by influenza virus-specific CD8+ T cells. Eur J Immunol. 2007;37(2):368–375. doi: 10.1002/eji.200636766. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159(7):3364–3371. [PubMed] [Google Scholar]
- Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280(7):5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178(4):2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187(9):1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T. Respective roles of TLR, RIG-I and NLRP3 in influenza virus infection and immunity: impact on vaccine design. Expert Rev Vaccines. 2010;9(11):1315–1324. doi: 10.1586/erv.10.118. [DOI] [PubMed] [Google Scholar]
- Ingulli E, Funatake C, Jacovetty EL, Zanetti M. Cutting edge: antigen presentation to CD8 T cells after influenza A virus infection. J Immunol. 2009;182(1):29–33. doi: 10.4049/jimmunol.182.1.29. [DOI] [PubMed] [Google Scholar]
- Jeisy-Scott V, Davis WG, Patel JR, Bowzard JB, Shieh WJ, Zaki SR, Katz JM, Sambhara S. Increased MDSC accumulation and Th2 biased response to influenza A virus infection in the absence of TLR7 in mice. PLoS One. 2011;6(9):e25242. doi: 10.1371/journal.pone.0025242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S, Thomsen AR. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J Virol. 2012;86(6):2900–2910. doi: 10.1128/JVI.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski MM, Ohnemus A, Cornitescu M, Staeheli P. Plasmacytoid dendritic cells and Toll-like receptor 7-dependent signalling promote efficient protection of mice against highly virulent influenza A virus. J Gen Virol. 2012;93(Pt 3):555–559. doi: 10.1099/vir.0.039065-0. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281(48):36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kilbourne ED. Future influenza vaccines and the use of genetic recombinants. Bull World Health Organ. 1969;41(3):643–645. [PMC free article] [PubMed] [Google Scholar]
- Kim TS, Hufford MM, Sun J, Fu YX, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207(6):1161–1172. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J Immunol. 2010;184(8):4470–4478. doi: 10.4049/jimmunol.0902485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, Kawai T, Akira S. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179(7):4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10(10):1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SU, Kwon HJ, Song JH, Byun YH, Seong BL, Kawai T, Akira S, Kweon MN. MyD88 signaling is indispensable for primary influenza A virus infection but dispensable for secondary infection. J Virol. 2010;84(24):12713–12722. doi: 10.1128/JVI.01675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe. 2010;7(6):440–451. doi: 10.1016/j.chom.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12(1):48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend AR, Rothbard J, Gotch FM, Bahadur G, Wraith D, McMichael AJ. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Wang JP, Asher DR, Chan M, Kurt-Jones EA, Finberg RW. Cutting Edge: Antibody-mediated TLR7-dependent recognition of viral RNA. J Immunol. 2007;178(6):3363–3367. doi: 10.4049/jimmunol.178.6.3363. [DOI] [PubMed] [Google Scholar]
- Wang JP, Cerny A, Asher DR, Kurt-Jones EA, Bronson RT, Finberg RW. MDA5 and MAVS mediate type I interferon responses to coxsackie B virus. J Virol. 2010;84(1):254–260. doi: 10.1128/JVI.00631-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Kurt-Jones EA, Cerny AM, Chan M, Bronson RT, Finberg RW. MyD88 intrinsically regulates CD4 T-cell responses. J Virol. 2009;83(4):1625–1634. doi: 10.1128/JVI.01770-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Interferon-γ (IFN-γ) or tumor necrosis factor-α (TNFα)-secreting CD4+ and CD8+ T-cells were gated as live/dead aqua (LDA)−/CD3+/CD4+ or CD8+ lymphocytes. Spleen and lung cell suspensions were stimulated for 6 hours with MHC class I or II-restricted IAV peptides, as described in previous figures, in the presence of Brefeldin A. One representative example of the gating strategy is shown.
MHC Class I peptide tetramers (PR/8 IAV nucleoprotein (NP)366–374/Db) were generated by the NIH Tetramer Facility (Atlanta, GA). Tetramer staining was performed on splenocytes for 30 min on ice, followed by staining for CD8+ T-cells. Live/Dead Aqua (LDA) was used to exclude nonviable cells from analysis. At least 200,000 events were collected for analysis. Data was analyzed using FlowJo software Treestar, Ashland, OR). Data shown are the relative frequencies of MHC class I tetramer positive splenic CD8+ T-cells following heterosubtypic IAV infections (see Figure 1).