Abstract
Purpose
A subset of patients with common variable immunodeficiency (CVID) develops granulomatous and lymphocytic interstitial lung disease (GLILD), a restrictive lung disease associated with early mortality. The optimal therapy for GLILD is unknown. This study was undertaken to see if rituximab and azathioprine (combination chemotherapy) would improve pulmonary function and/or radiographic abnormalities in patients with CVID and GLILD.
Methods
A retrospective chart review of patients with CVID and GLILD who were treated with combination chemotherapy was performed. Complete pulmonary function tests (PFTs) and high-resolution computed tomography (HRCT) scans of the chest were done prior to therapy and >6 months later. HRCT scans of the chest were blinded, randomized, and scored independently (in pairs) by two radiologists. The differences between pre- and post-treatment HRCT scores and PFT parameters were analyzed.
Results
Seven patients with CVID and GLILD met inclusion criteria. Post-treatment increases were noted in both FEV1 (p=0.034) and FVC (p=0.043). HRCT scans of the chest demonstrated improvement in total score (p=0.018), pulmonary consolidations (p=0.041), ground-glass opacities (p=0.020) nodular opacities (p=0.024), and both the presence and extent of bronchial wall thickening (p=0.014, 0.026 respectively). No significant chemotherapy-related complications occurred.
Conclusions
Combination chemotherapy improved pulmonary function and decreased radiographic abnormalities in patients with CVID and GLILD.
Keywords: Common variable immunodeficiency (CVID), primary immunodeficiency, lung disease, granulomatous and lymphocytic interstitial lung disease (GLILD), rituximab, azathioprine
Introduction
Common variable immunodeficiency (CVID) is the most common clinically significant primary immunodeficiency. [1] CVID is defined by the presence of low IgG and IgA or IgM, poor specific antibody response to vaccination, and exclusion of other causes of hypogammaglobulinemia. [2] Patients with CVID commonly present with recurrent sinopulmonary infections. [3] Treatment with immunoglobulin replacement markedly decreases the infectious complications of CVID. [4, 5] As a result, the non-infectious complications of CVID (e.g., lymphoproliferative disease, pulmonary complications, hepatic and gastrointestinal disease) are an increasingly important cause of morbidity and mortality. [6–13]
A subset (10–15 %) of patients with CVID develops granulomatous/lymphocytic interstitial lung disease (GLILD), which is frequently accompanied by splenomegaly, adenopathy, autoimmune cytopenias, and gastrointestinal and hepatic disease. [13–20] GLILD is a histologic diagnosis, defined as pulmonary tissue containing both granulomatous and lymphoproliferative histopathologic patterns (i.e. lymphocytic interstitial pneumonitis (LIP), follicular bronchiolitis, and/or lymphoid hyperplasia). [13] Previous studies suggest that patients with CVID and GLILD have poorer outcomes. [10, 13, 21] As such, interventions directed at patients with CVID and polyclonal lymphocytic infiltration, such as GLILD, may reduce the rates of disability and premature mortality. [22] Various treatments have been used, including corticosteroids, immunomodulators and biologics, but the efficacy of these therapies is unknown. [16] Consequently, there is no established standard of care for the treatment of patients with CVID and GLILD.
In the course of evaluating patients with CVID, we routinely obtain open lung biopsies when diffuse abnormalities are present on high-resolution computed tomography (HRCT) scans of the chest. In patients subsequently diagnosed with GLILD, we found that the lung biopsies contained infiltrates of T and B cells. The purpose of this study is to examine the effect of the administration of chemotherapy directed at eliminating T cells and B cells in the lung (e.g. azathioprine and rituximab) on the pulmonary function and radiographic abnormalities found on HRCT scans of the chest in patients with GLILD.
Methods
Patient Population
Following approval by the Children’s Hospital of Wisconsin Institutional Review Board, we retrospectively reviewed the charts of all patients with CVID and GLILD seen at our institution between 2006 and 2012, and abstracted demographic, immunologic, physiologic and radiographic data. Patient charts were also queried for previous immunosuppressive therapy. In all cases, the diagnosis of CVID was consistent with current guidelines. [2]
Criteria for inclusion in the study were: 1) Histological diagnosis of GLILD on pulmonary biopsy obtained by either open lung biopsy (Patients 1–3, 5, 7, Table I) or transbronchial biopsy, (Patient 6, Table I) as determined by current diagnostic criteria [13] or 2) radiographic findings on HRCT of the chest characteristic of GLILD with a mediastinal biopsy negative for B cell lymphoma [16, 23] (Patient 4, Table I, Fig. 1c), and 3) treatment with combination chemotherapy for at least 6 months. Exclusion criteria included non-adherence to therapy.
Table I.
Patient demographics: pre-treatment
| ID | Age/ Gender |
Method | GLILD Duration |
Comorbidities | CD3+ | CD4+ | CD8+ | HLA_DR/ CD4+% |
HLA_DR/ CD8+% |
CD4+/ CD45RO+% |
CD19+ | CD19+/ CD27+% |
CD19+/IgD−/ CD27+% |
CD56+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27y F | VATS | 4.9y | Adenopathy Anemia Thrombocytopenia |
0.92 | 0.81 | 0.12 | n/a | n/a | n/a | 0.04 | n/a | n/a | 0.27 |
| 2 | 21y F | VATS | 6.1y | ADEM/MS Hypothyroidism Chronic diarrhea |
0.72 | 0.47 | 0.18 | 5 | 4 | 75 | 0.03 | 11 | 4.0 | 0.12 |
| 3 | 18y M | VATS | 6.7y | Splenomegaly Adenopathy Thrombocytopenia |
0.38 | 0.27 | 0.10 | 5 | 5 | 46 | 0.03 | 11 | 7.0 | 0.05 |
| 4 | 31y M | MS | 2.6y | Splenomegaly Adenopathy Neutropenia |
1.98 | 0.91 | 0.96 | 6 | 20 | 83 | 0.09 | 6.0 | 1.0 | 0.13 |
| 5 | 42y M | VATS | 15.6y | Splenomegaly Adenopathy |
12.67 | 0.94 | 0.31 | 7 | 6 | 88 | 0.11 | 20 | 2.0 | 0.20 |
| 6 | 43y F | TBBX | 2.3y | Splenomegaly Adenopathy Lymphopenia Anemia Thrombocytopenia |
0.98 | 0.80 | 0.23 | n/a | n/a | 77 | 0.03 | 6.0 | 0.5 | 0.06 |
| 7 | 24y F | VATS | 1.5y | Splenomegaly Adenopathy |
0.77 | 0.52 | 0.20 | 11 | 5 | 79 | 0.11 | 5.0 | 1.0 | 0.22 |
Abbreviations: VATS (video-assisted thorascopic surgery), MS (mediastinoscopy), TBBX (transbronchial biopsy), ADEM (acute demyelinating encephalomyelitis), MS (multiple sclerosis), n/a (not-available)
Reference ranges: CD3+ (0.7–2.1/mm3 ), CD4+ (0.3–1.4/mm3 ), CD8+ (0.2–0.9/mm3 ), HLA_DR/CD4+ % (0–8.0 %), HLA_DR/CD8+ % (0–8.0 %), CD19+ (0.1–0.5/mm3 ), CD19+/CD27+ % (17.5–46.5 %), CD19+/IgD−/CD27+ % (8.3–27.8 %), CD56+ (0.09–0.6/mm3 )
Fig. 1.
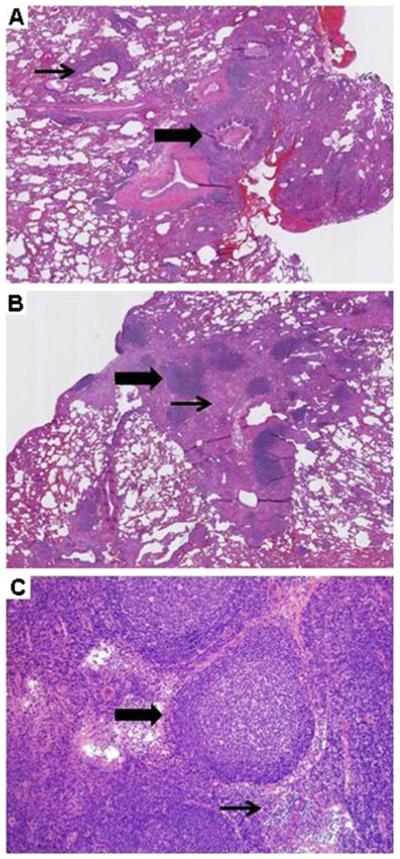
GLILD: Histologic findings. a Follicular bronchiolitis (thick arrow), with lymphocytic aggregates around an airway (thin arrow). (Lung, H&E, 100×, patient 2). b Focal lymphoid aggregates (thick arrow) within collagenized fibrous tissue (thin arrow). (Lung, H&E, 100×, patient 5). c Mediastinal biopsy reveals follicular hyperplasia with prominent germinal centers (thick arrow) and associated para-cortical hyperplasia. Interfollicular areas (thin arrow) are expanded. (Lymph node, H&E, 40×, patient 4)
The charts of 16 patients with CVID and GLILD were reviewed. Of these, seven patients met criteria for analysis (Table I). Of the nine patients excluded from the study, two patients were noncompliant with the combination chemotherapy regimen, one patient elected to forego treatment and pursue palliative care, one patient was recently initiated on combination chemotherapy with insufficient time elapsed for post-treatment imaging and PFTs, and five patients were seen for consultation only at the request of providers from outside institutions.
Combination Chemotherapy
After obtaining informed consent, patients with CVID and GLILD received weekly doses of IV rituximab (375 mg/M2/infusion) for 4 weeks, and began oral azathioprine (1.0– 2.0 mg/kg/day, 18 months duration). Rituximab infusions were repeated at 4–6 month intervals, for 3 or 4 total courses (12–16 infusions). In patients unable to tolerate oral azathioprine due to gastrointestinal side effects (nausea, anorexia) 6-mercaptopurine [(6-MP) 1.0–1.5 mg/kg/day], the active metabolite of azathioprine, was used (patients 2, 5.) One patient (patient 5) was unable to tolerate azathioprine or 6-MP due to hepatotoxicity, and so mycophenolate mofetil [(MMF) 1,000 mg twice daily] was substituted. Complete blood counts and liver function tests were monitored per current recommendations and thiopurine methyltransferase (TPMT) genotype and enzymatic activity were determined prior to the initiation of treatment with azathioprine. [24, 25] Lymphocyte enumeration was performed prior to initiating therapy, and at 6-months and 1-year post-treatment to ensure rituximab effect.
Pathology
Lung biopsy specimens were evaluated by two independent surgical pathologists for features characteristic of GLILD. [13] Histological examination and immunohistochemical studies were performed on paraffin-embedded tissue sections 3.5–4.0 μm thick. In cases of multiple specimens, representative sections were taken. Hematoxylin and eosin (H&E) staining was performed in all cases. Sections were evaluated for the presence, extent and distribution of interstitial and alveolar inflammation. The presence of lymphoid aggregates, with or without germinal centers, was assessed. Areas of lymphoid infiltrate were screened for the presence of lymphoepithelial lesions and the presence, frequency, distribution and appearance of granulomata. Fibrosing changes, including features of organizing pneumonia and interstitial fibrosis, with or without the presence of alveolar remodeling, were also assessed.
Immunohistochemical stains were performed for a panel of antibodies, including CD20 (B cells), CD3 (T cells), and CD68-KP1 (histiocytes). In-situ hybridization was performed for kappa and lambda immunoglobulin light chains. Special stains for microorganisms (Ziehl-Neelsen stain for acid fast bacilli, Gomori Methenamine stain for fungal organisms) were performed using established staining protocols.
Radiology
HRCT scans (1.25 or 2.5 mm) scans were obtained on each patient. The dates were blinded; the scans were randomized and scored by two chest radiologists using a published scoring system to quantify the extent of 12 types of radiographic abnormalities. The scoring system included: bronchiectasis (severity, extension, axial distribution), bronchial wall thickening (presence, extent), mucus plugging, nodular opacities, linear and/or irregular opacities, pulmonary consolidations, ground-glass opacities, honeycombing, and emphysema. The undated scans on each patient were then reviewed in pairs using the same scoring system. Scores ranged from 0 (no disease) to 3 (severe disease) for each category, resulting a minimum total score of 0 and a maximum total score of 36 for each HRCT scan. [26]
Pulmonary Function Testing
PFTs were performed as per American Thoracic Society/European Respiratory Society Task Force guidelines. [27, 28] Collected parameters included forced expiratory volume in one second (FEV1), forced vital capacity (FVC), ratio of FEV1 to FVC (FEV1/FVC), total lung capacity (TLC), residual volume (RV), carbon monoxide diffusion capacity (DLCO), and DLCO corrected for alveolar volume (DLCO/VA).
Flow Cytometry
Lymphocyte subset enumeration was performed by fluorescent antibody staining for CD3+ (total T cells), CD3+/CD4+ (helper T cells), CD3+/CD8+ (cytotoxic T cells), CD4+/ CD45RO + (memory CD4+ T-cells), CD19+ (total B cells), CD19+/CD27+ (memory B cells), CD19+/IgD−/CD27+ (switched memory B cells), and CD56+ (NK cells) and analyzed using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, New Jersey). Lymphocyte numbers were expressed as cells per cubic milliliter and compared to established control values. [29, 30]
DNA Sequence Analysis
DNA derived from PBMC from all patients was sequenced for mutations in the exons of the tumor necrosis factor (TNF) receptor superfamily member 13b (TNFRSF13b, more commonly known as TACI), using primers and conditions previously described. [31] Due to difficulties amplifying exon 3 with the published primer set, the forward primer used was 5′GATGTCCTTTGTGGTCAAACC CAG3′. The PCR products were sequenced with amplification primers and run on an ABI 3730×l DNA Sequencer with 50 cm arrays (PE Applied Biosystems). The data was analyzed using Geneious software, version 50.3 (Biomatters, Ltd., Auckland, New Zealand).
Data Analysis
Wilcoxon signed-rank tests were used to compare continuous variables in the pre- and post-treatment groups. Data analysis was performed using SPSS software, version 20. A p-value of <0.05 was considered statistically significant.
Results
Demographic Data
All seven patients were receiving supplemental immunoglobulin at doses of ≥500 mg/kg/month at the time of enrollment, and continued at doses in this range (individually titrated to keep patients free from significant infection) throughout their participation in this study. There was no increase in the incidence or severity of infection either prior to or after initiation of combination chemotherapy. No patient developed a serious or life-threatening infection (e.g. pneumonia, sepsis, CNS infection). Monthly trough IgG levels (in patients on IVIG) ranged from 650–1,000 mg/ dL, and steady-state IgG levels (in patients on SQIG) ranged from 1,000–1,300 mg/dL (data not shown). The duration of GLILD was estimated by determining the period of time from the date of the first HRCT of the chest demonstrating radiographic changes strongly suggestive of GLILD to the date of the initiation of combination chemotherapy. The mean duration of disease was 5.7 years, with a range of 1.5 to 15.6 years. As in previously published studies, all patients with GLILD and CVID had significant comorbidities, including splenomegaly, adenopathy, and autoimmune disease (particularly cytopenias). [10, 12, 32] Five patients (patients 1, 2, 5, 6 and 7) received oral steroids prior to initiation of combination chemotherapy for treatment of the GLILD. The duration of continuous oral steroid treatment ranged from 3 months (patient 2) to fifteen years (patient 5). Corticosteroid therapy did not induce a remission of the GLILD in any case. Other historical therapies included rituximab (single agent), methotrexate, cyclosporine, and sirolimus. Consistent with other studies, patients with GLILD had a low percentage of total memory and switched memory B cells. [10, 12, 32] In addition, there were an increased percentage of memory T cells (CD4+/CD45RO +) in all patients. Two patients (patients 3, 6) had markedly decreased numbers of natural killer (NK) cells. One patient (patient 6) was found to have a heterozygous missense mutation in TACI (c260T>A; I87N). This is a previously reported mutation associated with CVID, predicted to be deleterious by bioinformatic analysis only. [33]
Pathology
In the six patients who underwent open lung biopsy or transbronchial biopsy, tissue specimens demonstrated all of the characteristic histological findings of GLILD (e.g., LIP, follicular bronchiolitis, lymphoid hyperplasia, and non-necrotizing granuloma) (Fig. 1a, b). [13] Patient 4 underwent mediastinoscopy, and the representative lymph node specimen histologically showed reactive follicular hyperplasia with prominent germinal centers and associated paracortical hyperplasia (Fig. 1c). Immunohistochemistry demonstrated a variable mixture of T cells, B cells and macrophages (Fig. 2).
Fig. 2.
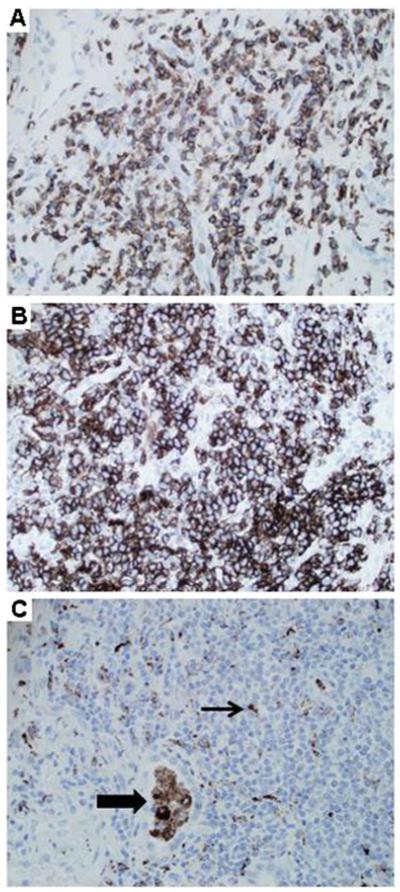
GLILD: Immunohistochemistry. a CD3 staining reveals frequent T cells. (Lung, 200×, patient 5). b CD20 staining demonstrates numerous B cells. (Lung, 200×, patient 5). c CD68 staining shows alveolar macrophages (thick arrow), and scattered dendritic cells (thin arrow) within lymphoid aggregates. (Lung, 200×, patient 5)
Response to Combination Chemotherapy
HRCT scans of the chest showed similar patterns in all patients, which included patchy areas of consolidation throughout the lung fields, with diffuse nodularity and ground-glass opacities (Fig. 3a). Scarring and/or bronchiectatic changes, as well as mediastinal adenopathy, were variably noted. Comparisons of pre- and post-treatment HRCT scans of the chest revealed improvements in total score (p=0.018), presence of pulmonary consolidations (p=0.041), ground-glass opacities (p=0.020), nodular opacities (p=0.024), and both the presence and extent of bronchial wall thickening (p=0.014, 0.026 respectively) (Table II, Online Resource Table E1). The improvements in radiographic abnormalities of the pulmonary parenchyma are readily apparent upon comparison of pre- and post-treatment HRCT scans of the chest (Figs. 3 and 4a, b).
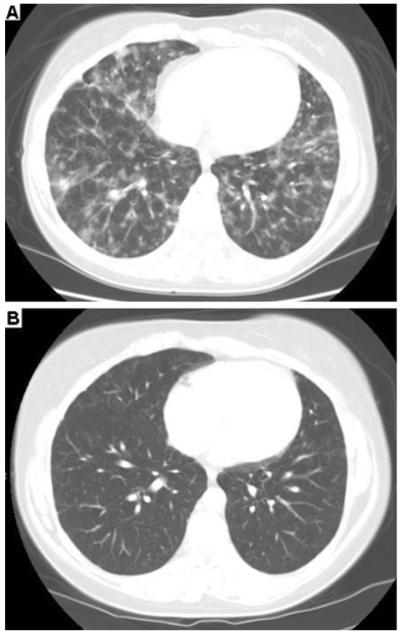
Fig. 3.
High-resolution computed tomography (HRCT) scans: pre-/ post-treatment. a High resolution CT scan of the chest demonstrates typical features of GLILD prior to initiation of combination chemotherapy, including diffuse ground glass and nodular opacities. (Patient 7). b Improvement of parenchymal abnormalities 17 months post-combination chemotherapy. (Patient 7)
Table II.
PFTs, Total High-resolution computed tomography (HRCT) Scores
| ID | Status | Date | FEV1 (L) (% predicted) |
FVC (L) (% predicted) |
FEV1/ FVC (%) |
TLC (L) (% predicted) |
RV (L) (% predicted) |
DLCO (% predicted) |
DLCO/VA (% predicted) |
Total HRCT Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pre | 12/14/2009 | 4.38 (114 %) | 5.19 (114 %) | 84.4 | 6.70 (106 %) | 1.67 (103 %) | 23.28 (84 %) | 3.89 (n/a) | 15 |
| 1 | Post | 4/11/2011 | 4.68 (123 %) | 5.61 (123 %) | 83.4 | 7.7 (122 %) | 2.20 (132 %) | 30.17 (111 %) | 4.46 (n/a) | 4 |
| 2 | Pre | 7/30/2009 | 3.21 (102 %) | 3.51 (98 %) | 91.0 | 4.42 (91 %) | 0.78 (58 %) | (n/a) | (n/a) | 16 |
| 2 | Post | 2/3/2011 | 3.33 (108 %) | 3.91 (111 %) | 85.0 | 5.00 (100 %) | 1.10 (83 %) | 22.1 (98 %) | 5.34 (112 %) | 6 |
| 3 | Pre | 1/19/2011 | 2.60 (61 %) | 3.19 (63 %) | 82.0 | 4.30 (82 %) | 0.80 (74 %) | 19.20 (67 %) | 4.86 (97 %) | 17 |
| 3 | Post | 8/18/2011 | 2.69 (63 %) | 3.30 (65 %) | 82.0 | 4.16 (63 %) | 0.77 (71 %) | 13.90 (48 %) | 3.67 (n/a) | 14 |
| 4 | Pre | 6/18/2010 | 3.77 (78 %) | 4.70 (79 %) | 80.0 | 6.42 (86 %) | 1.47 (71 %) | 22.90 (70 %) | 4.08 (91 %) | 18 |
| 4 | Post | 5/17/2011 | 4.17 (85 %) | 5.10 (84 %) | 82.0 | 6.60 (86 %) | 1.39 (65 %) | 25.80 (79 %) | 4.42 (99 %) | 7 |
| 5 | Pre | 3/22/2010 | 2.02 (47 %) | 2.31 (43 %) | 87.0 | 3.65 (51 %) | 1.42 (74 %) | 10.59 (31 %) | 3.20 (68 %) | 23 |
| 5 | Post | 5/20/2011 | 1.88 (45 %) | 2.09 (39 %) | 90.0 | 3.46 (49 %) | 1.25 (65 %) | 11.10 (34 %) | 3.39 (72 %) | 21 |
| 6 | Pre | 8/18/2010 | 2.94 (90 %) | 3.35 (82 %) | 87.7 | (n/a) | (n/a) | (n/a) | (n/a) | 12 |
| 6 | Post | 2/28/2011 | 3.91 (121 %) | 4.57 (115 %) | 86.0 | 5.67 (100 %) | 1.10 (59 %) | 19.00 (76 %) | 3.63 (80 %) | 2 |
| 7 | Pre | 5/19/2009 | 3.28 (93 %) | 3.91 (89 %) | 84.0 | 4.99 (83 %) | 1.04 (57 %) | 16.20 (58 %) | 3.85 (n/a) | 15 |
| 7 | Post | 2/18/2011 | 3.77 (109 %) | 4.64 (107 %) | 81.0 | 6.44 (108 %) | 1.74 (93 %) | 22.8 (78 %) | 4.17 (n/a) | 4 |
Abbreviations: n/a (not available)
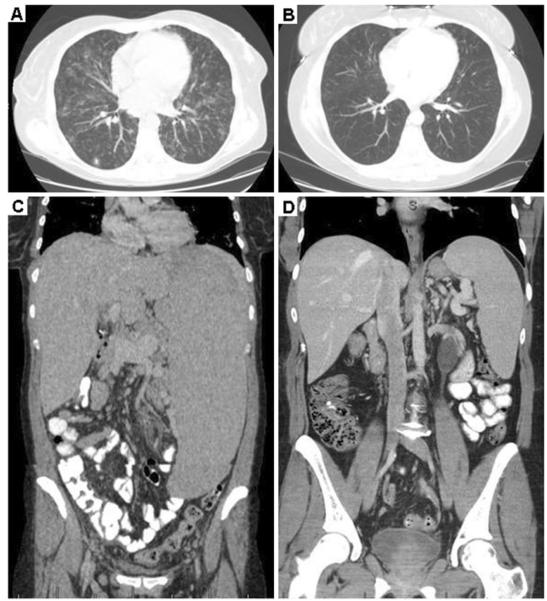
Fig. 4.
HRCT scans of the chest, abdomen in a patient with CVID, GLILD and a mutation in TACI. a Pre-treatment HRCT scan of the chest demonstrates diffuse pulmonary ground glass and nodular opacities. (Patient 6). b Improvement in pulmonary parenchymal abnormalities post-chemotherapy (Patient 6). c Pre-treatment splenomegaly extends to the iliac crest. (Patient 6; note: maximal spleen length shown). d Marked decrease in splenomegaly post-chemotherapy. (Patient 6; note: maximal spleen length shown)
In addition to improvement in the pulmonary parenchyma, combination chemotherapy led to a diminution of the splenomegaly in patients 3, 6 and 7. In particular, the patient with a mutation in TACI (patient 6) had marked splenomegaly at baseline that dramatically improved upon initiation of chemotherapy (Fig. 4c, d). In contrast, patients 4 and 5 did not have significant improvement of splenomegaly with combination chemotherapy (data not shown).
The severity of pulmonary function abnormalities in patients with GLILD varied considerably, with some patients having normal baseline PFTs despite marked radiographic abnormalities on HRCT of the chest (Table II). Consistent with the improvement of radiographic abnormalities in the chest, combination chemotherapy resulted in increases in both FEV1 (p=0.034) and FVC (p=0.043).
Discussion
Approximately 10–20 % of patients with CVID develop GLILD, a term used to reflect the characteristic histopathologic changes in the lung (lymphocytic interstitial pneumonitis, follicular bronchiolitis, granuloma). [13] GLILD is almost invariably accompanied by splenomegaly and diffuse adenopathy, with affected patients at increased risk to develop B cell lymphomas. [10, 13, 34] Laboratory studies in these patients often reveal cytopenias and evidence of immune dysregulation, with T cells skewed toward a memory phenotype (Table I). Histologic evaluation of pulmonary and lymphatic tissue is most consistent with dysregulated lymphoproliferation. GLILD, therefore, appears to be the pulmonary manifestation of a generalized, multisystemic lymphoproliferative disease. [10]
Prior studies suggest that the development of GLILD is associated with increased mortality. [10, 11, 13] Unfortunately, the optimal treatment for CVID-related GLILD is unknown and there remains no established standard of care for management of these patients. Some clinical immunologists have elected to follow patients longitudinally, to assess if GLILD led to a progressive, restrictive interstitial lung disease. Others have taken a more proactive approach, with the use of immunosuppressive medications. Corticosteroid therapy is the most commonly used medication, although in our experience, corticosteroids do not induce long lasting remission, and are associated with clinically significant side effects. For example, five of the seven patients in this series failed a trial of high dose corticosteroid therapy. Chemotherapeutic agents or biologics that antagonize the activities of tumor necrosis factor-alpha (TNF-α) have been used with some success in isolated case reports. [35–37] Additionally, over the course of several years, we have unsuccessfully used azathioprine or rituximab as single agent therapy (JMR, unpublished observations). This lack of response led us to modify our therapeutic approach to treat GLILD.
In lung biopsies from patients with GLILD, we consistently found pulmonary infiltrates consisting of T cells, B cells and macrophages (Fig. 2). We hypothesized that these inflammatory cells acted in concert, leading to progressive ILD and pulmonary fibrosis. Therefore, we reasoned that therapy directed at eliminating B cells (rituximab) and T cells (azathioprine) from the lung would lead to a significant improvement in pulmonary disease. The promising results reported here reflect our initial experience using combination therapy in seven patients with GLILD.
We found that combination chemotherapy with rituximab and azathioprine resulted in significant improvement in pulmonary function and parenchymal abnormalities found on HRCT scans of the chest (Table II). The improvement in radiographic findings was not altered by excluding patient 4, who did not have a formal tissue diagnosis of GLILD (data not shown). While four of the seven patients in this series had normal or near normal PFTs at baseline (FEV1, FVC >80 % of predicted; DLCO, DLCO >60 % of predicted), [38] they had markedly abnormal HRCT scans of the chest before therapy (Figs. 2 and 4, Table II and Online Resource Figures 5, 6, 7, 8, 9, 10 and 11). This strongly emphasizes that HRCT scans are a much more sensitive test for detecting GLILD than PFTs. As such, it is not surprising that an HRCT scan of the chest is more sensitive at measuring the effect of chemotherapy on GLILD (Table II). In addition to improving pulmonary abnormalities, the use of rituximab and azathioprine also improved splenomegaly in some patients, including a marked reduction of massive splenomegaly in a patient with a mutation in TACI (Fig. 4c, d). We could not evaluate the potential beneficial effects of combination chemotherapy on cytopenias, as all study patients had adequate cell counts at the time of enrollment, with stable levels during their treatment course.
Apart from reversible hepatotoxicity due to azathioprine in one patient, combination chemotherapy was well tolerated and did not result in an increased number or severity of infections. Although we do not have long-term outcome data on patients in this study, one patient (patient 7) has been off all chemotherapy for greater than 8 months and remains in remission, with stable PFTs and stable HRCT scans of the chest. It will be of interest to follow these patients longitudinally to determine if the clinical improvement is long-lived or if repeat therapy will be required. Additionally, it is possible that the elimination of B cells in patients with GLILD may secondarily decrease the incidence of B cell lymphomas and autoimmune complications.
In some patients, there was an incomplete response to the use of combination chemotherapy. As this is a retrospective study, it was not designed to ascertain factors that contribute to resistance to therapy. However, the two patients with the most severe restrictive lung disease (patients 3 and 5) also had the smallest reduction in the HRCT score following chemotherapy (Table I). These patients also had longstanding GLILD prior to the institution of therapy, and received repeated courses of systemic corticosteroids – despite continued deterioration of their lung function – prior to transferring care to our institution. Therefore, based on our observations, we suspect that early treatment (e.g., prior to the development of fibrosis/irreversible lung disease) may be of significantly greater benefit than treatment after these sequelae have developed. As significant GLILD may exist with a normal routine chest radiograph [13] and normal PFTs (Table II and Online Resource Figures 5, 6, 7, 8, 9, 10 and 11), our findings support a recommendation that a HRCT scan of the chest should be obtained in all patients with CVID at the time of diagnosis.
Our approach to patients with suspected GLILD is to obtain an open lung biopsy whenever feasible to exclude neoplastic (e.g. lymphoma) and non-neoplastic pulmonary disease [e.g. cryptogenic organizing pneumonia (COP)] that may mimic the radiographic findings of GLILD. The value of an open lung biopsy is illustrated by the clinical course of patient 2, who was diagnosed with COP after an initial transbronchial biopsy. While COP is typically responsive to corticosteroids, the patient failed to improve despite a 3-month course of high dose corticosteroids. Consequently, an open lung biopsy was obtained, which resulted in a diagnosis of GLILD. Subsequent therapy with rituximab and 6MP resulted in considerable radiographic improvement (Online Resource Table E1, Online Resource Figure 6).
While our study helps in establishing a protocol for evaluation and treatment of patients with CVID and GLILD, we recognize that it has several important drawbacks. First, all retrospective studies have inherent flaws and can be subject to bias. Second, due to insurance reasons or other logistical issues, serial radiographic and pulmonary function studies were often obtained at different institutions, which can result in increased variability. Third, the scoring system we utilized to quantify pulmonary parenchymal abnormalities found on HRCT of the chest is only semi-quantitative, and may not have adequately reflected improvement. For example, a collective 4-point decrease in nodular and ground-glass opacities may appear with our scoring system to be a minimal change in outcome, but these differences are quite striking when the actual pre-/post-treatment HRCTscans of the chest are viewed (Online Resource Figures 5, 6, 7, 8, 9, 10 and 11). Fourth, additional pulmonary tissue was not available in some cases, and therefore the diagnosis of GLILD was made by reviewing existing slides. Consequently, the ability to perform a more extensive phenotypic analysis of the inflammatory cells in the lungs of these patients was not possible. Collectively, these problems highlight the need for a proper blinded, prospective study to determine the best modality of therapy to treat CVID-associated GLILD.
Conclusions
In summary, this retrospective analysis of seven patients with CVID and GLILD represents the largest novel treatment protocol to date and demonstrates that combination chemotherapy improves pulmonary function and markedly decreases radiographic abnormalities in patients with CVID and GLILD. Additional prospective studies are needed to determine the optimal timing of therapy, preferred chemotherapeutic agents, and long-term effects on morbidity and mortality in this subset of patients with CVID.
Supplementary Material
Acknowledgements
We would like to acknowledge and thank Dr. Mitchell Grayson for his thoughtful review of the manuscript.
This research was supported by National Institutes of Health grant R01CA122539 (www.nih.gov), and the Children’s Research Institute of the Children’s Hospital of Wisconsin (www.chw.org/display/PPF/DocID/30477/router.asp).
Footnotes
Conflict of interest The authors of this manuscript declare that they have no financial conflicts of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s10875-012-9755-3) contains supplementary material, which is available to authorized users.
Contributor Information
Nicole M. Chase, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Allergy/Clinical Immunology, Medical College of Wisconsin, Milwaukee, WI, USA
James W. Verbsky, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Allergy/Clinical Immunology, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Rheumatology, Medical College of Wisconsin, Milwaukee, WI, USA
Mary K. Hintermeyer, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, USA
Jill K. Waukau, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Allergy/Clinical Immunology, Medical College of Wisconsin, Milwaukee, WI, USA
Aoy Tomita-Mitchell, Department of Surgery, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Cardiothoracic Surgery, Medical College of Wisconsin, Milwaukee, WI, USA.
James T. Casper, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Hematology/Oncology/BMT, Medical College of Wisconsin, Milwaukee, WI, USA
Sumit Singh, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Diagnostic Radiology, Medical College of Wisconsin, Milwaukee, WI, USA.
Kaushik S. Shahir, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Diagnostic Radiology, Medical College of Wisconsin, Milwaukee, WI, USA
William B. Tisol, Department of Surgery, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Cardiothoracic Surgery, Medical College of Wisconsin, Milwaukee, WI, USA
Melodee L. Nugent, Department of Quantitative Health Sciences, Medical College of Wisconsin, Milwaukee, WI, USA
R. Nagarjun Rao, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Pathology, Medical College of Wisconsin, Milwaukee, WI, USA.
A. Craig Mackinnon, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Pathology, Medical College of Wisconsin, Milwaukee, WI, USA.
Lawrence R. Goodman, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Diagnostic Radiology, Medical College of Wisconsin, Milwaukee, WI, USA
Pippa M. Simpson, Department of Quantitative Health Sciences, Medical College of Wisconsin, Milwaukee, WI, USA
John M. Routes, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, USA; Division of Allergy/Clinical Immunology, Medical College of Wisconsin, Milwaukee, WI, USA; 9000 W. Wisconsin Avenue, #440, Milwaukee, WI 53226, USA
References
- 1.Gathmann B, Grimbacher B, Beaute J, Dudoit Y, Mahlaoui N, Fischer A, et al. The European internet-based patient and research database for primary immunodeficiencies: results 2006–2008. Clin Exp Immunol. 2009;157(Suppl 1):3–11. doi: 10.1111/j.1365-2249.2009.03954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonilla FA, Bernstein IL, Khan DA, Ballas ZK, Chinen J, Frank MM, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94(5 Suppl 1):S1–S63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham-Rundles C. Common variable immunodeficiency. Curr Allergy Asthma Rep. 2001;1(5):421–9. doi: 10.1007/s11882-001-0027-1. [DOI] [PubMed] [Google Scholar]
- 4.Busse PJ, Razvi S, Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2002;109(6):1001–4. doi: 10.1067/mai.2002.124999. [DOI] [PubMed] [Google Scholar]
- 5.Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125(6):1354–60. e4. doi: 10.1016/j.jaci.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham-Rundles C, Lieberman P, Hellman G, Chaganti RS. Non-Hodgkin lymphoma in common variable immunodeficiency. Am J Hematol. 1991;37(2):69–74. doi: 10.1002/ajh.2830370202. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham-Rundles C, Siegal FP, Cunningham-Rundles S, Lieberman P. Incidence of cancer in 98 patients with common varied immunodeficiency. J Clin Immunol. 1987;7(4):294–9. doi: 10.1007/BF00915550. [DOI] [PubMed] [Google Scholar]
- 8.Kinlen LJ, Webster AD, Bird AG, Haile R, Peto J, Soothill JF, et al. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet. 1985;1(8423):263–6. doi: 10.1016/s0140-6736(85)91037-2. [DOI] [PubMed] [Google Scholar]
- 9.Sander CA, Medeiros LJ, Weiss LM, Yano T, Sneller MC, Jaffe ES. Lymphoproliferative lesions in patients with common variable immunodeficiency syndrome. Am J Surg Pathol. 1992;16(12):1170–82. doi: 10.1097/00000478-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112(2):277–86. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 11.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119(7):1650–7. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111(1):77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 13.Bates CA, Ellison MC, Lynch DA, Cool CD, Brown KK, Routes JM. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol. 2004;114(2):415–21. doi: 10.1016/j.jaci.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 14.Mechanic LJ, Dikman S, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Ann Intern Med. 1997;127(8 Pt 1):613–7. doi: 10.7326/0003-4819-127-8_part_1-199710150-00005. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto Y, Routes JM. Granulomatous disease in common variable immunodeficiency. Curr Allergy Asthma Rep. 2005;5(5):370–5. doi: 10.1007/s11882-005-0008-x. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Levinson AI. Granulomatous-lymphocytic interstitial lung disease (GLILD) in common variable immunodeficiency (CVID) Clin Immunol. 2010;134(2):97–103. doi: 10.1016/j.clim.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham-Rundles C. Autoimmune manifestations in common variable immunodeficiency. J Clin Immunol. 2008;28(Suppl 1):S42–5. doi: 10.1007/s10875-008-9182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniels JA, Lederman HM, Maitra A, Montgomery EA. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol. 2007;31(12):1800–12. doi: 10.1097/PAS.0b013e3180cab60c. [DOI] [PubMed] [Google Scholar]
- 19.Khodadad A, Aghamohammadi A, Parvaneh N, Rezaei N, Mahjoob F, Bashashati M, et al. Gastrointestinal manifestations in patients with common variable immunodeficiency. Dig Dis Sci. 2007;52(11):2977–83. doi: 10.1007/s10620-006-9736-6. [DOI] [PubMed] [Google Scholar]
- 20.Malamut G, Ziol M, Suarez F, Beaugrand M, Viallard JF, Lascaux AS, et al. Nodular regenerative hyperplasia: the main liver disease in patients with primary hypogammaglobulinemia and hepatic abnormalities. J Hepatol. 2008;48(1):74–82. doi: 10.1016/j.jhep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Yong PF, Thaventhiran JE, Grimbacher B. “A rose is a rose is a rose,” but CVID is Not CVID common variable immune deficiency (CVID), what do we know in 2011? Adv Immunol. 2011;111:47–107. doi: 10.1016/B978-0-12-385991-4.00002-7. [DOI] [PubMed] [Google Scholar]
- 22.Abolhassani H, Aghamohammadi A, Abolhassani F, Eftekhar H, Heidarnia M, Rezaei N. Health policy for common variable immunodeficiency: burden of the disease. J Investig Allergol Clin Immunol. 2011;21(6):454–8. [PubMed] [Google Scholar]
- 23.Torigian DA, LaRosa DF, Levinson AI, Litzky LA, Miller WT., Jr Granulomatous-lymphocytic interstitial lung disease associated with common variable immunodeficiency: CT findings. J Thorac Imaging. 2008;23(3):162–9. doi: 10.1097/RTI.0b013e318166d32f. [DOI] [PubMed] [Google Scholar]
- 24.MacDermott RP. Immunomodulator therapy in Crohn’s disease. In: Basow DS, editor. UpToDate. UpToDate; Waltham, MA: 2012. [Google Scholar]
- 25.MacDermott RP. 6-mercaptopurine (6-MP) metabolite monitoring and TPMT testing in the treatment of inflammatory bowel disease with 6-MP or azathioprine. In: Basow DS, editor. UpToDate. UpToDate; Waltham, MA: 2012. [Google Scholar]
- 26.Gregersen S, Aalokken TM, Mynarek G, Kongerud J, Aukrust P, Froland SS, et al. High resolution computed tomography and pulmonary function in common variable immunodeficiency. Respir Med. 2009;103(6):873–80. doi: 10.1016/j.rmed.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 28.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–22. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 29.Piatosa B, Wolska-Kusnierz B, Pac M, Siewiera K, Galkowska E, Bernatowska E. B cell subsets in healthy children: reference values for evaluation of B cell maturation process in peripheral blood. Cytometry B Clin Cyto. 2010;78(6):372–81. doi: 10.1002/cyto.b.20536. [DOI] [PubMed] [Google Scholar]
- 30.Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte sub-populations. J Pediatr. 1997;130(3):388–93. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 31.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37(8):820–8. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 32.Mouillot G, Carmagnat M, Gerard L, Garnier JL, Fieschi C, Vince N, et al. B-cell and T-cell phenotypes in CVID patients correlate with the clinical phenotype of the disease. J Clin Immunol. 2010;30(5):746–55. doi: 10.1007/s10875-010-9424-3. [DOI] [PubMed] [Google Scholar]
- 33.Salzer U, Bacchelli C, Buckridge S, Pan-Hammarstrom Q, Jennings S, Lougaris V, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2008;113(9):1967–76. doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chua I, Quinti I, Grimbacher B. Lymphoma in common variable immunodeficiency: interplay between immune dysregulation, infection and genetics. Curr Opin Hematol. 2008;15(4):368–74. doi: 10.1097/MOH.0b013e328302c7b6. [DOI] [PubMed] [Google Scholar]
- 35.Hatab AZ, Ballas ZK. Caseating granulomatous disease in common variable immunodeficiency treated with infliximab. J Allergy Clin Immunol. 2005;116(5):1161–2. doi: 10.1016/j.jaci.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 36.Lin JH, Liebhaber M, Roberts RL, Dyer Z, Stiehm ER. Etanercept treatment of cutaneous granulomas in common variable immunodeficiency. J Allergy Clin Immunol. 2006;117(4):878–82. doi: 10.1016/j.jaci.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 37.Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol. 2005;95(3):293–300. doi: 10.1016/S1081-1206(10)61228-8. [DOI] [PubMed] [Google Scholar]
- 38.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.