Abstract
Trans-tympanic injection into the middle ear has long been the standard for local delivery of compounds in experimental studies. Here we demonstrate the advantages of the novel method of intra-tympanic injection through the otic bone for the delivery of compounds or siRNA into the adult mouse cochlea. First, a fluorescently-conjugated scrambled siRNA probe was applied via intra-tympanic injection into the middle ear cavity and was detected in sensory hair cells and nerve fibers as early as 6 h after the injection. The fluorescent probe was also detected in other cells of the organ of Corti, the lateral wall, and in spiral ganglion cells 48 h after the injection. Furthermore, intra-tympanic delivery of Nox3 siRNA successfully reduced immunofluorescence associated with Nox3 in outer hair cells 72 h after injection by 20%. Drug or siRNA delivery via intra-tympanic injection does not compromise the tympanic membrane or interfere with noise-induced hearing loss, while trans-tympanic injections significantly altered the cochlear response to noise exposure. In summary, intra-tympanic injection through the otic bone into the middle ear cavity provides a promising approach for delivery of compounds or siRNA to cochlear hair cells of adult mice, relevant for the study of mechanisms underlying inner ear insults and, specifically, noise-induced hearing loss.
Keywords: tympanic membrane, local application, noise-induced hearing loss, fluorescent probe, siRNA delivery in vivo
1. Introduction
Local application of drugs to the inner ear has the potential to increase their efficacy in specific cell types and reduce side effects in comparison to systemic administration. For instance, local application of medication has been used clinically to treat inner ear disorders, such Meniere’s disease with gentamicin or of idiopathic sensorineural hearing loss with corticosteroids (Assimakopoulos et al., 2003; Seggas et al., 2011). Similarly, compounds have been delivered to the inner ear of guinea pigs, rats, chinchillas, and mice for laboratory research (Mikulec et al., 2009; Plontke et al., 2007; Roehm et al., 2007; Wagner et al., 2005; Xu et al., 2010). In addition, biodegradable gels loaded with therapeutic compounds and placed on the round window membrane reduce noise-induced hearing loss (NIHL) in guinea pigs, chinchillas, and rats (Coleman et al., 2007; Iwai et al., 2006; Lee et al., 2007). Among the types of local administration, the simplest and most frequently used is injection through the tympanic membrane (Borkholder, 2008), often using the mouse as a model as it offers an advantage in the availability of molecular data and genetic information. The kinetics of drug distribution in the inner ear after this procedure have been documented; gentamicin can be detected in the perilymphatic fluids of the mouse 1 day after intra-tympanic injection and its concentration drastically decreases after 3 days (Xu et al., 2010).
Short interfering RNA (siRNA) is a powerful tool for the study of molecular pathways and pathologies. In inner ear research, local application is required, as systemic administration would lead to dilution or degradation before significant concentrations in target cells can be achieved. Indeed, local delivery of siRNA into the rat cochlea has been successfully used for investigation of the mechanisms of cisplatin-induced hearing loss (Kaur et al., 2011; Mukherjea et al., 2010; Mukherjea et al., 2008). However, it remains to be established which method of siRNA application would be most suitable for the study of NIHL, which cells take up siRNA after the local application, and how long after delivery of siRNA the compounds are effective in the targeted cells.
To define these parameters, adult CBA/J mice at 3 months of age were used for investigation. We first characterized the effects of local injection through the tympanic membrane (trans-tympanic administration) or through the otic bone into the middle ear cavity (intra-tympanic administration) on subsequent auditory function as measured by auditory brain stem response (ABR) and on the response to noise exposure with broadband noise (BBN) from 2–20 kHz at 108 dB for 2 h. Next, the localization of a fluorescently-tagged scrambled siRNA probe delivered into the middle ear via the intra-tympanic route was determined at several time points after the delivery. Finally, the ability of Nox3 siRNA treatment to suppress Nox3-associated immunofluorescence in outer hair cells was assessed.
2. Materials and methods
2.1. Materials
Alexa 546-conjugated Nox3 siRNA (target sequence: 5′-AAGGTGGTGAGTCACCCATCT-3′), scrambled siRNA, Phalloidin, Hoechst 33342 and Alexa 488 secondary fluorescent antibody were purchased from Invitrogen (Carlsbad, CA). Polyclonal rabbit anti-Nox3 (# SC67005) was from Santa Cruz Biotechnology. Tissue adhesive was purchased from 3M Vetbond (#1469SB, St. Paul, MN). All other reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO).
2.2. Animals
Male CBA/J mice at 12 weeks of age (Harlan Sprague Dawley, Indianapolis, IN) were allowed free access to water and a regular mouse diet (Purina 5025, St. Louis, MO) and were kept at 22 ± 1 °C under a standard 12h:12 h light-dark cycle for one week of acclimation before the experiments. All research protocols were approved by either the University of Michigan (UM) Committee on Use and Care of Animals or by the Institutional Animal Care & Use Committee at the Medical University of South Carolina (MUSC). Animal care was under the supervision of either the Unit for Laboratory Animal Medicine at UM or the Division of Laboratory Animal Resources at MUSC.
2.3. Auditory brainstem response
Animals were anesthetized via intra-peritoneal injections of xylazine (7 mg/kg), ketamine (65 mg/kg), and acepromazine (2 mg/kg) and then placed in a sound-isolated and electrically shielded booth (Acoustic Systems, Austin, TX). Body temperature was monitored and maintained with a heating pad. Acoustic stimuli were delivered monaurally to a Beyer earphone attached to a customized plastic speculum inserted into the ear canal. Sub-dermal electrodes were inserted at the vertex of the skull, under the left ear, and under the right ear (ground). Tucker Davis Technology (TDT) System III hardware and SigGen/Biosig software were used to present the stimuli (15 ms duration tone bursts with 1 ms rise-fall time) and record the response. Up to 1024 responses were averaged for each stimulus level. ABR responses were determined for each frequency by reducing the intensity in 10 dB increments and then in 5 dB steps near threshold until no organized responses were detected. Thresholds were estimated at the lowest stimulus level at which a response was observed, identified by the presence of ABR waves I and II. All ABR measurements were evaluated by an expert blinded to the treatment conditions.
2.4. Noise exposure
Mice were exposed to a BBN from 2–20 kHz at 108 dB SPL for 2 h. Four mice were exposed at the same time in separate stainless steel wire cages (approximately 9 cm × 9 cm × 9 cm). The sound exposure chamber was fitted with a loudspeaker (model 2450H; JBL, Northridge, CA) driven by a power amplifier (model XLS 202D; Crown Audio, Elkhart, IN) fed from a CD player (model CD-200; Tascam TEAC American, Montebello, CA). Audio CD sound files were created and equalized with audio editing software (Audition 3; Adobe System, Inc., San Jose, CA). Sound levels were calibrated with a sound level meter (model 1200; Quest Technologies, Oconomovoc, WI) at multiple locations within the sound chamber to ensure uniformity of the sound field, and were measured before and after exposure to monitor stability.
2.5. Surgical procedure for intra-tympanic application
Catheter tubes 29.5 cm in length were prepared from two types of tubing. Twenty-eight cm of micro medical polyethylene tubing (#BB31695, Scientific Commodities Inc., Lake Havasu City, AZ) and 2 cm of medical grade polyimide tubing (#823400, A-M Systems Inc., Sequim, WA) were fitted with an overlap of 0.5 cm under a dissection microscope and bonded with one drop of Quick Bond (#72588, Electron Microscopy Sciences, Hatfield, PA). After bonding overnight, the catheter tubes were sterilized with ethylene oxide gas. Animals were anesthetized with an intra-peritoneal injection of xylazine (20 mg/kg) and ketamine (100 mg/kg). Body temperature was maintained with a heating pad during the surgical procedure. A retroauricular incision was made for the approach to the temporal bone (fig. 1). The facial nerve was identified deeply along the wall of the external auditory canal. The otic bulla was identified ventrally to the facial nerve and a small hole was made in the thin part of the otic bulla with a 30 G needle, located ventrally to the facial nerve (fig. 1 arrowhead). Injury of the stapedial artery can be avoided at this location. The catheter-tube described above was fed through the hole for about 0.5 cm and 10 μL of the experimental solution was slowly injected. After delivery, the hole was covered by the surrounding muscles. No seal was necessary to prevent leakage. The skin incision was closed with tissue adhesive.
Figure 1. Surgical approach for intra-tympanic injection into the tympanic cavity through the otic bulla.
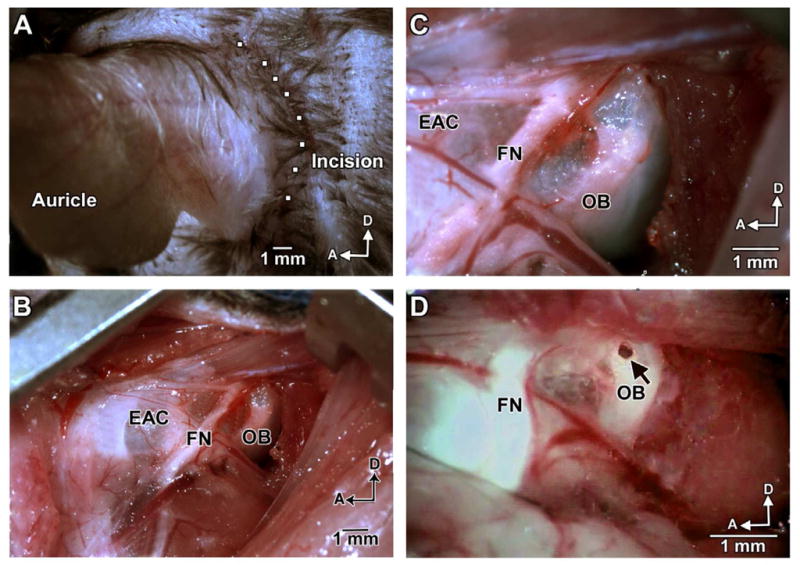
(A) A retroauricular incision of approximately 2 cm in length was made as indicated. (B) Lower magnification of the surgical area shows the otic bulla and facial nerve after incision through the dermis and hypodermis and separation of the muscles. (C) Enhanced magnification allows for careful inspection of structures and vasculature prior to the puncture of the otic bulla. (D) With a 30 G needle, a hole was made through the thin part of the otic bulla (arrow), which is located ventrally to the facial nerve. Ten microliters of a solution containing siRNA were injected into the middle ear through the hole using a customized catheter. OB: otic bulla, FN: facial nerve, EAC: external auditory canal, D: dorsal, A: anterior. Scale bar = 1 mm.
2.6. Immunocytochemistry for surface preparations or cryosections
Following decalcification with 4% EDTA for 72 h at 4 °C, cochleae were processed for immunocytochemistry on surface preparations or frozen sections. For surface preparations, the decalcified cochleae were dissected under a microscope by removing the softened otic capsule, stria vascularis, Reissner’s membrane, and tectorial membrane. The remaining tissue, including the modiolus and cochlear sensory epithelium, was permeabilized in 3% Triton X-100 solution for 30 min at room temperature. For cryosection analysis, sections of 8 μm thickness were incubated in 0.3% Triton X-100 in PBS for 30 min at room temperature. The specimens for either cryosections or surface preparations were washed three times with PBS, and incubated with phalloidin at a concentration of 1:100 for 1 h at room temperature. After the final wash with PBS, the tissue for surface preparations was dissected by removing the modiolus. The epithelia were divided into three segments (apex, middle, and base). Specimens were mounted on slides with anti-fade mounting media. Immunolabeled surface preparations were imaged with an Olympus laser confocal microscope (Olympus Fluoview 500 confocal microscope; Olympus Corporation, Tokyo). Immunolabeled cryosections were imaged with a Zeiss laser confocal microscope. For each group a sample size of 3 was used.
2.7. Quantification of the immunofluorescence signals in outer hair cells from surface preparations
Immunofluorescence of Nox3 in outer hair cells on surface preparations was quantified from original confocal images, each taken with a 63× magnification lens under identical conditions and equal parameter settings for laser gains and PMT gains, using ImageJ software (National Institute of Health, Bethesda, USA). The cochleae from the scrambled siRNA and Nox3 siRNA groups were fixed and immunolabled simultaneously with identical solutions and processed in parallel. All of the surface preparations were counter-stained with phalloidin to label hair cell structure in order to identify the comparable parts of the hair cells. The borders of each individual outer hair cell were outlined based on the phalloidin staining. The immunofluorescence of Nox3 in outer hair cells was measured in the upper-basal cochlear surface preparations in 0.12 mm segments, each containing about 60 outer hair cells. The intensity of the background fluorescence was subtracted and average fluorescence per cell was then calculated. The relative fluorescence was quantified by normalizing the ratio of the average Nox3-associated immunofluorescence after scrambled siRNA treatment in outer hair cells to the average Nox3-associated fluorescence after Nox3 siRNA treatment in outer hair cells. Each condition was replicated in 4 different animals.
2.8. Statistical analysis
Data was statistically evaluated by one-way ANOVA with Student-Newman-Keuls multiple comparison tests using Primer of Biostatistics software (McGraw-Hill Software, New York, NY) or two-tailed, one sample t-test using GraphPad Software (GraphPad Software Inc., San Diego, CA).
3. Results
3.1 Holes created by trans-tympanic injections heal slowly
Examination under a surgical microscope revealed that the tympanic membranes required several days for the hole created by a trans-tympanic injection to heal (table 1). In order to study this time course, five microliters of saline were injected with a 30 G needle through the pars tensa of the right and left tympanic membranes of four CBA/J mice. Two to three days after injection, holes in only 1 out of the 8 membranes had closed. Four and five days after injection, five and two remained unhealed, respectively. The two unhealed tympanic membranes remained open until the mice were euthanized 4 months after the injection.
Table 1. Tympanic membranes heal slowly after trans-tympanic injections.
The tympanic membranes of 4 mice, for a total of 8 membranes, were assessed after trans-tympanic injection of saline through the pars tensa of the tympanic membrane with a 30 G needle.
| Condition of the tympanic membrane | Number of tympanic membranes | |||
|---|---|---|---|---|
| Day after trans-tympanic injection | ||||
| 2nd | 3rd | 4th | 5th | |
| open | 7 | 7 | 5 | 2 |
| closed | 1 | 1 | 3 | 6 |
3.2 Route of injection affects hearing thresholds
The effect of trans-tympanic delivery on hearing was compared to intra-tympanic injections, which do not cause any damage to the tympanic membrane. Consistent with a slowly healing tympanic membrane, ABR thresholds at 24 kHz showed a progressive deficit from one to three days after trans-tympanic injection reaching a magnitude of about 10 dB (fig. 2). In contrast, ABR threshold shifts at both 12 and 24 kHz had significantly recovered by day 2 after intra-tympanic injection through the otic bulla and had completely recovered on day 3 (p < 0.01, n = 3, fig. 2).
Figure 2. Comparison of ABR threshold shifts after two different approaches to injection of saline into the middle ear cavity.
ABR thresholds were significantly improved 2 d after intra-tympanic injections through the otic bulla at both 12 and 24 kHz in comparison to trans-tympanic injections. Hearing was completely recovered to baseline levels by day 3 in the intra-tympanic group, while animals receiving trans-tympanic injections still showed significant ABR shifts. Data are presented as means + SD, n = 3, **p < 0.01.
3.3 Intra-tympanic injection through the otic bulla does not affect noise-induced permanent hearing loss
ABR threshold shifts in response to noise exposure were compared between three conditions: 1) trans-tympanic injection of saline; 2) intra-tympanic injection of saline; 3) no injection (control). Three days after injections, mice were exposed to BBN at 108 dB for 2 h. There was no difference in the magnitude of noise-induced hearing loss between the control animals and those subjected to the intra-tympanic injection (fig. 3). However, threshold shifts in the trans-tympanic injection group were significantly attenuated in comparison to the other two groups (p < 0.01, n = 3).
Figure 3. Unlike trans-tympanic injections, intra-tympanic injections through the otic bulla do not affect NIHL.
Mice were exposed to BBN for 2 h at 108 dB 3 d after injections. PTS in the trans-tympanic injection group (n = 7) were significantly attenuated in comparison to those of the controls (without any injection, n = 7) and of the intra-tympanic injection group (n = 5). Noise-induced threshold shifts of the control and intra-tympanic injection groups were almost identical. Data are presented as means + SD, **p < 0.01.
3.4 Fluorescence is detected in the cochlea after intra-tympanic injection
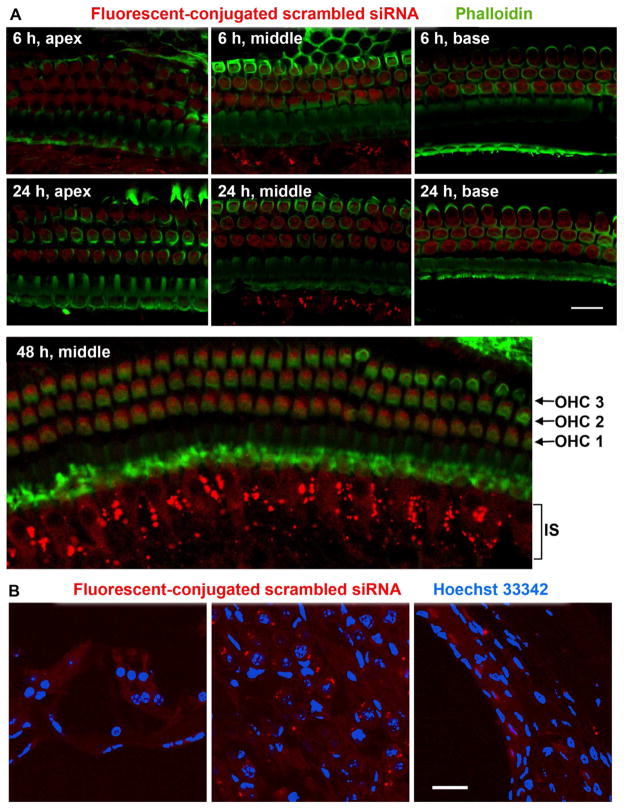
In order to evaluate how quickly the drug entered inner ear cells, we delivered an Alexa-546 fluorescence-conjugated scrambled siRNA probe via intra-tympanic injection. The red fluorescence was detected in outer and inner hair cells, as well as in the inner sulcus in the apical, middle, and basal turns of the epithelium at 6 and 24 h after injection (fig. 4A, 6–24 h). At 48 h after injection, fluorescence was still present in hair cells, but stronger fluorescence was observed in the inner sulcus (fig. 4A, 48 h). Fluorescence was also observed in the lateral wall and the spiral ganglion cells at the 48 h time point in cryosections (fig. 4B). Five days after injection, the majority of the fluorescent probe was eliminated from the whole-mount cochlear preparations (data not shown).
Figure 4. Distribution of a scrambled siRNA probe conjugated with Alexa 546 fluorescence.
(A) The red fluorescent siRNA was detected in both outer and inner hair cells in the apical turn through the basal turn of the cochlear spiral at 6 and 24 h after injection. In addition, fluorescence was also observed in a region corresponding to the inner sulcus (IS). Fluorescence was still detected in hair cells and stronger fluorescence was observed in the IS 48 h after injection. (B) Fluorescence was detected in the lateral wall and the spiral ganglion cells 48 h after injection on cryosections. n = 4, scale bars = 20 μm.
3.5 Nox3 siRNA delivered via intra-tympanic injection suppresses Nox3 associated-immunofluorescence in outer hair cells
Since the fluorescence probe was quickly taken up by outer hair cells, we examined how efficiently siRNA suppressed the expression of protein in these cells. We selected Nox3 siRNA because Nox3 is highly expressed in the inner ear (Banfi et al., 2004) and Nox3 siRNA has successfully suppressed the expression of Nox3 in rat cochleae via trans-tympanic injection (Mukherjea et al., 2010). Nox3 siRNA was injected into the left ear and scrambled siRNA (as control) into the right ear of each male CBA/J mouse at 3 months of age via intra-tympanic injection and the animals were euthanized 72 h after the siRNA delivery. The scrambled and Nox3 siRNA cochleae were processed in parallel for the immunofluorescence assay. The immunofluorescence associated with an antibody against Nox3 in outer hair cells was suppressed 72 h after injection of siRNA (fig. 5A). Quantification of Nox3-associated immunofluorescence in outer hair cells confirmed a significant decrease. The ratio of scrambled siRNA to Nox3 siRNA was 1:0.8 (p < 0.05, n = 4, fig. 5B).
Figure 5. Delivery of Nox3 siRNA via intra-tympanic injection reduces Nox3-associated immunofluorescence in hair cells.
(A) Surface preparations from the upper basal turns of cochlear epithelia from CBA/J mice were labeled with the Nox3 antibody 72 h after 0.3 μg Nox3 siRNA or scrambled siRNA delivery. Nox3-associated immunofluorescence in sensory hair cells was weaker in the Nox3 siRNA treated group than those of the scrambled siRNA group. These images are representative of 4 cochlear samples for each condition. Scale bar = 10 μm. Quantification of Nox3-associated immunofluorescence in outer hair cells revealed a significant decrease in the Nox3 siRNA group. The ratio of relative fluorescence in outer hair cells of scrambled to Nox3 siRNA treatment was 1:0.8 (p < 0.05, n = 4, fig. 5B).
4. Discussion
The salience of this study is the demonstration of a method for delivery of siRNA into the adult mouse cochlea, particularly (but not exclusively) appropriate for the study of NIHL. The study also provides documentation of the cell types in the cochlea that take up a fluorescently-conjugated siRNA probe and delineates the duration of the probe maintained within outer hair cells. Our data indicate that the fluorescently-conjugated siRNA probe can penetrate outer hair cells within hours and is eliminated from outer hair cells after 5 days. Consistent with the pattern of uptake, administration of Nox3 siRNA significantly diminished the immunofluorescence associated with Nox3 protein in outer hair cells 72 h after the injection. Such a time frame is highly suitable for acute studies of brief noise trauma or cisplatin injections and could be extended by repeat injections to study the chronic administration of aminoglycosides (Wu et al., 2001).
A major difference between trans-tympanic and intra-tympanic injections is the existence of slowly healing holes in the tympanic membrane after trans-tympanic injection. Since any transient injury to the tympanic membrane will affect its impedance, such delivery may lead to changes in sound and auditory processing. Our results demonstrate that injection through the tympanic membrane lead to a progressive ABR threshold deficit of about 10 dB at 24 kHz manifesting between one and three days after the injection. This may be due to the thickening of the tympanic membrane from scar tissue formation. We did not observe any signs of infection of the tympanic membrane. Specifically, our data demonstrated that tympanic membrane perforation could protect against NIHL, which is consistent with previous reports in the literature (Leidenfrost, 1976; Moneim, 1996). Such a protective effect might be due to the persisting hole in the tympanic membrane that reduces the sound energy conducted into the middle ear, attenuating noise-induced permanent threshold shifts (PTS). In contrast, intra-tympanic injection via the otic bulla avoids any damage to the tympanic membrane and, therefore, excludes the effects on NIHL. Although we did not observe signs of infection, the rate of middle ear infections may be higher after trans-tympanic injections. Many interventions against NIHL provide only partial protection or rescue, in particular, the attenuation of NIHL in mice is quite small (Oishi et al., 2011; Tamir et al., 2010). As local application of drugs holds the potential to increase the efficacy of the treatment in comparison to systemic administration, intra-tympanic injection of compounds may offer an advantage for the treatment of NIHL. In addition, local application of biodegradable gels via an intra-tympanic approach offers another opportunity to avert inner ear damage and provides sustained delivery because the compound is slow-released with the degradation of the gel (Heydt et al., 2004; Liu et al., 2005; Maeda et al., 2007; Xu et al., 2010).
In summary, we demonstrated that local application of compounds via trans-tympanic injection is not suitable for the study of NIHL. In its stead, intra-tympanic injection via the otic bone offers a suitable route for drug or siRNA delivery into the adult mouse cochlea for investigation of the mechanisms of NIHL.
Highlights.
Fluorescence-conjugated siRNA probes are taken up by cochlear sensory hair cells.
siRNA target protein is successfully suppressed in cochlear sensory hair cells.
Intra-tympanic delivery of siRNA or other compounds is suitable for study of NIHL.
Acknowledgments
The research project described was supported by grant R01 DC009222 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health. This work was partially conducted in the Walton Research Building in renovated space supported by grant C06 RR014516. Some animals used in this study were housed in MUSC CRI animal facilities supported by grant C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. We thank Dr. Jochen Schacht for his valuable comments on the manuscript.
Non-standard abbreviations
- ABR
auditory brain stem response
- BBN
broadband noise
- NIHL
noise-induced hearing loss
- PTS
noise-induced permanent threshold shifts
- siRNA
short interfering RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assimakopoulos D, Patrikakos G. Treatment of Meniere’s disease by intratympanic gentamicin application. J Laryngol Otol. 2003;117:10–6. doi: 10.1258/002221503321046586. [DOI] [PubMed] [Google Scholar]
- Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–72. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- Borkholder DA. State-of-the-art mechanisms of intracochlear drug delivery. Curr Opin Otolaryngol Head Neck Surg. 2008;16:472–7. doi: 10.1097/MOO.0b013e32830e20db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JK, Littlesunday C, Jackson R, Meyer T. AM-111 protects against permanent hearing loss from impulse noise trauma. Hear Res. 2007;226:70–8. doi: 10.1016/j.heares.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Heydt JL, Cunningham LL, Rubel EW, Coltrera MD. Round window gentamicin application: an inner ear hair cell damage protocol for the mouse. Hear Res. 2004;192:65–74. doi: 10.1016/j.heares.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Iwai K, Nakagawa T, Endo T, Matsuoka Y, Kita T, Kim TS, Tabata Y, Ito J. Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope. 2006;116:529–33. doi: 10.1097/01.mlg.0000200791.77819.eb. [DOI] [PubMed] [Google Scholar]
- Kaur T, Mukherjea D, Sheehan K, Jajoo S, Rybak LP, Ramkumar V. Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis. 2011;2:e180. doi: 10.1038/cddis.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Nakagawa T, Okano T, Hori R, Ono K, Tabata Y, Lee SH, Ito J. Novel therapy for hearing loss: delivery of insulin-like growth factor 1 to the cochlea using gelatin hydrogel. Otol Neurotol. 2007;28:976–81. doi: 10.1097/MAO.0b013e31811f40db. [DOI] [PubMed] [Google Scholar]
- Leidenfrost U. Middle ear deafness and noise trauma: Animal studies with the surface specimen technique. Laryngol Rhinol Otol (Stuttg) 1976;55:1005–10. [PubMed] [Google Scholar]
- Liu Y, Okada T, Sheykholeslami K, Shimazaki K, Nomoto T, Muramatsu S, Kanazawa T, Takeuchi K, Ajalli R, Mizukami H, Kume A, Ichimura K, Ozawa K. Specific and efficient transduction of Cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol Ther. 2005;12:725–33. doi: 10.1016/j.ymthe.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Fukushima K, Kawasaki A, Nishizaki K, Smith RJ. Cochlear expression of a dominant-negative GJB2R75W construct delivered through the round window membrane in mice. Neurosci Res. 2007;58:250–4. doi: 10.1016/j.neures.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Mikulec AA, Plontke SK, Hartsock JJ, Salt AN. Entry of substances into perilymph through the bone of the otic capsule after intratympanic applications in guinea pigs: implications for local drug delivery in humans. Otol Neurotol. 2009;30:131–8. doi: 10.1097/mao.0b013e318191bff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moneim IA. Audiometric pattern in a group of workers with one ear conductive deafness exposed to continuous noise. J Egypt Public Health Assoc. 1996;71:243–56. [PubMed] [Google Scholar]
- Mukherjea D, Jajoo S, Kaur T, Sheehan KE, Ramkumar V, Rybak LP. Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid Redox Signal. 2010;13:589–98. doi: 10.1089/ars.2010.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjea D, Jajoo S, Whitworth C, Bunch JR, Turner JG, Rybak LP, Ramkumar V. Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J Neurosci. 2008;28:13056–65. doi: 10.1523/JNEUROSCI.1307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi N, Schacht J. Emerging treatments for noise-induced hearing loss. Expert Opin Emerg Drugs. 2011;16:235–45. doi: 10.1517/14728214.2011.552427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plontke SK, Siedow N, Wegener R, Zenner HP, Salt AN. Cochlear pharmacokinetics with local inner ear drug delivery using a three-dimensional finite-element computer model. Audiol Neurootol. 2007;12:37–48. doi: 10.1159/000097246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm P, Hoffer M, Balaban CD. Gentamicin uptake in the chinchilla inner ear. Hear Res. 2007;230:43–52. doi: 10.1016/j.heares.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Seggas I, Koltsidopoulos P, Bibas A, Tzonou A, Sismanis A. Intratympanic steroid therapy for sudden hearing loss: a review of the literature. Otol Neurotol. 2011;32:29–35. doi: 10.1097/mao.0b013e3181f7aba3. [DOI] [PubMed] [Google Scholar]
- Tamir S, Adelman C, Weinberger JM, Sohmer H. Uniform comparison of several drugs which provide protection from noise induced hearing loss. J Occup Med Toxicol. 2010;5:26. doi: 10.1186/1745-6673-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N, Caye-Thomasen P, Laurell G, Bagger-Sjoback D, Thomsen J. Cochlear hair cell loss in single-dose versus continuous round window administration of gentamicin. Acta Otolaryngol. 2005;125:340–5. doi: 10.1080/00016480510026881. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res. 2001;158:165–78. doi: 10.1016/s0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]
- Xu L, Heldrich J, Wang H, Yamashita T, Miyamoto S, Li A, Uboh CE, You Y, Bigelow D, Ruckenstein M, O’Malley B, Li D. A controlled and sustained local gentamicin delivery system for inner ear applications. Otol Neurotol. 2010;31:1115–21. doi: 10.1097/MAO.0b013e3181eb32d1. [DOI] [PubMed] [Google Scholar]