Abstract
Fifty years ago, the causes of cancer were largely unknown. Since then, it has become clear that a strong relationship exists between obesity and many cancers, particularly postmenopausal breast cancer. A major challenge in understanding the link between obesity and cancer risk has been elucidating the biological basis underlying the association. Although this remains unresolved, the main candidate systems linking adiposity and cancer risk are (1) insulin and the insulin-like growth factor-I (IGF-I) axis, (2) endogenous reproductive hormones, and (3) chronic inflammation. The purpose of this paper is to provide a mechanistic overview of the hypothesized relationship between diet, physical activity, and obesity with breast cancer risk and progression. In addition, we will provide examples of recently funded randomized clinical trials examining metabolic risk factors in relation to breast cancer risk and survival. Additional research is warranted to validate the strength and consistency of the relationships among diet, these biomarkers, and breast cancer risk. As these relationships become clearer, future studies will be needed to develop effective intervention programs to prevent breast cancer and improve cancer prognosis by promoting a healthy lifestyle.
Keywords: Obesity, Diet, Physical Activity, Metabolism, Breast Cancer
Introduction
Fifty years ago, the causes of cancer were largely unknown. In 1963, Chester Southam wrote a review for Cancer Research indicating that the most widely recognized causes of human cancer were ionizing irradiation and carcinogenic chemicals.1 He also noted that hormones were associated with growth of prostate, breast and uterine cancer; that genetic factors influenced cancer genesis (“although most examples of ‘cancer families’ are readily explained as pure chance”), and that there was no proof of a viral etiology of any human cancer.
In 2002, the International Agency for Cancer Research concluded that there is “now sufficient evidence that excess body weight is an avoidable cause of cancer including colorectal in men, endometrial, post-menopausal breast, kidney, pancreatic, esophageal, and aggressive prostate cancers.”2 In 2003, a prospective study of more than 900,000 adults in the US estimated that 14 percent of all cancer deaths in men and 20 percent in women could be blamed on excess weight.3 As obesity became epidemic,4 a new scientific focus emerged directed to the study of diet, physical activity, obesity and disease risk; referred to as energetics.
As late as 1983, Moore et al. concluded that ionizing radiation, oophorectomy, hysterectomy, and hormone therapy were the principal iatrogenic factors that influence the incidence of breast cancer.5 It has since become clear that a strong relationship exists between obesity and postmenopausal breast cancer. Data from a prospectively studied population of 495,477 women revealed that a 2-fold increase in risk for death from breast cancer was significantly associated with a BMI >40 kg/m2.3 A recent review found that in women who have been diagnosed with either pre- or postmenopausal breast cancer, excess adiposity was associated with a 30% increased risk of mortality.6 Although the observational data for physical activity were not as consistent (or abundant), higher levels of activity appear to be associated with a 30% decreased risk of mortality. In a study of 1436 women diagnosed with breast cancer in 1996–1997, women who gained more than 10% weight after diagnosis had a worse survival (hazard ratio 2.7, 95% CI 1.4–5.0) compared to women who maintained their pre-diagnosis weight.7 The authors are not aware of any data on weight loss and breast cancer prognosis. Given that 70% of postmenopausal US women are overweight or obese,4 elucidation of the role of diet, physical activity, and excess adiposity in breast cancer risk and progression is a public health priority.
The objective of this paper is to provide a mechanistic overview of the hypothesized relationship of obesity with breast cancer risk and progression. In addition, examples of recently funded clinical trials examining metabolic risk factors in relation to breast cancer risk and survival will be provided. These trials are part of the National Cancer Institute’s (NCI) Transdisciplinary Research in Energetics and Cancer (TREC) Initiative and illustrate novel approaches to studying energetics and cancer risk.
Overweight/Obesity and Breast Cancer: Proposed Mechanisms
A major challenge in understanding the link between obesity and cancer risk has been elucidating the biological basis underlying the association. Although this remains unresolved, the main candidate systems linking adiposity and cancer risk are (1) insulin and the insulin-like growth factor-I (IGF-I) axis, (2) endogenous reproductive hormones, and (3) chronic inflammation. Below biological data supporting these hypotheses is outlined.
Insulin and the IGF-1 axis
Obesity and inactivity frequently lead to insulin resistance, which has been proposed as a central mechanism linking obesity and diabetes with increased breast cancer risk. Insulin has a variety of actions related to tumor development, including activation of the insulin receptor, which stimulates cell growth and cell division.8 Elevated glucose may also increase cancer risk. Neoplastic cells use glucose for proliferation and therefore higher circulating concentrations of glucose may favor selection of malignant cell clones.9
Hyperinsulinemia also impacts the insulin-like growth factors (IGFs). These multifunctional peptides regulate cell proliferation, differentiation, and apoptosis – cellular activities important in tumorigenesis.10 The insulin-cancer hypothesis postulates that prolonged hyperinsulinemia reduces the production of IGF binding protein-1 (IGFBP-1) and IGFBP-2. These binding proteins normally bind to and inhibit the action of IGF-1, with resultant increases in the levels of free IGF-1 and concomitant changes in the cellular environment that favor tumor development.11 In addition, IGFBP-3 is thought to be antiproliferative and proapoptotic through actions independent of the IGF-1 receptor.10
In support of the insulin-cancer hypothesis, it is notable that silencing of insulin signaling has been proposed as a novel strategy for cancer therapy.12 In particular, metformin, a drug used to treat type 2 diabetes mellitus, is being studied as an anticancer agent.13–17 Using the UK-based General Practice Research Database, long-term use of metformin (>5 years) was associated with an adjusted odds ratio of 0.44 (95% CI 0.24–0.82) for developing breast cancer compared with no use of metformin.18 A clinical effect of metformin on breast cancer prognosis is mechanistically plausible, either indirectly via reduced insulin levels or directly via mammalian target of rapamycin (mTOR) inhibition.19 Metformin has also been shown to reduce proliferation of most cultured breast cancer cell lines.20
Endogenous reproductive hormones
Sex steroid hormones mediate tumor promotion in breast cancer and have been a target for therapeutic intervention since George Beatson’s discovery of hormone-dependent breast cancer in the late 1800’s.21 There is abundant evidence that reproductive hormones play a primary role in the etiology and progression of breast cancer.22 For example, several variables that alter hormone status (e.g., early age at menarche, late age at menopause) are associated with an increased risk of breast cancer.23 Further, hormonal therapies such as selective estrogen receptor modulators (e.g., tamoxifen) reduce breast cancer incidence and recurrence.24–25
Overall, women with hormones in the top 20% of circulating levels of estrogen and androgens have a 2- to 3-fold higher risk of breast cancer compared to the bottom 20%.22 Obesity influences the synthesis and bioavailability of estrogen, androgen, and progestins. There are at least three mechanisms implicating these sex steroids in the development of cancer.11 First, adipose tissue expresses several sex-steroid metabolizing enzymes, including aromatase, that promote the formation of estrogens from androgenic precursors. Second, increased concentrations of insulin, which frequently occurs in obesity, result in reduced hepatic synthesis and blood concentrations of sex-hormone-binding globulin (SHBG). This leads to an increase in the fraction of bioavailable estrogen. Finally, higher insulin concentrations increase androgen synthesis in the ovaries and, to some extent, the adrenal glands.
Estrogens contribute to tumor growth by promoting the proliferation of cells with an existing mutation or increasing the opportunity for mutation.22,26 Androgens may increase breast cancer risk directly, by increasing cellular growth and proliferation, or indirectly via conversion to estrogen. However, less is known about how sex hormones interact with other risk factors to modulate breast cancer risk, although there is evidence linking estrogen with insulin resistance.27–29
Chronic Inflammation
Cancer and inflammation were first linked in the 19th century when tumors were observed to arise at sites of chronic inflammation and it was discovered that inflammatory cells were present in biopsied samples from tumors.30 Although this hypothesis fell out of favor for more than a century, there has been a recent resurgence in interest based on new lines of evidence. Inflammatory cells and inflammatory mediators (e.g., chemokines, cytokines, adipokines, prostaglandins) are present in the microenvironment of most, if not all, tumors.31
Adipose tissue is now recognized as having an endocrine function, secreting several hormones, notably leptin and adiponectin, and a diverse range of other protein signals and factors involved in lipid metabolism, insulin sensitivity, and the regulation of energy balance.32 In addition, there is a growing list of adipokines involved in inflammation (e.g., TNF-α, IL-1β, IL-6, IL-8, IL-10) and the acute-phase response (plasminogen activator inhibitor-1, haptoglobin, serum amyloid A). Production of these proteins by adipose tissue is increased in obesity, and raised circulating levels of several acute-phase proteins and inflammatory cytokines has led to the view that obesity is characterized by a state of chronic low-grade inflammation, which links causally to insulin resistance33 and cancers.34
Although a firm relationship between breast cancer and inflammation has not been established, indicators of inflammation are present in breast tumors. In addition, a number of epidemiologic studies have reported increased breast cancer risk or worse prognosis among women with signs of chronic inflammation or higher circulating levels of adipokines.35–40
Summary of Breast Cancer Mechanisms
The mechanistic summaries provided above are brief and therefore relatively cursory in nature – and interested readers may refer to numerous in-depth reviews of these topics.10, 31–32, 34–35, 37–38, 41–47 Although insulin resistance, sex hormones, and inflammation are frequently examined as independent risk factors; considerable evidence indicates that these three systems form an interrelated and interdependent network. As shown in Figure 1, insulin resistance is posited to have a central role in cancer risk because of the downstream effects on a host of obesity- and breast cancer-related biomarkers.
Figure 1.
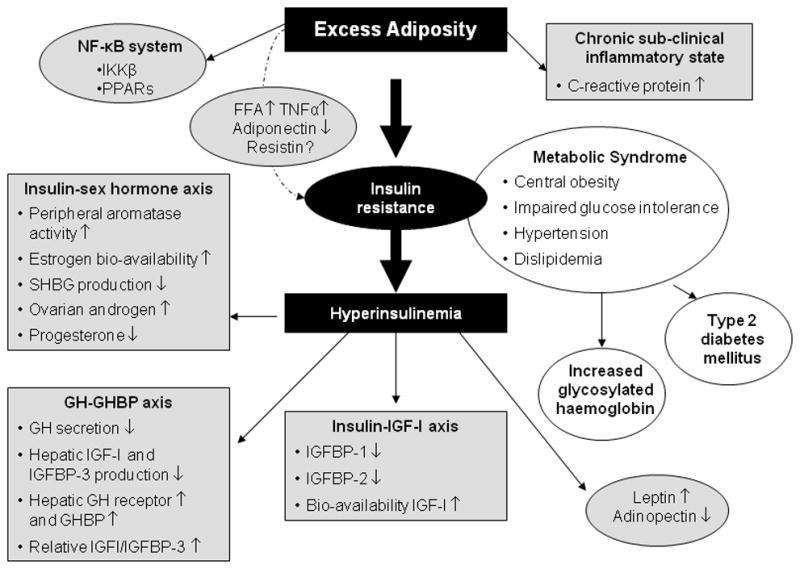
A Proposed Mechanistic Model in Which the Shaded Boxes Represent Pathways Hypothesized to Link Excess Adiposity (defined as BMI ≥ 25 kg/m2), Insulin Resistance, and Other Metabolic Factors with Cancer Risk (either Incidence or Recurrence).
Abbreviations: NF-κB=nuclear factor κB; IKKβ=IκB kinase β; PPARs= peroxisome proliferator-activated receptors; FFA=free fatty acids; TNF-α=tumor necrosis factor-alpha; GH=growth hormone; IGF=insulin-like growth factor; IGFBP=insulin-like growth factor binding protein; GHBP=growth hormone binding protein. Keys: ↑, increase in circulating concentrations; ↓ decrease in circulating concentrations.
Table 1 provides a mechanistic overview of major circulating biomarkers in the three systems summarized above.11, 31–32, 37, 43, 45, 47–49 A panel of 6 University of California, San Diego (UCSD) faculty with expertise in nutrition, breast cancer, and epidemiology reviewed the published studies and reviews to qualitatively assess the strength of the evidence linking circulating biomarkers and breast cancer risk, as shown in Table 1. To date, published data most strongly support fasting insulin, bioavailable estradiol, and C-reactive protein as biomarkers of breast cancer risk. Individually, these biomarkers are associated with an approximate two-fold increased risk of incident or recurrent breast cancer.39, 50–52 Fewer data are available on the association of other biomarkers with breast cancer. New research untangling the nuances of the mechanisms linking obesity and cancer is needed to more definitely relate these biomarkers with cancer risk.
Table 1.
Circulating Biomarkers Proposed as Linking Adiposity with Breast Cancer Risk10–11, 31–32, 34–35, 37–38, 41–49
| Biomarker | Evidence | Hypothesized Role in Cancer Risk | |
|---|---|---|---|
| Insulin –Glucose and the IGF Systm | Insulin | +++ | Obesity-related hyperinsulinemia is growth-promoting and antiapoptotic, inhibits hepatic secretion of SHBG and IGFBP-1, exerts mitogenic effects in breast cancer cells that may synergize with estrogen, and is associated with decreased adiponectin levels. |
| Glucose | + | Growth promoter. May favor selection of malignant cell clones. | |
|
| |||
| IGF1 | ++ | Mitogenic, antiapoptotic, proangiogenic, regulates cell size, increases cell migration, potentiates growth factors | |
|
| |||
| IGFBP-1 | ++ | Prolonged hyperinsulinemia reduces IGFBP-1 levels, leading to increased IGF1 bioactivity | |
|
| |||
| Endogenous sex hormones | Estradiol | +++ | Higher levels in postmenopausal women caused by conversion of androgenic precursors through increased aromatase enzyme activity in adipose tissue. Mitogenic in normal and neoplastic mammary tissues. May be associated with increased CRP levels. |
| Testosterone | ++ | May directly stimulate breast cell proliferation or serve as estrogen precursor. | |
| SHBG | ++ | Regulates the bioavailability of estradiol and testosterone. May have hormone independent effects on breast tissue. | |
|
| |||
| CRP | ++ | Marker of chronic inflammation at the vascular level associated with cancer risk. High levels of estradiol may correlate with CRP concentrations. | |
|
| |||
| TNF-α | + | Proinflammatory cytokine. The release of this protein from adipose tissue gives rise to insulin resistance. | |
|
| |||
| IL-6 | + | Proinflammatory cytokine. Highly correlated with adiposity and insulin resistance. Stimulates the growth and differentiation of malignant cells. Governs CRP production. | |
|
| |||
| SAA | + | A proinflammatory and lipolytic adipokine recently associated with poor breast cancer outcomes. | |
|
| |||
| Resistin | + | May induce insulin resistance or impair hepatic sensitivity to insulin. | |
|
| |||
| Adiponectin | + | Insulin-sensitizing, anti-diabetic, anti-inflammatory, proapoptotic and antiangiogenic. Lower levels linked to increased risk of breast cancer or more aggressive phenotype. | |
|
| |||
| Leptin | + | Mediator of energy balance and immune processes. Elevated levels are observed in obese individuals, induce the growth of breast cancer cells, and can mediate angiogenesis. Associated with SHBG and insulin levels. | |
Epidemiologic evidence for the association of the biomarker with cancer risk:
Supported by reviews, meta-analyses and/or clinical trials;
Association observed in numerous (3+) observational studies;
Association observed in small number of observational studies.
Abbreviations: IGF1=insulin-like growth factor-1; IGFBP1=IGF binding protein-1; SHBG=sex hormone binding protein; CRP=C-reactive protein; TNF-α=tumor necrosis factor-alpha; IL-6=interleukin 6; SAA=serum amyloid A.
Impact of Diet on Metabolic Markers Putatively Associated with Breast Cancer Risk
As indicated above, the presence of excess adiposity can result in insulin resistance, chronic inflammation, and higher levels of circulating estradiol; all of which may increase breast cancer risk. Therefore it is generally assumed that any diet resulting in loss of adipose tissue will normalize these risk factors. The Diabetes Prevention Trial found that a weight-loss dietary counseling intervention lead to significant improvements in insulin resistance.53 Weight loss is also associated with a reduction in inflammatory factors.54 For example, a randomized clinical trial of weight loss and chronic inflammation among obese adults found that an average diet-induced weight loss of 5.7% resulted in significant reductions in concentrations of IL-6 and TNF-α.55 Finally, In a 12-month, randomized weight loss controlled trial, overweight participants (n=439) were assigned to either reduced calorie, exercise, reduced calorie plus exercise, or usual care.56 Compared with controls, estradiol decreased 16.2% (P < .001) with diet, 4.9% (P = .10) with exercise, and 20.3% (P < .001) with diet plus exercise. Similar results were seen for estrone and testosterone. Taken together, published research supports the role of weight loss for risk reduction through lowering exposure to breast cancer biomarkers.
Metabolism-Cancer Hypothesis: Research Frontiers
Recognition of the complex, multidimensional relationship between excess adiposity and cancer risk has motivated the scientific community to seek new research models and paradigms. In response, NCI developed a concept to establish a centers grant mechanism in nutrition, energetics, and physical activity; referred to as TREC (http://grants.nih.gov/grants/guide/rfa-files/RFA-CA-10-006.html). The primary mission of the TREC Centers is to foster collaboration among transdisciplinary teams of scientists with the goal of accelerating progress towards reducing cancer incidence and morbidity and mortality associated with obesity, low levels of physical activity, and poor diet.
In 2011, UCSD was awarded a TREC Center focused on breast cancer. The objective of this Center is to enhance knowledge regarding insulin resistance and inflammation underlying the association of energetics with breast cancer carcinogenesis, from the cell to the community. Below an overview is provided of the human trials being conducted by the UCSD TREC Center, which illustrate innovative research designs capitalizing on the science of energetics and cancer risk. The UCSD Institutional Review Board approved the study protocols summarized below and all participants provide written informed consent. A fourth UCSD project using mouse models to explore the role of inflammation and insulin resistance in breast cancer is not summarized here.
Diet Composition and Genetics: Effects on Weight, Inflammation and Biomarkers
Optimal macronutrient distribution of weight loss diets has not been established. However, emerging evidence suggests that the optimal dietary composition for weight loss differs across individuals based on metabolic status.57 Specifically, differential effects of the dietary pattern on cancer-related mechanistic factors may occur in insulin-sensitive vs. insulin-resistant obese women. Effects of diet composition in weight loss interventions on hormonal and other factors linking obesity to breast cancer risk or progression have not been compared or examined. Further, polymorphisms in genes that determine cytokine production and inflammation, a key factor in the pathophysiology of insulin resistance, may modify the response to weight loss and diet modification.
This study in obese women is designed to examine the effect of three dietary approaches in a weight loss intervention. The three diets under investigation are (i) a higher carbohydrate (65% energy), lower fat (20% energy) diet; (ii) a lower carbohydrate (45% energy), higher monounsaturated fat (35% energy) diet; and (iii) a walnut-rich, lower carbohydrate (45%), higher fat (35% of energy) diet. The specific aims of this study are:
To examine whether there is a differential weight loss response to the three diets depending on insulin resistance status.
To examine whether hormonal and inflammatory response to the diets differs by insulin resistance status. Markers to be examined link obesity to breast cancer mortality: insulin, SHBG, estrogens, C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), and as a marker for gene expression, IL-6 and TNF-α gene methylation (DNA methylation is one of several mechanisms that cells use to control gene expression).
To identify whether the differential response of IL-6 and TNF- α to weight loss and diet composition are associated with polymorphisms (i.e., common genetic variations) in IL-6 and TNF-α genes.
These aims will be addressed in a randomized controlled study involving 234 obese women. Participants will be randomized to one of the three diets, stratified by menopausal status and insulin resistance as assessed using the homeostasis model assessment of insulin resistance.58 As summarized in Table 2, the intervention is a multifaceted cognitive-behavioral weight loss program that consists of closed group sessions and individualized diet counseling over a 12-month period. All intervention participants will also be counseled to increase their physical activity to 60 minutes per day of moderate-intensity exercise. Intervention adherence will be assessed by measured weight loss, 24-hour dietary recalls, and objective measures of physical activity energy expenditure (see below for more details).
Table 2.
Overview of Weight Loss Interventions in Two Clinical Trials on Obesity and Breast Cancer Conducted as part of the Transdisciplinary Research in Energetics and Cancer (TREC) Center at the University of California, San Diego (2011–2016)
| Key elements of the interventions | Diet Composition and Genetics: Effects on Weight, Inflammation and Biomarkers (n=234) | Obesity-Related Mechanisms and Mortality in Breast Cancer Survivors (n=340) |
|---|---|---|
| Participants | Non-diabetic obese women aged 21 and older with a BMI ≥30 k/m2 and ≤40 kg/m2. | Survivors of postmenopausal breast cancer with a BMI of ≥25 kg/m2. |
| Major theoretical foundations | The behavioral determinants model, which is based on social cognitive theory. This model posits there are personal, social, and physical environmental antecedents and consequences of behavior. | A stepwise, phased approach using strategies outlined by social cognitive theory that focus on (a) establishing a series of short-term goals and (b) reviewing performance on these goals in a way that builds self-efficacy. |
| Intervention goal | To lose ≥ 7% body weight | To lose ≥ 10% body weight |
| Intervention length | 12 months | 6 months |
| Intervention mode | Closed-groups of 12–15 women | Telephone counseling calls |
| Number of contacts | 26 group meetings plus 16–24 counseling calls or email contacts | 12 counseling calls plus 6 newsletters |
| Primary intervention goal | To promote a reduction in energy intake relative to expenditure, aiming for a deficit of 500–1000 kcal/day | To promote a caloric deficit of 500–1000 calories through a reduction in energy intake and an increase in energy expenditure. |
| Dietary intervention goals | Women randomized to: (i) a higher carbohydrate (65% energy), lower fat (20% energy) diet; (ii) a lower carbohydrate (45% energy), higher monounsaturated fat (35% energy) diet; or (iii) a walnut-rich, lower carbohydrate (45%), higher fat (35% of energy) diet. | Healthful changes such as consuming 5–9 servings/day vegetables and fruit, replacing refined grains with whole grains, choosing fish and legumes as a protein source, and replacing saturated fatty acids with monounsaturated fatty acids. |
| Physical activity goal | 60 minutes per day of planned exercise at a moderate level of intensity | 300 minutes per week of moderate-to-vigorous intensity exercise |
| Sample topics |
|
|
| Measures of intervention adherence | Measured weight loss, 24-hour dietary recalls, and objective measures of physical activity energy expenditure (accelerometers, GPS, and heart rate monitor) | Measured weight loss and objective measures of physical activity energy expenditure (accelerometers and GPS) |
It is hypothesized that compared to obese women who are not insulin resistant, those who are insulin resistant will demonstrate more favorable responses to the lower carbohydrate, higher fat diets compared to the higher carbohydrate, lower fat diet. A walnut-rich diet is likely to promote a similar response in cancer risk factors as a higher-fat diet in which monounsaturated fatty acids are the predominant source of fat. Results of this study will help to refine and individualize dietary guidance for optimal weight control and breast cancer prevention.
Obesity-Related Mechanisms and Mortality in Breast Cancer Survivors
The objective of this study is to investigate the degree to which metformin, a lifestyle intervention, or both, can reduce breast cancer mortality among overweight or obese, postmenopausal breast cancer survivors. The specific aims of the investigation are:
To use data from a large, well-characterized cohort to develop a Biomarker Risk Score that independently predicts breast cancer mortality based on the joint impact of circulating biomarkers related to the insulin-IGF axis, endogenous sex hormones, and inflammation.
To investigate the effects of metformin, lifestyle, and a combination metformin/lifestyle intervention on this Biomarker Risk Score.
To predict (by means of the Biomarker Risk Score) the effects of metformin, lifestyle, and a combination metformin/lifestyle intervention on breast cancer mortality.
These aims will be addressed in a 6-month, 2×2 randomized controlled trial with 340 overweight or obese, postmenopausal breast cancer survivors. Participants will be randomized in equal numbers to (i) placebo, (ii) metformin, (iii) weight loss intervention and placebo, or (iv) weight loss intervention and metformin. As summarized in Table 2, the intervention is a telephone-based counseling protocol that follows a stepwise, phased approach using strategies outlined by social cognitive theory59 that focus on (a) establishing a series of short-term goals and (b) reviewing performance on these goals in a way that builds self-efficacy.59–60 All intervention participants will also be counseled to increase their physical activity to 300 minutes per week moderate to vigorous physical activity. Intervention adherence will be assessed by measured weight loss and objective measures of physical activity energy expenditure (see below for more details). This trial will evaluate the degree to which each intervention changes a Biomarker Risk Score that will be developed to predict breast cancer mortality.
To develop the Biomarker Risk Score, biomarkers will be assayed in archived blood samples from overweight/obese, postmenopausal cancer survivors (125 cases [breast cancer death]:250 matched controls) who participated in the Women’s Healthy Eating and Living (WHEL) Study, which offers long-term follow-up for mortality.61 For each case, two obese/overweight postmenopausal controls who were alive and cancer-free at the time of death of the corresponding case62–63 will be matched on (i) time since diagnosis of original breast cancer, (ii) time of follow up after enrollment in the WHEL Study, (iii) age at diagnosis, (iv) the stage of breast cancer at initial diagnosis, and (v) clinical site.
The goal of the analysis is to identify a set of markers that are most predictive of breast cancer mortality. To this end, a conditional logistic regression model will be developed to examine the joint role of these markers on breast cancer prognosis. The biomarker risk score will be a weighted linear combination of the biomarkers in the model with corresponding log-odds ratios defining the weights. To avoid over-fitting and to get an unbiased estimate of the predictive power of the biomarkers,64 samples will be randomly separated into a training set containing 85 case-control samples and a validation set containing 40 case-control samples. The prediction models will be developed using the training set and tested for predictive accuracy using the validation set. Predictive accuracy will be gauged using area under the ROC curve.65 Further, within the training set, cross-validation techniques will be applied to reduce prediction error.66–67 Changes in this Risk Score should reflect intervention-related changes in these markers in an individual and therefore can be used to assess the clinical impact of the metformin and weight loss trial.
It is hypothesized that among overweight or obese postmenopausal breast cancer survivors, metformin and lifestyle interventions will reduce breast cancer mortality (as assessed by changes in the Biomarker Risk Score). These complementary investigations have the potential to provide robust, mechanistic data in support of clinical trials to reduce breast cancer mortality.
Advancing Assessment of Physical Activity Energy Expenditure in Women with Increased Cancer Risk
The TREC weight loss trials described above provide a unique opportunity to examine the impacts of objectively assessed physical activity on fasting insulin concentrations and insulin resistance. Accelerometers provide good quality objective estimates of physical activity when activities involve the whole body. However, the accuracy of these estimates declines substantially when movements are of light intensity or mainly involve peripheral limbs, such as cycling.68 Global Positioning System (GPS) and heart rate data may improve assessment of physical activity by incorporating data on speed of travel, location of activity, and heart rate.
The objective of this study is to improve the assessment of physical activity energy expenditure (PAEE) by applying novel computational techniques to accelerometer, heart rate monitor, and GPS device data. The specific aims are:
To compare traditional assessments of physical activity intensity measured by accelerometer counts alone with estimates of PAEE from multiple devices using Machine Learning techniques to identify types of physical activity and sedentary behavior contributing to PAEE.
To assess the relationship between PAEE (from traditional and novel estimation techniques) and insulin resistance over time, with and without adjustments for weight loss.
To investigate whether different patterns of PAEE accumulation are related to insulin resistance, independent of total PAEE.
For seven days at baseline and follow-up, obese women enrolled in the weight loss trials described above will wear accelerometers, GPS devices, and heart rate monitors1. Machine Learning algorithms will be applied to the data collected by these devices. Machine Learning is a process whereby computer algorithms are developed and validated in comparison to a training data set (i.e., a gold standard). The Machine learns how to recognize features from the data signals and how to classify them into behaviors (e.g., walking versus stationary cycling). The training data set will be developed in a separate study where obese women will wear physical activity assessment devices and a SenseCam (Microsoft, Redmond, Washington): a small device worn like a necklace that passively collects ~3000 images per day. These images will be coded to create the annotated truth file for comparison to the data collected via accelerometers, GPS, and heart rate monitors. Improving assessment of PAEE and understanding the pathway to cancer risk is vital to improving cancer prevention interventions.
Conclusions
The role of diet and physical activity in cancer prevention is considerably more complex then the classical energy-balance model. For example, recent research indicates that regular activity is associated with reduced risk of breast cancer or cancer-related mortality via interrelated biologic pathways that involve adiposity, sex hormones, insulin resistance, adipokines, chronic inflammation and immune function.69–71 Although the data are preliminary, research also suggests that certain foods and dietary patterns may generate an anti-inflammatory milieu, which may be an important mechanism linking poor quality diets to metabolic disease and cancer risk. It is generally believed that such an anti-inflammatory diet would be a low glycemic-load diet, relatively low in omega-6 fatty acids, and rich in omega-3 fatty acids.72–74 Ongoing research is focusing on numerous other foods, herbs, spices, and compounds that may have anti-inflammatory properties such as curcuma, ginger, and flavenoids.72, 75–76 Well-designed randomized trials are needed to better understand how and to what extent obesity, diet, and physical activity may affect cancer risk via modification of insulin resistance, sex hormone concentrations, and chronic inflammation.
Historically, the role of the dietetics professional in cancer has focused on providing nutrition support. For example, dietitians helped patients cope with anorexia, appetite changes, localized tumor effects, or treatment effects such as nausea.77 Nutrition guidelines for cancer survivors tended to reflect general health advice, such as to eat a variety of healthful foods with an emphasis on plant sources, adopt a physically active lifestyle, maintain a healthy weight, and limit consumption of alcoholic beverages.78 However, the paradigm shift relating metabolic risk factors with cancer has the potential to lead to new opportunities for dietitians in the realm of cancer prevention and survivorship. Additional research is warranted to validate the strength and consistency of the relationships among diet, these biomarkers, and breast cancer risk. As these relationships become clearer, future studies will be needed to develop effective intervention programs to prevent breast cancer and improve cancer prognosis by promoting a healthy lifestyle.
Footnotes
To minimize participant burden, women in the breast cancer survivor study will wear only accelerometers and GPS devices.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Southam CM. The Complex Etiology of Cancer. Cancer Res. 1963;23:1105–1115. [PubMed] [Google Scholar]
- 2.IARC. Weight Control and Physical Activity. Vol. 6. Lyon, France: International Agency for Research on Cancer; 2002. Handbook of Cancer Prevention. [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348 (17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307 (5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 5.Moore DH, Moore DH, 2nd, Moore CT. Breast carcinoma etiological factors. Adv Cancer Res. 1983;40:189–253. doi: 10.1016/s0065-230x(08)60681-8. [DOI] [PubMed] [Google Scholar]
- 6.Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 2010;66 (1):5–15. doi: 10.1016/j.maturitas.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw PT, Ibrahim JG, Stevens J, et al. Postdiagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology. 2012;23 (2):320–327. doi: 10.1097/EDE.0b013e31824596a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faulds MH, Dahlman-Wright K. Metabolic diseases and cancer risk. Curr Opin Oncol. 2012;24 (1):58–61. doi: 10.1097/CCO.0b013e32834e0582. [DOI] [PubMed] [Google Scholar]
- 9.Muti P, Quattrin T, Grant BJ, et al. Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. 2002;11 (11):1361–1368. [PubMed] [Google Scholar]
- 10.Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363 (9418):1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 11.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17 (8):328–336. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28 (6):1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampton T. Researchers probe targeted agents’ clinical potential for cancer prevention. JAMA. 2010;303 (2):115–116. doi: 10.1001/jama.2009.1894. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher EJ, LeRoith D. Diabetes, cancer, and metformin: connections of metabolism and cell proliferation. Ann N Y Acad Sci. 2011;1243:54–68. doi: 10.1111/j.1749-6632.2011.06285.x. [DOI] [PubMed] [Google Scholar]
- 15.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin: Response to Farooki and Schneider. Diabetes Care. 2006;29 (8):1990–1991. doi: 10.2337/dc06-0997. [DOI] [PubMed] [Google Scholar]
- 16.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330 (7503):1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32 (9):1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33 (6):1304–1308. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310 (5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8 (6):909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 21.Beatson CT. On treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. Lancet. 1896;2:104–107. [PMC free article] [PubMed] [Google Scholar]
- 22.Hankinson SE. Endogenous hormones and risk of breast cancer in postmenopausal women. Breast Dis. 2005;24:3–15. doi: 10.3233/bd-2006-24102. [DOI] [PubMed] [Google Scholar]
- 23.Velie EM, Nechuta S, Osuch JR. Lifetime reproductive and anthropometric risk factors for breast cancer in postmenopausal women. Breast Dis. 2005;24:17–35. doi: 10.3233/bd-2006-24103. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90 (18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 25.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361 (9354):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 26.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4 (8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 27.Kalyani RR, Franco M, Dobs AS, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94 (11):4127–4135. doi: 10.1210/jc.2009-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295 (11):1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 29.Golden SH, Dobs AS, Vaidya D, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92 (4):1289–1295. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- 30.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357 (9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454 (7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 32.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92 (3):347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 33.Kolb H, Mandrup-Poulsen T. The global diabetes epidemic as a consequence of lifestyle-induced low-grade inflammation. Diabetologia. 2010;53 (1):10–20. doi: 10.1007/s00125-009-1573-7. [DOI] [PubMed] [Google Scholar]
- 34.Heikkila K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health. 2007;61 (9):824–833. doi: 10.1136/jech.2006.051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48 (4):155–170. doi: 10.3109/10408363.2011.599831. [DOI] [PubMed] [Google Scholar]
- 36.Heikkila K, Harris R, Lowe G, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20 (1):15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 37.Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. J Cell Biochem. 2008;105 (4):956–964. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- 38.Vona-Davis L, Rose DP. Angiogenesis, adipokines and breast cancer. Cytokine Growth Factor Rev. 2009;20 (3):193–201. doi: 10.1016/j.cytogfr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Pierce BL, Ballard-Barbash R, Bernstein L, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27 (21):3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yigit E, Gonullu G, Yucel I, et al. Relation between hemostatic parameters and prognostic/predictive factors in breast cancer. Eur J Intern Med. 2008;19 (8):602–607. doi: 10.1016/j.ejim.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 41.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8 (12):915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 42.Lann D, LeRoith D. The role of endocrine insulin-like growth factor-I and insulin in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13 (4):371–379. doi: 10.1007/s10911-008-9100-x. [DOI] [PubMed] [Google Scholar]
- 43.Becker S, Dossus L, Kaaks R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch Physiol Biochem. 2009;115 (2):86–96. doi: 10.1080/13813450902878054. [DOI] [PubMed] [Google Scholar]
- 44.Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology. 2002;63 (4):317–332. doi: 10.1159/000066230. [DOI] [PubMed] [Google Scholar]
- 45.Nunez NP, Hursting SD, Yakar S, Fowler D, Vinson C. Obesity provides a permissive milieu in inflammation-associated carcinogenesis: analysis of insulin and IGF pathways. Methods Mol Biol. 2009;512:29–37. doi: 10.1007/978-1-60327-530-9_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. J Steroid Biochem Mol Biol. 2007;106 (1–5):24–30. doi: 10.1016/j.jsbmb.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94 (9):1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 49.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114 (1):71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 50.Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst. 2009;101 (6):384–398. doi: 10.1093/jnci/djp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20 (1):42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 52.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94 (8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 53.Kitabchi AE, Temprosa M, Knowler WC, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54 (8):2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17 (1):4–12. [PubMed] [Google Scholar]
- 55.Nicklas BJ, Ambrosius W, Messier SP, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79 (4):544–551. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 56.Campbell KL, Foster-Schubert KE, Alfano CM, et al. Reduced-Calorie Dietary Weight Loss, Exercise, and Sex Hormones in Postmenopausal Women: Randomized Controlled Trial. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pittas AG, Roberts SB. Dietary composition and weight loss: can we individualize dietary prescriptions according to insulin sensitivity or secretion status? Nutr Rev. 2006;64 (10 Pt 1):435–448. doi: 10.1301/nr.2006.oct.435-448. [DOI] [PubMed] [Google Scholar]
- 58.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27 (6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 59.Bandura A. Social Foundations of Thought and Action: a social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 60.Bandura A. Social Learning Theory. Englewood Cliffs, NJ: Prentice Hall; 1977. [Google Scholar]
- 61.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. Jama. 2007;298(3):289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldstein L, Messer K. Optimal plug in estimators for nonparametric functional estimation. Annals of Statistics. 1992;20:1306–1328. [Google Scholar]
- 63.Prentice RL. On the design of synthetic case-control studies. Biometrics. 1986;42:301–310. [PubMed] [Google Scholar]
- 64.Simon RJ. Planning and inference, development and validation of biomarker classifiers in treatment selection. J Stat Plan Inference. 2008;138:308–320. doi: 10.1016/j.jspi.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alonzo TA, Siegmund KD. Statistical methods for evaluating DNA methylation as a marker for early detection or prognosis. Dis Markers. 2007;23 (1–2):113–120. doi: 10.1155/2007/308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Breiman L. Random Forest. Machine Learning. 2001;45:5–32. [Google Scholar]
- 67.Zhang X, Lu X, Shi Q, et al. Recursive SVM feature selection and sample classification for mass-spectrometry and microarray data. BMC Bioinformatics. 2006;7:197. doi: 10.1186/1471-2105-7-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bassett DR., Jr Validity and reliability issues in objective monitoring of physical activity. Res Q Exerc Sport. 2000;71 (2 Suppl):S30–36. [PubMed] [Google Scholar]
- 69.Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res. 2011;186:13–42. doi: 10.1007/978-3-642-04231-7_2. [DOI] [PubMed] [Google Scholar]
- 70.Winzer BM, Whiteman DC, Reeves MM, Paratz JD. Physical activity and cancer prevention: a systematic review of clinical trials. Cancer Causes Control. 2011;22 (6):811–826. doi: 10.1007/s10552-011-9761-4. [DOI] [PubMed] [Google Scholar]
- 71.Ballard-Barbash R, Friedenreich CM, Courneya KS, et al. Physical Activity, Biomarkers, and Disease Outcomes in Cancer Survivors: A Systematic Review. J Natl Cancer Inst. 2012 doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106 (Suppl 3):S5–78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 73.Sears B. Anti-inflammatory diets for obesity and diabetes. J Am Coll Nutr. 2009;28 (Suppl):482S–491S. doi: 10.1080/07315724.2009.10718115. [DOI] [PubMed] [Google Scholar]
- 74.Esposito K, Giugliano D. Diet and inflammation: a link to metabolic and cardiovascular diseases. Eur Heart J. 2006;27 (1):15–20. doi: 10.1093/eurheartj/ehi605. [DOI] [PubMed] [Google Scholar]
- 75.Jungbauer A, Medjakovic S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas. 2012;71 (3):227–239. doi: 10.1016/j.maturitas.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 76.Baliga MS, Haniadka R, Pereira MM, et al. Update on the chemopreventive effects of ginger and its phytochemicals. Crit Rev Food Sci Nutr. 2011;51 (6):499–523. doi: 10.1080/10408391003698669. [DOI] [PubMed] [Google Scholar]
- 77.Shils ME, Olson JA, Shike M. Modern Nutrition in Health and Disease. 8. Vol. 2. Philadelphia: 1994. [Google Scholar]
- 78.Brown JK, Byers T, Doyle C, et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. Ca-Cancer J Clin. 2003;53 (5):268–291. doi: 10.3322/canjclin.53.5.268. [DOI] [PubMed] [Google Scholar]


