Abstract
Advances in regenerative medicine have improved the potential of using cellular therapy for treating several diseases. However, the effectiveness of new cellular therapies is largely limited by low cell engraftment and inadequate localization. To improve upon these limitations, we developed a novel delivery mechanism using cell-seeded biological sutures. Herein, we demonstrate the ability of cell-seeded biological sutures to efficiently implant human mesenchymal stem cells (hMSCs) to specific regions within the beating heart; a tissue known to have low cell retention and engraftment shortly after delivery. Cell-seeded biological sutures were developed by bundling discrete microthreads extruded from extracellular matrix proteins, attaching a surgical needle to the bundle, and seeding the bundle with hMSCs. Prior to cell preparation, hMSCs were loaded with quantum dot (QD) nanoparticles for cell tracking within the myocardium. Each biological suture contained an average of 5,903 ± 1,966 hMSCs/cm suture length. Delivery efficiency was evaluated by comparing cell-seeded biological suture implantation with intramyocardial cell injections (10,000 hMSCs in 35 µL) into the left ventricle of normal, non-infarcted rat hearts after one hour. Delivery efficiency of hMSCs by biological sutures (63.6 ± 10.6%) was significantly higher than intramyocardial injection (11.8 ± 6.2%; p < 0.05). Cell-tracking analysis indicated suture-delivered hMSCs were found throughout the thickness of the ventricular myocardium; along the entire length of the biological suture track, localizing closely with native myocardium. These results suggest cell-seeded biological sutures can deliver cells to the heart more efficiently than conventional methods, demonstrating an effective delivery method for implanting cells in soft tissue.
Keywords: Mesenchymal stem cells, Stem cell delivery, Biomaterials – biological scaffold, Regenerative medicine, Cell tracking
INTRODUCTION
Over the past decade, a strong focus in regenerative medicine has aimed at developing cell therapy strategies to treat multiple pathologies. However, the effectiveness of any cell therapy is dependent on being able to deliver an appropriate number of cells to a specific, localized region of interest. Current cell delivery methods face two major limitations in that they result in low cell engraftment and lack the capacity for targeted cell delivery.
In the heart, exogenous cellular therapies have the potential to regenerate infarcted myocardium.1–7 Several different cell types have been implanted to show modest improvements in cardiac function (i.e. mesenchymal stem cells, embryonic stem cells, cardiac stem cells, and induced pluripotent stem cells).1,5,8–11 In addition, recent studies suggest that a small percentage of biopsied cardiomyocytes can undergo proliferation,12 with the hope that newly generated myocytes can be re-delivered to the heart. However, if exogenous cells are to be used to regenerate tissue, new delivery techniques must overcome the limitations of current methods to efficiently place these cells in the desired region.
Cell delivery by intramyocardial (IM) injection results in only 11% cell retention in the myocardium one hour after delivery,13 likely due in part to cell death from the shear stress generated in a small diameter needle and extravasation after needle retraction.14 Four hours after intravenous (IV) cell delivery, only 1% of cells are retained in the infarcted myocardium,15 while a majority of delivered cells circulate in the bloodstream and accumulate in other organs (e.g. the lungs).16 Intracoronary (IC) delivery during angioplasty can target a specific region of interest, but only approximately 3% of delivered cells are retained.13 In addition, cell-graft constructs or cell-seeded biomaterials are commonly implanted onto the epicardial surface of the heart, but the cells do not effectively traverse the ventricular wall. As a result, less than 1% of delivered cells reach the endocardium,17 where many clinical infarcts occur.
Herein, we describe a novel method for delivering stem cells to a tissue of interest (the heart), implementing a relatively easy-to-use technique that allows for increased cell retention and targeted delivery. Based on our previous work, we created cell-seeded biological sutures developed from biopolymer microthreads made from extracellular matrix (ECM) proteins.18–20 We show that these biological sutures can be seeded with human mesenchymal stem cells (hMSCs) derived from adult bone marrow, a cell type chosen based on our previous work and their clinical safety.21,22 In addition, we show hMSC-seeded biological sutures can be implanted into the beating rat myocardium, a tissue of interest known to have low engraftment rates. Compared to conventional IM injection, our hMSC-seeded biological sutures have higher cell retention and demonstrate improved efficiency in cell delivery.
MATERIALS AND METHODS
Collagen and Fibrin Microthread Production and Bundling
Collagen microthreads were created as previously described.23 Briefly, type I collagen was extracted from rat tails, and prepared to a concentration of 10mg/ml. Using a syringe pump set to a flow rate of 0.23 mL/min (KD Scientific, New Hope, PA), type I collagen was extruded through 0.86 mm diameter polyethylene tubing (Becton Dickinson, Inc., Franklin, NJ) into fiber formation buffer maintained at 37°C (135 mM NaCl, 30 mM TrizmaBase, and 5 mM NaPO4 dibasic; pH 7.42; Sigma Aldrich, St. Louis, MO). After 24 hours in formation buffer, collagen microthreads were transferred into fiber incubation buffer maintained at 37°C (135 mM NaCl, 30 mM Tris, and 5 mM sodium phosphate dibasic; pH 7.42; Sigma) for another 24 hours. After fiber incubation, collagen microthreads were transferred into 37°C distilled water for another 24 hours. Lastly, the collagen microthreads were removed from the water bath, air dried, and stored in a room temperature desiccator before use.
Fibrin microthreads were created as previously described.19 Briefly, a 1 mL syringe was filled with thrombin (150µL of an 8 U/200µL thrombin solution, diluted in 850µL of calcium chloride; Sigma Aldrich, St. Louis, MO) and another 1 mL syringe was filled with fibrinogen (70 mg/mL; Sigma Aldrich, St. Louis, MO), both derived from bovine plasma. A blending applicator tip was used to conjoin the two 1 mL syringes, which were then placed in a syringe pump set to a flow rate of 0.23 mL/min. The combined thrombin/fibrinogen solution was extruded through 0.38 mm polyethylene tubing (Becton Dickinson) into a 10 mM HEPES bath (pH 7.4, room temperature). After 15 minutes of formation, fibrin microthreads were removed from the bath, air-dried, and stored in a desiccator at room temperature before use.
To create bundles, 12 microthreads were arranged adjacently onto an opaque background (all microthreads ~8 cm long). One end of the thread-grouping was taped down, and the free end was lifted at an angle. Droplets of distilled water were placed onto the free end of the grouping to hydrate the length. During hydration, the free end was slightly twisted to form a single entwined bundle of 12 microthreads, approximately 8 cm long. Bundles comprised of 12 microthreads consisted of either 12-fibrin microthreads or a combination of 8-fibrin/4-collagen microthreads.
Quantum-Dot Loading of hMSCs
Prior to seeding microthread sutures, human mesenchymal stem cells (hMSCs; Lonza Inc, Walkersville, MD) were passively loaded with quantum dot nanoparticles.24 Passage 4–8 hMSCs were grown in monolayers until they reached 70–80% confluence, then treated with growth medium (MSCGM; Lonza Inc) supplemented with 8.2 nM QDot 655 ITK™ carboxyl quantum dots (Invitrogen, Carlsbad, CA). Treated hMSCs were incubated for 24 hours in an incubator set at 37°C, 5% CO2, for quantum dots to be endocytosed by the cells. Quantum dot loading was verified with fluorescent microscopy. After 24 hours, hMSCs were gently washed with sterile phosphate-buffered saline (PBS), and maintained in unsupplemented MSCGM until use.
Suture Formation, Sterilization, and Cell Seeding
Each microthread bundle (consisting of 12 entwined microthreads, ~8 cm long) was cut to 4 cm lengths. Four centimeter bundles were then threaded through the eye of a surgical suture needle (size #20, 3/8” circle, tapered; Securos Surgical, Fiskdale, MA), and the needle was positioned at the middle of the bundle. The two free ends of the microthread bundle were folded onto each other, creating a 2 cm suture of 24 threads in the body of the construct, with 12 threads looping through the contact-point of the needle (Figure 1). The 24-thread suture body was hydrated with distilled water and gently twisted to make an entwined suture.
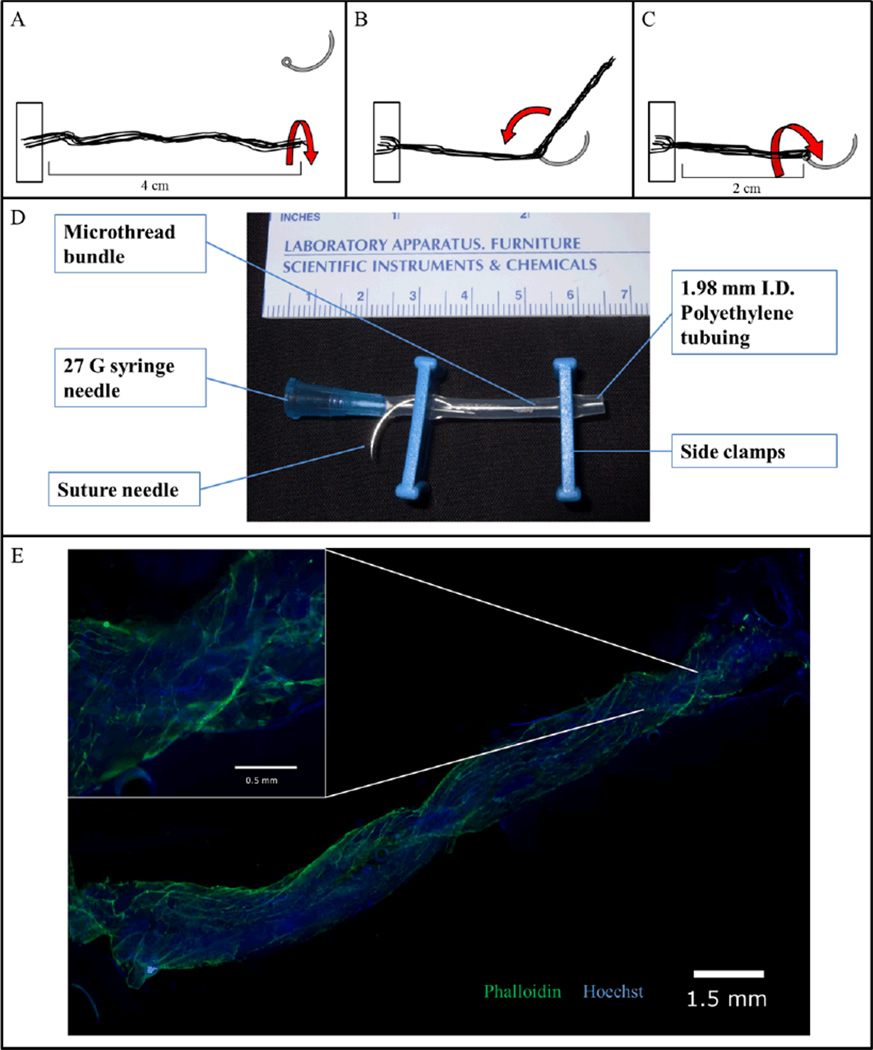
Figure 1. Cell-seeded biological sutures.
(A) Microthreads are anchored at one end and then twisted into a bundle. (B) The bundle is threaded through the eye of a 27 gauge needle and doubled over at the midpoint. (C) The thread is then twisted again to tighten the bundle, forming a biological suture. (D) The biological suture is placed in a bioreactor tube where the cell solution can be added via the needle. The bioreactor tube is then placed in a rotator and incubated for 24 hours. (E) A 2 cm length biological microthread bundle after 24 hours of seeding with hMSCs. (Inset) Nine 5× images merged; Hoechst-dyed nuclei are blue, Phalloidin stained f-actin filaments are green.
Prior to cell seeding, microthread sutures were placed into gas-permeable Silastic™ tubing (1.98 mm ID, Dow Corning, Midland, MI). In the free-end of the suture construct (opposite to the end with the surgical needle), a 27-gauge needle was inserted into the tubing (Becton Dickinson and Co., Franklin Lakes, NJ) to facilitate cell seeding, and clamps were fixed to both ends of the tubing to seal the chamber. Constructs were then ethylene-oxide gas sterilized for a 12 hour cycle before cell seeding.
For cell seeding, 100 µL of a 1×106 cell/mL suspension was drawn into a sterile disposable 1mL syringe (Becton Dickinson). The syringe was attached to the 27-gauge needle of the suture construct, and the 100 µL of cell suspension (consisting of ~100,000 hMSCs) was dispensed into the tube with the suture. The seeded suture constructs were then placed into vented 50 mL conical tubes (2 constructs per conical tube), and the tubes were placed in a slow-speed MACSmix™ rotator (Miltenyi Biotec, Bergisch Gladbach, Germany). The portable rotator was place into a 37°C incubator (5% CO2, atmospheric gas concentrations), and the sutures were seeded for 24 hours.
Delivering hMSCs to the Rat Heart: Biological Suture Implantation and Intramyocardial Injection
All procedures that were performed on animal subjects were approved and overseen by Worcester Polytechnic Institute’s Institutional Animal Care and Use Committee (IACUC). Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used for both suture implantation and intramyocardial (IM) injection procedures. Rats were anesthetized with an intraperotineal injection of a mixture of Ketamine (100 mg/kg, from a 100 mg/mL stock; Manufactured by Bioniche Teoranta, Galway, Ireland; Distributed by Vedco Inc, St. Joseph, MO) and Xylazine (10 mg/kg, from a 100 mg/mL stock; Manufactured by IVX Animal Health Corporation, St. Joseph, MO; distributed by Phoenix Pharmaceuticals, St. Joseph, MO), intubated with a 16 gauge angiocatheter, and then maintained under a mixture of isoflurane (Webster Veterinary, Devens, MA) and oxygen for the duration of the procedure. Heart rates were maintained between 200–250 beats per minute. Hearts were exposed by making a left-side thoracotomy between the 4th and 5th intercostal space to access the thoracic cavity, then resecting the pericardium.
Stem cell-seeded biological sutures were passed through the myocardial wall of the left ventricle, from the base of the heart towards the apex of the heart. The biological sutures were then cut from the suture needle, and excess suture was cut and removed from both the entry and exit points.
IM injections were performed by delivering a bolus of hMSCs in solution into the left ventricle of the rat heart. Cell suspensions were prepared at concentrations of 300,000 cells/mL. Thirty-five µL of cell suspension (~10,000 hMSCs) was drawn into a 100 µL syringe (Hamilton Company, Reno, NV). A 27-gauge needle was then attached (Becton Dickinson and Co., Franklin Lakes, NJ) to the syringe, and the 35 µL bolus was injected into the left ventricular wall of the rat heart.
After cell delivery by either suture implantation (n=5) or IM injection (n=5), rats were maintained under anesthesia for one hour. At the end of procedure, rats were euthanized (0.25 mL/kg, Beuthanasia-D Special, Schering-Plough Animal Health, Kenilworh, NJ) and hearts were excised for histological analysis.
Histology
Hearts were removed from the thoracic cavity and cut in half between the base and apex, bisecting the delivered microthread or IM injection site. Both halves of the heart were rinsed with PBS to remove excess blood, and then fixed in 4% paraformaldehyde for 24 hours. After 24 hour fixation, the heart halves were placed in 30% sucrose until they were processed for sectioning. For sectioning, each half of the rat heart was embedded in O.C.T. Compound (Sakura Finetek USA Inc., Torrance, CA). Eight-micrometer sections were cut from the embedded hearts using a Leica CM3050 cryostat (Leica Microsystems, Bannockburn, IL), and sections were collected on positively-charged glass slides (3 sections/slide; VWR® Micro Slides; VWR International, West Chester, PA). The slides were then stored in a freezer (≤ −20°C) to preserve the heart sections.
For histological analysis, the first slide from either heart half (first 3 sections taken proximal to the bisection) was stained with Hoechst dye (1:6000 in PBS, 5 min; Invitrogen, Carlsbad, CA), to counterstain nuclei and co-localize the quantum dot signal within delivered hMSCs. For analysis through the heart, the next sixty heart sections were skipped (20 slides; approximately 480 micrometers) and the same staining procedure was continued, until QD loaded hMSCs were no longer detected in the heart sections. Sections were also stained for alpha-actinin in order to mark the native myocardium and allow visualization of the engraftment of hMSCs into the heart. Paraformaldehyde-fixed 8 µm-thick heart cross sections were first washed with PBS and then treated with 0.25% Triton X-100 for 10 minutes. After three 5 minute PBS washes, sections were blocked with 5% normal rabbit serum for 30 minutes at room temperature. The sections were then treated overnight with anti-α-actinin antibody (1:100 in 1.5% Normal Rabbit Serum, Host: mouse; Sigma Aldrich, St. Louis, MO) at 4°C. After three, 5 minute PBS washes, sections were incubated with an Alexa Fluor 488-conjugated secondary antibody (1:400 in 1.5% Normal Rabbit Serum, Host: rabbit; Invitrogen, Carlsbad, CA). The sections were again washed 3 times with PBS and then counterstained with Hoechst and coverslipped with Cytoseal-60 (Richard Allan Scientific).
Heart sections stained with Hoechst dye were first washed with PBS to equilibrate sections to room temperature. Sections were treated with Hoechst (1:6000 in PBS, for 5 min; Cambrex Bio Science, Charles City, IO), washed in PBS (3× for 5 min), and then coverslipped using CytoSeal-60 mounting media (Thermo Scientific, Fremont, CA).
Counting and Calculating Cells Delivered to the Heart
Staining the heart sections allowed the visualization of the cell nuclei and microthread implantation, as well as co-localization of nuclei with QD-signal and approximation of QD-signal with host myocardium. Co-localization of QD-signal with Hoechst-stained nuclei confirmed that QD-loaded hMSCs were being delivered to the heart, and retained after one hour in vivo. QD-loaded hMSCs were manually counted from Hoescht-stained sections, until a section was reached in which QD-signal could not be detected. To calculate the total number of cells delivered to the heart, a linear relationship between the counted slides was assumed. Once the total number of cells delivered to the heart was determined, the delivery efficiency was calculated by dividing the total number of delivered cells by the normalized number of cells on the implanted suture. To determine the number of cells on the suture, delivered suture length was divided by the length of the total suture construct (2 cm), which was then multiplied by the average number of hMSCs that could be seeded onto the total suture construct over 24 hours. The delivery efficiency calculation is shown as follows:
The implant length was the distance between where the implanted biological microthread entered and exited the heart, as determined from the Hoescht-stained sections.
The delivery efficiency of each intramyocardial injection was calculated as follows:
Statistical Analysis
Statistical differences for engraftment were determined using unpaired, two-tailed, unequal variance t-tests for two groups. Statistical differences for cell delivery distribution to different locations in the heart were determined using a one-way ANOVA on ranks, with a Tukey post-hoc analysis. Statistical differences for comparing cell delivery methods at defined regions of the heart were determined using unpaired, two-tailed, unequal variance t-tests for two groups. All data was reported as mean ± SEM (standard error of the mean), and statistical differences were determined to be significant with a p-value < 0.05.
RESULTS
Implantable biological sutures can be made with biopolymer microthreads seeded with hMSCs
Previous structural studies indicated that the mechanical properties of single fibrin or collagen microthreads,18,19 or small bundles of 4 microthreads,20 were insufficient to withstand the force needed for surgical implantation into rat myocardium. Therefore, discrete fibrin and collagen microthreads were made as previously described18,19 and then 12 microthreads (either 12-fibrin or combinations with 8-fibrin/4-collagen) were twisted together into a fiber bundle to form a composite suture (Figure 1a). Each microthread bundle was threaded through the eye of a suture needle (size #20, 3/8”, ½-circle tapered; Figure 1b), and then folded back onto itself so that the body of the suture became a total of 24 microthreads with 12 microthreads looping through the contact-point of the needle (Figure 1c). The processes of bundling and needle attachment to produce a biological suture effectively increased the total surface area available for cell attachment, while increasing the tensile strength of the suture, allowing for successful implantation.
For cell attachment, biological sutures were placed into gas-permeable Silastic™ tubing, which was seeded with 100,000 hMSCs (100 µL of a 1×106 cell/mL suspension) and sealed with side clamps (Figure 1D). Seeded biological sutures were placed in a slow-speed rotator, and placed in an incubator for a 24 hour period. Hoechst and Phalloidin staining revealed that hMSCs attached to the biological suture, with uniform distribution along the entire length and around the circumference (Figure 1E). By digesting cell-seeded sutures with trypsin and counting detached cells with a hemocytometer, it was determined that 11,806 ± 3,932 hMSCs were attached to each biological suture (n=20; Average suture length of 2 cm).
Human MSC-seeded biological sutures can be delivered to the beating rat heart
To determine whether biological sutures could deliver cells to the normal non-infarcted heart in vivo, we implanted cell-seeded sutures into normal Sprague-Dawley rat hearts. Briefly, hearts were exposed and then stem cell-seeded biological sutures were implanted through the left ventricular myocardial wall (base to apex). One hour after suture implantation, animals were euthanized and hearts were excised for histological analysis. Masson’s Trichrome staining of serial sections allowed observation of the location and depth of fibrin/collagen microthread suture bundles within the myocardium (Figure 2). Cell-seeded biological sutures entered the heart through the epicardial surface at the base of the left ventricle, traversed the mid-myocardium to the endocardium, and then exited through the epicardial surface near the apex, thereby accessing the entire thickness of the heart (Figure 2).
Figure 2. Cell-seeded biological suture implantation tracking.
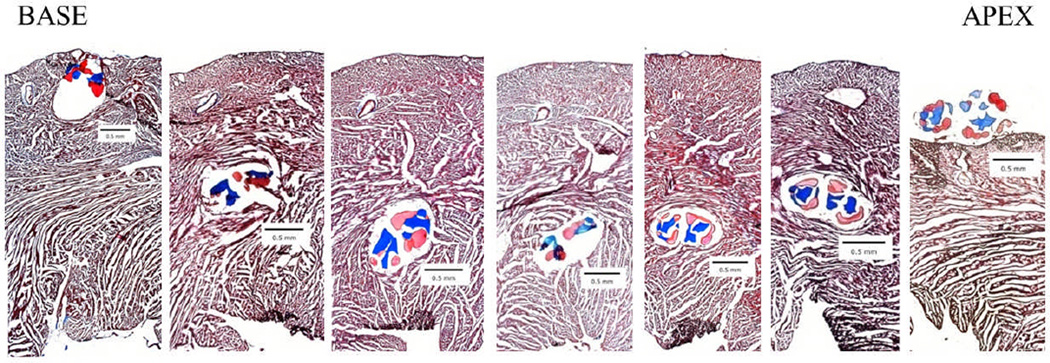
hMSC-seeded biological sutures were implanted from the base to the apex of the left ventricle. These images were used to determine the distance of the suture from the innermost section of the endocardium. Each section is 480 µm apart. (5× magnification, Masson’s Trichrome staining, collagen microthreads are blue, fibrin microthreads are pink).
Biological sutures increase cell delivery efficiency compared to intramyocardial injection
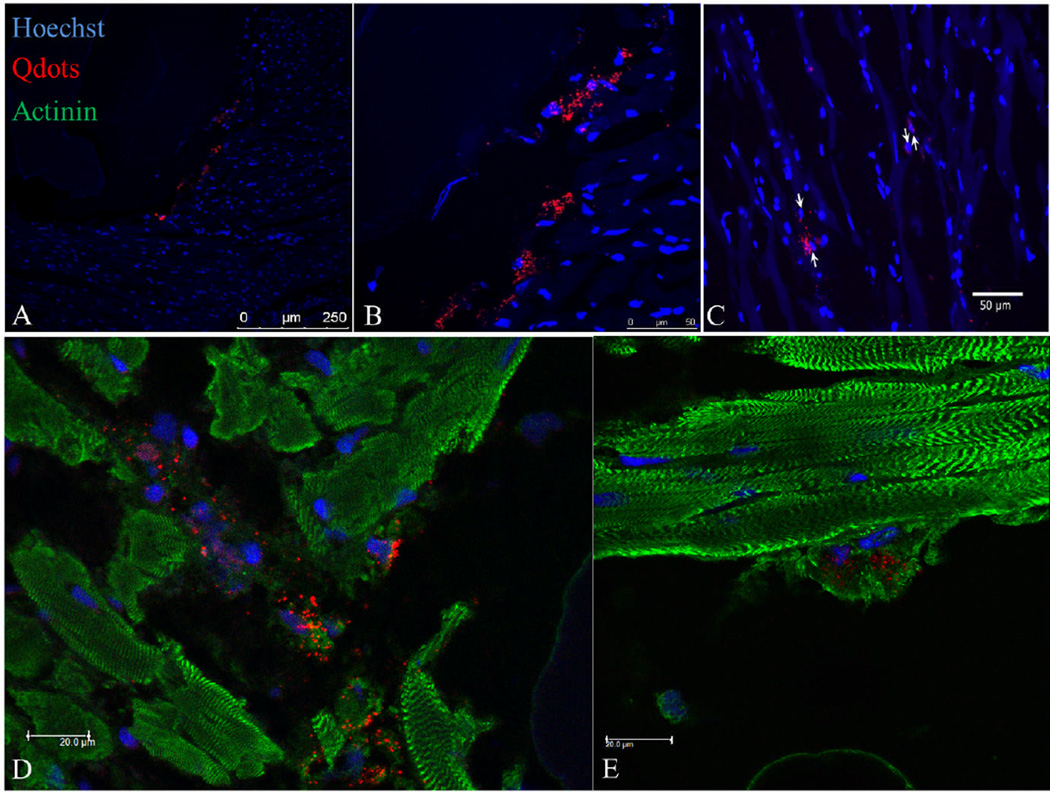
Cell delivery with biological sutures was compared to intramyocardial injection, which is the clinical delivery method that shows the best engraftment efficiency. In order to track delivered cells, hMSCs were passively loaded with quantum dot (QD) nanoparticles prior to implantation, as previously described.24 Cell-seeded sutures were prepared with QD-loaded hMSCs and implanted for an hour, as described above. For intramyocardial injections, 35 µL (300,000 cells per mL) of cell suspension was injected into the left ventricular myocardial wall (midway between the base and apex). Rats were sustained for 1 hour before being euthanized. Cell delivery was confirmed by the observation of QD signal within histological sections (Figure 3). For cell-seeded biological sutures, hMSCs were found along the entire length of the suture track. Qualitative observations indicated that QD signal was present in all myocardial layers localized closely to the biological suture, although a large mass of hMSCs was found at the initial insertion point of the suture possibly due to cell shearing upon entry. Cells delivered on the microthread sutures and by IM injection engrafted within the myocardium, with some of the hMSCs showing close proximity to the surrounding cardiomyocytes (Figure 3).
Figure 3. Examples of quantum dot loaded hMSCs delivered to the rat heart.
(A) Quantum dot loaded hMSCs injected into rat heart. (B) hMSCs delivered in close proximity to one of the biological microthreads that is part of the biological suture (20×). (C) hMSCs delivered in close proximity to biological microthread bundle (63×). (D) Quantum dot positive cells engrafted in the myocardium surrounding an implanted microthread. (E) Quantum dot positive cell in close proximity with a cardiac myofibers.
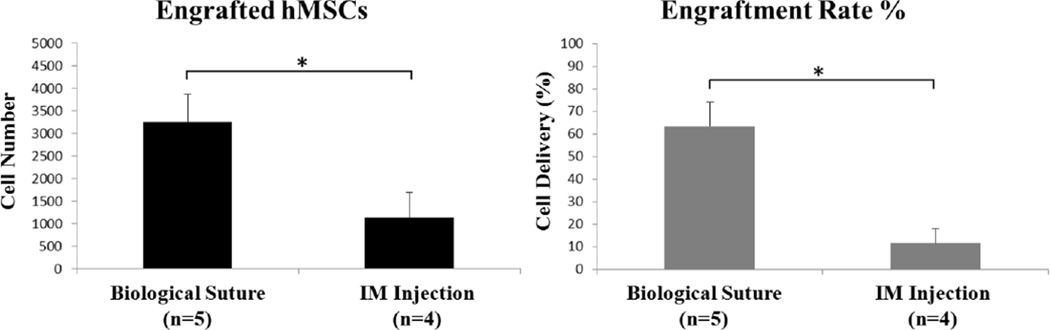
To compare the efficiency of cell delivery by microthread sutures with direct IM injection, cells were quantified by counting Hoechst-stained nuclei that co-localized with QD signal. QD-loaded hMSCs implanted by IM injection were observed within the myocardium in 4 of the 5 hearts receiving injections, with the cells localized only at the injection site. Quantitatively, the average number of cells delivered by IM injection was 1,146 ± 547 (n=4; the heart without QD signal was not included in analysis), which was significantly lower than the average number of cells delivered by biological suture implantation (3,527 ± 624; p<0.05; Figure 4A). Based on the number of cells delivered and the number of cells counted post-procedure, the cell delivery efficiency of cell-seeded biological sutures (63.6 ± 10.6%) was significantly higher than IM injection (11.8 ± 6.2%; p<0.05; Figure 4B).
Figure 4. Biological sutures result in better cell engraftment than intramyocardial injection.
(A) Total number of hMSCs engrafted in rat hearts, determined by counting QD-loaded cells in histological sections. (B) hMSC engraftment rate (%) was calculated based on the total number of cells engrafted in each heart and the starting cell number delivered. Asterisks indicate significance with a p-value less than 0.05.
Biological sutures increase delivery distribution of implanted hMSCs
To assess the coverage area of delivered hMSCs, additional quantitative measurements were taken from histological sections of hearts from both the cell-seeded biological suture group and the IM injection group. The length of heart tissue throughout which QD-loaded hMSCs were found (from base to apex) was 0.55 ± 0.06 cm for the biological suture group and 0.33 ± 0.13 cm for the IM injection group (p<0.05).
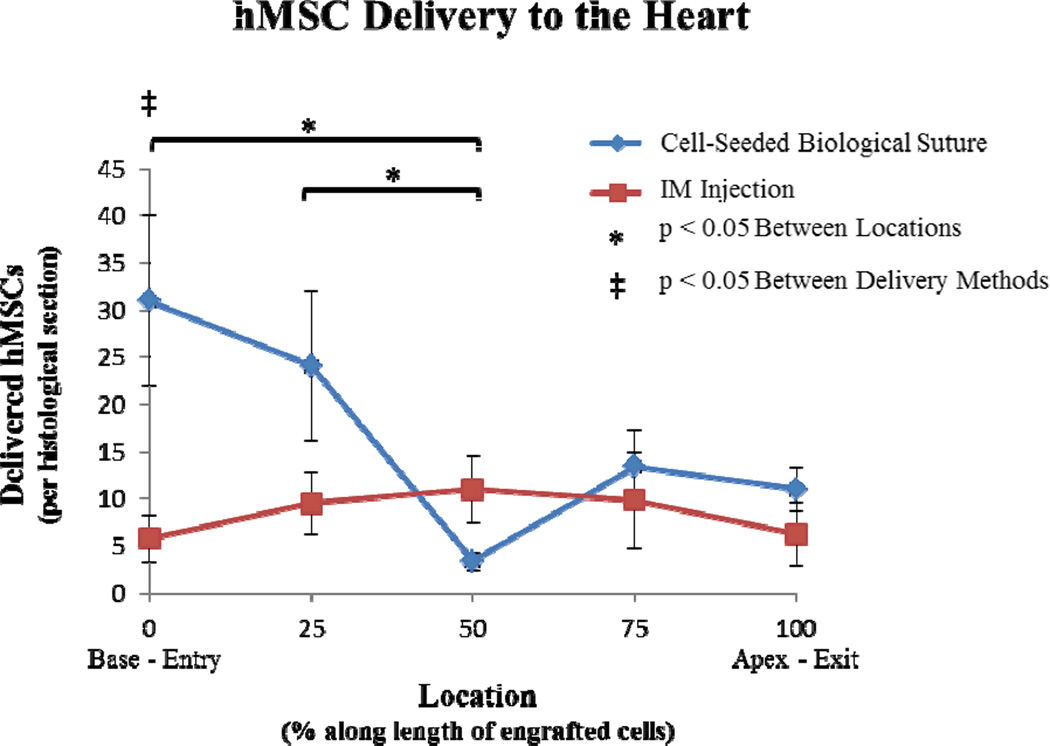
The cell distribution of QD-loaded hMSCs along the determined delivery length (from base to apex) was calculated by averaging cell counts per slide within established segments of the length. For both treatment groups, serial sections were analyzed and areas of cell delivery were segmented into 5 regions (0, 25, 50, 75, and 100%). For cell-seeded sutures, the 0% region denoted the suture entry site (at the base of the heart) and the 100% region denoted the suture exit site (at the apex of the heart). For IM injected cells, the 0% region was determined by identification of the first QD-loaded cells located proximal to the base of the heart and the 100% region was determined by the last QD-loaded cells located proximal to the apex of the heart. For the hMSC-seeded biological sutures, a high percentage of the cells were delivered close to the entry point (Figure 5, blue data points). Statistical analysis of the cell distributions of the 5 regions for cell-seeded sutures showed that both the 0% and 25% regions contained significantly more cells than the 50% region (p<0.05). At 50% of the delivery length, there was a reduction in the cell delivery percentage in the cell-seeded biological suture group, which we attributed to complete transmural suture entry into the left ventricular cavity in two of the five hearts. However, re-entry of the cell-seeded biological sutures into the endocardium showed delivery of cells in the 75% and 100% regions, as well. In the IM injected hearts, a majority of the cells were delivered at the 50% point; which coincided with the location where the syringe needle injected the hMSC suspension into the heart wall (Figure 5, red data points). Fewer cells were delivered near the apex and the base of the heart (away from the 50% point).
Figure 5. Distribution of delivered hMSCs.
Distributions of delivered hMSCs were compared between cell-seeded suture implantation and IM injection. The cell delivery areas were divided into 5 regions (0, 25, 50, 75, and 100%). For the cell-seeded sutures, 0% corresponded with the entry site and 100% corresponded with the exit site. For IM injections, 0% denoted the first QD-loaded cells identified at the base of the heart and 100% denoted the last QD-loaded cells found at the apex of the heart. Cells were found over 0.55 ± 0.06 cm for the biological suture group and 0.33 ± 0.13 cm for the IM injection group. For cell-seeded sutures, both the 0% and 25% regions contained significantly more cells than the 50% region. Comparing delivery methods at different locations, the cell-seeded suture modality delivered significantly more cells to the 0% entry-site region compared to IM injection.
DISCUSSION
Current cell delivery methods often result in low engraftment rates and lack the ability to localize cell delivery to specific regions within the target tissue. Intravenous and intra-arterial delivery methods offer minimally-invasive techniques, but require cells to migrate through the bloodstream and hone to the infarction site, which contributes to the problem of low engraftment.13,15,16 Intra-muscular injection is an option, but this is a more invasive procedure. In delivering cells to the heart, injection depth, cell retention, and cell distribution remain uncontrolled with intra-muscular injection.13 Without targeted delivery, the distribution and quantity of cells implanted in and around the region of interest is difficult to control. Cell-seeded biological sutures offer a novel method to overcome these limitations, by delivering cells on a stable and provisional biopolymer matrix, while utilizing a needle-based delivery mechanism that is commonly used in surgery.
Previously, we demonstrated that hMSCs seeded on fibrin microthreads maintain their pluripotency.20 Herein, biological sutures were developed by bundling our novel microthreads made of ECM proteins (i.e. fibrin and collagen), which provide a sturdy biological construct for cell attachment and delivery. While we showed that we can seed biological sutures with hMSCs, it is likely that other cell types can be seeded onto the sutures and delivered to other tissues in a similar fashion. The attachment of a surgical needle defines the “suture” modality of our constructs, which we chose for its surgical simplicity and targeting ability. This modality allows for controlled, targeted delivery of cells within the suture track.
Seeding hMSCs onto Biological Sutures
By bundling biological microthreads into sutures (Figure 1), it is possible to increase the number of cells that can be seeded onto microthreads. Previously, we showed that bundles of four fibrin microthreads can be seeded with an average of 3,344 hMSCs/bundle after 1 day of seeding.20 In the previous study, we also demonstrated that increasing the incubation time to 3 days correlated with an increased quantity of cells. However, increased seeding times of 5 or more days caused the 4-fibrin microthread bundles to lose mechanical integrity, most likely due to cell-mediated degradation of the matrix. For implantation into soft tissue, the material integrity of the biological sutures has to be strong enough to prevent them from breaking during delivery. Therefore, we opted to implant biological sutures after only 24 hours of cell seeding. We increased the number of microthreads in our sutures and increased the quantity of cells seeded on microthreads by using a rotary bioreactor. Suture bundles consisted of either 12-fibrin microthreads or a combination of 8-fibrin/4-collagen microthreads, which folded over a surgical needle for sutures of 24 total microthreads. We found that by increasing the number of microthreads, we increased the available surface area of the biological construct to allow for increased cell seeding. With our new biological sutures, we improved cell seeding to an average of 11,806 ± 3,932 hMSCs/suture within a 24 hour period, which represents a greater than 3-fold improvement over our previous cell seeding techniques.
The size of an individual microthread is approximately 80 microns (hydrated). When they are bound together, the diameter of the bundle of 24 microthreads is approximately 0.64 mm, which is similar in size to a 2-0 or 3-0 suture. As can be seen in Figure 2, this diameter suture is approximately 20% of the thickness of the left ventricular wall. By varying the diameter and number of microthreads per suture, the diameter of the suture can be altered, also providing the ability to deliver different numbers of cells to a targeted tissue.
Cardiac Applications
Of the millions of Americans living with heart disease, nearly half experience a myocardial infarction (MI).25 Novel surgical and pharmaceutical interventions aiming to treat diseased myocardium fail to address the fundamental need to replace dead cardiomyocytes with new contractile cells in order to restore active cardiac mechanical function.26 Exogenous cell delivery strategies have been explored with the intention of replacing necrotic myocardium with new contractile cells for the purpose of restoring mechanical function. These strategies will likely be more effective if a critical mass of delivered cells can engraft within the tissue; ideally, in a specific region of interest within the infarct site. Current methods for delivering cells to the heart, such as IM injection, result in low cell engraftment.13 To overcome poor engraftment rates, larger numbers of cells are required to be cultured and delivered, which subsequently increases the resources and costs of treatment. Improving delivery methods will allow for better engraftment, so that a greater percentage of the delivered cells can be retained to provide a therapeutic benefit.
hMSC delivery efficiency and distribution
To assess delivery efficiency, cell-seeded biological sutures were compared to cells implanted by IM injection. Suspensions of 10,000 hMSCs were delivered by IM injection, as a comparable number of total cells that we found could be seeded on the biological sutures. As stated in the previous section, we found an average of 11,806 ± 3,932 hMSCs could attach along the 2 cm suture length. However, only 0.63 cm of suture length was actually delivered to the heart upon implantation. Therefore, the amount of cells delivered to the heart by biological suture was less than the number of hMSCs that could be seeded, and consequently was less than the 10,000 hMSCs delivered by IM injection. However, comparisons could be made by normalizing the number of suture-implanted cells with the implanted suture length. Even by implanting a shorter suture length into the myocardium, the biological suture delivery showed better than 3-fold engraftment of hMSCs compared to IM injection, for an engraftment efficiency of 63.6%. We also observed that hMSC-seeded microthreads traversed the entire thickness of the ventricular wall and penetrated into the ventricular cavity in two hearts. The cells implanted to the ventricular cavity were not included in the quantified number of cells delivered in these hearts, thereby further reducing the number of hMSCs delivered by biological sutures.
We showed that QD-loaded hMSCs were distributed along the length of the suture track, which can be targeted to pass through the layers of the myocardium for different depths and lengths. In addition, the implanted hMSCs localize closely with native cardiomyocytes. The improved delivery method using biological sutures is likely to be applicable for multiple cell types. In addition, it is likely that cell-seeded biological sutures can be used to deliver cells for other regenerative medicine applications beyond cardiac cell therapy. Taken together, our work provides evidence to suggest that biological sutures can improve the efficiency of cell delivery.
As a proof of concept, we chose to deliver hMSC-seeded biological sutures to normal rat hearts for one hour. While the endpoint of this study is relatively short, we chose the one-hour time point post cell delivery in order to be consistent with a previous study that evaluated IM injection, IC injection, and interstitial retrograde coronary venous cell delivery methods.13 Our results for IM engraftment efficiency (11.8 ± 6.2%) were consistent with the IM engraftment efficiency reported in this previous study (11 ± 3%).13 In addition, we believed the one-hour endpoint was long enough for non-engrafted cells to be washed away by extravasation or perfusion, but short enough to eliminate confounding factors including cell proliferation or rejection. The results presented here provide a basis for longer-term studies on cell retention, cell viability, inflammatory response to the biological sutures, and immunogenicity of the implanted cells. Our study shows that biological sutures can more efficiently deliver hMSCs to the heart, an organ that is known to have poor cell engraftment and retention. Future long term studies will address the delivery of hMSC-seeded biological sutures to normal and infarcted hearts, to assess cell engraftment, cell viability, therapeutic benefits of delivered hMSCs, including improvements in cardiac function.
Poor engraftment efficiencies resulting from current delivery methods have confounded the examination of how the implanted cells contribute to restoring physiological function to the tissue of interest. For example, in the heart it remains unclear how many new cells must be delivered in order to generate a sustainable improvement in heart function. As few as 3,000–12,000 cells may be needed to improve function in the rat heart;20 an amount easily achievable with a single cell-seeded biological suture. With increased engraftment efficiency of cells to a targeted area, it may be possible to improve regional analysis, to understand how cell number may affect mechanical function, electrophysiology, or cardioprotection. Future studies will determine whether we can deliver a similar number of cells to an infarcted heart, and whether the delivered cells can improve cardiac mechanical function.
With respect to using biological sutures to deliver stem cells to other tissues and organs, the suture modality offers a wide range of applications that may be more practical than current cell delivery methods. Targeted delivery of cells to a disease site may help to isolate or mitigate the affliction. Using cell-seeded biological sutures for wound closures may improve the healing time, reduce the inflammatory response, and reduce scar formation. The provisional matrix proteins used in the biological suture may provide additional structural integrity to cells delivered for soft tissue repair. In addition, biological sutures may also provide directionality to implanted cells that align parallel to the suture track, which may be important in some applications (e.g. skeletal muscle, ligament, or nerve repair).27
In order to develop this technique for delivering cells in the hearts of large animals or patients, several aspects should be considered. In this study, we successfully delivered an average of 3,527 ± 624 hMSCs to rat hearts. However, larger animals and other tissues may necessitate significantly more cells. Increasing cell delivery with biological sutures could be achieved by either optimizing the cell-seeding process (e.g. bundle size, seeding time, seeding concentration), or by increasing the number of biological sutures that are implanted. In addition, biological sutures can be customized for different lengths and gauges depending on the application. Due to the inefficiencies in current delivery methods, it remains unclear how many cells are needed to achieve a “therapeutic dose”. Recent studies report improved cardiac function and decreased infarct size, with only 1–11% delivery efficiencies from initial cell numbers ranging from 106 – 107 in pigs.13,28 This suggests that a “therapeutic dose” for infarcted hearts may be on the order of between 10,000 – 1,000,000 engrafted cells. Based on our findings in the rat heart, a therapeutic dose may only require as little as 3 or 4 cell-seeded biological sutures to achieve improved cardiac function in a pig. More realistically, biological sutures may need to be modified using the parameters mentioned above, in order to achieve engraftment of close to 1 million cells.
CONCLUSION
Biological sutures were developed into a novel cell delivery vehicle that can be implanted using a simple surgical technique. To demonstrate the effectiveness of this novel cell delivery method, hMSC-seeded biological sutures were successfully driven through the heart wall, and hMSCs were engrafted into the surrounding myocardium. Cell-seeded biological sutures delivered a higher percentage of hMSCs to the heart compared to IM injection; and the percentage of engrafted cells by biological sutures was higher than what has been reported for other conventional delivery techniques. The higher rate of engraftment associated with the biological sutures indicates that fewer hMSCs were lost during cell delivery. The use of a surgical needle permits targeting a specific region of injured tissue. By engrafting more cells to site-specific regions, this system can improve the effectiveness of cell therapy for a variety of applications and tissues.
Acknowledgments
Funding: This work is supported in part by National Institutes of Health (HL093639 to GRG) and American Heart Association (0635013N to GRG).
Footnotes
Author Contributions: GRG conceived and supervised the study. GDP, MWR, and GRG designed the research plan. JPG, MF, EJB, ZT, and GRG performed the experiments and analyzed the data. JPG, MF, EJB, ZT, GDP, MWR, and GRG wrote the manuscript.
REFERENCES
- 1.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, Gaussin V, Homsy C, Bartunek J, Terzic A. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010;56(9):721–734. doi: 10.1016/j.jacc.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93(1):32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 4.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108(3):407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118(5):507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 6.Kreutziger KL, Murry CE. Engineered human cardiac tissue. Pediatr Cardiol. 2011;32(3):334–341. doi: 10.1007/s00246-011-9888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 9.Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, Grünwald F, Aicher A, Urbich C, Martin H, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106(24):3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 10.Potapova IA, Doronin SV, Kelly DJ, Rosen AB, Schuldt AJT, Lu ZJ, Kochupura PV, Robinson RB, Rosen MR, Brink PR, et al. Enhanced recovery of mechanical function in the canine heart by seeding an extracellular matrix patch with mesenchymal stem cells committed to a cardiac lineage. American Journal of Physiology-Heart and Circulatory Physiology. 2008;295(6):H2257–H2263. doi: 10.1152/ajpheart.00219.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 12.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 13.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112(9 Suppl):I150–I156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 14.Dow J, Simkhovich BZ, Kedes L, Kloner RA. Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc Res. 2005;67(2):301–307. doi: 10.1016/j.cardiores.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 16.Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112(10):1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson D, Liu H, Fan TH, Nerem R, Dudley SC., Jr A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25(9):2350–2357. doi: 10.1634/stemcells.2007-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornwell KG, Lei P, Andreadis ST, Pins GD. Crosslinking of discrete self-assembled collagen threads: Effects on mechanical strength and cell-matrix interactions. J Biomed Mater Res A. 2007;80(2):362–371. doi: 10.1002/jbm.a.30893. [DOI] [PubMed] [Google Scholar]
- 19.Cornwell KG, Pins GD. Discrete crosslinked fibrin microthread scaffolds for tissue regeneration. J Biomed Mater Res A. 2007;82(1):104–112. doi: 10.1002/jbm.a.31057. [DOI] [PubMed] [Google Scholar]
- 20.Proulx MK, Carey SP, Ditroia LM, Jones CM, Fakharzadeh M, Guyette JP, Clement AL, Orr RG, Rolle MW, Pins GD, et al. Fibrin microthreads support mesenchymal stem cell growth while maintaining differentiation potential. J Biomed Mater Res A. 2011;96(2):301–312. doi: 10.1002/jbm.a.32978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potapova I, Doronin S, Kelly D, Rosen A, Schuldt A, Lu J, Guo Y, Raptis N, Towner A, Robinson R, et al. Functional Regeneration of the Canine Ventricle Using Adult Human Mesenchymal Stem Cells Committed In Vitro to a Cardiac Lineage. Circ Res. 2006;99(5):E19. [Google Scholar]
- 22.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornwell KG, Downing BR, Pins GD. Characterizing fibroblast migration on discrete collagen threads for applications in tissue regeneration. J Biomed Mater Res A. 2004;71(1):55–62. doi: 10.1002/jbm.a.30132. [DOI] [PubMed] [Google Scholar]
- 24.Rosen AB, Kelly DJ, Schuldt AJ, Lu J, Potapova IA, Doronin SV, Robichaud KJ, Robinson RB, Rosen MR, Brink PR, et al. Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells. 2007;25(8):2128–2138. doi: 10.1634/stemcells.2006-0722. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 26.Gaudette GR, Cohen IS. Cardiac regeneration: materials can improve the passive properties of myocardium, but cell therapy must do more. Circulation. 2006;114(24):2575–2577. doi: 10.1161/CIRCULATIONAHA.106.668707. [DOI] [PubMed] [Google Scholar]
- 27.Page RL, Malcuit C, Vilner L, Vojtic I, Shaw S, Hedblom E, Hu J, Pins GD, Rolle MW, Dominko T. Restoration of skeletal muscle defects with adult human cells delivered on fibrin microthreads. Tissue Eng Part A. 2011;17(21–22):2629–2640. doi: 10.1089/ten.TEA.2011.0024. [DOI] [PubMed] [Google Scholar]
- 28.Wolf D, Reinhard A, Seckinger A, Katus HA, Kuecherer H, Hansen A. Dose-dependent effects of intravenous allogeneic mesenchymal stem cells in the infarcted porcine heart. Stem Cells Dev. 2009;18(2):321–329. doi: 10.1089/scd.2008.0019. [DOI] [PubMed] [Google Scholar]