Abstract
Advanced free energy perturbation molecular dynamics (FEP/MD) simulation methods are available to accurately calculate absolute binding free energies of protein-ligand complexes. However, these methods rely on several sophisticated command scripts implementing various biasing energy restraints to enhance the convergence of the FEP/MD calculations, which must all be handled properly to yield correct results. Here, we present a user-friendly web interface, CHARMM-GUI Ligand Binder (http://www.charmm-gui.org/input/gbinding), to provide standardized CHARMM input files for calculations of absolute binding free energies using the FEP/MD simulations. A number of features are implemented to conveniently setup the FEP/MD simulations in highly customizable manners, thereby permitting an accelerated throughput of this important class of computations while decreasing the possibility of human errors. The interface and a series of input files generated by the interface are tested with illustrative calculations of absolute binding free energies of three non-polar aromatic ligands to the L99A mutant of T4 lysozyme and three FK506-related ligands to FKBP12. Statistical errors within individual calculations are found to be small (~1 kcal/mol), and the calculated binding free energies generally agree well with the experimental measurements and the previous computational studies (within ~2 kcal/mol). CHARMM-GUI Ligand Binder provides a convenient and reliable way to setup the ligand binding free energy calculations and can be applicable to pharmaceutically important protein-ligand systems.
Keywords: Free Energy Perturbation, Molecular Dynamics, Protein-ligand Interactions, T4 Lysozyme, FKBP12
INTRODUCTION
Molecular recognition of small molecules by specific receptor proteins is one of the most important biological processes. Binding of a small molecule to a target protein is required to change the receptor’s dynamics and/or conformations allosterically, or to interfere with the protein-protein interactions, aiming at controlling the protein’s functions. Specific protein-ligand interactions can also be modulated by other small molecules that have similar physicochemical properties, begging for a quantitative assessment of binding specificity at the molecular level. For this reason, there is great interest in the concept of rational drug design based on atomic structures of receptor proteins or receptor-ligand complexes (reviewed in 1–3).
Arguably, accurate binding free energy calculations could be used to help develop novel drug molecules and improve the potency of existing ones. Considerable efforts have been made to reliably calculate the receptor-ligand association free energy at the atomic level using various computational methods, such as: linear interaction energy (LIE)4; molecular mechanics with Poisson-Boltzmann or generalized Born/surface area (MM-PBSA/GBSA)5, 6; or alchemical free energy calculation (reviewed in 7–9). LIE utilizes the energies calculated from atomic simulations, such as molecular dynamics (MD) or Monte Carlo (MC) simulations, to estimate the relative free energy difference upon changes in van der Waals (vdW) and/or electrostatic contributions from the reference. MM-PBSA/GBSA utilizes the trajectories from MD simulations to capture the dynamical aspect of protein-ligand interactions, but the produced results are sometimes not predictive.10
Alchemical free energy calculations, particularly, free energy perturbation molecular dynamics (FEP/MD) simulations, represent the most rigorous computational approach to calculate receptor-ligand binding free energy. FEP/MD simulations have shown to accurately calculate the binding affinities in various biologically important systems.11–14 The effect from explicit solvent molecules and flexibility of the molecules can be incorporated into the calculation, which is shown to be important in macromolecule-ligand association.15 To improve the robustness and computational efficiency of FEP/MD simulations, Roux and co-workers have developed a staged FEP/MD simulation protocol based on a step-by-step decomposition of the total reversible work through various computational techniques.12, 14 First, the absolute binding free energy is decomposed into several intermediate steps in which the ligand-surrounding environment interactions as well as the orientational, translational, and conformational sampling of the ligand are gradually turned “on” (or “off” depending on the setup). The staged protocol effectively and rigorously breaks down the complete free energy calculation into several independent MD simulations, which can be easily distributed over independent computer nodes. Second, the ligand conformational sampling is explicitly taken into account by umbrella sampling of ligand conformations based on root-mean-squared deviation (RMSD) from the bound state of the ligand. Third, Lennard-Jones (LJ) interactions between the ligand and the surrounding environment are decomposed into repulsive and dispersive interactions using the Weeks-Chandler-Andersen (WCA) separation, which improves the convergence of the calculation. Fourth, to reduce the computational cost and complexity, only a small region of interest near the receptor-ligand binding site is explicitly considered using solvent boundary potential methods.16, 17
The performance of the staged FEP/MD simulation method has been already tested with various biologically important systems and demonstrated that binding free energy can be calculated accurately with errors less than 2 kcal/mol (compared to experimental values).12, 14, 18 However, the setup of the staged FEP/MD simulation protocol is not trivial even for expert researchers due to the many restraint potentials and large number of intermediate windows. In addition, selection of a set of anchoring atoms for orientational/translational restraint potentials could be arbitrary and requires prior knowledge of the ligand system.
In this work, we have generalized the staged FEP/MD simulation protocol12, 14 and implemented CHARMM-GUI Ligand Binder (http://www.charmm-gui.org/input/gbinding), a web interface that provides standardized input files for calculations of absolute binding free energies. In addition, Ligand Binder has a number of features that can help users to setup the FEP/MD simulations with less human intervention. For example, automatic ligand force field generation, symmetric group detection, system size estimation, and anchoring atom selection/validation are implemented to quickly generate reliable FEP/MD simulation systems and inputs. To test the efficacy of the interface, we have calculated absolute binding free energies of three non-polar small aromatic ligands to the L99A mutant of T4 lysozyme and three FK506-related ligands to FKBP12. The calculated binding free energies generally agree well with the experimental measurements and the previous computational studies. Therefore, Ligand Binder provides a convenient and reliable way to setup the ligand binding free energy calculations and can be applicable to pharmaceutically important protein-ligand systems.
METHODS
Theoretical Background
The theoretical framework for calculation of absolute binding free energy used in this work is well formulated in the previous studies12, 14 and is closely related to the double decoupling method.19, 20 According to this framework, a step-by-step FEP/MD simulation protocol with restraining potentials is carried out to break down the complete alchemical reaction path into a number of intermediate physical states (FEP windows). The ordered step-by-step FEP/MD can be briefly summarized as: (1) switch-on the RMSD restraint potential on the ligand conformation in the binding site, (2) switch-on the relative ligand-receptor positional/rotational restraint potentials (in the presence of the RMSD restraint), (3) switch-off the ligand interactions with the binding site environment (in the presence of the RMSD and positional/rotational restraints), (4) switch-off the positional/orientational restraint potentials (this contribution is evaluated analytically), (5) switch-on the ligand interactions with the bulk solution environment (in the presence of the RMSD restraint), (6) switch-off the conformational RMSD restraint potential on the ligand in bulk solution. The various restraining potentials introduced in the successive FEP windows are used to maintain the position and orientation of the ligand around the “pose” adopted in the bound complex. These restraining potentials help enhance configurational sampling and also serve to correctly handle the decoupled ligand states. In the following sections, we will focus on its generalization and implementation in CHARMM-GUI Ligand Binder.
General FEP/MD setup using CHARMM-GUI Ligand Binder
The CHARMM input files generated by Ligand Binder are arranged in six distinct steps for clarity and convenience (Table 1). Ligand Binder produces both inputs and outputs for STEP 1 and STEP 2 for a given protein-ligand complex, but only generates the inputs files of the remaining equilibration and production steps of the FEP/MD simulations because they generally require extensive computational resources. In this section, the role of each step and the corresponding outcomes are discussed in detail. As shown in Table 1, for easy recognition the generated input files have a corresponding step number in their filename followed by the two different tags, “BULK” and “SITE”, to represent the FEP/MD simulation systems in bulk solvent and in the protein binding site, respectively. Hereinafter, for brevity, the simulation systems with protein/ligand complex and the ligand molecule in the bulk solvent are referred to as SITE and BULK, respectively. Various accessory scripts that are compatible with UNIX and portable batch system (PBS) are also provided to perform actual FEP/MD simulations conveniently.
Table 1.
Important input and output files generated by Ligand Binder and during the FEP/MD simulations.
| Filename | Description | ||
|---|---|---|---|
| STEP1 | Input | step1_pdbreader.inp | To read a protein-ligand complex structure |
|
| |||
| Output | step1_pdbreader.pdb | Structure after initialization in CHARMM | |
| @lig/@lig.rtf | Ligand topology file | ||
| @lig/@lig_g.rtf | Ligand topology file (with atom groups) | ||
| @lig/@lig.prm | Ligand parameter file | ||
| @lig/ndihe.str | Symmetric groups found in the ligand if any | ||
|
| |||
| STEP2 | Input | step2.1_site_solvator.inp | To solvate the binding site with a water sphere (SITE) |
| step2.2_bulk_solvator.inp | To solvate the ligand with a water sphere (BULK) | ||
|
| |||
| Output | step2.1_site_solvator.pdb | Solvated simulation system for SITE | |
| step2.2_bulk_solvator.pdb | Solvated simulation system for BULK | ||
|
| |||
| STEP3 | Input | step3.1_gsbp_setup.inp | To setup GSBP simulation system for SITE |
| step3.2_ssbp_setup.inp | To setup SSBP simulation system for BULK | ||
| config.py | Contains several (user-defined) configuration variables | ||
| step3_job.pbs | Example PBS script for batch system | ||
|
| |||
| Output | step3.1_gsbp_setup.pdb | SITE simulation system after GSBP setup | |
| step3.1_gsbp_setup.mij | Calculated multipolar reaction field | ||
| step3.1_gsbp_setup.phix | Calculated external reaction field | ||
| step3.2_ssbp_setup.pdb | BULK simulation system after SSBP setup | ||
|
| |||
| STEP4 | Input | step4.1_site_gcmc.inp | To adjust the number of water molecules in GSBP |
| step4.2_site_equil.inp | To equilibrate SITE system | ||
| step4.3_restraint_setup.inp | To setup anchoring atoms and restraint parameters | ||
| step4.3_restraint_geo.str | To determine anchoring atoms | ||
| step4.3_restraint_geotest.str | To test validity of anchoring atoms | ||
| step4.3_restraint_ref.str | To determine the restraint parameters | ||
| step4.4_bulk_equil.inp | To equilibrate BULK system | ||
| step4_job.pbs | Example PBS script for batch system | ||
|
| |||
| Output | step4.1_site_gcmc.crd | SITE system after adjustment of number of water | |
| step4.2_site_equil.dcd | Trajectory of SITE system equilibration | ||
| step4.2_site_equil.crd | Last snapshot of equilibrated SITE system | ||
| step4.3_restraint_geo.prm | Anchoring atom definition | ||
| step4.3_restraint_geo.pdb | Anchoring atoms (named as DUM) | ||
| step4.3_restraint_ref.prm | Reference values for restraint potential | ||
| step4.3_restraint_consdihe.str | Symmetric group restraints | ||
| step4.3_restraint_ligave.pdb | Average structure of bound ligand during equilibration | ||
| step4.4_bulk_equil.pdb | Last snapshot of equilibrated BULK system | ||
|
| |||
| STEP5 | Input | step5.1_site_fes.inp | FEP/MD input for SITE system (including PMF input) |
| step5.1_site_gconst.str | To setup orientational/translational restraints | ||
| step5.2_bulk_fes.inp | FEP/MD input for BULK system (including PMF input) | ||
| step5_jobmanager.com | Script for submitting batch jobs | ||
| step5_template.pbs | Example PBS script for batch system | ||
|
| |||
| STEP6 | Input | step6.1_wham_fep.inp | WHAM input for FEP/MD simulations |
| step6.1_wham_fep.pbs | Example PBS script for batch system | ||
| step6.2_wham_rmsd.inp | WHAM input for PMF umbrella sampling simulations | ||
| step6.2_wham_rmsd.pbs | Example PBS script for batch system | ||
| step6_wham.com | Script for WHAM calculation | ||
| step6_table.py | Example script to tabulate the binding free energy | ||
STEP 1: Reading of a protein-ligand complex into CHARMM
In this step, the web interface generates inputs to read a protein-ligand complex into the molecular dynamics simulation program CHARMM.21 A user can upload a PDB format file or provide a PDB entry ID from the RCSB protein databank22 that contains the protein-ligand complex. Because a molecular force field (FF) is not generally available for many small molecules, the web interface provides several options: (1) if the small molecule’s topology and parameter is already included in the CHARMM standard FF, a user simply needs to leave the residue name as it is or rename it to match the residue name in the CHARMM FF; (2) if a user already has a CHARMM FF customized for the user’s small molecule, the topology and parameter files can be uploaded and used; (3) if no FF is available, Ligand Binder provides an option for automated FF generation based on the CHARMM general FF (CGenFF)23 or general AMBER force field (GAFF)24, 25 to generate the ligand CHARMM FF (see below for details). Symmetric groups (currently, planar ring or t-butyl-groups) are algorithmically recognized after the topology generation and used in the later steps to apply flat-bottom dihedral restraints for preventing exchanges between physically identical rotameric states. Simple protein structure modifications (e.g., disulfide bond generation, terminal group changes, phosphorylation, and protonation) can also be made during this step (see ref. 26 for details).
STEP 2: Solvation of the systems
Once a protein-ligand structure is successfully initialized in CHARMM, the protein-ligand system is solvated in a water box. The current interface supports a spherical water box for a reduced system with solvent boundary potentials and an orthorhombic water box for the full system with periodic boundary conditions (PBC). While one can generate the FEP/MD simulation inputs for the full system with PBC, only the reduced system is used for the illustrative purposes in this work. The size of the spherical water box (Rwat) from the ligand’s center of mass (COM) can be specified by a user-defined value. For the user’s convenience in determining the size, the extent of the ligand (Rlig) is provided on the interface. Based on our experience and extensive testing, Rwat that is at least (Rlig + 1Å) for BULK and (Rlig + 5Å) for SITE are recommended for stable simulations. Nonetheless, Rwat may need to be optimized for specific problems. If a ligand molecule is composed of a small number of atoms, it may not be necessary to calculate the conformational free energy change upon binding. Therefore, the option for the conformational free energy simulation is unchecked by default if Rlig is smaller than 5 Å, but it can be turned on by the user.
With the given option in this step, Ligand Binder generates the input and output files for STEP 2, as well as the input files for the rest of the FEP/MD simulations (STEP 3–6). The user can download the generated files in an archived file and carry out the FEP/MD simulations on user’s machine. It should be noted that although many parts of the input files are similar for different ligands, some system-dependent parameters are inserted during the input generation. Thus, it is advised to re-generate the input files through Ligand Binder instead of copying and modifying the input files for different ligands if the user is not confident.
STEP 3: Setup of the solvent boundary systems
In this step, the solvent boundary potentials are set up to generate the reduced atomic systems. For SITE, the generalized solvent boundary potential (GSBP)17 is used. Ligand Binder uses the following setup for the GSBP system as a default. A spherical inner region is defined by Rwat from the ligand’s COM. All the atoms within the inner region are allowed to move during the equilibration and FEP/MD simulations. The inner region is extended by 3 Å to define a smooth spherical dielectric cavity (Rsphere). The protein atoms in the extended region (Rwat < R < Rsphere) as well as the atoms in the inner region linked via 1–3 bonds with the protein atoms in the outer region (> Rsphere) are fixed according to a group-based criterion. The water molecules in the outer region are removed and treated implicitly, and the inner region water molecules that overlap with the ligand and the protein are also deleted. The long-range electrostatic influence of the protein atoms in the outer region on the atoms in the inner region is represented in terms of the solvent-shielded static field and the solvent-induced reaction field. The reaction field due to the variations of the charge distribution in the inner region is expressed in terms of a generalized multipolar expansion using 11 spherical harmonic functions by default. The solvent-shielded static field and the reaction field matrix are calculated by the finite-difference Poisson-Boltzmann (PB) method using the PBEQ module27 in CHARMM, assuming a dielectric constant of 1 inside the protein as well as the inner and extended regions, and 80 otherwise. A non-polar cavity potential is used to keep the water molecules inside the inner region.
For BULK, the spherical solvent boundary potential (SSBP)16 is used together with the spherical water box generated in the previous step. The water box and the ligand molecules are centered at the origin, and water molecules that overlap with the ligand are deleted.
STEP 4: Equilibration of the systems
Equilibration of SITE (steps 4.1 and 4.2)
To overcome the problem that the size of the inner system is invariant during the subsequent simulations, the grand canonical Monte Carlo (GCMC) method is used to hydrate the inner region properly (step 4.1).28 In this step, 20 cycles of MC and MD simulations are performed. Each cycle is comprised of 10,000 MC moves followed by 10,000 MD steps with a time-step of 2 fs. The MC steps include rigid body translation, rotation, and GCMC insertion/deletion of water molecules, and each move type has the equal probability. A harmonic restraint potential with a force constant of 5.0 kcal/(mol·Å2) is applied to the protein and the ligand molecule throughout the MD simulations. All bonds involving hydrogen atoms are fixed with the SHAKE algorithm.29 The simulation is carried out using Langevin dynamics at 300 K with a friction coefficient of 5 ps−1 assigned to all non-hydrogen atoms. During the simulation, nonbonded interactions within 14.5 Å are explicitly accounted and the rest is treated with an extended electrostatics method. After 20 cycles of GCMC/MD simulations, the protein-ligand complex is equilibrated (step 4.2) for 200 ps at 300 K using Langevin dynamics with the same option as in step 4.1, but without the positional harmonic restraint.
Geometric and conformational restraint setup (step 4.3)
In this step, a set of anchoring atoms for the translational and orientational restraints are determined and the detailed procedures are described below. After the anchoring atoms are determined, the last 190-ps equilibration trajectory is used to calculate the average reference distances, angles, and dihedrals for the translational/orientational restraints. If the ligand has a symmetric group, the average dihedral angle around the symmetric group is also calculated. The average ligand conformation during the equilibration simulation is first calculated followed by a short energy minimization. The resulting averaged and minimized ligand structure is used as the reference configuration for the conformational restraint during FEP/MD simulations.
Equilibration of BULK (step 4.4)
The ligand molecule in bulk solvent is equilibrated for 200 ps at 300 K using the SSBP method and Langevin dynamics with the same option as in step 4.1. A weak positional restraint potential with a force constant of 0.5 kcal/(mol·Å2) is applied to the ligand’s COM to prevent the ligand from drifting away from the origin. The conformational restraint potential using the averaged ligand structure calculated in step 4.3 is applied to keep the reference conformation during the equilibration if the conformational free energy calculation option is selected in STEP 2.
STEP 5: FEP/MD and RMSD-umbrella sampling simulations
In this step, the FEP/MD simulations are carried out to calculate free energies associated with restriction of ligand conformation to the reference conformation (ΔGc), restriction of ligand orientation and translation (ΔGt,r), and interactions with surrounding environments (ΔGint). In the current scheme, the FEP/MD simulations are divided into a total of 137 independent (FEP/RMSD window) simulations that can be distributed over independent computer nodes. During the simulations, the perturbation energies for each state are collected for the later analysis. All simulations are carried out using Langevin dynamics with the same option as in step 4.1. An accessory script (step5_jobmanager.com) is provided to automatically check finished jobs and to (re)submit the FEP/MD and RMSD-umbrella sampling simulations (see Table 1). If necessary, optional GCMC simulation can be performed to adjust the solvation of reduced system during the FEP/MD simulations (the GCMC simulation option has to be turned on in the STEP 2).
Conformational free energy
To calculate the free energy associated with the restriction of the ligand conformation in the bound state, the conformational free energies ( and ) are estimated by calculating the potential of mean force (PMF) as a function of ligand RMSD using umbrella sampling simulations.30 Simulations are carried out using the quadratic biasing potential, kc(ξ − ξi)2, where kc is the force constant, ξ is the instantaneous RMSD with respect to the average ligand conformation in the bound state that is calculated from the SITE equilibration, and ξi is the RMSD offset value of each window. A total of 21 biasing windows are used with the RMSD offset value from 0.0 to 5.0 Å in steps of 0.25 Å for the ligand in SITE and BULK. The initial configurations for the 21 umbrella sampling windows are generated using a short initial run (20 ps) with a strong force constant of 500 kcal/(mol·Å2). Then, each window is simulated using a force constant of 10 kcal/(mol·Å2) for 110 ps and the data from the last 100-ps simulations are collected, which corresponds to one cycle.
During the umbrella sampling simulation for SITE, dissociation of the ligand from the binding pocket may occur for some windows with a large RMSD offset value due to large distortion of the ligand conformation. According to the strict step-by-step free energy decomposition scheme adopted in the present work, the translational degrees of freedom of the ligand should not be restricted at this stage. Nevertheless, to avoid the spurious contributions to the PMF from an unbound ligand, a flat-bottomed translational restraint with a small force constant (1.0 kcal/(mol·Å2)) is applied. This restraint, which allows free diffusion of the ligand’s COM within a spherical region of 2 Å radius centered around the initial position of ligand’s COM, is sufficient to prevent the ligand from leaving the binding pocket.
If symmetric groups (e.g., planar ring group or t-butyl group) are present in the ligand, interconversion between the physically equivalent conformations will make the ligand conformational sampling more challenging. Insufficient sampling will eventually lead to an inaccurate estimation of loss of conformational freedom upon ligand binding. To avoid this problem, a steep flat-bottom dihedral restraint potential with a force constant of 500 kcal/(mol·rad2) is applied to each of the symmetric unit of the ligand during all the calculations in BULK and SITE.
Translational and rotational free energy
The free energies corresponding to restriction of the ligand’s translational and rotational degrees of freedom near the binding site ( ) are calculated using FEP/MD simulations. The six point positions in the protein (P1, P2, and P3) and the ligand (L1, L2, and L3) determined in step 4.3 (see below for details) are used to define the relative position and orientation of the ligand with respect to the protein. A total of three translational (1 distance, 1 angle, and 1 dihedral restraints) and three rotational restraints (1 angle, 2 dihedral restraints) are applied with the reference values determined in step 4.3. The translational and rotational restraints are gradually turned on via the linear coupling parameters λt,r (Table 2) to final force constants of 10 kcal/(mol·Å2), 200 kcal/(mol·rad2), and 200 kcal/(mol·rad2) for the distance, angle, and dihedral restraints, respectively. For each set of coupling parameters, 50-ps simulations are performed and the data from the last 40-ps simulations are collected, which corresponds to one cycle.
Table 2.
The coupling parameters in the FEP/MD calculations.
| λrep | 0.0 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | |||||
| λdisp | 0.0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | ||||
| λelec | 0.0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | ||||
| λt,r | 0.0 | 0.025 | 0.005 | 0.0075 | 0.01 | 0.02 | 0.04 | 0.06 | 0.08 | 0.1 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 |
Interactions with surrounding environments
The contributions from the interactions of the ligand with its surrounding environments ( and ) are calculated with FEP/MD simulations. The Lennard-Jones (LJ) potential is separated into repulsive and dispersive free energies using the WCA separation method.31 Associated coupling parameters, λrep, λdisp, and λelec are introduced to control the repulsive, dispersive, and electrostatic interactions, respectively (Table 2). For each set of coupling parameters, 110-ps simulations are performed and the data from the last 100-ps simulations are collected, which corresponds to one cycle.
STEP 6: Calculation of the binding free energy
In the current staged FEP/MD simulation scheme, the final binding free energy can be expressed as
| (1) |
where represents the free energy change due to non-bonded interactions between the ligand and its environment. and represent the free energy changes due to the loss of conformational freedom and the translational/rotational freedom, respectively. The ligand’s translational (Ft) and rotational (Fr) freedoms in BULK are calculated analytically.12,14
In this step, the data generated in STEP 5 are aggregated and each term in the standard binding free energy is computed. The weighted histogram analysis method (WHAM)32, 33 is used to evaluate the PMF as a function of RMSD from the umbrella sampling simulations in STEP 5; a Fortran WHAM code is provided. The free energy change due to the loss of conformational freedom (ΔGc) is calculated by integration of the Boltzmann factor of the RMSD PMF. For BULK, is given by
| (2) |
where is the ligand PMF as a function of its RMSD with respect to the reference ligand conformation (i.e., average structure calculated in step 4.3). A similar expression is used for .
The data from the STEP 5 FEP/MD simulations (for and ) are unbiased using the WHAM facility in CHARMM. The individual free energy components are calculated for the ligand in SITE and BULK, respectively. Once individual free energy components are computed, a table of free energy can be printed using an accessory script (step6_table.py).
Automatic Generation of Ligand Force Field
Ligand Binder provides automatic FF generation options for small molecules using CGenFF23 or GAFF.24, 25 The initial structure of a ligand molecule with proper protonation states and explicit hydrogen atoms is required. When the CGenFF option is selected, Ligand Binder communicates with the ParamChem server (http://www.paramchem.org), which automatically generates the ligand FF based on analogy to known small molecular FF parameters. The ParamChem server assigns the charge and parameter penalty values, which could be used as indicative values for the parameter quality, and it is advisable to check if further optimization is needed before performing the FEP/MD simulations. Although it is not generally recommended due to a compatibility issue, a ligand FF based on GAFF can be generated. When the GAFF option is selected, the topology and parameters of the ligand are generated in the CHARMM format using the Antechamber toolkit.25 The charges of the ligands are from AM1/BCC,34 and an explicit charge state can be specified in the case that the ligand molecule is not neutral.
Automatic Determination of Anchoring Atoms
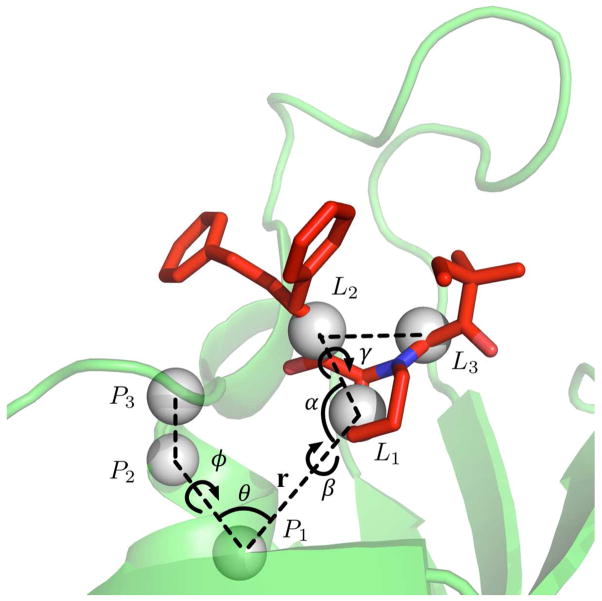
The translational and orientational restraint potentials applied to the ligand in the binding site are constructed from six anchoring atoms: three point positions in the protein (P1, P2, and P3) and three point positions in the ligand (L1, L2, and L3) (Figure 1). Ligand Binder provides a protocol for the automatic selection of reasonable anchoring points for any protein-ligand complex in step 4.3. Although it is referred to as an anchoring “atom”, the average coordinates of a group of atoms are used to define each anchoring position. Specifically, L1 is defined by the COM of a non-hydrogen ligand atom (L0) closest to the ligand’s COM and the ligand atoms that are bonded to L0. P1 is defined by the receptor backbone heavy atoms of a residue that is closest to the receptor’s COM. If the distance between P1 and L1 is too close (< 5Å) or too far (> 10 Å), P1 is randomly reselected from the receptor backbone heavy atoms of a residue within a distance of 5 – 10 Å from L1. P2 is defined by the receptor backbone heavy atoms of a residue that satisfy the condition of 60° ≤ ∠L1P1P2 ≤ 120°. P3 is defined by the receptor backbone heavy atoms of a residue that satisfy the condition of 60° ≤ ∠P1P2P3 ≤ 120°. L2 (and L3) is defined by the COM of a ligand heavy atom and the atoms bonded to the heavy atom with the following conditions; 30° ≤ ∠P1L1L2 ≤ 150° and 30° ≤ ∠L1L2L3 ≤ 150°. These conditions were empirically established through extensive testing to ensure consistent FEP/MD results.
Figure 1.
Illustration of translational and orientational restraints on SB3 ligand. Anchoring atoms are represented as grey sphere. r (P1−L1), θ (∠P2P1L1), φ (∠P3P2P1L1) are defined for the translational restraint, and α (∠P1L1L2), β (∠P2P1L1L2), and γ (∠P1L1L2L3) are defined for the orientational restraint.
The translational restraint is defined as Ut = 1/2[kt(r − r0)2 + ka(θ − θ0)2 + kd(φ − φ0)2], where r is the distance between P1 and L1, θ is the angle ∠P2P1L1, and φ is the dihedral angle ∠P3P2P1L1. kt, ka, and kd are the force constants for distance, angle, and dihedral angle restraints, respectively, and r0, θ0, and φ0 are the reference restraint values taken from the equilibration simulation in SITE (step 4.3). Similarly, the orientational restraint is defined as , where α is the angle ∠P1L1L2, β is the dihedral angle ∠P2P1L1L2, and γ is the dihedral angle ∠P1L1L2L3. In principle, the six anchoring atoms could be chosen arbitrarily without affecting the outcome of the FEP/MD calculations. However, poorly chosen anchoring atoms may yield geometric restraints that are ineffective to maintain the relative ligand-receptor orientation and cause undesired instabilities during the FEP/MD simulations. For this reason, it is important to select an adequate set of anchoring atoms.
Computational Details
In order to test a series of input files generated by Ligand Binder, illustrative calculations of absolute binding free energies were preformed for three non-polar aromatic ligands to the L99A mutant of T4 lysozyme and three FK506-related ligands to FKBP12 (see Figure 2 for the ligand structures) in the reduced simulation systems (Figure 3 and Table 3). The protein-ligand complex structures from the PDB in Table 3 were used to initialize the structures in Ligand Binder. The FF for each ligand was automatically generated by CGenFF with ligand structure files obtained from the PDB without modification. As mentioned above, the ParamChem server annotates the charge and parameter penalties as an indicative value for the parameter quality. The three nonpolar ligands used in T4 lysozyme L99A mutant had zero penalties for charge and parameter values. The three FK506-related ligands had a charge penalty of about 100 and a parameter penalty of about 141. Although further optimizations for the entries with a penalty greater than 50 are recommended by the authors of the ParamChem server, such an optimization was not attempted in this work.
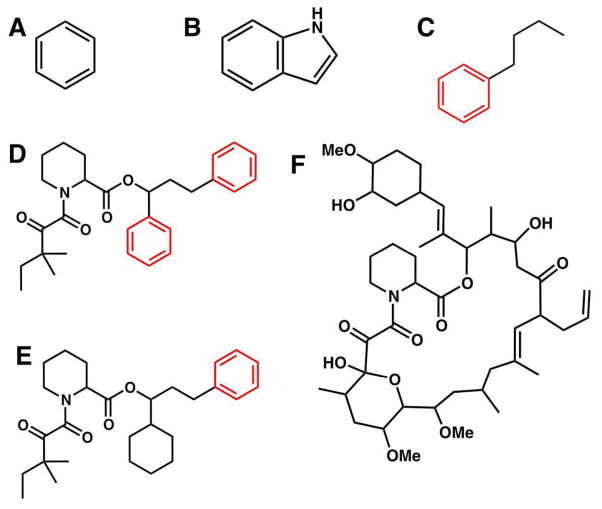
Figure 2.
Structures of ligand molecules used in this work. (A) Benzene, (B) Indole, (C) n-butylbenzene, (D) 1,3-diphenyl-1-propyl-1-(3,3-dimethyl-1,2-dioxypentyl)-2-piperidine carboxylate (SB3), (E) 1-cyclohexyl-3-diphenyl-1-propyl-1-(3,3-dimethyl-1,2-dioxypentyl)-2-piperidine carboxylate (SBX), and (F) K506 (FK5). Symmetric groups detected by Ligand Binder are colored in red.
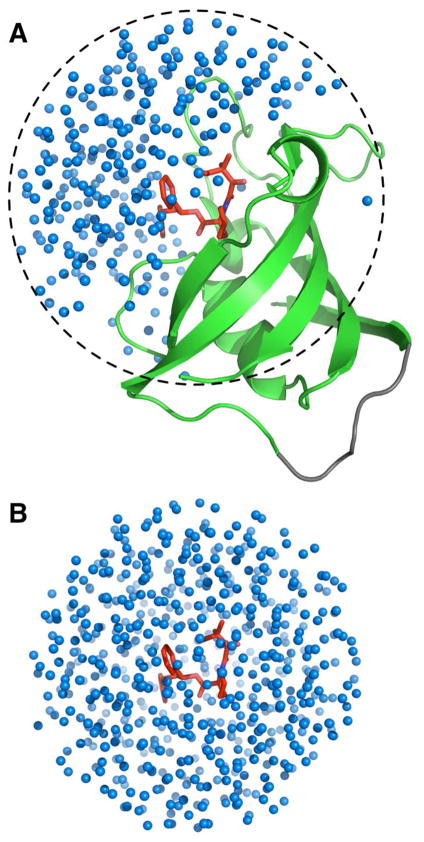
Figure 3.
Reduced simulation systems for (A) SITE and (B) BULK using GSBP and SSBP boundary potentials.
Table 3.
Simulation parameters for each FEP/MD simulation system.
| Ligand | PDB ID | Rlig |
|
|
|
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| benzene | 181L | 2.3 Å | 14 Å | 13 Å | 46 (155) | 288 | ||||
| indole | 185L | 3.3 Å | 14 Å | 14 Å | 42 (150) | 351 | ||||
| n-butylbenzene | 186L | 4.1 Å | 14 Å | 15 Å | 45 (152) | 439 | ||||
| SB3 | 1FKG | 6.6 Å | 14 Å | 17 Å | 130 (208) | 630 | ||||
| SBX | 1FKH | 6.6 Å | 14 Å | 17 Å | 128 (208) | 628 | ||||
| FK5 | 1FKJ | 8.1 Å | 14 Å | 19 Å | 147 (235) | 865 |
and are the numbers of water molecules in SITE and BULK systems, respectively. The numbers in the parenthesis is the number of water molecules in SITE system after 20 cycles of GCMC/MD simulations (averaged over three independent simulations).
All the MD simulations were carried out using CHARMM.21 All the simulation system setup and FEP/MD calculations were performed using the input files generated by Ligand Binder without further modification. For the non-polar ligands in T4 lysozyme L99A mutant, the option for the conformational free energy calculation was not selected due to their small sizes by default. Figure 3 shows a typical reduced protein/ligand complex system for SITE and BULK using GSBP17 and SSBP16 boundary potentials. The number of water molecules is given in Table 3 before and after the hydration of SITE using GCMC/MD simulations. Each protein/ligand complex system was equilibrated for 200 ps with Langevin dynamics without any biasing restraints. After equilibration was done for SITE, the anchoring atoms for the translational and orientational restraints were automatically chosen and stored in a stream file (step4.3_restraint_geo.prm) for the FEP/MD simulations. The equilibration trajectory was also used to determine the reference values for the distance (r), angle (θ and α), and dihedral (φ, β, and γ) restraints (Figure 1). The list of anchoring atoms used in the simulation as well as the reference values are given in Supporting Information Table S1. The BULK systems were also equilibrated for 200 ps with Langevin dynamics with ligand conformation restrained using the average ligand conformation from the SITE equilibration as a reference.
For each protein-ligand complex in Table 3, 10 cycles of FEP/MD simulations were carried out for better convergence. The conformational free energy term was not calculated for T4 lysozyme ligands due to small ligand size. Each cycle consisted of 10-ps equilibration and 100-ps production except for the translational/rotational free energy contribution (10-ps equilibration and 50-ps production). Each cycle was started using the last coordinates of the previous cycle with random initial velocities. The free energy values and the errors are presented using the average and the standard deviation of the last five cycles, respectively. Two additional (independent) 10-cycle FEP/MD simulations were performed using different sets of automatically chosen anchoring atoms to illustrate the robustness of our scheme for anchoring atom selections.
RESULTS AND DISCUSSION
To examine and illustrate the FEP/MD simulation protocol adopted in CHARMM-GUI Ligand Binder, the 18 test FEP/MD simulations using six different protein-ligand complexes were performed (Table 3). The calculated standard binding free energies are given in Table 4. In this section, we first discuss the FEP/MD calculation results, and a general discussion on the binding free energy calculation results is then followed.
Table 4.
Binding free energies¶ of T4 lysozyme ligands and FKBP ligands.
| Ligand | #§ |
|
|
ΔΔGc | ΔΔGt,r | ΔGb | ||
|---|---|---|---|---|---|---|---|---|
| benzene | C1 | −9.9 ± 0.2 | 0.5 ± 0.1 | - | 4.5 ± 0.4 | −5.9 ± 0.4 | ||
| C2 | −11.3 ± 0.1 | 0.4 ± 0.3 | - | 6.0 ± 0.5 | −5.7 ± 0.6 | |||
| C3 | −8.1 ± 0.2 | 0.5 ± 0.2 | - | 2.2 ± 0.3 | −6.4 ± 0.4 | |||
|
| ||||||||
| indole | C1 | −16.5 ± 0.1 | −6.3 ± 0.2 | - | 7.6 ± 0.1 | −2.7 ± 0.3 | ||
| C2 | −17.2 ± 0.1 | −6.3 ± 0.2 | - | 8.7 ± 0.1 | −2.2 ± 0.2 | |||
| C3 | −18.0 ± 0.2 | −6.2 ± 0.5 | - | 9.1 ± 0.2 | −2.7 ± 0.6 | |||
|
| ||||||||
| n-butylbenzene | C1 | −17.2 ± 0.3 | 1.9 ± 0.5 | - | 8.8 ± 0.1 | −10.2 ± 0.6 | ||
| C2 | −18.8 ± 0.2 | 1.8 ± 0.4 | - | 9.3 ± 0.1 | −11.4 ± 0.4 | |||
| C3 | −16.7 ± 0.4 | 1.6 ± 0.3 | - | 8.5 ± 0.0 | −9.8 ± 0.5 | |||
|
| ||||||||
| SB3 | C1 | −33.9 ± 0.8 | −8.0 ± 1.2 | 2.8 ± 1.4 | 9.9 ± 0.0 | −13.3 ± 2.0 | ||
| C2 | −33.5 ± 1.3 | −8.5 ± 1.3 | 3.0 ± 1.1 | 9.9 ± 0.0 | −12.1 ± 2.0 | |||
| C3 | −34.8 ± 1.6 | −9.1 ± 1.6 | 2.5 ± 1.0 | 9.2 ± 0.1 | −14.0 ± 2.3 | |||
|
| ||||||||
| SBX | C1 | −32.5 ± 1.1 | −7.3 ± 0.7 | 4.9 ± 1.3 | 9.6 ± 0.1 | −10.4 ± 1.8 | ||
| C2 | −32.5 ± 1.3 | −7.8 ± 1.0 | 5.6 ± 1.3 | 9.9 ± 0.0 | −9.2 ± 2.0 | |||
| C3 | −33.3 ± 1.7 | −6.5 ± 2.1 | 5.3 ± 1.0 | 10.0 ± 0.0 | −11.5 ± 2.8 | |||
|
| ||||||||
| FK5 | C1 | −60.0 ± 1.3 | −35.0 ± 1.9 | 2.3 ± 0.7 | 9.8 ± 0.0 | −12.9 ± 2.4 | ||
| C2 | −59.4 ± 1.3 | −35.3 ± 1.1 | 3.2 ± 0.9 | 10.1 ± 0.1 | −10.8 ± 1.9 | |||
| C3 | −59.3 ± 2.0 | −34.4 ± 1.3 | 4.2 ± 1.2 | 9.9 ± 0.1 | −10.7 ± 2.9 | |||
Units of free energies are kcal/mol. The errors of all the free energy values are standard deviation of the last five cycles of FEP/MD, each started with the last snapshot of previous cycle and random velocities.
C1, C2, and C3 represent independent FEP/MD simulations for each ligand.
Illustration of FEP/MD simulations using CHARMM-GUI Ligand Binder
For each ligand, the reduced systems were copied to make three independent simulation systems. The GCMC/MD simulations and the equilibrations were performed independently. In addition, different sets of anchoring atoms were used for each independent simulation. By doing so, the independent simulations serve to estimate the impact of slightly different initial conditions and different selections of anchoring atoms on the final binding free energies (C1, C2, and C3 in Table 4).
The reduced GSBP representation of the binding site is hydrated with GCMC/MD simulations in the presence of the ligand molecule during the equilibration step 4.1, and the number of water molecules was fixed during the FEP/MD simulations. Although Ligand Binder provides an option to allow the number of water molecules to fluctuate during the FEP/MD simulations, such option was not activated in this study to reduce the computational time. Based on additional tests, the final binding free energies of the test systems in the current study are not significantly affected by allowing the number of water to fluctuate during the FEP/MD simulations, which is in agreement with the observation recently made by Lee et. al.35 However, rehydration of the binding site could be potentially important to accurately calculate the binding free energy when the size of the reduced system is small or the size of the ligand is large.28
In general, the smaller size of T4 lysozyme non-polar ligands had more consistent results for each independent calculation with better convergence compared to larger FKBP ligands. Better convergence of smaller non-polar ligands is expected due to the small size of the ligand and well-defined hydrophobic binding pocket in the receptor. In contrast, FKBP ligands showed relatively larger standard deviations (1–2 kcal/mol) between the cycles of FEP/MD simulations. In addition, the calculated binding free energy of an independent system showed about 1–2 kcal/mol differences.
When compared to the previous computational results12, 14 and experimental data36, 37 in Table 5, our results generally agree well within statistical errors, although there are differences for specific systems. For example, the calculated binding free energy of indole is about 2 kcal/mol less favorable than the previous calculation,12 and the calculated free energy of FKBP ligands in this work showed more statistical errors compared to the previous calculations.14 Although the current protocol implemented in Ligand Binder is based on the previous work done by Roux and co-workers,12, 14 there are several reasons for such discrepancy. Certainly, longer equilibration and careful adjustments of ligand FF parameters could enhance the accuracy of the calculation results. For instance, a different FF parameter set was used (CHARMM PARAM2238 FF in ref 12 and Antechamber toolkit25 in ref 14) and/or the sampling time was much longer in the previous calculations (about 1–2 ns in ref 14 versus about 100 ps in this work for each cycle). Because the purpose of the current work is to illustrate the standardized and automated protocol for absolute binding free energy calculations, further optimization is not performed. Certainly, some optimization by user (e.g., force field parameters and equilibration/sampling times) could be beneficial.
Table 5.
Comparison of binding free energies of T4 lysozyme ligands and FKBP ligands with experiments36, 37 and previous calculations.
| Ligand | ΔGb (kcal/mol)
|
|||
|---|---|---|---|---|
| This work§ | Deng et. al.12 | Wang et. al.14 | Experiments36, 37 | |
| benzene | −6.0 ± 0.3 | −6.0 ± 0.2 | - | −5.2± 0.2 |
| indole | −2.5 ± 0.2 | −4.2 ± 0.2 | - | −4.9± 0.1 |
| n-butylbenzene | −10.5 ± 0.5 | −8.7 ± 0.4 | - | −6.7± 0.0 |
| SB3 | −13.1 ± 0.8 | - | −10.3 ± 0.4 | −10.9± 0.1 |
| SBX | −10.4 ± 1.0 | - | −11.7 ± 1.0 | −11.1± 0.2 |
| FK5 | −11.5 ± 1.0 | - | −10.1 ± 1.2 | −12.7± 0.2 |
The free energies for this work are calculated by taking averages from the values in Table 4, and the errors are standard errors from the 3 independent calculations (C1, C2, and C3).
Validation of anchoring atoms for orientational restraints
In the current FEP/MD simulation protocol, the restraint potentials are used to maintain the position and orientation of the ligand around the “pose” adopted in the bound complex. These restraints are also used to calculate the contributions from the restriction of orientational and translational degrees of freedom of the ligand molecule upon its binding to a receptor protein. A set of six anchoring atoms (three from the receptor protein and three from the ligand molecule) is selected to define the ligand position and orientation in terms of distance, angle, and dihedral angles (Figure 1).
In principle, the final binding free energy should not be affected by the choice of anchoring atoms. The translational and orientational restraints have been widely adopted in the double decoupling method12, 13, 20, 39 and the choice of anchoring atoms were shown to have only minimal influences on the final binding free energy.20 However, it is easy to imagine possible sets of anchoring atoms that are problematic. For example, if a set of anchoring atoms in the ligand yields a co-linear reference angle (e.g., ∠L1L2L3 ~ 180°), the orientation of the ligand relative to the receptor will be poorly defined. Poor choices can be avoided when the anchoring atoms are selected manually, but objective criteria must be implemented when the anchoring atoms are selected by an automated protocol. Ligand Binder provides an automated anchoring atom selection protocol, which carries out some basic tests to prevent a poor choice of anchoring atoms.
Table 6 shows the sum of and ΔΔGt,r from independent FEP/MD simulations with different sets of anchoring atoms. Because the orientational and translational restraint potentials were applied during the FEP/MD simulations of and ΔΔGt,r, the individual free energy terms calculated using different sets of anchoring atoms could have different values, but the sum of these terms should remain unchanged regardless of the choice of the anchoring atoms. As shown in the sum of and ΔΔGt,r from the independent FEP/MD simulations, different sets of anchoring atoms automatically chosen by Ligand Binder have only minimal impacts on the final free energy, illustrating that the automatic anchoring atom selection in Ligand Binder is reliable.
Table 6.
Comparison of free energy terms¶ with different selections of the anchoring atoms.
| Ligand | #§ |
|
|
|---|---|---|---|
| benzene | C1 | −5.4 ± 0.3 | |
| C2 | −5.3 ± 0.4 | ||
| C3 | −5.9 ± 0.2 | ||
|
| |||
| indole | C1 | −9.0 ± 0.1 | |
| C2 | −8.5 ± 0.1 | ||
| C3 | −8.8 ± 0.2 | ||
|
| |||
| n-butylbenzene | C1 | −8.4 ± 0.2 | |
| C2 | −9.5 ± 0.2 | ||
| C3 | −8.2 ± 0.3 | ||
|
| |||
| SB3 | C1 | −24.0 ± 0.5 | |
| C2 | −23.6 ± 1.4 | ||
| C3 | −25.7 ± 1.0 | ||
|
| |||
| SBX | C1 | −22.7 ± 0.7 | |
| C2 | −22.3 ± 0.8 | ||
| C3 | −23.0 ± 1.1 | ||
|
| |||
| FK5 | C1 | −50.6 ± 0.8 | |
| C2 | −49.2 ± 1.0 | ||
| C3 | −49.4 ± 1.5 | ||
Units of free energies are kcal/mol. The errors of all the free energy values are standard deviation of the last five cycles of FEP/MD, each started with the last snapshot of previous cycle and random velocities.
C1, C2, and C3 represent independent FEP/MD simulations for each ligand.
CONCLUSIONS
In this work, we have developed a web interface, CHARMM-GUI Ligand Binder (http://www.charmm-gui.org/input/gbinding), to provide standardized CHARMM input files for absolute binding free energy calculations using the FEP/MD simulations. The sophisticated staged FEP/MD simulation protocol based on the step-by-step decomposition of the total reversible work representing the binding process12, 14 is adopted in Ligand Binder. The method is closely related to the double decoupling method,19, 20 and it has been applied to calculate absolute binding free energies in a number of biologically important systems.11–14
Ligand Binder has a number of features that help the user to setup the FEP/MD simulations in straightforward manners. First, a ligand FF can be easily generated and incorporated into the simulation inputs. Although a careful parameterization may need to be carried out by the user for improved results, a ligand FF can be generated automatically using either CGenFF23 or GAFF.24, 25 In addition, Ligand Binder can automatically detect symmetric groups based on the ligand topology (e.g., currently planar ring and t-butyl group) and generate all the necessary input files. Another important feature of Ligand Binder is to provide a robust method to select anchoring atoms without knowing the ligand molecule a priori. All input files generated by Ligand Binder can be downloaded, which allows customization of the FEP/MD simulation protocol if necessary.
The current implementation of Ligand Binder supports reduced system representation using SSBP for the ligand in solution, and GSBP for the protein binding site. While these approaches provide useful approximations, it is important to note the situations for which there can be problems. Usage of GSBP, in particular, could result in systematic deviations and inaccuracies of the calculated binding free energies when the receptor protein is highly flexible or undergoes large conformational change upon ligand binding.35, 40 To alleviate the problem, Ligand Binder also supports fully explicit system representation with PBC and comprehensive testing is underway. To enhance sampling and improve convergence, the replica-exchange molecular dynamics (REMD) method is also adopted41, 42 and can be activated by the user. As high performance computing resources become increasingly available, state-of-the-art free energy methodologies are expected to eventually migrate toward the full replica-exchange FEP/MD simulations with PBC. Consequently, Ligand Binder will support these trends by generating all the necessary input files for the highly scalable simulation programs with built-in parallel/parallel features such as NAMD in the future.
To test the efficacy of Ligand Binder, we have performed a set of illustrative absolute binding free energy calculations for three non-polar aromatic ligands to the L99A mutant of T4 lysozyme and three FK506-related ligands to FKBP12. Without any further modification of the current protocol (i.e., generated input files), statistical errors within individual calculations are found to be small (~1–2 kcal/mol), and the calculated binding free energies generally agree well with the experimental measurements and the previous computational studies. It is likely that statistical precision and convergence of the calculations could be improved by carrying out multiple FEP/MD runs or by increasing the sampling times of all the FEP windows. A larger statistical error is observed in FK506-related ligands. Thus, slight modification based on the input files generated by Ligand Binder for better sampling would be advisable for medium to large sized ligand molecules.
Ligand Binder allows one to quickly setup and perform FEP/MD simulations, thus providing an ideal platform for accurate calculations of the absolute binding free energy. By providing standardized input scripts containing sophisticated commands for various biasing potentials, Ligand Binder could facilitate robust calculations of absolute binding free energy without intimate knowledge of ligand molecule or lengthy trial simulations. Such an automated and standardized protocol in alchemical free energy can also be used in evaluation of the binding affinity of a large number of molecules or in a lead optimization process.
Supplementary Material
Acknowledgments
This work was supported by the University of Kansas General Research Fund allocation #2301388-003, NSF ABI-1145987, NIH U54 GM087519-01, TeraGrid/XSEDE resources (TG-MCB070009) (to W.I.), and NSF MCB-0920261 (to B.R.).
Footnotes
Supporting Information. Anchoring atom and reference restraint values used for the FEP/MD simulations in this study is available in Table S1. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Carlson HA, McCammon JA. Accommodating protein flexibility in computational drug design. Mol Pharmacol. 2000;57:213–218. [PubMed] [Google Scholar]
- 2.Gane PJ, Dean PM. Recent advances in structure-based rational drug design. Curr Opin Struct Biol. 2000;10:401–404. doi: 10.1016/s0959-440x(00)00105-6. [DOI] [PubMed] [Google Scholar]
- 3.Huggins DJ, Sherman W, Tidor B. Rational Approaches to Improving Selectivity in Drug Design. J Med Chem. 2012;55:1424–1444. doi: 10.1021/jm2010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aqvist J, Medina C, Samuelsson JE. A new method for predicting binding affinity in computer-aided drug design. Protein Eng. 1994;7:385–391. doi: 10.1093/protein/7.3.385. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan J, Cheatham T, Cieplak P, Kollman P, Case D. Continuum solvent studies of the stability of DNA, RNA, and phosphoramidate - DNA helices. J Am Chem Soc. 1998;120:9401–9409. [Google Scholar]
- 6.Kollman PA, Massova I, Reyes C, Kuhn B, Huo S, Chong L, Lee M, Lee T, Duan Y, Wang W, Donini O, Cieplak P, Srinivasan J, Case DA, Cheatham TE. Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc Chem Res. 2000;33:889–897. doi: 10.1021/ar000033j. [DOI] [PubMed] [Google Scholar]
- 7.Deng Y, Roux B. Computations of standard binding free energies with molecular dynamics simulations. J Phys Chem B. 2009;113:2234–2246. doi: 10.1021/jp807701h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mobley DL, Dill KA. Binding of small-molecule ligands to proteins: “what you see” is not always “what you get”. Structure. 2009;17:489–498. doi: 10.1016/j.str.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chodera JD, Mobley DL, Shirts MR, Dixon RW, Branson K, Pande VS. Alchemical free energy methods for drug discovery: progress and challenges. Curr Opin Struct Biol. 2011;21:150–160. doi: 10.1016/j.sbi.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearlman DA. Evaluating the molecular mechanics poisson-boltzmann surface area free energy method using a congeneric series of ligands to p38 MAP kinase. J Med Chem. 2005;48:7796–7807. doi: 10.1021/jm050306m. [DOI] [PubMed] [Google Scholar]
- 11.Fujitani H, Tanida Y, Ito M, Jayachandran G, Snow CD, Shirts MR, Sorin EJ, Pande VS. Direct calculation of the binding free energies of FKBP ligands. J Chem Phys. 2005;123:084108. doi: 10.1063/1.1999637. [DOI] [PubMed] [Google Scholar]
- 12.Deng Y, Roux B. Calculation of Standard Binding Free Energies: Aromatic Molecules in the T4 Lysozyme L99A Mutant. J Chem Theory Comput. 2006;2:1255–1273. doi: 10.1021/ct060037v. [DOI] [PubMed] [Google Scholar]
- 13.Mobley DL, Chodera JD, Dill KA. On the use of orientational restraints and symmetry corrections in alchemical free energy calculations. J Chem Phys. 2006;125:084902. doi: 10.1063/1.2221683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Deng Y, Roux B. Absolute binding free energy calculations using molecular dynamics simulations with restraining potentials. Biophys J. 2006;91:2798–2814. doi: 10.1529/biophysj.106.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoichet BK, Leach AR, Kuntz ID. Ligand solvation in molecular docking. Proteins. 1999;34:4–16. doi: 10.1002/(sici)1097-0134(19990101)34:1<4::aid-prot2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Beglov D, Roux B. Finite Representation of an Infinite Bulk System - Solvent Boundary Potential for Computer-Simulations. J Chem Phys. 1994;100:9050–9063. [Google Scholar]
- 17.Im W, Bernèche S, Roux B. Generalized solvent boundary potential for computer simulations. J Chem Phys. 2001;114:2924–2937. [Google Scholar]
- 18.Ge X, Roux B. Absolute binding free energy calculations of sparsomycin analogs to the bacterial ribosome. J Phys Chem B. 2010;114:9525–9539. doi: 10.1021/jp100579y. [DOI] [PubMed] [Google Scholar]
- 19.Gilson MK, Given JA, Bush BL, McCammon JA. The statistical-thermodynamic basis for computation of binding affinities: a critical review. Biophys J. 1997;72:1047–1069. doi: 10.1016/S0006-3495(97)78756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boresch S, Tettinger F, Leitgeb M, Karplus M. Absolute binding free energies: A quantitative approach for their calculation. J Phys Chem B. 2003;107:9535–9551. [Google Scholar]
- 21.Brooks BR, Brooks CL, Mackerell AD, Jr, Nilsson L, Petrella RJ, Roux B, Won Y, Archontis G, Bartels C, Boresch S, Caflisch A, Caves L, Cui Q, Dinner AR, Feig M, Fischer S, Gao J, Hodoscek M, Im W, Kuczera K, Lazaridis T, Ma J, Ovchinnikov V, Paci E, Pastor RW, Post CB, Pu JZ, Schaefer M, Tidor B, Venable RM, Woodcock HL, Wu X, Yang W, York DM, Karplus M. CHARMM: the biomolecular simulation program. J Comput Chem. 2009;30:1545–1614. doi: 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanommeslaeghe K, Hatcher ER, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Guvench O, Lopes P, Vorobyov I, Mackerell AD., Jr CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem. 2010;31:671–690. doi: 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of a general amber force field. J Comput Chem. 2004;25:1157–1174. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Wang W, Kollman PA, Case DA. Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graphics Modell. 2006;25:247–260. doi: 10.1016/j.jmgm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Jo S, Kim T, Iyer VG, Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 27.Im W, Beglov D, Roux B. Continuum Solvation Model: computation of electrostatic forces from numerical solutions to the Poisson-Boltzmann equation. Comput Phys Commun. 1998;111:59–75. [Google Scholar]
- 28.Deng Y, Roux B. Computation of binding free energy with molecular dynamics and grand canonical Monte Carlo simulations. J Chem Phys. 2008;128:115103. doi: 10.1063/1.2842080. [DOI] [PubMed] [Google Scholar]
- 29.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical-Integration of Cartesian Equations of Motion of a System with Constraints - Molecular-Dynamics of N-Alkanes. J Comput Phys. 1977;23:327–341. [Google Scholar]
- 30.Woo HJ, Roux B. Calculation of absolute protein-ligand binding free energy from computer simulations. Proc Natl Acad Sci US A. 2005;102:6825–6830. doi: 10.1073/pnas.0409005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weeks JD, Chandler D, Andersen HC. Role of Repulsive Forces in Determining Equilibrium Structure of Simple Liquids. J Chem Phys. 1971;54:5237–5247. [Google Scholar]
- 32.Kumar S, Bouzida D, Swendsen R, Kollman P, Rosenberg J. The weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J Comput Chem. 1992;13:1011–1021. [Google Scholar]
- 33.Souaille M, Roux B. Extension to the weighted histogram analysis method: combining umbrella sampling with free energy calculations. Comput Phys Commun. 2001;135:40–57. [Google Scholar]
- 34.Jakalian A, Jack DB, Bayly CI. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J Comput Chem. 2002;23:1623–1641. doi: 10.1002/jcc.10128. [DOI] [PubMed] [Google Scholar]
- 35.Lee HS, Jo S, Lim H-S, Im W. Application of Binding Free Energy Calculations to Prediction of Binding Modes and Affinities of MDM2 and MDMX Inhibitors. J Chem Inf Model. 2012 doi: 10.1021/ci3000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holt D, Luengo J, Yamashita D, Oh H, Konialian A, Yen H, Rozamus L, Brandt M, Bossard M, Levy M, Eggleston D, Liang J, Schultz L, Stout T, Clardy J. Design, Synthesis, and Kinetic Evaluation of High-Affinity Fkbp Ligands and the X-Ray Crystal-Structures of Their Complexes with Fkbp12. J Am Chem Soc. 1993;115:9925–9938. [Google Scholar]
- 37.Morton A, Matthews BW. Specificity of ligand binding in a buried nonpolar cavity of T4 lysozyme: linkage of dynamics and structural plasticity. Biochemistry. 1995;34:8576–8588. doi: 10.1021/bi00027a007. [DOI] [PubMed] [Google Scholar]
- 38.MacKerell A, Bashford D, Bellott M, Dunbrack R, Evanseck J, Field M, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau F, Mattos C, Michnick S, Ngo T, Nguyen D, Prodhom B, Reiher W, Roux B, Schlenkrich M, Smith J, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 39.Hermans J, Wang L. Inclusion of loss of translational and rotational freedom in theoretical estimates of free energies of binding. Application to a complex of benzene and mutant T4 lysozyme. J Am Chem Soc. 1997;119:2707–2714. [Google Scholar]
- 40.Lau AY, Roux B. The hidden energetics of ligand binding and activation in a glutamate receptor. Nat Struct Mol Biol. 2011;18:283–287. doi: 10.1038/nsmb.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang W, Hodoscek M, Roux B. Computation of Absolute Hydration and Binding Free Energy with Free Energy Perturbation Distributed Replica-Exchange Molecular Dynamics. J Chem Theory Comput. 2009;5:2583–2588. doi: 10.1021/ct900223z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodcock HL, 3rd, Hodoscek M, Gilbert AT, Gill PM, Schaefer HF, 3rd, Brooks BR. Interfacing Q-Chem and CHARMM to perform QM/MM reaction path calculations. J Comput Chem. 2007;28:1485–502. doi: 10.1002/jcc.20587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.