Abstract
Objective
To determine the effect of a 12-month community-based tango dance program on activity participation among individuals with Parkinson disease (PD).
Design
Randomized controlled trial with assessment at baseline, 3, 6, and 12 months.
Setting
The intervention was administered in the community; assessments were completed in a university laboratory.
Participants
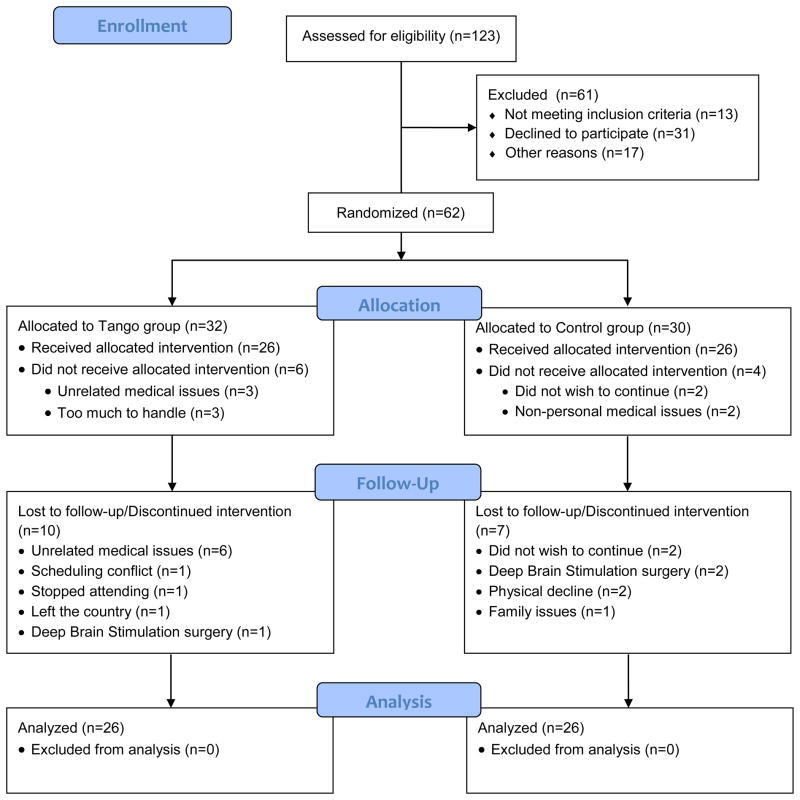
Sixty-two volunteers with PD enrolled in the study and were randomized to treatment group. Ten participants did not receive the allocated intervention, so the final analyzed sample included 52 participants.
Intervention
Participants were randomly assigned to the Tango group, which involved 12 months of twice weekly Argentine tango dance classes, or to the no intervention Control group (n = 26 per group).
Main Outcome Measures
Current, new and retained participation in instrumental, leisure and social activities as measured by the Activity Card Sort (with the “dance” activity removed).
Results
Total Current participation in the Tango group was higher at 3, 6, and 12 months compared to baseline (ps ≤ 0.008), while the Control group did not change (ps ≥ 0.11). Total Activity Retention (since onset of PD) in the Tango group increased from 77% to 90% (p = 0.006) over the course of the study, whereas the Control group remained around 80% (p = 0.60). These patterns were similar in the separate activity domains. The Tango group gained a significant number of New Social activities (p = 0.003), but the Control group did not (p = 0.71).
Conclusions
Individuals with PD who participated in a community-based Argentine tango class reported increased participation in complex daily activities, recovery of activities lost since the onset of PD, and engagement in new activities. Incorporating dance into the clinical management of PD may benefit participation and subsequently quality of life for this population.
Keywords: Parkinson disease, exercise, rehabilitation, social participation, quality of life
Parkinson disease (PD) is a neurodegenerative disorder that affects over one million North Americans 1. In PD, degeneration of dopamine-producing neurons in the substantia nigra disrupts basal ganglia functioning. This results in motor dysfunction, most prominently tremor, rigidity, akinesia, bradykinesia, and postural instability. In addition, individuals with PD can experience a variety of non-motor problems such as sensory disturbances, cognitive impairment, autonomic dysfunction, and psychological changes 2, 3. As PD progresses, it can hinder the individual’s ability to perform activities of daily living (ADL), leading to dependency on others 4, 5. As such, PD is associated with reduced quality of life 6–8 and significant socioeconomic costs 9.
Although loss of independence in ADL (i.e., disability) is thought to occur in the middle or moderate stages of disease progression 4, emerging research 10 suggests that PD can negatively impact participation early in the disease. The International Classification of Functioning, Disability and Health (ICF) defines participation as involvement in life situations and the extent to which individuals are engaged in a societal context 11. Individuals with early and mild PD, for whom motor dysfunction is not yet sufficient to cause physical disability, report reduced participation in instrumental, low physical-demand leisure and social activities compared to healthy, age-matched peers 10, 12. Furthermore, participation restrictions among individuals with PD are associated with reduced health-related quality of life 12, 13. Participation is positively correlated with functional status and life satisfaction 14, 15 and may be protective against physical and cognitive decline as people age 16, 17. These links highlight the importance of pursuing interventions targeted at improving participation for those with PD. Dopaminergic medication does not adequately address many of the factors which contribute to reduced participation in this population including impaired mobility, depressive symptoms, and cognitive dysfunction 10, 13, 18. There is a need for more comprehensive approaches to manage the complex manifestations of PD and their effects on individuals’ participation.
One adjunct to medical treatment that is beneficial for PD patients is exercise. Exercise has been found to improve physical function, mobility, cognition, and health-related quality of life among individuals with PD 19–21. Unfortunately, more than half of all American seniors do not engage in the recommended amount of exercise, and those with PD engage in 15% less exercise than their healthy peers 22. Because of this, the identification of alternative forms of exercise that are at least as effective as traditional exercise programs but foster better adherence are needed. Individuals with PD report that social support and social interaction positively influence their exercise adherence, with one of the most salient motivators being spousal participation 23, 24. Dance is a form of partnered exercise that provides social engagement and, importantly, has been found to have a higher adherence rate than other forms of exercise among individuals with PD 25. At the end of a study comparing traditional exercise to dance 26, nearly half of the participants in the dance group chose to continue to participate in the dance classes while no participants from the traditional exercise class continued their form of exercise. Instead, some of the participants from the traditional exercise group chose to attend the dance classes after the study was complete.
In addition to promoting better adherence, dance may improve upon the beneficial physical effects of traditional exercise. In the study described above 25, the dance group showed significant improvements in balance, whereas the traditional exercise group did not. Argentine tango was found to be a particularly effective form of dance, resulting in larger improvements in balance, mobility, movement initiation, and attention to movement control compared to other forms of dance (waltz and foxtrot) 27. The positive effects of Argentine tango on physical function (e.g., motor dysfunction severity, balance, gait) and progression of motor symptoms were recently confirmed in a randomized controlled trial of a long-term community-based tango dancing program 28.
The benefits of tango dancing may extend beyond physical improvements to stimulate broader participation in life activities and roles for those with PD 29. For example, partnered exercise programs can facilitate the development of social support networks, thereby increasing opportunities for social participation outside of the formal program 23. Interventions for improving participation among individuals with PD have previously been unexplored. The purpose of this study was to determine whether the community-based tango dance program that reduced disease severity and improved physical function in a group of PD patients 28 affected activity participation. We hypothesized that PD participants who engaged in the dance program would report increased activity participation over the course of the study relative to a control group of participants with PD who did not engage in the dance program.
METHODS
Participants
Participants were recruited from the clinical research database of the Washington University School of Medicine (WUSM) Movement Disorders Center, the WUSM Research Participant Registry, neurologists in the St. Louis area, and advertisements in the newsletter of the Greater St. Louis Chapter American Parkinson Disease Association. All participants were diagnosed with idiopathic PD using published clinical diagnostic criteria 30, were classified as Hoehn and Yahr stages I–IV 31, and experienced clear motor benefit from levodopa. Participants had to be able to walk independently for 10 feet with or without an assistive device. Individuals were excluded if they had a history of neurological deficit other than PD, serious medical problem(s), evidence of abnormality other than PD-related changes on brain imaging, or history or evidence of musculoskeletal or psychological problems. This study was approved by the institution’s Human Research Protection Office, and all participants provided written informed consent.
Intervention
This was a single-blind randomized controlled trial. Upon enrollment, participants were randomly assigned to the Tango or Control group. The protocol for the tango intervention was based on prior investigations of the effects of Argentine tango for individuals with PD 32. Tango participants attended one-hour dance classes two times per week for 12 months. Dance classes were taught by an experienced dance instructor who was trained and supervised by the principal investigator (G.E.) to ensure appropriateness and safety for individuals with PD. The classes consisted of progressive Argentine tango lessons in which participants learned a new step in each class. This form of dance involved flexible, improvisational step patterns composed of small step elements, spontaneous multi-directional changes, and rhythmic variation. Individuals with PD were paired with individuals who did not have PD. These dance partners were caregivers (e.g., spouses, family members) who accompanied PD participants to the classes and healthy young volunteers recruited from health-related graduate and undergraduate departments at Washington University in St. Louis (volunteers received special training on fall prevention and safety). All individuals, regardless of gender, were asked to dance in both the leader and follower roles to ensure that everyone spent similar amounts of time moving forward and backward. In addition, participants changed partners every ten minutes, a practice commonly used in dance classes to facilitate learning. Control participants were asked to continue the normal life routine that they had engaged in before enrolling in the study.
Assessment Procedure
Control and Tango participants were evaluated at baseline and then at 3, 6, and 12 months post-baseline. All assessments were conducted while participants were off their regular anti-parkinsonian medications (12 hour washout period) by a blinded rater (R.D.) at the WUSM Program in Physical Therapy. The full evaluation consisted of a variety of measures to characterize physical function and mobility, including the Unified Parkinson’s Disease Rating Scale sections 1–3 33 (for a complete description of study measures, see 28). The Beck Depression Inventory-II 34, 35 was administered to assess depressive symptoms. Participation, the primary outcome for this analysis, was measured using the Activity Card Sort (ACS) 36. The ACS is a standardized assessment that measures perceived level of participation in daily life activities as well as changes in participation in relation to certain events (e.g., the onset of disease or disability, beginning a new treatment regimen) or over specified periods of time (e.g., in the past five years). It consists of 89 cards containing pictures of people involved in activities that fall into one of four domains: (1) Instrumental activities (20 items; e.g. grocery shopping, doing laundry, household maintenance), (2) Low-demand Leisure activities (35 items; e.g. cooking as a hobby, playing table games, reading, watching movies or television), (3) High-demand Leisure activities (17 items; e.g. swimming, woodworking, hiking, fishing, gardening), and (4) Social activities (17 items; e.g. traveling, eating at a restaurant, volunteer work, spending time with friends). Participants sorted the cards into one of five categories with the corresponding numerical point values: Continue to Do since Illness [at pre-illness level] = 1, Do Less since Illness = 0.5, Given Up Due to Illness = 0, New Activity since Illness = 1, or Not Done Prior to Illness = 0. Parkinson disease was the “Illness” to which the categories referred. As per published scoring criteria 36, Current participation scores were calculated by summing the point values for the activities sorted into the Continue to Do, Do Less, and New Activity categories. Activities sorted into the Continue to Do, Do Less, and Given Up categories each also received 1 point for Previous participation. Activity Retention since PD Onset was calculated by dividing Current by Previous participation scores. These calculations were completed for all activities (Total) and for the separate activity domains (Instrumental, Low-demand Leisure, High-demand Leisure, Social). The ACS includes “dancing” as a social activity; however, this item was removed from analysis so it would not inflate the Tango participants’ scores. In the present study, Total Current and Previous scores could range from 0 to 88, and separate activity domain scores could range as follows: Instrumental: 0–20, Low-demand Leisure: 0–35, High-demand Leisure: 0–17, Social: 0–16. Higher scores indicate participation in more activities. Activity Retention scores could be above 0%, with higher scores indicating proportionately more activities retained since the onset of PD. The ACS has strong psychometric properties in community-dwelling samples of healthy adults 14, 36–37 and those with various neurological conditions (i.e., Alzheimer’s disease, multiple sclerosis, stroke) 37, including good internal consistency, test-retest reliability, and construct validity. The ACS demonstrated sensitivity to change in a sample of individuals with stroke in response to a community rehabilitation program 38.
Statistical Analyses
Descriptive statistics were calculated for all variables. Group characteristics at baseline were compared using independent samples t-tests, Wilcoxon signed rank tests for ordinal scales (e.g., Hoehn and Yahr stage), and Chi-squared tests for categorical data (e.g., gender). Longitudinal analyses were conducted using mixed-model 2×4 analyses of variance with group (Tango, Control) as the between-subjects factor and time (baseline, 3, 6, and 12 months) as the within-subjects factor. Planned pairwise comparisons were used to characterize change over time within each group. An intent-to-treat analysis was employed including all participants who completed the baseline and 3-month evaluation and carrying forward last recorded data for those who dropped out after this point. A significance level of 0.05 was used in all statistical analyses, and p-values < 0.10 were considered trends.
RESULTS
Participant characteristics
Sixty-two individuals with idiopathic PD (30 Control, 32 Tango) enrolled in this study, were randomized to treatment group and completed the baseline evaluation. Fifty-two participants (26 Control, 26 Tango) completed the 3-month evaluation and were included in the current analysis (Figure 1). Sample characteristics are presented in Table 1. There were no differences between the Tango and Control groups at baseline with regards to gender, age, duration and severity of PD, or depressive symptoms (all p ≥ 0.25)
Figure 1.
CONSORT flow diagram illustrating participant recruitment, randomization, and attrition. All participants retained through 3 months were included in the final analyzed sample; last observations from those who dropped out after 3 months were carried forward for intent-to-treat analysis.
Table 1.
Demographic and clinical characteristics for each group at baseline (N = 52).
| Control | Tango | |
|---|---|---|
| n | 26 | 26 |
| Male/Female ratio (n) | 15/11 | 15/11 |
| Age in years | 69.0 (7.8) | 69.3 (9.4) |
| Hoehn & Yahr Stage (n) | ||
| 2 | 9 | 11 |
| 2.5 | 11 | 10 |
| 3 | 5 | 5 |
| 4 | 1 | 0 |
| UPDRS 1 | 12.5 (6.7) | 10.7 (4.9) |
| UPDRS 2 | 13.8 (7.8) | 13.2 (7.9) |
| UPDRS 3 | 48.0 (9.0) | 44.5 (11.9) |
| Years since diagnosis | 7.0 (4.8) | 5.8 (5.4) |
| BDI–II | 11.4 (7.0) | 9.3 (6.2) |
Numbers represent means (standard deviation) or number of participants. UPDRS = Unified Parkinson’s Disease Rating Scale; BDI–II = Beck Depression Inventory II.
Activity Participation
Current Participation
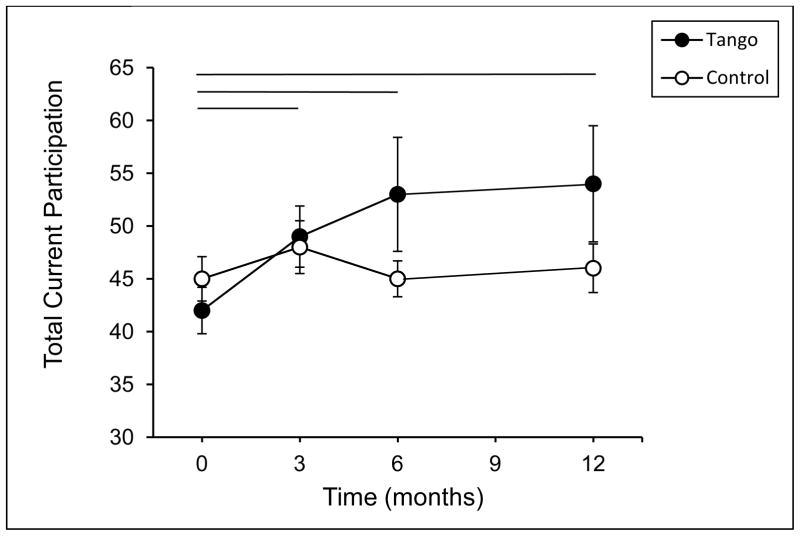
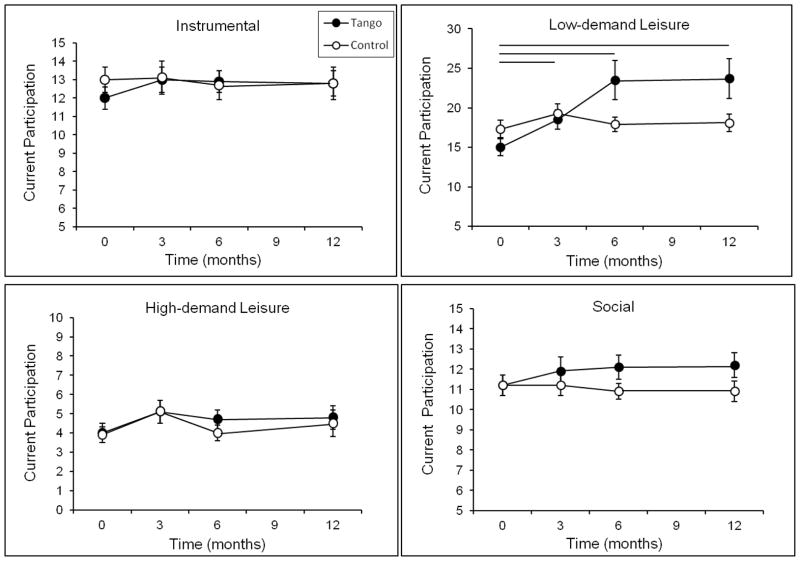
There were no group differences in Current participation at baseline for Total activities or for the separate activity domains (all p ≥ 0.15). There was an effect of time, F(3, 48) = 2.78, p = 0.04, and a trend for a time by group interaction in Total Current participation, F(3, 48) = 2.55, p = 0.06 (Figure 2). Planned comparisons indicated that there was a main effect of time in the Tango group, F(3, 48) = 4.05, p = 0.01, but not the Control group, F(3, 48) = 0.96, p = 0.42. Specifically, the Total Current participation of the Tango group was higher at 3, 6, and 12 months compared to baseline (all p ≤ 0.008), while the Control group did not change (all p ≥ 0.11). Analysis of the separate activity domains (Figure 3) showed a similar pattern for Low-demand Leisure activities, such that there was a main effect of time for the Tango group, F(3, 48) = 4.75, p = 0.006, but not for the Control group, F(3, 48) = 1.36, p = 0.27. Low-demand Leisure participation was higher in the Tango group at 3, 6, and 12 months compared to baseline (all p ≤ 0.03), while the Control group did not change (all p ≥ 0.50). There were no significant effects for High-demand Leisure, Instrumental or Social activities (all p ≥ 0.11).
Figure 2.
Total Current Participation scores on the Activity Card Sort (ACS) at baseline, 3-, 6-, and 12-month evaluations for the Tango and Control groups. Values are means ± SEs. Horizontal lines indicate a significant difference within the Tango group between the time points spanned by the line.
Figure 3.
Current Participation scores on the Activity Card Sort (ACS) for each activity domain at baseline, 3-, 6-, and 12-month evaluations for the Tango and Control groups. Values are means ± SEs. Horizontal lines indicate a significant difference within the Tango group between the time points spanned by the line.
Activity Retention since PD Onset
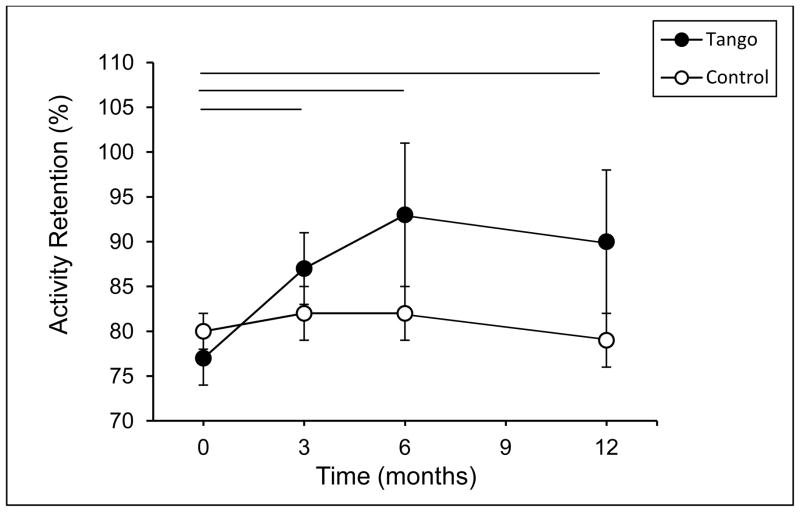
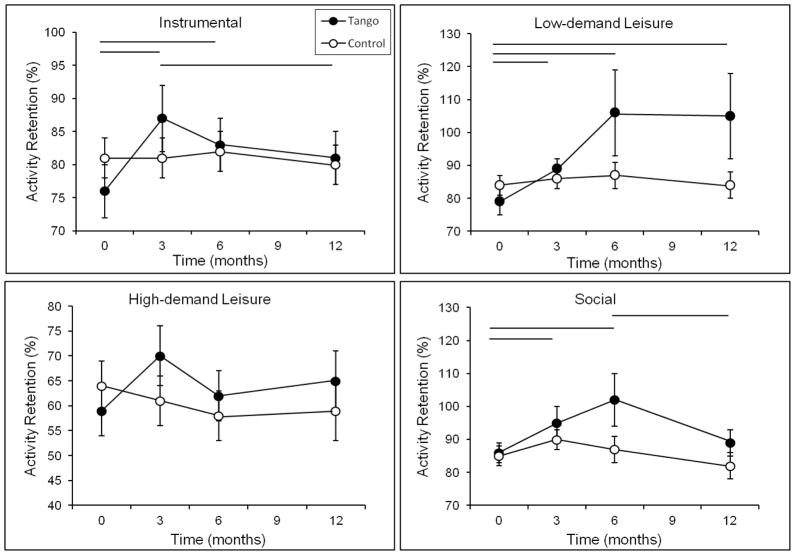
There was a main effect of time for Total Activity Retention, F(3, 48) = 3.70, p = 0.02. The time by group interaction did not reach significance (p = 0.15); however, as with Total Current Participation, planned comparisons revealed a significant effect of time within the Tango group, F(3, 48) = 4.68, p = 0.006, but not the Control group, F(3, 48) = 0.631, p = 0.60 (Figure 4). Over the course of the study, the percentage of pre-PD activities engaged in by the Tango group increased from 77% to 90%, whereas the Control group remained around 80%. Analysis of the separate activity domains (Figure 5) revealed significant effects of time on Instrumental and Low-demand Leisure Activity Retention in the Tango group, Fs ≥ 3.7, ps ≤ 0.02, but not the Control group, Fs < 0.47, p ≥ 0.70. Percentage of pre-PD Instrumental activities increased from 76% at baseline to 87% at 3 months before declining to 81% at 12 months in the Tango group but remained around 80% in the Control group. Percentage of pre-PD Low-demand Leisure activities increased from 79% to 106% in the Tango group but remained at 84% in the Control group. This indicates that the Tango participants reported engaging in more Low-demand Leisure activities at the end of the study compared to before the onset of PD. There was an effect of time for Social Activity Retention, F(1, 50) = 13.75, p = 0.001. Percentage of pre-PD Social activities in the Tango group increased from 85% at baseline to 94% and 102% at 3 and 6 months, respectively, before declining to 89% at 12 months, F(3, 48) = 4.83, p = 0.005. After an initial increase from 85% at baseline to 90% at 3 months in the Control group, there was a decline to 82% at 12 months, F(3, 48) = 2.61, p = 0.06. There were no significant effects for High-demand Leisure Activity Retention (all p ≥ 0.25).
Figure 4.
Total Activity Retention scores on the Activity Card Sort (ACS) at baseline, 3-, 6-, and 12-month evaluations for the Tango and Control groups. Activity Retention scores represent the proportion of pre-PD activities currently engaged in, calculated as Current Participation/Previous Participation. Values are means ± SEs. Horizontal lines indicate a significant difference within the Tango group between the time points spanned by the line.
Figure 5.
Activity Retention scores on the Activity Card Sort (ACS) for each activity domain at baseline, 3-, 6-, and 12-month evaluations for the Tango and Control groups. Values are means ± SEs. Horizontal lines indicate a significant difference within the Tango group between the time points spanned by the line.
New Activities
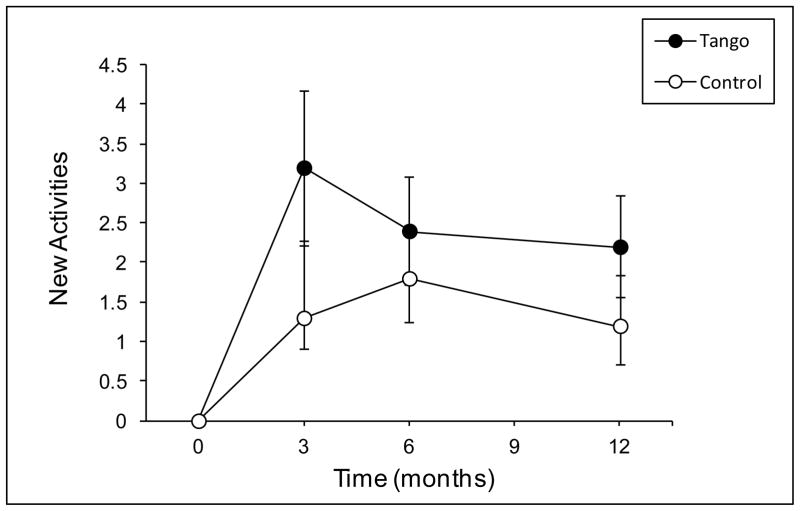
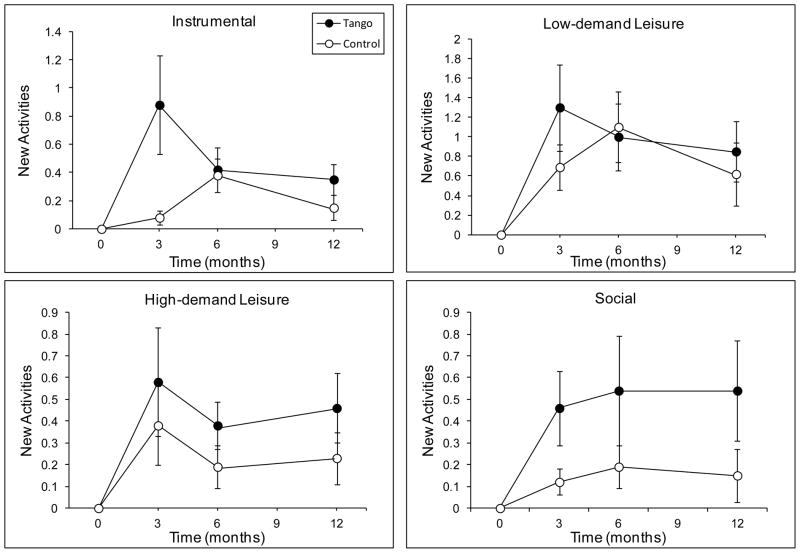
There was a main effect of time on New Activity participation, F(3, 48) = 9.83, p < 0.001, such that both groups reported more new activities at 3, 6, and 12 months compared to baseline (all p ≤ 0.001) (Figure 6). On average, the Tango group reported 2.6 (SD = 3.9) new activities at each time point and the Control group reported 1.4 (SD = 3.6). The effect of time was significant for each activity domain, Fs ≥ 5.46, ps < 0.01 (Figure 7). However, for New Social activities, it was qualified by a time by group interaction, F(3, 48) = 4.26, p = 0.03, such that there was a significant effect of time in the Tango group, F(3, 48) = 5.38, p = 0.003, but not the Control group, F(3, 48) = 0.47, p = 0.71. The Tango group gained a significant number of new Social activities from baseline to 3, 6, and 12 months (M = 0.5, SD = 1.1; ps < 0.001) but the Control group did not (M = 0.15, SD = 0.48; ps ≥ 0.31).
Figure 6.
Total New Activity scores on the Activity Card Sort (ACS) at baseline, 3-, 6-, and 12-month evaluations for the Tango and Control groups. Values are means ± SEs. Both groups reported more new activities at 3-, 6-, and 12-months compared to baseline.
Figure 7.
New Activity scores on the Activity Card Sort (ACS) for each activity domain at baseline, 3-, 6-, and 12-month evaluations for the Tango and Control groups. Values are means ± SEs.
DISCUSSION
The purpose of this study was to examine the effects of a community-based Argentine tango dance program on activity participation among individuals with PD. Volunteers with PD were randomized to participate in 12 months of Argentine tango classes (Tango group) or to continue their daily routine as usual (Control group). As hypothesized, participants in the Tango group reported increased activity participation over the course of the study. Moreover, they recovered a significant proportion of the activities they had lost since the onset of PD. Activity participation among individuals in the Control group remained relatively stable.
Our results extend previous work 28 and suggest that in addition to improving physical function among individuals with PD, socially engaging and functional, skill-based exercise promotes participation in instrumental, leisure and social activities. The changes in participation observed in the Tango group appear to be driven by a combination of increased engagement in prior activities done less or given up since the onset of PD as well as engagement in novel activities. The only activity domain for which participation did not increase in the Tango group relative to the Control group was High-demand Leisure. Tango participants were required to attend the dance classes and, as such, were engaging in a high-demand leisure activity that was not captured by our outcome measure (because this item was removed); however, they did not report participating in additional exercise activities outside of the dance classes. There are a number of potential reasons for this, including lack of opportunities or access to exercise or being satisfied with the level of exercise offered by the dance classes. Regardless, by engaging in more instrumental, low-demand leisure and social activities, participants in the Tango group did boost their overall level of physical activity, which simply requires bodily movement that increases energy expenditure above the basal level and can include occupational, household, transportation, and leisure activities 39.
Of the separate activity domains, Low-demand Leisure showed the most consistent improvements, with Tango participants engaging in more of these types of activities by the end of the study than they had before the onset of PD. This outcome is significant, as participation in low-demand leisure activities has been associated with improved mental health in older adults 14. It is important to note that while the activities in this domain are not as physically demanding as those in the High-demand Leisure domain, many place significant demand on mobility (e.g., going to the museum, recreational shopping) or other functions affected by PD such as cognition and fine motor coordination (e.g., games, puzzles, needlecrafts). Some also involve social interaction. Thus, rather than representing the adoption of a more sedentary lifestyle, increased low-demand leisure participation may reflect a higher level of daily challenge and engagement for individuals with PD.
There are a number of ways in which Argentine tango could positively influence activity participation in PD. As a form of physical exercise, it benefits PD-related mobility impairments and may even slow disease progression (for a discussion of these effects, refer to 28), which could result in improved capacity for daily performance and participation. Importantly, progressive tango classes have features that may additively benefit participation compared to traditional exercise. For example, tango requires working memory, control of attention and multitasking to integrate newly learned and previously learned dance elements, stay in rhythm with the music, and maneuver around others on the dance floor. Leading requires self-initiated movements and motor planning while following requires reading and responding appropriately to the leader’s body cues 25. These cognitive challenges may further improve capacity for daily performance and result in increases in, or maintenance of, participation.
The social interaction, social support and social influences that emerged from the tango classes likely also had positive effects on participation. The group setting provided an opportunity for social modeling, the establishment and reinforcement of social norms regarding health-promoting behavior, and the development of social networks 40. In fact, participants in the Tango group reported engaging in social activities together outside of class, including attending a play, the symphony, and a social dance. On an individual level, the presence of a partner may have helped those with PD to feel more comfortable challenging themselves in the complexity and difficulty of movements 27, thereby providing the opportunity for mastery experiences, a primary source of self-efficacy 41. Improvements in self-efficacy that occurred during the tango classes could have translated to daily life, cultivating the desire to go out and engage in more or new activities, re-try activities that had been given up, or devote the necessary effort and persistence required to maintain one’s current level of activity.
Study Limitations
There was no control for attention and social interaction across groups, so it is possible that the changes in participation observed in the Tango group were due to non-specific effects of socializing. However, studies in older adults have shown that exercise outcomes are more strongly predicted by the social cognitive factors associated with exercise, such as improved self-efficacy, rather than by social interaction alone 42. Thus, we propose that the improvements in participation in the Tango group are larger than what would occur from socialization alone. Future studies should incorporate a social control group and measure social cognitive factors to provide stronger support for this conclusion.
Our attrition rate was relatively high compared to previous exercise studies in PD 43, 44. Attrition may have been a function of the research study rather than of the intervention itself. Participation in the study required four off all antiparkinsonian medication evaluations at a separate location from the dance class. This feature likely added a level of burden or discomfort that would not be present in a community-based dance program alone. Importantly, the Tango participants who completed the entire study were actively engaged in the intervention as evidenced by an average of nearly 80% attendance to all classes. This adherence rate is good compared to other 12-month exercise trials in older adults e.g., 45. Furthermore, most of these participants (13/16) chose to continue attending the dance classes after the study was over. Thus, dance appears to be an enjoyable and highly motivating form of physical activity for some people with PD. Formalized follow up, perhaps using the ACS with the “dancing” item re-incorporated, would provide support for this notion and help to determine longer term effects of the intervention. Consistent with the notion that a one-size-fits-all approach to promoting exercise will not be efficacious, but instead, that exercise recommendations should be tailored to individuals’ needs, desires and barriers 46, these findings suggests the need for continued identification of alternative, effective forms of exercise for the diverse population of individuals with PD.
While the ACS provides a broad picture of a person’s perceived participation in complex daily activities, it may not have fully captured all changes in participation in our sample. For example, it allows for the measurement of New Activities but does not include a “Do More” category, and therefore cannot account for increased participation in existing activities. In addition, the ACS does not provide information regarding factors such as length of time spent engaged in activities, the relative importance of activities to people, satisfaction with participation, or difficulty experienced while performing activities. Participation is a complex and multidimensional construct. The present study has provided initial support for the efficacy of community-based dance for improving participation among individuals with PD. Future work can investigate the nature of changes in participation that are occurring as well as the relative importance of the various facets of participation to overall health and well-being in PD.
CONCLUSIONS
In summary, we found that engagement in a community-based Argentine tango dance class is associated with increased activity participation among individuals with PD. To our knowledge, this is the first study to test the effect of any intervention on activity participation in this population. Rehabilitation research in PD primarily focuses on motor impairment and physical disability, measuring outcomes at the level of functional mobility and self-care ADL. Given its importance for health and well-being 14, 16, 47, optimal participation in all the activities and roles of daily life should be the ultimate goal of rehabilitation for PD and should be a primary outcome in intervention studies. Our findings suggest that dance, a socially-engaging form of exercise, should be included in the clinical management of PD. Future work should examine longer term effects of the intervention and investigate the potential biological and psychosocial mechanisms underlying the benefits of dance for individuals with PD.
Acknowledgments
This work was supported by a grant from the Parkinson’s Disease Foundation. Additional support was provided by the Greater St. Louis American Parkinson Disease Association (APDA) and the APDA Center for Advanced PD Research at Washington University in St. Louis. Thanks to Ruth Porter, DPT, and John Michael Rotello for tango instruction and to Vanessa Heil-Chapdelaine for assistance with data management.
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, if applicable, we certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript.
Abbreviations
- ADL
Activities of daily living
- ACS
Activity Card Sort
- PD
Parkinson disease
- UPDRS
Unified Parkinson’s Disease Rating Scale
Footnotes
Reprints will be available from the corresponding author.
The trial was registered on ClinicalTrials.gov as “PD4PD: Partnered Dance for Parkinson Disease,” CT0138856.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998;339(15):1044–53. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 2.Adler CH. Nonmotor complications in Parkinson’s disease. Mov Disord. 2005;20 (Suppl 11):S23–9. doi: 10.1002/mds.20460. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee G, et al. Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord. 2007;22(11):1623–9. doi: 10.1002/mds.21586. [DOI] [PubMed] [Google Scholar]
- 4.Shulman LM, Gruber-Baldini AL, Anderson KE, Vaughan CG, Reich SG, Fishman PS, et al. The evolution of disability in Parkinson disease. Mov Disord. 2008;23(6):790–6. doi: 10.1002/mds.21879. [DOI] [PubMed] [Google Scholar]
- 5.Tison F, Barberger-Gateau P, Dubroca B, Henry P, Dartigues JF. Dependency in Parkinson’s disease: a population-based survey in nondemented elderly subjects. Mov Disord. 1997;12(6):910–5. doi: 10.1002/mds.870120612. [DOI] [PubMed] [Google Scholar]
- 6.Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord. 2008;23(10):1428–34. doi: 10.1002/mds.21667. [DOI] [PubMed] [Google Scholar]
- 7.Schrag A, Jahanshahi M, Quinn N. How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov Disord. 2000;15(6):1112–8. doi: 10.1002/1531-8257(200011)15:6<1112::aid-mds1008>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;69(3):308–12. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huse DM, Schulman K, Orsini L, Castelli-Haley J, Kennedy S, Lenhart G. Burden of illness in Parkinson’s disease. Mov Disord. 2005;20(11):1449–54. doi: 10.1002/mds.20609. [DOI] [PubMed] [Google Scholar]
- 10.Foster ER, Hershey T. Everyday Executive Function Is Associated With Activity Participation in Parkinson Disease Without Dementia. OTJR: Occupation, Participation and Health. 2011;31(1):16–22. doi: 10.3928/15394492-20101108-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) International classification of functioning, disability, and health (ICF) Geneva, Switzerland: WHO; 2001. [Google Scholar]
- 12.Connor LT, Wolf TJ, Foster ER, Hildebrand M, Baum C. Participation and engagement in occupation in adults with disabilities. In: Pierce D, editor. Occupational Science for Occupational Therapy. Thorofare, NJ: SLACK Inc; in press. [Google Scholar]
- 13.Duncan RP, Earhart GM. Measuring participation in individuals with Parkinson disease: relationships with disease severity, quality of life, and mobility. Disabil Rehabil. 2011;33(15–16):1440–14466. doi: 10.3109/09638288.2010.533245. [DOI] [PubMed] [Google Scholar]
- 14.Everard KM, Lach HW, Fisher EB, Baum MC. Relationship of activity and social support to the functional health of older adults. J Gerontol B Psychol Sci Soc Sci. 2000;55(4):S208–12. doi: 10.1093/geronb/55.4.s208. [DOI] [PubMed] [Google Scholar]
- 15.Edwards DF, Hahn M, Baum MC, Dromerick AW. The impact of mild stroke on meaningful activity and life satisfaction. Journal of Stroke and Cerebrovascular Diseases. 2006;15 (4):151–7. doi: 10.1016/j.jstrokecerebrovasdis.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Buchman AS, Boyle PA, Wilson RS, Fleischman DA, Leurgans S, Bennett DA. Association between late-life social activity and motor decline in older adults. Arch Intern Med. 2009;169(12):1139–46. doi: 10.1001/archinternmed.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. AmJEpidemiol. 2002;155(12):1081–7. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- 18.Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc JL. Limitations of current Parkinson’s disease therapy. Ann Neurol. 2003;53(Suppl 3):S3–12. doi: 10.1002/ana.10513. discussion S-5. [DOI] [PubMed] [Google Scholar]
- 19.Cruise KE, Bucks RS, Loftus AM, Newton RU, Pegoraro R, Thomas MG. Exercise and Parkinson’s: benefits for cognition and quality of life. Acta Neurol Scand. 2010;123(1):13–9. doi: 10.1111/j.1600-0404.2010.01338.x. [DOI] [PubMed] [Google Scholar]
- 20.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2008;23(5):631–40. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K, Quadros AC, Jr, Santos RF, Stella F, Gobbi LT, Gobbi S. Benefits of physical exercise on executive functions in older people with Parkinson’s disease. Brain Cogn. 2009;69(2):435–41. doi: 10.1016/j.bandc.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Toth MJ, Fishman PS, Poehlman ET. Free-living daily energy expenditure in patients with Parkinson’s disease. Neurology. 1997;48(1):88–91. doi: 10.1212/wnl.48.1.88. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien M, Dodd KJ, Bilney B. A qualitative analysis of a progressive resistance exercise programme for people with Parkinson’s disease. Disabil Rehabil. 2008;30(18):1350–7. doi: 10.1080/09638280701614546. [DOI] [PubMed] [Google Scholar]
- 24.Ravenek MJ, Schneider MA. Social support for physical activity and perceptions of control in early Parkinson’s disease. Disabil Rehabil. 2009;31(23):1925–36. doi: 10.1080/09638280902850261. [DOI] [PubMed] [Google Scholar]
- 25.Hackney ME, Kantorovich S, Levin R, Earhart GM. Effects of tango on functional mobility in Parkinson’s disease: a preliminary study. J Neurol Phys Ther. 2007;31(4):173–9. doi: 10.1097/NPT.0b013e31815ce78b. [DOI] [PubMed] [Google Scholar]
- 26.Hackney ME, Kantorovich S, Earhart GM. A study on the effects of Argentine tango as a form of parnered dance for those with Parkinson disease. Amer J Dance Ther. 2007;29:109–27. [Google Scholar]
- 27.Hackney ME, Earhart GM. Effects of dance on gait and balance in Parkinson’s disease: a comparison of partnered and nonpartnered dance movement. Neurorehabil Neural Repair. 2010;24(4):384–92. doi: 10.1177/1545968309353329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26(2):132–43. doi: 10.1177/1545968311421614. [DOI] [PubMed] [Google Scholar]
- 29.Earhart GM. Dance as therapy for individuals with Parkinson disease. Eur J Phys Rehabil Med. 2009;45(2):231–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. Am J Med Genet. 1999;88(5):539–43. [PubMed] [Google Scholar]
- 31.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 32.Hackney ME, Earhart GM. Recommendations for implementing partnered tango classes for persons with Parkinson disease. Amer J Dance Ther. 2010;32(1):41–52. doi: 10.1007/s10465-010-9086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov Disord. 2007;22(1):41–7. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- 34.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 35.Schrag A, Barone P, Brown RG, Leentjens AF, McDonald WM, Starkstein S, et al. Depression rating scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2007;22(8):1077–92. doi: 10.1002/mds.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baum C, Edwards DF. Activity Card Sort (ACS) 2. Bethesda, MD: AOTA Press; 2008. [Google Scholar]
- 37.Katz N, Karpin H, Lak A, Furman T, Hartman-Maeir A. Participation in occupational performance: Reliability and validity of the Activity Card Sort. OTJR: Occupation, Participation and Health. 2003;23:10–7. [Google Scholar]
- 38.Hartman-Maeir A, Eliad Y, Kizoni R, Nahaloni I, Kelberman H, Katz N. Evaluation of a long-term community based rehabilitation program for adult stroke survivors. NeuroRehabilitation. 2007;22:295–301. [PubMed] [Google Scholar]
- 39.Hui EK, Rubenstein LZ. Promoting physical activity and exercise in older adults. J Am Med Dir Assoc. 2006;7(5):310–4. doi: 10.1016/j.jamda.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 40.McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63(4):1011–22. doi: 10.1016/j.socscimed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 42.Brassington GS, Atienza AA, Perczek RE, DiLorenzo TM, King AC. Intervention-related cognitive versus social mediators of exercise adherence in the elderly. Am J Prev Med. 2002;23(2 Suppl):80–6. doi: 10.1016/s0749-3797(02)00477-4. [DOI] [PubMed] [Google Scholar]
- 43.Allen NE, Canning CG, Sherrington C, Lord SR, Latt MD, Close JC, et al. The effects of an exercise program on fall risk factors in people with Parkinson’s disease: a randomized controlled trial. Mov Disord. 2010;25(9):1217–25. doi: 10.1002/mds.23082. [DOI] [PubMed] [Google Scholar]
- 44.Sage MD, Almeida QJ. Symptom and gait changes after sensory attention focused exercise vs aerobic training in Parkinson’s disease. Mov Disord. 2009;24(8):1132–8. doi: 10.1002/mds.22469. [DOI] [PubMed] [Google Scholar]
- 45.Fielding RA, Katula J, Miller ME, Abbott-Pillola K, Jordan A, Glynn NW, et al. Activity adherence and physical function in older adults with functional limitations. Med Sci Sports Exerc. 2007;39(11):1997–2004. doi: 10.1249/mss.0b013e318145348d. [DOI] [PubMed] [Google Scholar]
- 46.Brawley LR, Rejeski WJ, King AC. Promoting physical activity for older adults: the challenges for changing behavior. Am J Prev Med. 2003;25(3 Suppl 2):172–83. doi: 10.1016/s0749-3797(03)00182-x. [DOI] [PubMed] [Google Scholar]
- 47.Law M. Participation in the occupations of everyday life. Am J Occup Ther. 2002;56(6):640–9. doi: 10.5014/ajot.56.6.640. [DOI] [PubMed] [Google Scholar]