SUMMARY
Knowledge of human T cells derives chiefly from studies of peripheral blood, whereas their distribution and function in tissues remains largely unknown. Here, we present a unique analysis of human T cells in lymphoid and mucosal tissues obtained from individual organ donors, revealing tissue-intrinsic compartmentalization of naive, effector and memory subsets conserved between diverse individuals. Effector-memory CD4+ T cells producing IL-2 predominated in mucosal tissues and accumulated as central-memory subsets in lymphoid tissue, whereas CD8+ T cells were maintained as naïve subsets in lymphoid tissues and IFN-γ-producing effector-memory CD8+ T cells in mucosal sites. The T cell activation marker, CD69, was constitutively expressed by memory T cells in all tissues, distinguishing them from circulating subsets, with mucosal memory T cells exhibiting additional distinct phenotypic and functional properties. Our results provide an assessment of human T cell compartmentalization as a new baseline for understanding human adaptive immunity.
INTRODUCTION
Immunological memory is essential for maintaining immunity to ubiquitous pathogens, and for achieving protection from vaccines. The importance of T cell memory has been demonstrated in mouse models of infection, where multiple memory subsets and their diverse distribution in lymphoid, mucosal and other non-lymphoid sites are integral to their protective capacity and long-term maintenance (Bevan, 2011; Masopust and Picker, 2012; Sheridan and Lefrancois, 2011; Teijaro et al., 2011). However, mouse models cannot recapitulate the human memory immune response and the effects of decades-long exposure to multiple pathogens. In humans, studies on T cell activation and memory have been largely limited to T cells isolated from peripheral blood, and very little is known regarding human T cell activation and differentiation in lymphoid and mucosal tissue sites. Moreover, there are no strategies to target memory T cells for promoting long-term immunity in humans, despite worldwide efforts to develop effective vaccines against endemic pathogens such as HIV and malaria, chronic viruses such as HSV and hepatitis, and emerging pandemic strains of influenza. In order to break new ground in the study of human immunology and develop effective vaccines and therapies that specifically target immune responses at the sites where they are needed, it is essential to move beyond conventional studies of human peripheral blood and study immune responses in the tissue sites.
Activation of T lymphocytes occurs in lymphoid tissue and generates functionally diverse subsets of effector T cells with the capacity to migrate to multiple tissue sites (Campbell et al., 2003). While most activated effector cells die after a brief lifespan, a subset of primed T cells develop into long-lived memory T cells which persist as heterogeneous populations in lymphoid and mucosal sites. Studies in mice have revealed an important role for tissue-resident memory CD4+ and CD8+ T cells in protective immunity to site-specific pathogens in the lung and skin (Gebhardt et al., 2009; Liu et al., 2010; Teijaro et al., 2011), and that mucosal sites such as lung and intestine contain tissue-retained memory populations that do not recirculate (Jiang et al., 2012; Klonowski et al., 2004; Teijaro et al., 2011). These studies suggest that the protective efficacy of T cell responses cannot be measured or approximated from studying peripheral blood. In humans, studies on immune cells in tissue sites are limited to using individual tissues surgically excised due to disease. It is not known how circulating T cells are related to those in lymphoid and mucosal tissue sites within an individual, and how memory T cell responses are organized and maintained throughout the body.
Through the collaboration of the New York Organ Donor Network (NYODN), the organ procurement organization (OPO) for the greater New York metropolitan area, we have obtained access to multiple lymphoid and mucosal tissues from individual organ donors with a healthy immune system. We present here a multi-dimensional analysis of T cells throughout the human body from 24 different donors aged 15–60years, revealing distinct compartmentalization of naïve, effector and memory CD4+ and CD8+ T cell subsets intrinsic to the tissue site that is remarkably consistent in diverse individuals. Memory CD4+ T cells represent the majority subset in mucosal tissues and accumulate in lymphoid tissue throughout life. CD8+ T cell subsets, by contrast, are maintained as naïve cells in lymphoid compartments over decades, with memory CD8+ T cells mainly in mucosal sites and terminal effector cells confined to circulation. Importantly, memory T cells in all tissues specifically upregulate CD69 expression, a marker of T cell receptor (TCR)-mediated signaling, which distinguishes tissue-resident from circulating populations. Functionally, the majority of tissue-resident T cells were quiescent or IL-2-producing memory CD4+ T cells, followed by IFN-γ-producing memory CD8+ T cells, with IL-17 production confined to memory CD4+ T cells in mucosal compartments. Our results provide a three-dimensional analysis of human T cell immunity from which to interpret and study disease- and pathogen-specific immune responses, and to design new strategies for improving human health through vaccination and immunomodulation.
RESULTS
Tissue-specific compartmentalization of human lymphocytes
In collaboration with the New York Organ Donor Network (NYODN), we set up a research protocol to obtain multiple lymphoid and mucosal tissues from individual organ donors at the time of acquisition for life-saving transplantation. The 24 donors in this study comprise a broad sampling of the population between the ages of 15–60yrs (median age 38), all of whom died suddenly of traumatic causes, such as cerebrovascular stroke (33%), head trauma due to motor vehicle accident or homicide (33%), or anoxia due to suicide, drug-related deaths or other causes (33%) (Table 1). All donors were HIV-negative, free of cancer and other chronic or immunological diseases (data not shown). From each donor, we obtained blood and eight different healthy tissues including multiple lymphoid tissues (spleen, inguinal, mesenteric and bronchial/lung-draining lymph nodes (LNs)) and mucosal tissues including the lung, small intestine regions (jejunum, ileum) and colon (Figure 1).
Table 1.
Profile of organ donors from whom tissues were obtained for this study
| Donor code | Age | Gender | Cause of Death |
|---|---|---|---|
| #1 | 17 | M | Cerebrovascular stroke |
| #2 | 15 | F | Head Trauma |
| #3 | 55 | F | Cerebrovascular stroke |
| #4 | 32 | M | Anoxia |
| #7 | 31 | M | Head Trauma (MVA) |
| #8 | 17 | M | Anoxia/suicide |
| #9 | 60 | F | Cerebrovascular stroke |
| #10 | 39 | F | Cerebrovascular stroke |
| #11 | 46 | M | Anoxia/suicide |
| #12 | 39 | M | Head trauma (homicide) |
| #13 | 48 | M | Cerebrovascular stroke |
| #16 | 19 | M | Anoxia/drugs |
| #18 | 51 | M | Anoxia/cardiovascular |
| #26 | 33 | F | Anoxia/drugs |
| #27 | 52 | M | Cerebrovascular stroke |
| #29 | 23 | F | Anoxia/suicide |
| #33 | 55 | F | Cerebrovascular stroke |
| #39 | 32 | M | Anoxia/drugs |
| #40 | 30 | M | Cardiac arrest |
| #41 | 22 | M | Head trauma (MVA) |
| #42 | 54 | F | Cerebrovascular stroke |
| #43 | 60 | F | Anoxia/brain edema |
| #44 | 50 | M | Cerebrovascular stroke |
| #45 | 37 | M | Head Trauma (Fall) |
Figure 1. Lymphocyte subset frequency is intrinsic to the tissue site within and between individuals.
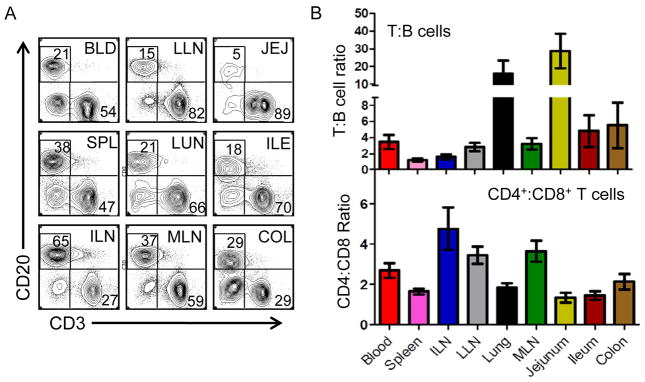
Lymphocytes were isolated from the tissues indicated in Table 1 and analyzed by flow cytometry. (A) Representative flow cytometry plots showing CD3 and CD20 expression, indicating T and B cell subsets, respectively, gated on forward and side scatter lymphocyte populations. Tissue designations from left to right: BLD, blood; LLN, lung lymph node; JEJ, jejunum; SPL, spleen; LUN, lung; ILE, ileum; ILN, inguinal lymph node; MLN, mesenteric lymph node; COL, colon. Data derive from Donor 3. (B) Upper: Graph shows relative T and B cell frequency in the nine tissue sites compiled from 14 donors (donor #’s 1–4, 7–10, 12, 13, 20, 27, 33, 40), plotted as the ratio of T:B cell frequency in each tissue site ±SEM. Lower: CD4+ and CD8+ T cell subset composition in different human tissue sites calculated as the mean ratio CD4+:CD8+ T cell frequency ±SEM within CD3+ cells from 17 individual donors (donor #’s 1–4, 7–10, 12, 13, 16, 18, 26, 27, 33, 39, 40). Individualized datasets for each donor are shown in Table S1.
We isolated mononuclear lymphocytes from all nine tissue sites (see Experimental Procedures), and obtained high lymphocyte yield and excellent sample integrity shown by representative flow cytometry analysis of CD3 and CD20 expression indicating frequency of T and B cells, respectively, in each site (Figure 1A). Within an individual, lymphocyte subset composition as assessed by the T:B cell ratio, differed in blood, lymphoid and mucosal compartments; whereas spleen contained a similar frequency of T and B cells, the frequency of T cells outnumbered B cells in all other sites-- by 2–4-fold in blood and LNs, 5–8 fold in ileum and colon and >15–20 fold in the lung and jejunum (Figure 1A, B, upper). These tissue-specific lymphocyte distribution patterns were remarkably similar between individuals despite differences in age, cause of death and lifestyle, as shown in the compiled ratio plots with limited variance among 17 donors (Figure 1B and see Table S1 for individual data for each donor). In addition, the T cell compartment is consistently dominated by CD4+ T cells throughout the body, except for intestines where comparable proportions of CD8+ and CD4+ T cells were found (Figure 1B, lower). Our results indicate that lymphocytic composition in humans is intrinsic to the tissue site regardless of background or antigenic exposure, and that tissues from organ donors can provide a unique snapshot into steady state adaptive immunity in humans that is highly conserved between individuals.
Memory T cells predominate throughout the body and accumulate with age
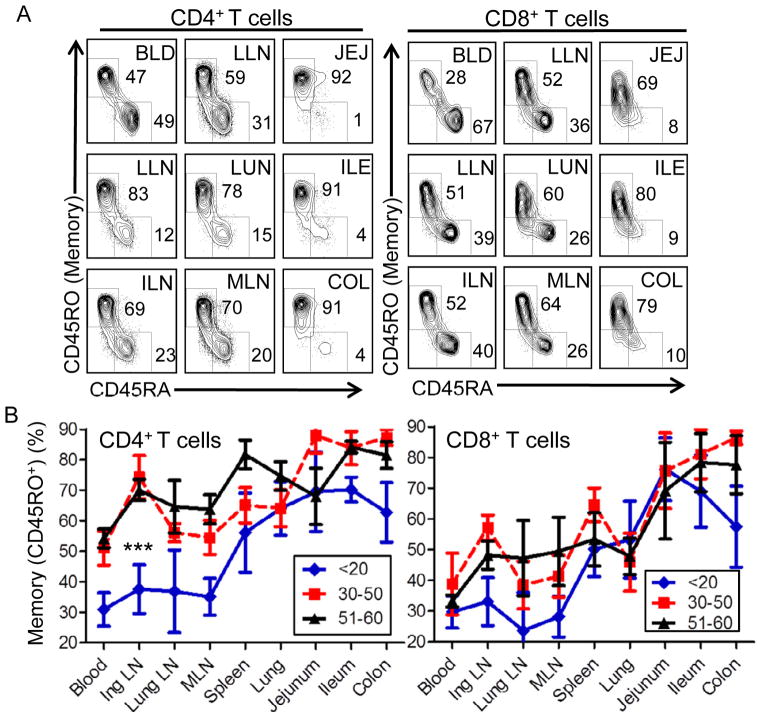
Expression of the CD45RO isoform is a well-known marker used to phenotypically identify memory CD4+ and CD8+ T cells (Dutton et al., 1998; Merkenschlager and Beverley, 1989), and conversely, CD45RA isoforms are expressed by naïve and terminally differentiated effector cells (Sallusto et al., 2004; Sallusto et al., 1999). We analyzed the proportion of CD45RO+ memory CD4+ and CD8+ T cells in each tissue compared to CD45RA subsets from multiple donors. As shown in a representative CD45RO vs CD45RA staining profile of 9 tissue sites from a donor near the median age (Donor 39, age 32), most of the tissues have a preponderance of CD45RO+ memory T cells, whereas peripheral blood contains a comparable proportion of CD45RO+ and CD45RA+ subsets (Figure 2A). CD45RO+ memory T cells predominate in mucosal sites including lung, jejunum, ileum and colon (>90% of total T cells); whereas their frequency in lymphoid tissue was reduced compared to mucosal sites (60–70%), it still exceeded that of CD45RA-expressing subsets (Figure 2A). Similar results were observed in adult donors aged 30 or older, with a representative older donor shown in Figure S1B. In a representative younger donor, CD45RA+ cells were at a higher frequency than CD45RO+ cells in lymphoid tissues, ranging from 40–70%, whereas the CD45RO+ subset still predominated in mucosal sites representing 70–95% of total T cells (Figure S1A). These results demonstrate that human memory T cells predominate throughout the body and particularly in mucosal sites of all individuals, whereas their frequency in lymphoid tissue may vary with age.
Figure 2. Memory T cells predominate throughout the body and vary with age in lymphoid compartments.
Lymphocytes isolated from indicated donor tissues were analyzed for CD45RO expression to delineate memory cells from CD45RA-expressing subsets representing either naïve or terminal effector cells. (A) Representative flow cytometry analysis of CD45RA and CD45RO expression from CD4+ T cells (left) and CD8+ T cells (right) from Donor 39, aged 32. Tissue designations from left to right: BLD, blood; LLN, lung lymph node; JEJ, jejunum; SPL, spleen; LUN, lung; ILE, ileum; ILN, inguinal lymph node; MLN, mesenteric lymph node; COL, colon. (B) Change in memory T cell frequency and distribution in different age groups. Plots show the mean frequency (±SEM) of memory (CD45RO+) CD4+ (left) and CD8+ (right) T cells in different tissues compiled from individuals of 3 age groupings: young (<20yrs; n=5), middle (30–50yrs, n=7) and older (50–60yrs, n=5). Differences in the overall proportion of memory CD4 T cells is highly significant (***) comparing the <20 to the 30–50yr (p=3×10−7) or comparing the <20 to the 51–60yr group (p=0.0005), but not significant comparing the 30–50yr to the 51–60yr groups (p=0.57). For CD8+ T cells, differences in the proportion of memory T cells was significant (**) comparing the <20yr to the 30–50yr (p=0.003), or to the 51–60yr group (p=0.04), but not significant between the 30–50yr and 51–60yr groups (p=0.33). CD45RO frequency for each of the 17 donors analyzed (donor #’s 1, 2, 3, 7, 8, 9, 10, 12, 13, 16, 18, 20, 27, 29, 33, 39, 40) is shown in Table S1.
We further investigated the age-dependence of memory T cell frequency in the body by compiling results from three donor age groups: one representing teens and young adults (<25yrs), a second representing individuals in middle-years (30–50yrs), and a third comprising those in the sixth decade of life (50–60yrs) (Figure 2B with individual datasets shown in Table S1). In mucosal sites and in spleen, both CD4+ and CD8+ T cells were predominantly memory (60–90%) throughout all stages of life, with intestines containing a higher proportion of memory T cells compared to lung and spleen (Figure 2B). In blood and lymph nodes, the frequency of memory CD4+ T cells differed significantly between the youngest and two older groups (p<0.0005, Figure 2B), being present in low frequency (average 30%) in younger donors, and comprising >50–70% in the two older adult groups, aged 30–60 (Figure 2B, left). By contrast, memory CD8+ T cells remained a minority population (<40%) in blood and lymph nodes in all age groups with CD45RA+ CD8+ T cells prevailing in these sites (Figure 2B). Our findings therefore show that over decades of life, memory CD4+ T cells accumulate in lymphoid tissue to proportions seen in mucosal sites, whereas memory CD8+ T cells retain their biased predominance in mucosal sites and do not accumulate over time in lymphoid compartments.
Distinct composition and compartmentalization of CD4+ and CD8+ effector and memory subsets
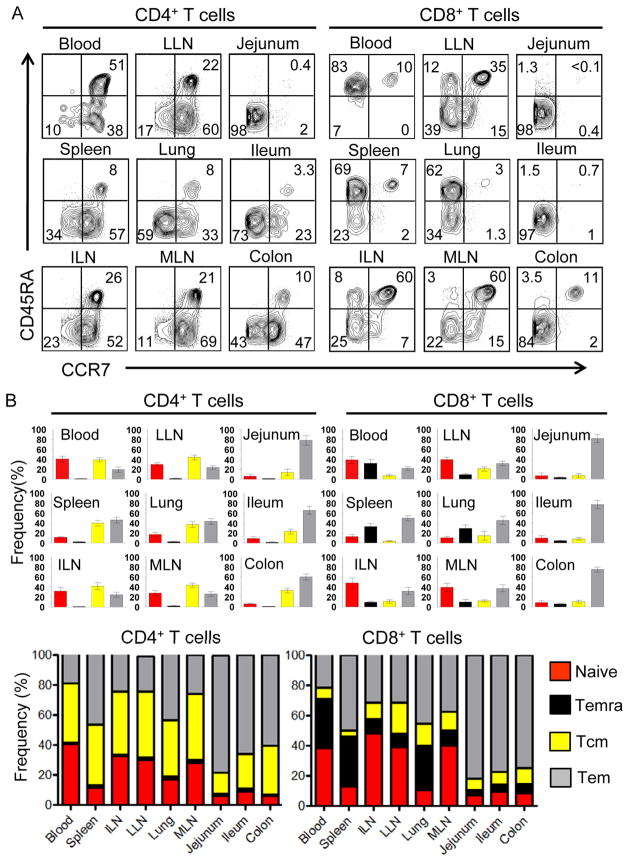
In humans, heterogeneous expression of the lymph node homing receptor CCR7 defines additional functional subsets of CD45RA+ and CD45RO+ T cells. Naïve T cells are primarily CD45RA+CCR7 +. There is also a subset of CD45RA+ T cells that are CCR7-negative, which are designated terminally differentiated effector T cells (designated Temra) (Sallusto et al., 2004). CD45RO+ memory T cells are subdivided into CCR7+ “central-memory” (Tcm) cell subsets which migrate to lymphoid tissue and CCR7− “effector-memory” (Tem) cell subsets which circulate to non-lymphoid sites (Masopust et al., 2001; Sallusto et al., 2004; Sallusto et al., 1999). We used coordinate analysis of CCR7 and CD45RA expression by CD4+ and CD8+ T cells in each site to precisely define how naïve, Temra, Tcm and Tem subsets are organized within an individual (Figure 3A) and whether tissue-specific patterns are conserved between individuals (compiled results from nine donors in Figure 3B, with individual datasets shown in Table S2). Analysis of CD4+ T cells subsets revealed that they comprise naïve, Tcm and Tem cell populations with tissue-intrinsic distribution: Blood and lymph nodes contained naïve, CD4+ Tcm and Tem cells in similar proportions, spleen and lung had mostly Tem cells followed by Tcm cell subsets, whereas intestinal sites contained predominantly Tem cells, with Tcm CD4+ T cells also present in colon (Figure 3B, left). The composition and distribution of CD8+ T cells, however, differed in several ways from that of CD4+ T cells. First, CD8+ Tcm existed in very low frequencies throughout the body –even in lymphoid tissue; second, the Temra subset was found only among CD8+ T cells and only significantly in blood, spleen and lung and not in other sites; third, naïve phenotype-CD8+ T cells were present in higher frequencies in blood and lymph nodes compared to their frequency among CD4+ T cells, and finally, CD8+ Tem completely predominated in intestinal tissues (Figure 3B, right). A frequency composite of the tissue-specific subset composition of CD4+ and CD8+ T cells (Figure 3B, bottom) reveals that CD4+ T cells were maintained as naïve, Tcm and cell subsets, whereas CD8+ T cells persisted as naive, Temra and Tem populations. For both CD4+ and CD8+ T cells, the proportion and distribution of these subsets in each tissue is likewise consistent between diverse individuals (Figure 3B and Table S2). Our results therefore demonstrate that the differentiation and maintenance of CD4+ and CD8+ T cells within an individual are intrinsic to the tissue compartment and cell lineage.
Figure 3. Distinct tissue distribution and subset composition of naïve, memory and terminal effector CD4+ and CD8+ T cell subsets.
(A) Coordinate expression of CD45RA and CCR7 by CD4+ (left) and CD8+ (Right) T cells in blood and 8 tissue sites delineates four subsets corresponding to naïve (CD45RA+CCR7+, upper right quadrant), terminal effector (Temra, CD45RA+CCR7−, upper left quadrant), central memory (Tcm, CD45RA−CCR7+, lower right quadrant), effector-memory (Tem, CD45RA−CCR7−, lower left quadrant). Results shown are from Donor 41, representative of nine donors. (B) Frequency of naïve (red), Temra (black), Tcm (yellow) and Tem (grey) CD4+ (left) and CD8+ (right) T cell subsets in each tissue site, compiled from nine donors (donor #’s 3, 15, 39, 40, 41, 42, 43, 44, 45; individualized datasets for each donor shown in Table S2). Small individual graphs show frequency of each subset in each site expressed as mean±SEM, calculated from dot plots as in (A). Lower: Large composite graphs show average frequency of each subset (naïve, Temra, Tcm, Tem) within CD4+ (left) or CD8+ (right) T cells in each tissue site.
CD69 expression distinguishes tissue-resident from circulating memory and effector subsets
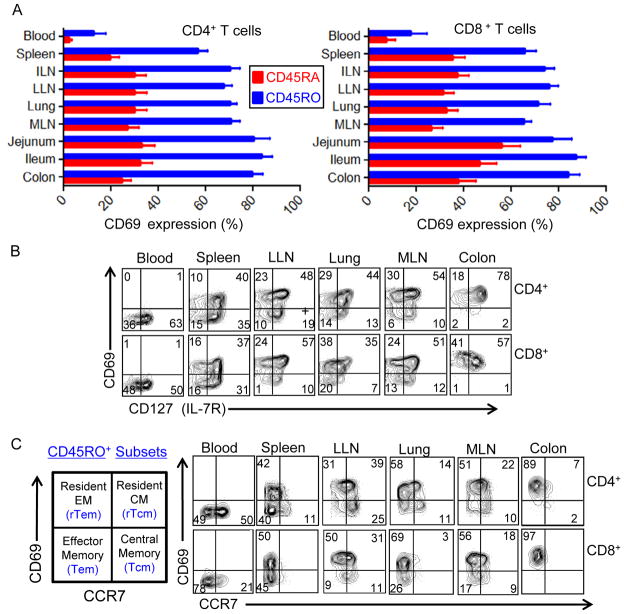
In all donors examined, memory (CD45RO+) CD4+ and CD8+ T cells in lymphoid and mucosal tissue sites exhibited constitutive upregulation of the early TCR-coupled activation marker CD69, with 60–100% of memory T cells in tissues being CD69+ (Figure 4A, Figure S2, and Table S3). By contrast, the majority of memory T cells in blood were CD69-negative, with CD69 expression found on only 1–20% of circulating memory T cells in 16/19 donors analyzed (Table S3). CD45RA+ T cells in all sites were also predominantly CD69-negative, including 100% of naïve T cells in blood, and 70–95% CD45RA+ T cells in tissues (Figure 4A, Figure S2, and Table S3). CD69hi memory T cells in lymphoid and mucosal sites were largely IL-7R+ (Figure 4B), indicative of a resting memory T cell phenotype, as IL-7R is downregulated on activated effector T cells (Dooms et al., 2007; Kaech et al., 2003; Paiardini et al., 2005; Seddiki et al., 2006). Together, these results show specific upregulation of CD69 on resting memory T cells in lymphoid and mucosal tissues.
Figure 4. CD69 expression distinguishes tissue-resident from circulating memory T cell subsets.
(A) Mean frequency (±SEM) of CD69+ T cells gated on CD45RA+ (red bars) and CD45RO+ (blue bars) CD4+ (left) and CD8+ (right) T cells in blood and tissues compiled from 19 donors (donor #’s 1, 7, 8, 9, 10, 12, 13,14,15, 27, 29, 33, 39, 40, 41, 42, 43, 44, 45; individual frequencies from each tissue of each donor are shown in Table S3, top portion). (B) IL-7 receptor (CD127) is expressed by the majority of CD69+ tissue-resident memory T cells in lymphoid and mucosal sites. Flow cytometry plots show CD127 expression as a function of CD69 expression gated on CD45RO+ CD4+ (upper) or CD8+ (lower) T cells in blood and indicated tissue sites. Results are from Donor 41 and representative of five donors. (C) CCR7 and CD69 expression by CD45RO+ CD4+ (upper row) and CD8+ (lower row) T cells delineates four subsets: circulating central memory (Tcm:CCR7+CD69−), circulating effector-memory (Tem:CCR7−CD69−) cells, resident Tcm (rTcm:CCR7+CD69+) and resident Tem (rTem:CCR7−CD69+) subsets (see leftmost quadrant diagram). Results are from Donor 39 and representative of seven donors.
We combined multiparameter analysis of memory subset markers (CD45RO and CCR7) with CD69 as a marker of tissue residence to obtain a composite picture of circulating and tissue-resident memory CD4+ and CD8+ T cell subsets throughout the body (Figure 4C). Memory T cells in the whole body comprise circulating central-memory (Tcm;CCR7+CD69−), circulating effector-memory (Tem;CCR7−CD69−), resident central-memory (rTcm; CCR7+CD69+) and resident effector-memory (rTem;CCR7−CD69+) subsets (Figure 4C). The composition of resident and circulating memory subsets was specific to each compartment within an individual, and differed in blood, spleen, LN and mucosal sites. Blood contained circulating Tem and Tcm equivalently represented among CD4+ T cells and mostly Tem for CD8+ T cells, whereas spleen was enriched for Tem and rTem CD4+ and CD8+ T cells. Lymph nodes contained rTcm and rTem CD4+ T cell subsets and rTem CD8+ T cells, with a small proportion of circulating Tcm cells (Figure 4C). Mucosal sites likewise differed in subset composition: Lung contained a predominant rTem subset, a low frequency rTcm fraction, and minority populations of circulating Tcm and Tem subsets (Figure 4C). By contrast, all intestinal sites examined including jejunum, ileum and colon contained only rTem CD4+ and CD8+ T cells (Figure 4C and data not shown). These results indicate that circulating subsets are present in low frequencies only in certain tissue sites such as spleen, LN and lungs, are completely absent from intestines, and that resident memory T cells predominate in lymphoid and mucosal sites and do not circulate in blood.
CD103 expression defines mucosal memory CD8+ T cells
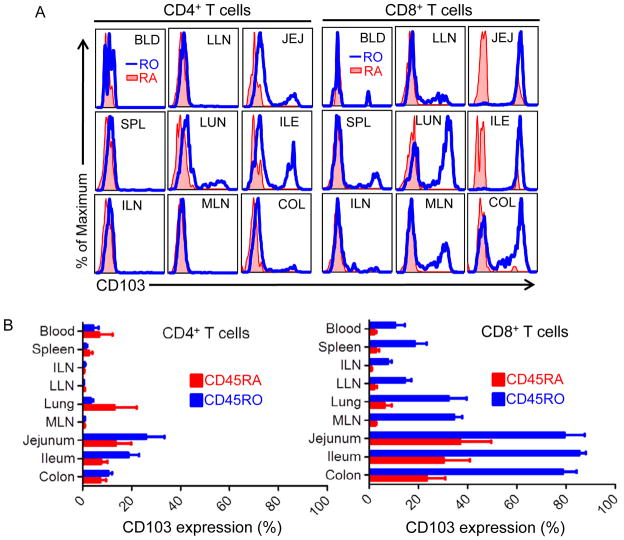
CD103 expression is associated with intestinal immune cell populations and tissue-infiltrating effector CD8+ T cells in mice (Schon et al., 1999; Sheridan and Lefrancois, 2011). We found that rTem CD8+ T cells in mucosal sites were further distinguished by expression of the integrin CD103 (Figure 5). Within an individual, all memory CD8 T cells in small intestine and colon were CD103+, with significant fractions of CD103+ memory CD8 T cells in lung and intestinal-draining LN, while blood, spleen and peripheral LN memory CD8 T cells were uniformly CD103− (Figure 5A). A proportion of CD8 Temra cells in intestines showed upregulated CD103 expression, although not to the same extent observed by memory T cells in these sites (Figure 5A). This compartmentalized expression of CD103 on mucosal memory CD8 T cells was consistent between all donors examined (Figure 5B and Table S3). Together, these results show that intestinal CD8+ rTem are not equivalent to rTem in other tissue sites, and suggest tissue-specific influences on resident Tem.
Figure 5. The integrin CD103 (αEβ7) specifically marks mucosal CD8 T cells.
(A) Expression of CD103 by CD45RO+ (blue histogram) and CD45RA+(red-filled histogram) CD4+ (left) and CD8+ (right) T cells from blood and 8 tissue sites. Results are shown from Donor 40 and are representative of 11 donors. (B) CD103 expression on CD45RO+ and CD45RA+ CD4+ (left) and CD8+ (right) T cells shown as percent CD103+ for each subset from each tissue site compiled from 11 donors (donor #’s 1, 7, 8, 9, 10, 12, 13, 14, 15, 27, 29, 33; individual frequencies for each donor in each site are presented in Table S3, lower part).
Functional profile of T cells resident in different tissue sites
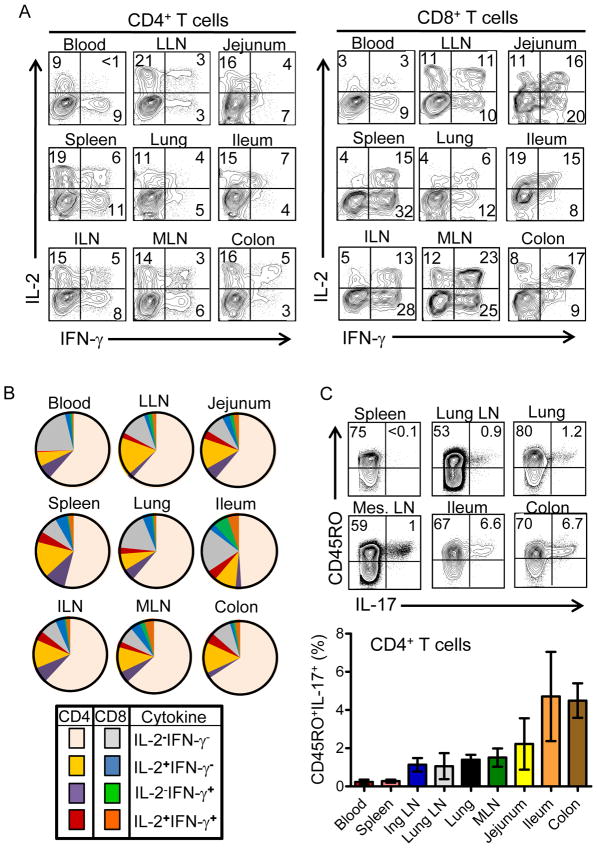
A hallmark of memory T cells is their rapid functional recall within hours of stimulation. We examined whether functional capacity of memory CD4+ or CD8+ T cells was differentially compartmentalized in tissue sites. Following short-term stimulation with PMA+ionomycin, the two most abundant cytokines produced by human CD4+ and CD8+ T cells in all sites were IFN-γ and IL-2, (Figure 6A), followed by TNF-α with minimal IL-4 and IL-10 at rapid times (data not shown). CD4+ T cells had comparable proportions of IL-2 and IFN-γ producers in the blood; however, in all tissues, the predominant cytokine secreted by CD4 T cells was IL-2 (Figure 6A and Figure S3A). In contrast, CD8+ T cells showed a preponderance of rapid IFN-γ-producers in the blood and all tissue sites, with dual IFN-γ/IL-2-producers found only in tissue sites, representing 30–50% of the total IFN-γ producers (Figure 6A). This differential pattern of IL-2 and IFN-γ secretion by CD4+ and CD8+ T cells in tissue sites was conserved between donors (Figure S3A and Table S4). Memory CD4+ and CD8+ T cells in each site were the main source of rapid IL-2 and IFN-γ production, respectively, with a small proportion of CD45RA+ CD8+ T cells producing IFN-γ in spleen, lungs and intestines (Figure S3B), likely derived from Temra cells in these sites. Comparing the absolute frequency of IL-2, IFN-γ- and dual-producing CD4+ and CD8+ T cells in the nine sites, reveals that quiescent CD4+ T cells, which did not secrete rapid cytokines, were the most abundant population throughout the body, followed by memory CD4+ T cells producing IL-2, dual IFN-γ and IL-2-producing CD4+ T cells and IFN-γ-producing CD8+ T cells (Figure 6B).
Figure 6. Functional profile of T cells in lymphoid and mucosal tissues.
T cells purified from indicated tissue sites were stimulated for 4hrs with PMA/ionomycin and cytokine production was assessed by intracellular cytokine staining (ICS). (A) IFN-γ and IL-2 production by CD4+ and CD8+ T cells in different tissue sites, with gates drawn based on unstimulated controls. Numbers in quadrants indicates percent of CD4+ T cells (left) or CD8+ T cells (right) producing IFN-γ, IL-2 or both cytokines. Results are from Donor 29, representative of four donors (#18, 26, 27, and 29) from which coordinate analysis of IFN-γ and IL-2 production was accomplished. (B) Individual pie chart diagrams showing the frequency of CD4+ and CD8+ T cells with rapid cytokine producing capacity in each tissue site from one donor, representative of four donors, showing a high proportion of quiescent CD4+ T cells in each site and a minority population of effector-memory CD4+ or CD8+ T cells. (C) IL-17 production is confined to memory CD4+ T cells in mucosal sites. Upper: Representative ICS analysis of IL-17 production from spleen, mucosal draining LN, lung and intestinal sites from Donor 9. Lower: Mean IL-17 production (±SEM) from blood and tissue of five donors where we analyzed IL-17 expression (Donor #’s 9, 10, 18, 26, 27).
We also assessed production of the pro-inflammatory cytokine IL-17 known to be associated with T cells in mucosal and inflammatory sites (Dubin and Kolls, 2008; Korn et al., 2009; Marks and Craft, 2009), following short-term stimulation of T cells in blood, lymphoid and mucosal sites. We detected IL-17 production only from PMA+ionomycin-stimulated memory CD4+ T cells in mucosal sites (and not from CD8+ T cells, data not shown) with the highest proportion from colon and ileum, lower frequencies in lung and mucosal-draining LN and negligible production from T cells in blood and spleen (Figure 6C and Table S4). When taken together, our functional analysis of T cell responses throughout the body show that a limited population of resident T cells has the capacity for immediate effector responses, with the majority of T cells in the body being quiescent or IL-2-producing memory CD4+ T cells, and IL-17 production showing tissue-specific and subset-specific compartmentalization.
DISCUSSION
We have taken a major step in elucidating fundamental and heretofore undefined aspects of human adaptive immunity by studying T cells isolated from human tissues obtained from organ donors. The resultant phenotypic and functional analysis of CD4 and CD8 T cells from blood, lymphoid and mucosal sites of 24 individuals reveals new insights into how T cell subsets are distributed and functionally maintained throughout the body. Specifically, CD4 T cells are the most abundant subset in tissue sites, with effector-memory CD4 T cells prevalent in mucosal tissues and accumulating as central-memory subsets in lymphoid sites. CD8 T cells are distributed as predominant effector-memory populations in mucosal tissue and spleen, as terminal effector cells in circulation, and are maintained as naïve cells in lymphoid tissue. Importantly, all memory T cells in lymphoid and mucosal sites constitutively express CD69, contrasting circulating memory T cells and all naïve subsets, and defining resident memory populations, with CD103 expression specific to mucosal memory CD8 T cells. Functionally, the majority of tissue-resident T cells were quiescent or IL-2 producing memory CD4 T cells with lower frequencies of effector CD8 T cells, and IL-17 production confined to mucosal tissue. Our results provide a new view of how human T cells are compartmentalized throughout the human body important for targeting tissue-resident immune responses and defining disease-specific immune pathologies.
Previous studies on T cells isolated from human tissues have been limited to individual tissues surgically excised due to disease or chronic inflammation, including tonsils obtained from tonsillectomies (Bentebibel et al., 2011), lungs from patients with lung cancer (Purwar et al., 2011) and intestines from IBD (Hovhannisyan et al., 2011). The steady state of T cells in healthy tissues within a single individual has not previously been investigated or defined. Here, brain-dead organ donors who died of traumatic, non-immunological causes were used as source of tissues for immunological analysis, which were obtained coincident with organ acquisition for clinical transplantation. The immediate isolation of cells with high viability from these tissues enabled an in-depth ex vivo analysis of human T lymphocytes from multiple anatomic compartments, in ways only previously possible to achieve in mice. However, in mice, tissue immune responses have been examined following infection of inbred mouse strains with a single pathogen, and it is not known whether infection with many diverse pathogens and maintenance of the immune system for decades of life will result in large variations in T cell subset composition throughout and within tissue sites. From our analysis of tissues from 24 different donors, we found notable consistencies in the compartmentalization of T cell subsets between donors despite different causes of death, diverse lifestyle, and the known heterogeneity of the human population. These findings indicate that the organization, differentiation and maintenance of human T cells in strikingly tissue-intrinsic, and that tissue from organ donors as obtained here is a valuable source for analyzing steady-state immune responses.
We found striking disparities in human CD4 and CD8 T cell subset compartmentalization throughout the body. CD4 T cells are maintained as naïve, Tcm and Tem subsets with no appreciable Temra subsets, whereas CD8 T cells persist as naive, Temra and Tem populations, with CD8+ Tcm present in low frequencies in lymphoid tissue and negligible frequencies in other sites. The low proportion of CD8+ Tcm compared to CD4+ Tcm was not predicted from analysis of peripheral blood (Gattinoni et al., 2011; Sallusto et al., 1999), and is most striking when comparing whole body subset delineation as accomplished here. Interestingly, the Temra subset is only found among CD8+ T cells in blood, spleen and lung, where circulating populations are found. These results suggest that CD8+ T cells may be actively responding to antigenic stimulation through interaction with MHC Class I-expressing cells in tissues and resulting in effector differentiation and migration to the periphery. An in-depth analysis of gene expression in CD4+ and CD8+ T cell subsets in the tissues will be essential to dissect their lineage relationship and origin.
In addition to differences in composition, CD4+ and CD8+ T cell subsets also exhibit distinct patterns of maintenance in lymphoid tissues. In younger individuals, CD4+ T cells were comparably apportioned into naïve and memory subsets in lymphoid tissues; however, in donors aged 30 or older, memory CD4+ T cells represented the majority population in lymphoid tissue. By contrast, naïve CD8+ T cells were the majority population in blood and lymph nodes throughout six decades of life. Maintenance of a high proportion of naïve CD8+ T cells in lymphoid tissue could arise from a lack of significant recycling of memory CD8+ T cells back to lymphoid compartments due to the paucity of Tcm among CD8+ T cells. Alternately, differences in homeostatic turnover between CD4+ and CD8+ T cells could contribute to differences in memory accumulation, as phenotypic conversion of mouse and human naïve T cells to memory cells can occur due to homeostatic expansion independent of effector differentiation (Onoe et al., 2010; Purton et al., 2007; Surh and Sprent, 2008; Tan et al., 2002). Thus, the dynamic accumulation of IL-2-producing memory CD4+ T cells in LN may arise by homeostatic turnover, while human CD8+ T cells may be less susceptible to homeostasis-driven alterations compared to CD4+ counterparts, resulting in more stable maintenance of naïve CD8+ T cells in lymphoid tissue. Conversely, the compartmentalization of effector-memory CD8+ T cells in mucosal sites, could indicate antigen-driven differentiation and stable retention of differentiated memory T cells in mucosal compartments. An evaluation of TCR clonality and repertoire in future studies will provide insights into how CD4+ and CD8+ T cells are maintained and circulate throughout the body.
In all donors, we found that the majority (>80%) of memory T cells in lymphoid and mucosal tissue had constitutive CD69 expression, while circulating memory T cells in blood were predominantly CD69-negative. CD69 is a well-known early marker of TCR signaling in humans and mice (Krishnan et al., 2001; Ziegler et al., 1994) and can also be upregulated by mouse CD8 T cells in response to type I IFN (Sun et al., 1998). CD69+ memory T cells have been identified in the skin, lungs and intestines of humans and mice (Clark et al., 2012; Jiang et al., 2012; Masopust et al., 2010; Masopust et al., 2006; Teijaro et al., 2011), although mouse memory T cells in spleen and LN are known to be CD69lo (Casey et al., 2012; Teijaro et al., 2011). However, we found that human memory T cells in LN and spleen were CD69+, indicating a key difference in either the maintenance or signaling state of human lymphoid compared to mouse lymphoid memory T cells. Tissue-resident CD69+ human memory T cells appeared quiescent as they maintained expression of IL-7R and were CD25-negative. Moreover, CD69 upregulation identified here was not due to tissue digestion or incubation as T cells isolated from LN and mucosal sites by mechanical, non-enzymatic disruption were likewise CD69+ (data not shown). We propose that CD69 upregulation by tissue memory T cells may derive from low level TCR engagement, responses to cytokines, and/or tonic signaling by interaction with tissue-resident DC or tissue accessory cells. This heightened activation state of tissue memory T cells may facilitate responses to low antigen doses or to costimulation-based second signals, increasing protective responses to site-specific pathogens.
Molecules expressed by tissue-resident memory T cells may also play roles in their retention. For example, CD69 upregulation by activated mouse T cells was shown to trigger retention in LN (Shiow et al., 2006). In addition, CD103, shown here to be expressed specifically by human mucosal memory CD8 T cells, is also known to play a role in retention in mouse intestines due to its interaction with E-cadherin on epithelial cells (Schon et al., 1999) (Sheridan and Lefrancois, 2011). In addition, T cell specificity may also mediate retention of certain T cell clonal populations. IL-17 production is has been associated with responses to intestinal microbiota in mice (Ivanov et al., 2009; Ivanov et al., 2008), and our finding that IL-17 production is confined to human memory CD4 T cells in mucosal sites suggests active retention of this functional subset through mucosal-specific factors. Moreover, the presence of IL-17+ memory CD4 T cells in mucosal-draining lymphoid tissues suggests that lymphoid tissue may be active sites of local priming for IL-17+ memory CD4 T cells.
Our findings indicate that the subset composition and phenotype of peripheral blood T cells does not reflect that of spleen, lymph nodes or mucosal tissues, suggesting that blood is a distinct compartment. Our results also suggest potential trafficking patterns for migration of circulating and tissue-resident naïve, effector and memory T cell subsets in blood, lymphoid and mucosal tissues. Blood-borne T cell subsets (naïve, Temra, Tcm, Tem) can enter certain tissue sites such as spleen, lung, and LN in low frequency, but are never found in intestinal sites. Moreover, the major subsets in all tissue sites are resident memory, with rTem populations predominating in lung, intestines, and spleen, CD4+ rTcm (CD4+) predominating in peripheral and tissue-draining LN along with CD4+ and CD8+ rTem. The origin of tissue-resident cells is not clear, although in mucosal sites, tissue-specific factors may act on its T cell inhabitants to maintain their residence and prevent egress, through the expression of molecules such as CD103. Molecular profiling of resident versus circulating subsets in future studies will provide keys to their lineage relationships and tissue-specific profiles.
Basic knowledge of the properties of human tissue T cells throughout the body, for which the findings in the paper represent an important first step, can contribute to a better understanding of a wide range of human diseases. Already, the findings presented here establish a new fundamental baseline for steady state human T cell immunity which can be applied to define more precisely the immune dysfunctions associated with site-specific and systemic autoimmune and inflammatory diseases. Understanding the origin and identity of tissue-resident T cell populations can also lead to therapies that target memory T cells to the specific sites of pathogen entry and persistence for protection. In conclusion, our approach to study human immune cells in tissue sites will open the door to fundamental studies on multiple aspects of human immunity, and will improve our ability to translate and optimize immunotherapies.
EXPERIMENTAL PROCEDURES
Acquisition of human tissues
Tissues were acquired from deceased organ donors in the OR at the time of organ acquisition for clinical transplantation through an approved research protocol and MTA with the NYODN. At the time of death, all donors were free of chronic disease and cancer, were HIV-negative and aged 60yrs or younger. Because tissues were obtained from deceased (and not living) individuals, the study does not qualify as “human subjects” research, as confirmed by the Columbia University IRB. Consent for use of donor tissues for research was obtained by NYODN transplant coordinators.
Lymphocyte isolation from human lymphoid and non-lymphoid tissues
Tissue samples were maintained in cold saline and brought to the lab within 4–6 hours following organ procurement. In order to achieve high quality, viable lymphocytes we developed a rapid cell isolation protocol combining enzymatic digestion and mechanical dissociation. We avoided DTT and EDTA steps and longer incubation periods for non-lymphoid tissue to minimize isolation time without impacting sample quality. Spleen (1–2cm2), Inguinal LN (2–3 nodes), Mesenteric LN (5–10nodes) and Bronchial LN (2–5 nodes) were chopped into small pieces and placed into 50ml conical tubes and filled up to 50mls with enzymatic digestion solution (RPMI containing 10% FBS, L-glutamate, sodium pyruvate, non-essential amino acids, penicillin-streptomycin, collagenase D (1mg/ml), trypsin inhibitor (1mg/ml) and DNase I (50–100μg/ml)), and incubated for at 37°C for 2 hours. Tissue digests were then mechanically disrupted using the gentleMACS™ tissue dissociator (Milteyni Biotech), residual tissue fragments were removed through a stainless steel tissue sieve (10 to 150 mesh size), and the resultant cells were pelleted by centrifugation and resuspended into 25mls RPMI. For RBC removal, 1ml of HetaSep (StemCell technologies) was added to 5mls of cell suspension and centrifuged at 50g for 5 minute at RT. The resulting supernatant containing lymphocytes was carefully removed and washed with PBS, with residual RBCs lysed using AKC lysis buffer. In order to remove debris, fat and dead cells, pellets were resuspended into 30% percoll (GE Healthcare Life Sciences) and centrifuged at 50g for 10 minute at RT. The top layer and pellet were discarded and cells were washed twice to remove percoll.
Intestines and lungs were processed as above with the following modifications. Intestinal samples were dissected into three parts, Jejunum, terminal ileum and ascending colon after isolation of mesenteric LN. Tissues were cleaned of fat, Peyer’s patches were removed, and the subsequent intestinal tissue segments were washed with sterile PBS. We injected 10–20mls enzymatic digestion solution submucosally into intestinal segments and directly into lung pieces, prior to chopping into small pieces and placement into 50mls enzymatic digestions solution. An additional DNAse step (50μg/ml of DNAse for 15 minutes incubation at 37°C) was inserted after mechanical disruption for lungs and intestines. For PBMC isolation, samples were processed for RBC removal steps as described above. The resultant lymphocyte enriched cells from all samples were resuspended into complete RPMI media containing antibiotics resulting in > 95% viability.
Flow Cytometry Analysis
We used 8–12 color flow cytometry analysis for phenotypic analysis of human immune cells from tissue sites. The following fluorochrome-conjugated antibodies were used: anti-human CD3 (eFluor Nanocrystal 650, OKT3, eBioscience), CD4 (Qdot705, S3.5, Invitrogen), CD8 (Allophycocyanin/Cy7, SK1, Biolegend), CD45RA (Qdot605, MEM-56, Invitrogen), CD45RO (PerCP-eFluor 710, UCHL1, eBioscience), CD28 (Phycoerythrin/Cy7, CD28.2, Biolegend), CCR7 (Alexa Fluor 488, TG8/CCR7, Biolegend), CD69 (Brilliant Violet 488, FN50, Biolegend), CD20 (Phycoerythrin, 2H7, Biolegend), CD103 (Alexa Fluor 488 or Phycoerythrin, Ber-ACT8, Biolegend), CD127 (Allophycocyanin, A019D5, Biolegend), CD25 (brilliant violet, BC96, Biolegend). SYTOX blue (Invitrogen) was used for live-dead cell analysis. Cells were stained with fluorochrome-conjugated antibodies (8–12 color panel), and were acquired on a 6-laser LSR II analytical flow cytometer (BD Biosciences, San Jose, CA) using FACSDiva software in the Columbia Center for Translational Immunology (CCTI) flow cytometry core. Controls samples included unstained and single fluorochrome stained cells for accurate compensation and data analysis. Results were analyzed using FlowJo software.
Functional analysis
For T cell stimulation, 1–2×106 total cells in 200μl of complete RPMI medium containing PMA (50ng/ml) plus ionomycin (1μg/ml) were plated in 96 well round bottom plates and incubated at 37 °C for 4–5hrs in the presence of BD Golgi plug (Brefeldin A). The cells were washed with PBS and fixed at RT using 1.6% PFA and stored at 4 °C. The cells were stained for surface phenotypic marker followed by permeabilization (saponin) and intracellular cytokine staining. Following cytokine antibodies were used: IL-2 (Brilliant Violet 421, 17H12, Biolegend), IL-17A (Alexa fluor 488, BL168, Biolegend), TNF-α (Allophycocyanin, MA611, Biolegend), and IFN-γ (Phycoerythrin, 4S.B3, BD Pharmingen)
Statistical Analysis
The statistical and graphical analyses were done using GraphPad prism software and Microsoft excel. One tailed t tests were used to assess significance.
Supplementary Material
Acknowledgments
This work was supported by NIH “Challenge” grant RC1AI086164 awarded to D.L.F. We wish to gratefully acknowledge the generosity of the donor families and the outstanding efforts of the NYODN transplant coordinators and staff for making this study possible. We also wish to thank David Corrigan and Minjun Yu for help with the tissue processing, Dr. Siu-Hong Ho for help with the flow cytometry, and Dr. Steven Reiner for critical reading of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bentebibel SE, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci U S A. 2011;108:E488–497. doi: 10.1073/pnas.1100898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MJ. Memory T cells as an occupying force. Eur J Immunol. 2011;41:1192–1195. doi: 10.1002/eji.201041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DJ, Debes GF, Johnston B, Wilson E, Butcher EC. Targeting T cell responses by selective chemokine receptor expression. Semin Immunol. 2003;15:277–286. doi: 10.1016/j.smim.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. Antigen-Independent Differentiation and Maintenance of Effector-like Resident Memory T Cells in Tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N, Dorosario AA, Chaney KS, Cutler CS, Leboeuf NR, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4:117ra117. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med. 2007;204:547–557. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, de Frutos RL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Warke VG, Nambiar MP, Wong HK, Tsokos GC, Farber DL. Generation and biochemical analysis of human effector CD4 T cells: alterations in tyrosine phosphorylation and loss of CD3ζ expression. Blood. 2001;97:3851–3859. doi: 10.1182/blood.v97.12.3851. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks BR, Craft J. Barrier immunity and IL-17. Semin Immunol. 2009;21:164–171. doi: 10.1016/j.smim.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol. 2012;188:5811–5817. doi: 10.4049/jimmunol.1102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M, Beverley PC. Evidence for differential expression of CD45 isoforms by precursors for memory-dependent and independent cytotoxic responses: human CD8 memory CTLp selectively express CD45RO (UCHL1) Int Immunol. 1989;1:450–459. doi: 10.1093/intimm/1.4.450. [DOI] [PubMed] [Google Scholar]
- Onoe T, Kalscheuer H, Chittenden M, Zhao G, Yang YG, Sykes M. Homeostatic expansion and phenotypic conversion of human T cells depend on peripheral interactions with APCs. J Immunol. 2010;184:6756–6765. doi: 10.4049/jimmunol.0901711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiardini M, Cervasi B, Albrecht H, Muthukumar A, Dunham R, Gordon S, Radziewicz H, Piedimonte G, Magnani M, Montroni M, et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J Immunol. 2005;174:2900–2909. doi: 10.4049/jimmunol.174.5.2900. [DOI] [PubMed] [Google Scholar]
- Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions [see comments] Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;131:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 Jointly Regulate Homeostatic Proliferation of Memory Phenotype CD8(+) Cells but Are Not Required for Memory Phenotype CD4(+) Cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells. 1994;12:456–465. doi: 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.