Abstract
Epinephrine enhances memory in young adult rats, in part, by increasing blood glucose levels needed to modulate memory. In old rats, epinephrine is deficient at raising blood glucose levels and thus is only moderately effective at enhancing memory. In contrast, systemic glucose injections improve memory in old rats, with resulting memory performance equal to that of young rats. The diminished response of glucose to training in old rats may blunt downstream neurochemical and molecular mechanisms needed to upregulate memory processes. In the first experiment, young adult and old rats were trained on an inhibitory avoidance task with immediate post-training injections of aCSF or glucose into the dorsal hippocampus. Old rats had significant memory impairments compared to young rats 7 days after training. Intrahippocampal injections of glucose reversed age-related deficits, improving memory scores in old rats to values seen in young rats. A second experiment examined age-related changes in activation of the transcription factor CREB, which is widely implicated in memory formation and may act downstream of hormonal and metabolic signals. Activation was assessed in response to training with systemic injections of epinephrine and glucose at doses known to enhance memory. Young adult and old rats were trained on inhibitory avoidance with immediate post-training systemic injections of saline, epinephrine, or glucose. After training, old rats had significant impairments in CREB phosphorylation in area CA1 and the dentate gyrus region of the hippocampus, and in the basolateral and lateral amygdala. Epinephrine and glucose attenuated age-related deficits in CREB phosphorylation, but were more effective in the amygdala and hippocampus, respectively. Together, these results support the view that age-related changes in blood glucose responses to epinephrine contribute to memory impairments, which may be related to alterations in regional patterns of CREB phosphorylation.
Keywords: Epinephrine, glucose, CREB, memory, aging, hippocampus
1. INTRODUCTION
Memory impairments accompany healthy aging in a variety of species. The impairments often take the form of rapid forgetting of recently learned information in both humans (Gagnon and Belleville, 2011; Huppert and Kopelman, 1989; Munro Cullum et al., 1990; Park et al., 1988) and rodents (Barnes, 1991; Countryman and Gold, 2007; Foster, 1999; Gold, 2005, 2008; Gold et al., 1982; Korol, 2002; Quartermain et al., 1988; Salinas and Gold, 2005; Winocur, 1988; Zornetzer et al., 1982). Previous work in rodents suggests that rapid forgetting may reflect deficits in neurobiological mechanisms of memory formation initiated during or soon after training. For example, old rodents have training-related alterations in neurotransmitter release, calcium signaling, and gene expression within the brain, all of which may be interrelated and contribute to memory impairments (Burke and Barnes, 2006; Dickstein et al., 2007; Kelly et al., 2006; Mora et al., 2007; Toescu and Verkhratsky, 2007; Welsh and Gold, 1984; Yankner et al., 2008).
Changes outside of the brain may also make important contributions to age-related impairments in memory processes. Substantial evidence suggests that alterations in peripheral hormones and neuroendocrine systems during aging can alter central neurobiological processes, producing deficits in memory and synaptic plasticity (Conrad and Bimonte-Nelson, 2010; Foy, 2011; Frick, 2009; Janowsky, 2006; Korol and Gold, 2007; Lupien et al., 2009). In particular, numerous studies indicate that deficits in rising blood glucose levels after peripheral epinephrine release contribute to age-related impairments in memory processes (Gold, 2005; Gold and Korol, 2010; Korol, 2002; Korol and Gold, 1998, 2007; Mabry et al., 1996; Messier, 2004). Epinephrine is a hormone released from the adrenal medulla as a response to arousal. In young adult rats, endogenous release of epinephrine facilitates stable memory formation for temporally associated events. Likewise, peripheral injections of epinephrine can convert short-lasting memories and the early phase of long-term potentiation (LTP) to more lasting forms (Korol and Gold, 2008). Since peripheral epinephrine cannot readily enter the brain from blood (Axelrod et al., 1959), epinephrine mediates its effects on memory peripherally, largely by liberating glucose from liver glycogen stores, thus increasing the supply of glucose to the brain during cognitively demanding times (Gold, 2005, 2008; Gold and Korol, 2010; Korol and Gold, 2007). The additional provision of glucose to the brain provides energy to support memory processes, possibly by contributing to lactate production in astrocytes with provision to neurons during cognitive processing (Newman et al., 2011; Suzuki et al., 2011).
Several studies show that epinephrine is deficient at raising blood glucose levels in old rats. Though old rats exhibit higher circulating epinephrine levels following training or exposure to a novel environment, they do not show the parallel increases in blood glucose levels seen in young rats (Mabry et al., 1995a,b,c). Likewise, peripheral injections of epinephrine fail to increase blood glucose levels in old rats to the levels seen in young rats (Morris et al., 2010). These age-related deficits in blood glucose responses to epinephrine can affect downstream neurochemical and behavioral memory processes. For example, one mechanism by which glucose may enhance memory is by increasing behaviorally-elicited release of the neurotransmitter acetylcholine (Gold, 2003; Kopf et al., 2001; Ragozzino and Gold, 1995; Ragozzino et al., 1996, 1998).
Age-related impairments in blood glucose responses to epinephrine may also affect downstream molecular processes, particularly those activated by cholinergic signaling. Several studies suggest that acetylcholine modulates phosphorylation of cAMP response element-binding protein (CREB) in the brain, most likely through activation of nicotinic acetylcholine receptors (Chang and Berg, 2001; Dajas-Bailador and Wonnacott, 2004; Hu et al., 2002) and possibly via activation of α7 nicotinic receptors (Morris et al., 2012). CREB is a transcription factor widely implicated in activity-dependent neuronal plasticity and the formation of durable memories (Alberini, 2009; Benito and Barco, 2010; Carlezon et al., 2005; Colombo et al., 2003; Silva et al., 1998; Taubenfeld et al., 1999; Yin and Tully, 1996). In young adult rats, interfering with CREB function via mutations or inhibitors generally disrupts long-term memory while leaving short-term processes intact (Bourtchuladze et al., 1994; Brightwell et al., 2005, 2008; Guzowski and McGaugh, 1997; Josselyn et al., 2004), mimicking age-related rapid forgetting. In old rats, there are significant age-related deficits in CREB activation after training in a variety of behavioral tasks, and these deficits correlate with memory impairments and rapid forgetting (Countryman and Gold, 2007; Kudo et al., 2005; Monti et al., 2005; Porte et al., 2008a; Xu et al., 2010). Several recent studies have shown that long-term exercise programs or chronic administration of various exogenous compounds can attenuate age-related impairments in CREB activation while improving performance in behavioral tasks (Aguiar et al., 2011; Assunção et al., 2010; Li et al., 2009; Trofimiuk et al., 2010; Xu et al., 2010). However, it is difficult to tell if these effects are specific to memory processes, given that the treatments were administered for several weeks or months prior to training.
Of particular relevance to the current experiments, a recent study found that old rats are significantly impaired in CREB phosphorylation following inhibitory avoidance training, even when the foot shock intensity is raised to promote stable memory formation (Morris and Gold, 2012). Similarly, other work has shown that old rats do not exhibit a foot shock-related rise in blood glucose levels, even with relatively high foot shock intensities (Mabry et al., 1995c). Together, these results suggest that old rats are significantly impaired in their ability to initiate CREB-mediated transcriptional processes, which may reflect deficits in blood glucose rises and associated impairments in acetylcholine release soon after training.
The present experiments examined how post-training administration of endogenous memory enhancing agents, such as epinephrine and glucose, may modulate memory and CREB function in old rats. The first experiment tested the hypothesis that direct brain injections of glucose after inhibitory avoidance training could reverse age-related memory impairments. The second experiment examined the hypothesis that glucose would be more effective than epinephrine at modulating downstream CREB phosphorylation in the brain, similar to glucose's greater efficacy in previously tested neurochemical and behavioral measures.
2. METHODS
2.1. Subjects
Young adult (3 to 4 mo.) and old (24 to 25 mo.) male Fischer-344 rats (Taconic Farms, Germantown, NY) were individually housed in translucent cages with a 12-h light/dark cycle (lights on at 07:00 h) and ad libitum access to food and water. Animal pain and discomfort were minimized. All experiments were conducted at the University of Illinois, which is fully accredited (AAALAC), in accordance with animal care guidelines established by the National Institute of Health.
2.2. Surgery
Rats were anesthetized with isoflurane and placed in a stereotaxic apparatus. Stainless steel guide cannulae (Plastics One, Roanoke, VA) were implanted bilaterally into the dorsal hippocampus of young [coordinates: - 3.2 mm from bregma; ± 3 mm lateral; - 1.9 mm deep from dura] and old rats [coordinates: - 3.4 mm from bregma; ± 3.1 mm lateral; - 2.1 mm deep from dura], according to the atlas of Paxinos and Watson (2003). Rats were monitored and allowed to recover for 7 days after surgery before further experimentation.
2.3. Inhibitory Avoidance Training
Rats were handled for 3-4 min each day on 5 consecutive days prior to inhibitory avoidance training. All training took place between 12:00 and 16:00 h. The inhibitory avoidance apparatus was a trough-shaped alleyway (91 cm long, 22.9 cm wide at the top, 7.6 cm wide at the bottom, and 15.2 cm deep) divided into lit (31 cm) and dark (60 cm) compartments by a sliding door that could be lowered through the floor. Each rat was placed in the lit chamber facing the door. When the rat turned completely around, the door was lowered to a height approximately 2 cm above the floor. When the rat again turned toward the door, a timer was started to record the training latency, defined as the time taken to enter (cross all four limbs into) the dark chamber. Rats that failed to enter the dark chamber within two minutes were not included in the study. Upon entering the dark chamber, the rat received a single foot shock (0.2 mA, 0.4 sec) and the door was closed to prevent reentry into the lit chamber. These training conditions were those with which we have seen rapid forgetting in senescent rats and stable memory in young adult rats in past experiments (Gold et al., 1982; Morris et al., 2010; Morris and Gold, 2012; Sternberg et al., 1985). In these past experiments, memory in aged rats was not impaired during the first hours after training but forgetting emerged within 1-7 days after training in aged rats, at times when young rats do not exhibit forgetting for the inhibitory avoidance experiences. Also, past tests revealed that aged Fischer-344 rats do not have higher foot shock thresholds than do young adult rats (Gold et al., 1982). Thus, the conditions used in the present experiment were appropriate for tests of the neurobiological concomitants of rapid forgetting in aged rats.
2.4. Microinfusions
In the microinfusion experiments, rats received immediate post-training infusions of artificial cerebral spinal fluid (aCSF) or glucose and were then returned to the holding cage. Memory was assessed by measuring latencies (max of 600 sec) on a test trial 7 days later using the same procedure as above, but without the foot shock.
Microinfusion probes (Plastics One) were inserted through the guide cannulae to a point 1 mm below the guide cannulae tips. A solution of aCSF (128 mM NaCl, 2.5 mM KCl, 1.3 mM CaCl2, 2.1 mM MgCl2, 0.9 mM NaH2PO4, 2.0 mM Na2HPO4, pH 7.4) containing either 1.0 or 33.4 mM glucose was infused bilaterally into the dorsal hippocampus at a rate of 0.25 μl / min for 2 min. The 1.0 mM glucose concentration in control aCSF is based on evidence that this level is seen at baseline in hippocampal CSF (McNay and Gold, 1999). Infusion probes were left in place for an additional 1 min to allow solutions to diffuse away from the probe tips. In contrast to glucose, epinephrine does not cross readily from blood to brain and neither circulating nor central epinephrine appears to act at adrenergic receptors in the hippocampus. Therefore, intrahippocampal injections of epinephrine were not tested here.
2.5. Systemic injections
For the immunohistochemistry experiments, rats received an immediate post-training subcutaneous injection of saline (0.9%), glucose (250 mg/kg), or epinephrine (0.1 mg/kg) and were then returned to the holding cage. These doses of glucose and epinephrine have been shown to restore memory in aged rats to levels seen in young rats (Sternberg et al., 1985; Salinas and Gold, 2005; McNay and Gold, 2001; Morris et al., 2010). The use of systemic injections, rather than microinfusions into the hippocampus, was chosen to avoid having damage to brain areas that would be measured using immunohistochemistry methods. In addition, systemic injections permit a comparison of the effects of a weak – epinephrine – and strong – glucose – enhancer of memory in aged rats (Morris et al., 2010). This comparison cannot be accomplished with direct brain infusions because circulating epinephrine would not normally reach the brain areas of interest (Axelrod et al., 1959).
2.6. Perfusion and Brain Slicing
To measure CREB and pCREB levels, rats were euthanized 30 min after training and immunostaining procedures were used at a later time. There are differences across studies in the optimal time to assess maximal pCREB responses to training. The 30-min time point was chosen based on prior work showing robust increases in CREB phosphorylation in young adult rats at that time under similar training conditions (Morris and Gold, 2012). We also hypothesized that 30 min would allow sufficient time for post-training treatments to enhance cell signaling processes related to CREB phosphorylation. In past experiments, glucose effects on the brain were evident only under conditions of training or pharmacological manipulation (Degroot et al, 2003; Durkin et al., 1992; Kopf et al., 2001; Messier et al., 1990; Ragozzino et al., 1996, 1998). Therefore, glucose and epinephrine groups in the absence of training were not tested here.
Rats were deeply anesthetized with an overdose i.p. injection of sodium pentobarbital (Sigma-Aldrich, St. Louis, MO) and then perfused intracardially with 80 ml of 0.1 M phosphate-buffered saline (PBS) followed by 80 ml of 4% paraformaldehyde in 0.1 M phosphate buffer. Rats were decapitated and the brains were removed and placed into 4% paraformaldehyde in 0.1 M PB for ~72 hrs. The brains were transferred to 20% glycerol in 0.1 M PBS for ~48 hrs. Frozen sections (40 μM) were collected at -30° C with a Leica 1800 cryostat (Leica Microsystems, Wetzlar, Germany). For the immunohistochemistry experiments, slices through the dorsal hippocampus were collected and stored in a cryopreservative solution (250 mM 40 KD polyvinylpyrrolidone, 880 mM sucrose, 30% v/v ethylene glycol, 50 mM sodium phosphate) at -20° C. For the microinfusion experiments, sections containing the guide cannulae tracts were mounted on slides, dried, stained with cresyl violet, and visualized under a light microscope. Behavioral data were discarded for those rats with probe sites outside of the dorsal hippocampus.
2.7. Immunohistochemistry
All steps took place at room temperature. All reactions were performed in duplicate, using alternating slices for pCREB and CREB staining ~3.3 mm posterior to bregma. Slices were washed three times for 10 min each time in 0.05 M PBS initially and in between all subsequent steps. Slices were first incubated in blocking solution (1% H2O2, 1% normal goat serum (NGS), 0.02% triton x-100, 0.05 M PBS) for 10 min. They were transferred to a pre-incubation solution (2% NGS, 0.4% triton x-100, 0.05 M PBS) for 20 min and then incubated overnight in a solution (1% NGS, 0.4% triton x-100, 0.05 M PBS) containing a rabbit primary antibody for Ser-133 phosphorylated CREB or total CREB (Millipore, Billerica, MA) diluted 1:4000. The next day, the slices were placed for 1 hr in a solution (1% NGS, 0.2% triton x-100, 0.05 M PBS) containing a goat anti-rabbit biotinylated secondary antibody (Santa Cruz, Santa Cruz, CA). They were next incubated for 30 min with ABC reagent (Vector, Burlingame, CA) in 0.05 M PBS, followed by incubation with DAB substrate (Vector) for 4 min. Slices were mounted onto slides and allowed to dry overnight. The next morning, slices were dehydrated with a graded ethanol series of washes, then coverslipped using DPX mountant (Sigma-Aldrich).
2.8. Image Acquisition and Analysis
Sections were imaged using a Leica DM 6000B/CTR6000 light microscope and a Leica DFC350 FX video camera, which was interfaced to a PC computer. This system was used in conjunction with Image-Pro software (Media Cybernetics, Inc., Bethesda, MD) for image acquisition and to correct for unevenness in illumination across images. Image J software (NIH, Bethesda, MA) was used to quantify the optical density of pCREB and CREB staining. The six brain regions analyzed were the dentate gyrus and areas CA3 and CA1 of the hippocampus, the basolateral and lateral nuclei of the amygdala, and the piriform cortex. The hippocampus and amygdala regions were selected because of prior evidence that training, as in the present experiment, elicited clear phosphorylation of CREB in young rats but not in old rats. The piriform cortex was selected as a control region based on evidence that CREB phosphorylation in this brain area is not altered by age or by training in either young or old rats (Morris and Gold, 2012). Morris and Gold (2012) provides an illustration of the specific targeted areas as well as a detailed discussion of CREB activation in these brain regions. The auto-thresholding method in Image J was used to ensure that only specifically labeled cells were being measured. For each image, the optical density of a nearby region with no or little specific staining was calculated and used for background subtraction.
2.9. Statistical Analyses
All analyses were performed using Statview software. Behavioral training and retention latencies were analyzed using a Kruskal-Wallis one-way analysis of variance. This non-parametric test was used since many rats had a maximum retention testing latency of 600 sec, which created a non-normal distribution of data. If Kruskal-Wallis analysis revealed a significant main effect, non-parametric Mann-Whitney U-tests for individual comparisons were performed.
The immunohistochemistry experiments were run in two cohorts. In the first cohort, rats were trained on the inhibitory avoidance task with a post-training injection of saline or glucose. These groups were compared to untrained rats that were handled but not trained on the task. In the second cohort, rats were trained with a post-training injection of saline or epinephrine, and these groups were also compared to untrained rats. To facilitate comparison between glucose and epinephrine, the data from the two cohorts were analyzed together by normalizing to the values obtained in the untrained rats. The optical densities of CREB and pCREB immunostaining, as well as pCREB:CREB ratios, were analyzed using two-way ANOVAs (age x training/treatment group). If the two-way ANOVAs revealed significant age or training/treatment effects, post hoc Fisher PLSD tests were performed.
3. RESULTS
3.1. Inhibitory avoidance training with intrahippocampal glucose injections
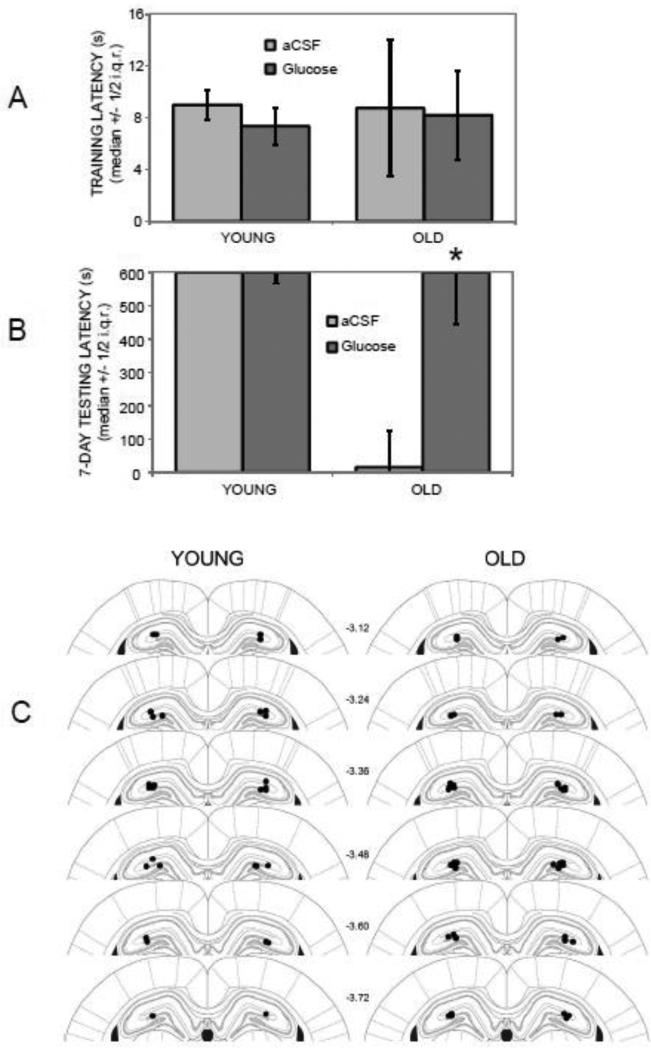
Figure 1 (A and B) shows training and 7-day retention latencies in young and old rats following inhibitory avoidance training and post-training intrahippocampal microinfusions of aCSF (Ns = 7 and 9 for young and old rats, respectively) or glucose (Ns = 8 and 11 for young and old rats, respectively). Kruskal–Wallis analyses of variance revealed a significant group effect on retention latencies (p < .01) but not on training latencies. Post-hoc Mann-Whitney U-tests showed that the old rats injected with glucose had significantly higher 7-day retention latencies compared to those of old rats that had received aCSF injections (p < .05). The higher latencies seen in the old rats treated with glucose were similar to those of young rats, with the group medians reaching the ceiling 600 sec maximum in each case. Figure 1C illustrates infusion cannula tip placements in the dorsal hippocampus for young and old rats.
Figure 1.
Effects of age and intrahippocampal glucose administration on 7-day inhibitory avoidance retention latencies. (A) There were no significant differences in training latencies across groups. (B) Old rats receiving post-training intrahippocampal injections of aCSF had significantly lower 7-day retention latencies compared to young rats receiving either aCSF or glucose injections. Post-training glucose injections reversed age-related impairments, improving retention latencies in old rats to levels seen in young rats. (*) p < .05 vs. old aCSF group. i.q.r. = interquartile range. (C) Infusion sites targeting the dorsal hippocampus in young and old rats. Filled circles represent tips of infusion tracts. Numbers refer to distance in mm posterior to bregma. Adapted with permission from Paxinos and Watson (2003).
3.2. Inhibitory avoidance training for immunohistochemistry experiments
For the immunohistochemistry experiments, retention testing latencies were not assessed since rats were euthanized 30 min after training. There were no significant differences in training latencies; the medians ranged from 5.5 – 7 sec across all groups (data not shown). Two old rats failed to cross into the dark chamber within two minutes and were not included in the study. Only two other rats had training latencies greater than 20 sec (40 and 120 sec in the young epinephrine and old glucose groups, respectively).
3.3. CREB and pCREB immunostaining following training
Immunostaining levels of pCREB and total CREB, as well as pCREB:CREB ratios, were examined in young and old rats 30 min after inhibitory avoidance training. Rats were analyzed in four treatment groups: untrained rats (Ns = 16 for both young and old) and trained rats receiving post-training injections of saline (Ns = 16), epinephrine (Ns = 8), or glucose (Ns = 8).
3.3.1. Hippocampus: Dentate Gyrus
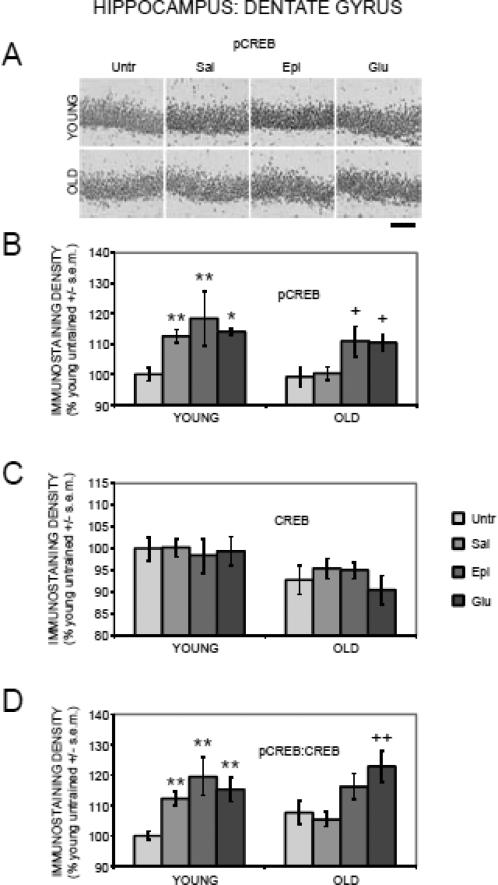
Figure 2 (A and B) shows the results for pCREB immunostaining in the dentate gyrus region of the hippocampus. A two-way ANOVA revealed a main effect of training/treatment on pCREB staining (F(3,88) = 7.51, p < .001). In young rats, training increased pCREB staining as compared to values in untrained rats. The increase was evident in all three trained groups that received post-training injections of saline, epinephrine, or glucose (ps < .05 vs. untrained where ps refer to the results of post-hoc comparisons). In old rats, training did not result in increased pCREB staining, as evident in the comparison of trained rats that received saline vs. untrained rats. Of particular interest here is the finding that post-training administration of glucose and epinephrine in aged rats significantly enhanced pCREB staining compared to both the untrained and saline groups (ps < .05).
Figure 2.
Age-, training-, and treatment-associated differences in pCREB and CREB immunoreactivity in the dentate gyrus of the hippocampus. (A) Representative photomicrographs of pCREB immunostaining. Scale bar = 100 microns. (B) Old rats had training-related deficits in pCREB activation, which were attenuated by epinephrine and glucose. (*) p < .05 vs. young untrained group. (**) ps < .01 vs. young untrained group. (+) ps < .05 vs. old untrained and saline groups. (C) There were significantly lower CREB levels in old compared to young rats. (D) Old rats had training-related deficits in pCREB:CREB ratios, which were attenuated by glucose. (**) ps < .01 vs. young untrained group. (++) ps < .01 vs. old untrained and saline groups.
There was a main effect of age (F(1,88) = 7.90, p < .01) on CREB immunostaining in the dentate gyrus (Figure 2C). However, post-hoc tests revealed no significant differences between young and old untrained rats. Neither training nor treatments with epinephrine or glucose significantly altered CREB staining in young or old rats (F(3,88) = 0.31, p > 0.2).
There was a main effect of training/treatment (F(3,88) = 8.60, p < .0001) on pCREB:CREB ratios in the dentate gyrus (Figure 2D). In young rats, training resulted in increased pCREB:CREB ratios, with higher pCREB:CREB ratios in rats receiving post-training injections of saline, epinephrine, or glucose (ps < .01 vs. untrained). However, training had no effect in old rats, as evident by similar pCREB:CREB ratios between untrained rats and those receiving post-training saline injections. Interestingly, post-training administration of glucose but not epinephrine led to increased pCREB:CREB ratios compared to both untrained and saline-injected rats (ps < .01).
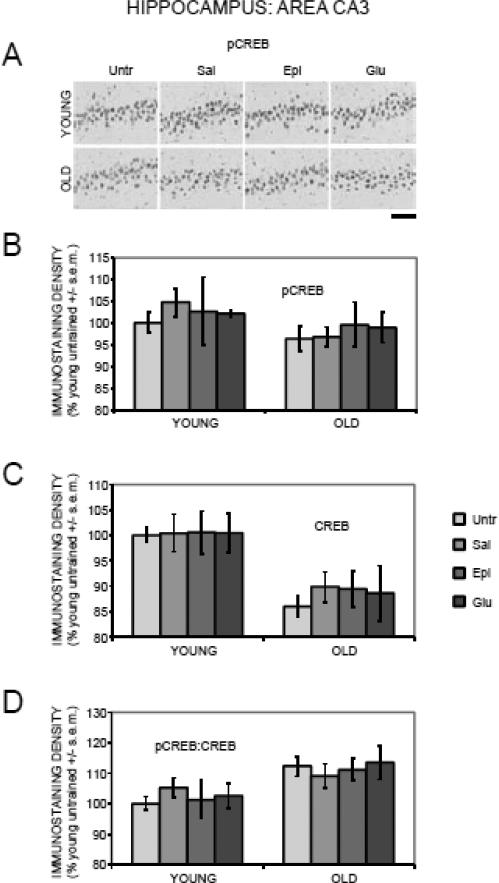
3.3.2. Hippocampus: Area CA3
In contrast to the results seen in dentate gyrus, there were no effects of training or treatments with epinephrine or glucose on pCREB staining (F(1,88) = 2.99, p > 0.2; Figure 3A and 3B) or on pCREB:CREB ratios (F(3,88) = 0.10, p > 0.2; Figure 4D) in area CA3. CREB staining in area CA3 decreased with age (F(1,88) = 24.21, p < .0001), with significantly lower levels in old compared to young untrained rats (p < .0001), but did not vary by training or treatments (F(3,88) = 0.23, p > 0.2; Figure 3C).
Figure 3.
Age-, training-, and treatment-associated differences in pCREB and CREB immunoreactivity in area CA3 of the hippocampus. (A) Representative photomicrographs of pCREB immunostaining. Scale bar = 100 microns. (B) There were no significant age-, training-, or treatment-related differences in pCREB levels. (C) There were significantly lower CREB levels in old compared to young rats. (D) pCREB:CREB ratios were significantly elevated in old compared to young rats.
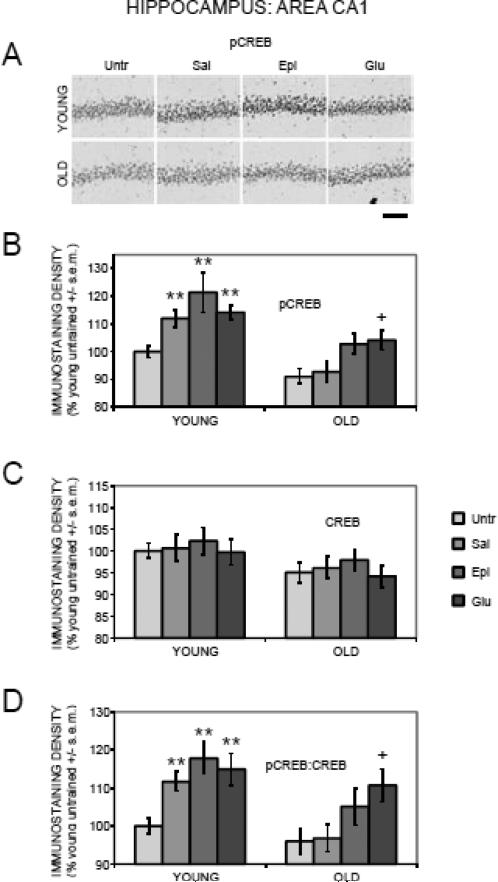
Figure 4.
Age-, training-, and treatment-associated differences in pCREB and CREB immunoreactivity in area CA1 of the hippocampus. (A) Representative photomicrographs of pCREB immunostaining. Scale bar = 100 microns. (B) Old rats had training-related deficits in pCREB activation, which were attenuated by epinephrine and glucose. (**) ps < .01 vs. young untrained group. (+) ps < .05 vs. old untrained and saline groups. (C) There were significantly lower CREB levels in old compared to young rats. (D) Old rats had training-related deficits in pCREB:CREB ratios, which were attenuated by glucose. (**) ps < .01 vs. young untrained group. (+) ps < .05 vs. old untrained and saline groups.
3.3.3. Hippocampus: Area CA1
The results for area CA1 (Figure 4) were very similar to those for the dentate gyrus, and thus are not presented in detail here. The major exception was in the pCREB results for old rats. Specifically, glucose significantly enhanced pCREB staining compared to both the untrained and saline groups (ps < .05). Epinephrine was less effective, significantly enhancing pCREB staining compared to untrained (p < .05) but not saline-injected rats.
3.3.4. Basolateral Amygdala
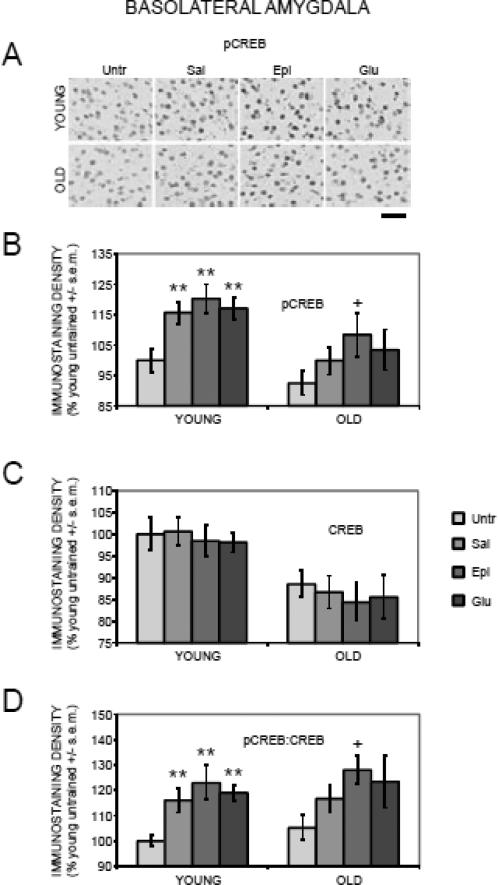
Figure 5 (A and B) shows the results for pCREB immunostaining in the basolateral nucleus of the amygdala. There was a main effect of training/treatment on pCREB staining (F(3,88) = 5.87, p < .01). In young rats, training significantly enhanced pCREB staining; the trained groups that received saline, glucose or epinephrine all had higher pCREB staining compared to untrained rats (ps < .01). Epinephrine and glucose did not enhance pCREB levels beyond those attained after training plus saline. In old rats, the trained saline rats had slightly higher levels of pCREB expression than did untrained rats, but this was not a significant difference. Interestingly, in old rats, epinephrine but not glucose augmented pCREB expression to levels significantly above those of untrained rats (p < .05).
Figure 5.
Age-, training-, and treatment-associated differences in pCREB and CREB immunoreactivity in the basolateral nucleus of the amygdala. (A) Representative photomicrographs of pCREB immunostaining. (B) Old rats had training-related deficits in pCREB activation, which were attenuated by epinephrine. (**) ps < .01 vs. young untrained group. (+) p < .05 vs. old untrained group. Scale bar = 50 microns. (C) There were significantly lower CREB levels in old compared to young rats. (D) Old rats had training-related deficits in pCREB:CREB ratios, which were attenuated by epinephrine. (**) ps < .01 vs. young untrained group. (+) p < .05 vs. old untrained group.
There was a main effect of age (F(1,88) = 21.41, p < .0001) on CREB immunostaining, with significantly lower CREB staining in old compared to young untrained rats (p < .05; Figure 5C). However, neither training nor treatments significantly altered CREB staining in young or old rats (F(3,88) = 0.23, p > 0.2).
The results obtained with pCREB:CREB ratios (Figure 5D) were similar to those seen with pCREB alone. There was a main effect of training/treatment (F(3,88) = 7.04, p < .001). Compared to the ratios of untrained young rats, pCREB:CREB ratios increased with training followed by saline, glucose or epinephrine (ps < 01). In old rats, the ratios also increased above untrained values but only significantly so for the epinephrine-treated group (p < .05), though there was a trend toward enhancement in the glucose group (p < .06).
3.3.5. Lateral Amygdala
The results in the lateral amygdala (data not shown) were very similar to those in the basolateral amygdala for pCREB, CREB, and pCREB:CREB ratios, and are thus not presented in detail here.
3.3.6. Piriform cortex
There were no main effects of age (Fs(1,88) < 0.52), training, or treatment (Fs(3,88) < 0.09) on pCREB, CREB, or pCREB:CREB ratios in the piriform cortex (data not shown).
3.3.7. Summary of immunohistochemistry results
Table 1 summarizes the effects of epinephrine and glucose on modulating CREB phosphorylation across brain regions.
Table 1.
Effects of epinephrine and glucose on modulating training-related CREB phosphorylation
| OLD RATS | ||
|---|---|---|
| EPINEPHRINE | GLUCOSE | |
| pCREB | ||
| DG | ✓ | ✓ |
| CA3 | ||
| CA1 | ✓ | ✓ |
| BLA | ✓ | |
| LA | ✓ | |
| PIR | ||
| pCREB:CREB | ||
| DG | ✓ | |
| CA3 | ||
| CA1 | ✓ | |
| BLA | ✓ | |
| LA | ✓ | |
| PIR | ||
✓ denotes significant effect versus untrained rats. Brain regions analyzed were dentate gyrus (DG), area CA3, and area CA1 of the hippocampus, basolateral (BLA) and lateral (LA) amygdala, and piriform cortex (PIR).
4. DISCUSSION
4.1. Age-Related memory impairments and reversal by intrahippocampal glucose
There are two main behavioral findings reported here. The first is that memory was impaired in old rats relative to young rats at 7 days after inhibitory avoidance training. These findings replicate past results, which showed that age-related impairments in memory after training on inhibitory avoidance training emerge with time (Gold et al., 1982; Zornetzer et al., 1982), i.e. old rats forget more rapidly than do young rats. Of note, differences in perception of the foot shock do not account for the findings (Foster and Kumar, 2007; Frye et al., 2010; Gold et al., 1982; Morris et al., 2010). The training conditions utilized in the present studies are the same as those for which systemic administration of epinephrine or glucose enhances memory (Morris et al., 2010; Sternberg et al., 1985). Thus, the training conditions used to evaluate CREB activation were appropriate for showing age-related impairments in memory and in modulation of memory.
The second main behavioral finding is that post-training injections of glucose directly into the hippocampus enhance later memory in aged rats. When drugs are administered after training, as here, effects on later memory for the learned experience cannot be attributed to alterations in non-mnemonic factors such as sensory, motor, or motivational variables (Gold, 2008; Gold and Korol, 2010; McGaugh, 1989, 2000). These results complement our previous findings that systemic epinephrine or glucose administration can enhance memory in aged animals in several settings, including when administered after training in both rodents (Morris et al., 2010; Sternberg et al., 1985) and humans (Manning et al., 1992).
To our knowledge, this is the first example of a direct brain injection enhancing memory in aged rats using a post-training design. Previous work in young rats has shown that direct infusions of glucose into the lateral ventricles or into specific brain areas, including the medial septum, hippocampus, and amygdala, enhance memory (Canal et al., 2005; Pych et al., 2006; Ragozzino et al., 1996, 1998; Schroeder and Packard, 2003; Stefani and Gold, 1998; Stefani et al., 1999). Together with the present results, these findings are consistent with the idea that glucose acts directly in the brain, rather than in the periphery, to enhance memory in young and old rats.
4.2. Age-Related Differences in CREB and pCREB Expression at Baseline and in Response to Training
Compared to levels in young rats, baseline CREB levels were significantly depressed in area CA3 and in the basolateral and lateral amygdala of old compared to young untrained rats. The age-related decreases in baseline CREB levels were not evident in area CA1, the dentate gyrus, or piriform cortex. These results replicate those we described previously, where age-related decreases in CREB at baseline were evident in area CA3 and the amygdala, but not other brain regions tested (Morris and Gold, 2012). More generally, age-related decreases in CREB expression observed here are also consistent with the findings of previous studies (Brightwell et al., 2004; Countryman and Gold, 2007; Trofimiuk et al., 2010).
In addition to decreases in CREB expression in some brain areas, there were also significant age-related differences in CREB phosphorylation in response to training. In young rats, training with a post-training saline injection enhanced CREB phosphorylation compared to untrained controls. The increased pCREB values after training were region-specific, occurring in the dentate gyrus, area CA1, and the basolateral and lateral amygdala, but not in area CA3 or the piriform cortex. This same pattern of anatomical localization of CREB activation was seen previously in response to training without post-training injection of saline or other treatment (Morris and Gold, 2012). Thus, it appears that the pCREB activation after training observed here in the saline controls is a response to training and not to the stress of injection.
In contrast to the results seen in young rats, pCREB levels in old rats were not responsive to training in any brain region tested. These results match those described previously, in which there were age-related deficits in CREB phosphorylation in the hippocampus and amygdala in response to training (Morris and Gold, 2012). The findings are also similar to the age-dependent deficits in hippocampal CREB activation and associated memory impairments after training on other hippocampus-sensitive tasks (Countryman and Gold, 2007; Kudo et al., 2005; Monti et al., 2005; Porte et al., 2008a; Xu et al., 2010). Together, these results suggest that old rats are deficient in their ability to activate CREB in the hippocampus and amygdala in response to training. These are brain regions associated with inhibitory avoidance learning and modulation of that learning (Cammarota et al., 2008; Canal and Gold, 2007; Izquierdo et al., 1992, 2002; Jobim et al., 2012; McGaugh et al., 2002; McIntyre et al., 2005; McReynolds et al., 2010; Milekic et al., 2007; Rossato et al., 2004). Using compromised CREB activation as a measure of functional integrity, the hippocampus and amygdala are therefore brain regions in which function impaired by age may contribute to the rapid forgetting seen in aged rats.
4.3. Modulation of CREB Phosphorylation by Epinephrine and Glucose
In young rats, neither epinephrine nor glucose resulted in activation of CREB in the hippocampus or amygdala beyond the levels produced by training alone. Apparently, then, in young rats, the foot shock used during training was adequate to increase pCREB to levels that were not sensitive to further enhancement by these modulators of memory formation. The findings that a single foot shock was sufficient to increase pCREB expression in the hippocampus and amygdala are comparable to findings reported by others after inhibitory avoidance training (Bernabeu et al., 1997; Cammarota et al., 2000; Izquierdo et al., 2002; Taubenfeld et al., 1999; Viola et al., 2000) and after training in other avoidance tasks (Bilang-Bleuel et al., 2002; Impey et al., 1998; Kogan and Richter-Levin, 2008; Porte et al., 2008b; Stanciu et al., 2001; Trifilieff et al., 2006). The question of whether activation of CREB is associated with enhancement of memory in young rats was not addressed in the present experiments. Note that the training conditions used here produced maximal memory scores (600 sec) in young rats with or without treatments. Because a ceiling effect interfered with demonstrations of possible enhancement of memory, the experimental conditions did not permit assessment of the possibility that modulators of memory might activate CREB in a manner associated with enhancement of memory. Although these treatments did not augment CREB activation beyond that attained with training alone in young rats, it will be important to determine whether CREB activation plays a role in enhancement of memory with epinephrine or glucose in young rats.
In old rats, significant increases in pCREB after training per se were not evident in the hippocampus or amygdala. However, treatment with either epinephrine or glucose augmented those responses under conditions where the treatments would enhance memory (Morris et al., 2010; Sternberg et al., 1985). While both epinephrine and glucose enhance memory in aged rats, the effects are generally more robust with glucose than with epinephrine (Morris et al., 2010). The pCREB results in the dentate gyrus and CA1 region of the hippocampus reflect this best. In these brain regions, glucose was somewhat more effective than was epinephrine at augmenting CREB activation. These findings suggest that activation of CREB in the hippocampus parallels and may contribute to memory enhancement by epinephrine and glucose in aged rats.
Related evidence indicates that glucose augments training-related release of acetylcholine in the hippocampus of young adult rats (Gold, 2003; Kopf et al., 2001; Morris et al., 2010; Ragozzino and Gold, 1995; Ragozzino et al., 1996, 1998) and is more effective than epinephrine at increasing acetylcholine release during training in the hippocampus of aged rats (Morris et al., 2010). Thus, one possibility is that glucose enhances CREB phosphorylation through activation of the cholinergic system. Related to this possibility is evidence that fimbria-fornix lesions, which disrupt cholinergic innervation to the hippocampus (Erb et al., 1997; Nilsson and Björklund, 1992), also disrupt hippocampal CREB activation and impair memory for inhibitory avoidance (Taubenfeld et al., 1999, 2001). Acetylcholine can result in phosphorylation of CREB through binding to muscarinic 3 (M3) receptors (Greenwood and Dragunow, 2002, 2010) or nicotinic α7 receptors (Bitner et al., 2007, 2010; Gubbins and Gopalakrishnan, 2010; Tietje et al., 2008). Actions mediated by nicotinic α7 receptors are somewhat more likely because these receptors are highly expressed in the hippocampus (Séguéla et al., 1993; Tribollet et al., 2004), while M3 receptors are expressed at low levels in the hippocampus (Buckley et al., 1988; Levey et al., 1994; Vilaró et al., 1993). Recent pharmacological evidence also suggests that, in young rats, glucose enhancement of memory may be mediated through hippocampal α7 vs. α4β2 nicotinic receptors (Morris et al., 2012b).
Importantly, Morris et al. (2010) examined acetylcholine release in the ventral hippocampus, whereas the present study examined CREB phosphorylation in the dorsal hippocampus. Thus, some of the differences in the effects of epinephrine and glucose on hippocampal acetylcholine release versus CREB phosphorylation may relate to differences in how the dorsal and ventral hippocampus modulate performance in the inhibitory avoidance task. For example, there is evidence that nicotine enhances contextual fear conditioning when injected into the dorsal hippocampus, but impairs performance when injected into the ventral hippocampus, with the impairing effects potentially mediated by activation of nicotinic α7 receptors (Kenney et al., 2012). In the present study, the choice to focus on the dorsal hippocampus was to allow for concurrent examination of CREB phosphorylation in the basolateral and lateral amygdala, including comparisons to our recent work (Morris and Gold, 2012). We also wanted to minimize tissue damage, which is more extensive with direct ventral hippocampal injections and with immunohistochemical processing of ventral hippocampal slices (i.e. due to the presence of large lateral ventricles in old rats).
While post-training injections of either epinephrine or glucose increased pCREB activation in old rats, only epinephrine did so significantly in the amygdala. Thus, the rank order of efficacy of epinephrine and glucose in augmenting pCREB levels in the amygdala does not match their effects on memory (Morris et al., 2010). These findings suggest that activation of the hippocampus may contribute more than does activation of the amygdala to the enhancement of memory in old rats by epinephrine and glucose. Epinephrine, like glucose, may enhance CREB phosphorylation through activation of the cholinergic system. However, because epinephrine is less effective than glucose at enhancing acetylcholine release in the hippocampus of old rats, epinephrine may work through additional mechanisms to activate CREB. Particularly in the amygdala, epinephrine may enhance CREB phosphorylation through the activation of neurotransmitters other than acetylcholine, such as norepinephrine. Noradrenergic mechanisms in the amygdala appear to be important for memory modulation generally and for enhancement of memory by epinephrine in particular (Ferry and McGaugh, 2000; McGaugh, 2004; McGaugh et al., 2002; McIntyre et al., 2003). In young adult rats, epinephrine or foot shock produces rapid and sustained increases in norepinephrine release in the amygdala (Canal et al., 2008; Gold and van Buskirk, 1978a,b; McIntyre et al., 2002; McReynolds et al., 2010; Quirarte et al., 1998; Williams et al., 1998). In addition, injections of adrenergic antagonists into the amygdala attenuate the memory-enhancing effects of epinephrine (Liang et al., 1986; Liang et al., 1990; McIntyre et al., 2005; McReynolds et al., 2010; Williams and McGaugh, 1993). Importantly, it is not likely that noradrenergic signaling mediates enhancement of memory by glucose. Peripheral glucose administration prior to spontaneous alternation testing significantly improved working memory scores but had no effect on hippocampal norepinephrine release (Men et al., 1999). In contrast, glucose significantly enhanced hippocampal acetylcholine release and improved memory under similar and related conditions (Ragozzino et al., 1996, 1998; Stefani and Gold, 2001). Noradrenergic signaling in the amygdala and other brain areas leads to activation of cAMP-mediated signaling pathways, including CREB phosphorylation (Barros et al., 1999; Chen et al., 2007; Davies et al., 2004; Ferry et al., 1999; Patel et al., 2010; Yuan et al., 2000). These findings suggest that epinephrine, unlike glucose, may modulate CREB phosphorylation by increasing norepinephrine release in the amygdala. This hypothesis fits well with the current results, in which epinephrine significantly enhanced CREB phosphorylation in the amygdala of old rats, presumably without associated increases in blood glucose. However, examining norepinephrine release in the amygdala and hippocampus of old rats is an important future direction.
The hypothesis that epinephrine and/or glucose may attenuate or reverse age-related memory impairments by altering regional patterns of CREB phosphorylation could be strengthened by several additional experiments. Examining CREB phosphorylation following post-training intrahippocampal injections of glucose into the various hippocampal subregions would help establish the regional specificity of glucose's effects, both on memory and on CREB phosphorylation. Likewise, examining CREB phosphorylation following post-training intraamygdalar injections of norepinephrine would help establish whether epinephrine-mediated norepinephrine release in the amygdala contributes to memory and CREB phosphorylation. These experiments would also further clarify the relationship between CREB and memory in old rats (see Morris and Gold, 2012).
4.4. Conclusions
The present experiments support the hypothesis that age-related deficits in blood glucose responses to endogenous epinephrine release may alter neurobiological processes related to memory formation. In old rats, increases in circulating epinephrine in response to training or stress may represent an impaired physiological mechanism in the liver for producing increases in blood glucose levels. The alterations in this neuroendocrine response could change the relative contributions of neurotransmitters and memory systems involved in cognitive processing. Although both epinephrine and glucose can support improved memory in old rats, both peripheral and intrahippocampal glucose administration are particularly effective in this regard. Thus, examining the neural mechanisms underlying glucose-mediated memory processes may provide greater insight into strategies for reversing age-related cognitive decline.
Highlights.
Old rats exhibit memory impairments following inhibitory avoidance training.
Post-training intrahippocampal glucose injections reverse these memory impairments.
Old rats exhibit deficits in CREB activation after inhibitory avoidance training.
Epinephrine attenuates CREB deficits, particularly in the amygdala.
Glucose attenuates CREB deficits, particularly in the hippocampus.
Acknowledgments
Supported by an NIH NRSA F30AG034803 (KAM), by NIH grants AG07648 and NSF grants IOS-08-43175 and IOS 10-52464, and by an award from the Alzheimer's Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aguiar AS, Jr., Castro AA, Moreira EL, Glaser V, Santos AR, Tasca CI, Latini A, Prediger RD. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech. Ageing Dev. 2011;132:560–567. doi: 10.1016/j.mad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assunção M, Santos-Marques MJ, Carvalho F, Andrade JP. Green tea averts age-dependent decline of hippocampal signaling systems related to antioxidant defenses and survival. Free Radic. Biol. Med. 2010;48:831–838. doi: 10.1016/j.freeradbiomed.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory changes with age: neurobiological correlates. In: Martinez JL, Kesner RP, editors. Learning and Memory: A Biological View. Academic Press; New York: 1991. pp. 259–296. [Google Scholar]
- Barros DM, Izquierdo LA, Sant'Anna MK, Quevedo J, Medina JH, McGaugh JL, Izquierdo I. Stimulators of the cAMP cascade reverse amnesia induced by intra-amygdala but not intrahippocampal KN-62 administration. Neurobiol. Learn. Mem. 1999;71:94–103. doi: 10.1006/nlme.1998.3830. [DOI] [PubMed] [Google Scholar]
- Benito E, Barco A. CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33:230–240. doi: 10.1016/j.tins.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl. Acad. Sci. U S A. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilang-Bleuel A, Rech J, De Carli S, Holsboer F, Reul JM. Forced swimming evokes a biphasic response in CREB phosphorylation in extrahypothalamic limbic and neocortical brain structures in the rat. Eur. J. Neurosci. 2002;15:1048–1060. doi: 10.1046/j.1460-9568.2002.01934.x. [DOI] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, Curzon P, Decker MW, Frost JM, Gronlien JH, Gubbins E, Li J, Malysz J, Markosyan S, Marsh K, Meyer MD, Nikkel AL, Radek RJ, Robb HM, Timmermann D, Sullivan JP, Gopalakrishnan M. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J Neurosci. 2007;27:10578–10587. doi: 10.1523/JNEUROSCI.2444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Decker MW, Drescher KU, Kohlhaas KL, Markosyan S, Marsh KC, Nikkel AL, Browman KE, Radek R, Anderson D, Buccafusco J, Gopalakrishnan M. In vivo pharmacological characterization of a novel selective {alpha}7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimer's disease. J. Pharmacol. Exp. Ther. 2010;334:875–886. doi: 10.1124/jpet.110.167213. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Brightwell JJ, Gallagher M, Colombo PJ. Hippocampal CREB1 but not CREB2 is decreased in aged rats with spatial memory impairments. Neurobiol. Learn. Mem. 2004;81:19–26. doi: 10.1016/j.nlm.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Brightwell JJ, Smith CA, Countryman RA, Neve RL, Colombo PJ. Hippocampal overexpression of mutant creb blocks long-term, but not short-term memory for a socially transmitted food preference. Learn. Mem. 2005;12:12–17. doi: 10.1101/lm.85005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightwell JJ, Smith CA, Neve RL, Colombo PJ. Transfection of mutant CREB in the striatum, but not the hippocampus, impairs long-term memory for response learning. Neurobiol. Learn. Mem. 2008;89:27–35. doi: 10.1016/j.nlm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J. Neurosci. 1988;8:4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Ardenghi P, Paratcha G, Levi de Stein M, Izquierdo I, Medina JH. Learning-associated activation of nuclear MAPK, CREB and Elk-1, along with Fos production, in the rat hippocampus after a one-trial avoidance learning: abolition by NMDA receptor blockade. Brain Res. Mol. Brain Res. 2000;76:36–46. doi: 10.1016/s0169-328x(99)00329-0. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Rossato JI, Lima RH, Medina JH, Izquierdo I. Parallel memory processing by the CA1 region of the dorsal hippocampus and the basolateral amygdala. Proc. Natl. Acad. Sci. U S A. 2008;105:10279–10284. doi: 10.1073/pnas.0805284105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Chang Q, Gold PE. Intra-amygdala injections of CREB antisense impair inhibitory avoidance memory: role of norepinephrine and acetylcholine. Learn. Mem. 2008;15:677–686. doi: 10.1101/lm.904308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Gold PE. Different temporal profiles of amnesia after intra-hippocampus and intra-amygdala infusions of anisomycin. Behav. Neurosci. 2007;121:732–741. doi: 10.1037/0735-7044.121.4.732. [DOI] [PubMed] [Google Scholar]
- Canal CE, Stutz SJ, Gold PE. Glucose injections into the hippocampus or striatum of rats prior to T-maze training: modulation of learning rates and strategy selection. Learn. Mem. 2005;12:367–374. doi: 10.1101/lm.88205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chang KT, Berg DK. Voltage-gated channels block nicotinic regulation of CREB phosphorylation and gene expression in neurons. Neuron. 2001;32:855–865. doi: 10.1016/s0896-6273(01)00516-5. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Nguyen TV, Pike CJ, Russo-Neustadt AA. Norepinephrine induces BDNF and activates the PI-3K and MAPK cascades in embryonic hippocampal neurons. Cell Signal. 2007;19:114–128. doi: 10.1016/j.cellsig.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Brightwell JJ, Countryman RA. Cognitive strategy-specific increases in phosphorylated cAMP response element-binding protein and c-Fos in the hippocampus and dorsal striatum. J. Neurosci. 2003;23:3547–3554. doi: 10.1523/JNEUROSCI.23-08-03547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Bimonte-Nelson HA. Impact of the hypothalamic-pituitary-adrenal/gonadal axes on trajectory of age-related cognitive decline. Prog. Brain Res. 2010;182:31–76. doi: 10.1016/S0079-6123(10)82002-3. [DOI] [PubMed] [Google Scholar]
- Countryman RA, Gold PE. Rapid forgetting of social transmission of food preferences in aged rats: relationship to hippocampal CREB activation. Learn. Mem. 2007;14:350–358. doi: 10.1101/lm.524907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signaling. Trends Pharmacol. Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Davies MF, Tsui J, Flannery JA, Li X, DeLorey TM, Hoffman BB. Activation of alpha2 adrenergic receptors suppresses fear conditioning: expression of c-Fos and phosphorylated CREB in mouse amygdala. Neuropsychopharmacology. 2004;29:229–239. doi: 10.1038/sj.npp.1300324. [DOI] [PubMed] [Google Scholar]
- Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007;6:275–284. doi: 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb C, Klein J, Köppen A, Löffelholz K, Jeltsch H, Cassel JC. Modulation of hippocampal acetylcholine release after fimbria-fornix lesions and septal transplantation in rats. Neurosci. Lett. 1997;231:5–8. doi: 10.1016/s0304-3940(97)00504-1. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Role of amygdala norepinephrine in mediating stress hormone regulation of memory storage. Acta Pharmacol. Sin. 2000;21:481–493. [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha1-adrenoceptors. J. Neurosci. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res. Rev. 1999;30:236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiol. Learn. Mem. 2007;87:522–535. doi: 10.1016/j.nlm.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR. Ovarian hormones, aging and stress on hippocampal synaptic plasticity. Neurobiol. Learn. Mem. 2011;95:134–144. doi: 10.1016/j.nlm.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm. Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Edinger KL, Lephart ED, Walf AA. 3alpha-androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety, and depressive behavior of male rats. Front. Aging Neurosci. 2010;2:15. doi: 10.3389/fnagi.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon LG, Belleville S. Working memory in mild cognitive impairment and Alzheimer's disease: contribution of forgetting and predictive value of complex span tasks. Neuropsychology. 2011;25:226–236. doi: 10.1037/a0020919. [DOI] [PubMed] [Google Scholar]
- Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol. Learn. Mem. 2003;82:230–242. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Gold PE. Glucose and age-related changes in memory. Neurobiol. Aging. 2005;26(Suppl 1):60–64. doi: 10.1016/j.neurobiolaging.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Gold PE. Memory enhancing drugs. In: Eichenbaum H, Byrne J, editors. Memory Systems, vol. 3 of Learning and Memory: A Comprehensive Reference. Elsevier Science; Oxford: 2008. pp. 555–576. [Google Scholar]
- Gold PE, Korol DL. Hormones and memory. In: Koob G, Le Moal M, Thompson RF, editors. Encyclopedia of Behavioral Neuroscience. Vol. 2. Academic Press; Oxford: 2010. pp. 57–64. [Google Scholar]
- Gold PE, McGaugh JL, Hankins LL, Rose RP, Vasquez BJ. Age-dependent changes in retention in rats. Exp. Aging Res. 1982;8:53–58. [Google Scholar]
- Gold PE, van Buskirk R. Posttraining brain norepinephrine concentrations: correlation with retention performance of avoidance training and with peripheral epinephrine modulation of memory processing. Behav. Biol. 1978a;23:509–520. doi: 10.1016/s0091-6773(78)91614-0. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk R. Effects of alpha- and beta-adrenergic receptor antagonists on post-trial epinephrine modulation of memory: relationship to post-training brain norepinephrine concentrations. Behav. Biol. 1978b;24:168–184. doi: 10.1016/s0091-6773(78)93045-6. [DOI] [PubMed] [Google Scholar]
- Greenwood JM, Dragunow M. Muscarinic receptor-mediated phosphorylation of cyclic AMP response element binding protein in human neuroblastoma cells. J. Neurochem. 2002;82:389–397. doi: 10.1046/j.1471-4159.2002.00992.x. [DOI] [PubMed] [Google Scholar]
- Greenwood JM, Dragunow M. M3 muscarinic receptors promote cell survival through activation of the extracellular regulated kinase (ERK1/2) pathway. Eur. J. Pharmacol. 2010;640:38–45. doi: 10.1016/j.ejphar.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Gubbins EJ, Gopalakrishnan M, Li J. Alpha7 nAChR-mediated activation of MAP kinase pathways in PC12 cells. Brain Res. 2010;1328:1–11. doi: 10.1016/j.brainres.2010.02.083. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc. Natl. Acad. Sci. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Liu QS, Chang KT, Berg DK. Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Mol. Cell. Neurosci. 2002;21:616–625. doi: 10.1006/mcne.2002.1202. [DOI] [PubMed] [Google Scholar]
- Huppert FA, Kopelman MD. Rates of forgetting in normal ageing: a comparison with dementia. Neuropsychologia. 1989;27:849–860. doi: 10.1016/0028-3932(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat. Neurosci. 1998;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, da Cunha C, Rosat R, Jerusalinsky D, Ferreira MB, Medina JH. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Learn. Mem. 1992;15:677–686. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- Izquierdo LA, Barros DM, Vianna MR, Coitinho A, deDavid e Silva T, Choi H, Moletta B, Medina JH, Izquierdo I. Molecular pharmacological dissection of short- and long-term memory. Cell Mol. Neurobiol. 2002;22:269–287. doi: 10.1023/a:1020715800956. [DOI] [PubMed] [Google Scholar]
- Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006;138:1015–1020. doi: 10.1016/j.neuroscience.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Jobim PF, Pedroso TR, Christoff RR, Werenicz A, Maurmann N, Reolon GK, Roesler R. Inhibition of mTOR by rapamycin in the amygdala or hippocampus impairs formation and reconsolidation of inhibitory avoidance memory. Neurobiol. Learn. Mem. 2012;97:105–112. doi: 10.1016/j.nlm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Kida S, Silva AJ. Inducible repression of CREB function disrupts amygdala-dependent memory. Neurobiol. Learn. Mem. 2004;82:159–163. doi: 10.1016/j.nlm.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Nadon NL, Morrison JH, Thibault O, Barnes CA, Blalock EM. The neurobiology of aging. Epilepsy Res. 2006;68(Suppl 1):S5–20. doi: 10.1016/j.eplepsyres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Raybuck JD, Gould TJ. Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus. 2012;22:1681–1690. doi: 10.1002/hipo.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan I, Richter-Levin G. Activation pattern of the limbic system following spatial learning under stress. Eur. J. Neurosci. 2008;27:715–722. doi: 10.1111/j.1460-9568.2008.06034.x. [DOI] [PubMed] [Google Scholar]
- Kopf SR, Buchholzer ML, Hilgert M, Löffelholz K, Klein J. Glucose plus choline improve passive avoidance behaviour and increase hippocampal acetylcholine release in mice. Neuroscience. 2001;103:365–371. doi: 10.1016/s0306-4522(01)00007-0. [DOI] [PubMed] [Google Scholar]
- Korol DL. Enhancing cognitive function across the life span. Ann. N.Y. Acad. Sci. 2002;959:167–179. doi: 10.1111/j.1749-6632.2002.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gold PE. Glucose, memory, and aging. Am. J. Clin. Nutr. 1998;67:764S–771S. doi: 10.1093/ajcn/67.4.764S. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gold PE. Modulation of learning and memory by adrenal and ovarian hormones. In: Kesner RP, Martinez JL, editors. Neurobiology of Learning and Memory. Elsevier Science; New York: 2007. pp. 243–268. [Google Scholar]
- Korol DL, Gold PE. Epinephrine converts long-term potentiation from transient to durable form in awake rats. Hippocampus. 2008;18:81–91. doi: 10.1002/hipo.20372. [DOI] [PubMed] [Google Scholar]
- Kudo K, Wati H, Qiao C, Arita J, Kanba S. Age-related disturbance of memory and CREB phosphorylation in CA1 area of hippocampus of rats. Brain Res. 2005;1054:30–37. doi: 10.1016/j.brainres.2005.06.045. [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Heilman CJ, Desmond TJ, Frey KA. Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain. Neuroscience. 1994;63:207–221. doi: 10.1016/0306-4522(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, Cai MY, Li Y. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience. 2009;159:1208–1215. doi: 10.1016/j.neuroscience.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Liang KC, Juler R, McGaugh JL. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Liang KC, McGaugh JL, Yao H. Involvement of amygdala pathways in the influence of posttraining amygdala norepinephrine and peripheral epinephrine on memory storage. Brain Res. 1990;508:225–233. doi: 10.1016/0006-8993(90)90400-6. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mabry TR, Gold PE, McCarty R. Age-related changes in plasma catecholamine responses to acute swim stress. Neurobiol. Learn. Mem. 1995a;63:260–268. doi: 10.1006/nlme.1995.1030. [DOI] [PubMed] [Google Scholar]
- Mabry TR, Gold PE, McCarty R. Age-related changes in plasma catecholamine responses to chronic intermittent stress. Physiol. Behav. 1995b;58:49–56. doi: 10.1016/0031-9384(94)00387-k. [DOI] [PubMed] [Google Scholar]
- Mabry TR, Gold PE, McCarty R. Age-related changes in plasma catecholamine and glucose responses of F-344 rats to footshock as in inhibitory avoidance training. Neurobiol. Learn. Mem. 1995c;64:146–155. doi: 10.1006/nlme.1995.1054. [DOI] [PubMed] [Google Scholar]
- Mabry TR, McCarty R, Gold PE, Foster TC. Age and stress history effects on spatial performance in a swim task in Fischer-344 rats. Neurobiol. Learn. Mem. 1996;66:1–10. doi: 10.1006/nlme.1996.0038. [DOI] [PubMed] [Google Scholar]
- Manning CA, Parsons MW, Gold PE. Anterograde and retrograde enhancement of 24-h memory by glucose in elderly humans. Behav. Neural Biol. 1992;58:125–130. doi: 10.1016/0163-1047(92)90351-4. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Dissociating learning and performance: drug and hormone enhancement of memory storage. Brain Res. Bull. 1989;23:339–345. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiol. Learn. Mem. 2002;78:539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur. J. Neurosci. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc. Natl. Acad. Sci. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Power AE, Roozendaal B, McGaugh JL. Role of the basolateral amygdala in memory consolidation. Ann. N.Y. Acad. Sci. 2003;985:273–293. doi: 10.1111/j.1749-6632.2003.tb07088.x. [DOI] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Extracellular glucose concentrations in the rat hippocampus measured by zero-net-flux: effects of microdialysis flow rate, strain and age. J. Neurochem. 1999;72:785–790. doi: 10.1046/j.1471-4159.1999.720785.x. [DOI] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. J. Gerontol.: Biol. Sci. 2001;56A:B66–B71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- McReynolds JR, Donowho K, Abdi A, McGaugh JL, Roozendaal B, McIntyre CK. Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiol. Learn. Mem. 2010;93:312–321. doi: 10.1016/j.nlm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men D, McCarty R, Gold PE. Enhanced release of norepinephrine in rat hippocampus during spontaneous alternation tests. Neurobiol. Learn. Mem. 1999;71:289–300. doi: 10.1006/nlme.1998.3880. [DOI] [PubMed] [Google Scholar]
- Messier C. Glucose improvement of memory: a review. Eur. J. Pharmacol. 2004;490:33–57. doi: 10.1016/j.ejphar.2004.02.043. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Pollonini G, Alberini CM. Temporal requirement of C/EBPbeta in the amygdala following reactivation but not acquisition of inhibitory avoidance. Learn. Mem. 2007;14:504–511. doi: 10.1101/lm.598307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti B, Berteotti C, Contestabile A. Dysregulation of memory-related proteins in the hippocampus of aged rats and their relation with cognitive impairment. Hippocampus. 2005;15:1041–1049. doi: 10.1002/hipo.20099. [DOI] [PubMed] [Google Scholar]
- Mora F, Segovia G, del Arco A. Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res. Rev. 2007;55:78–88. doi: 10.1016/j.brainresrev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Morris KA, Chang Q, Mohler EG, Gold PE. Age-related memory impairments due to reduced blood glucose responses to epinephrine. Neurobiol. Aging. 2010;31:2136–2145. doi: 10.1016/j.neurobiolaging.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KA, Gold PE. Age-related impairments in memory and in CREB and pCREB expression in hippocampus and amygdala following inhibitory avoidance training. Mech. Ageing Dev. 2012 doi: 10.1016/j.mad.2012.03.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KA, Li S, Bui DD, Gold PE. Glucose attenuates impairments in memory and CREB activation produced by an α4β2 but not an α7 nicotinic receptor antagonist. 2012. Submitted. [DOI] [PMC free article] [PubMed]
- Munro Cullum C, Butters N, Tröster AI, Salmon DP. Normal aging and forgetting rates on the Wechsler Memory Scale-Revised. Arch. Clin. Neuropsychol. 1990;5:23–30. doi: 10.1016/0887-6177(90)90004-9. [DOI] [PubMed] [Google Scholar]
- Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One. 2011;6:e28427. doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson OG, Björklund A. Behaviour-dependent changes in acetylcholine release in normal and graft-reinnervated hippocampus: evidence for host regulation of grafted cholinergic neurons. Neuroscience. 1992;49:33–44. doi: 10.1016/0306-4522(92)90074-c. [DOI] [PubMed] [Google Scholar]
- Patel NJ, Chen MJ, Russo-Neustadt AA. Norepinephrine and nitric oxide promote cell survival signaling in hippocampal neurons. Eur. J. Pharmacol. 2010;633:1–9. doi: 10.1016/j.ejphar.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Park DC, Royal D, Dudley W, Morrell R. Forgetting of pictures over a long retention interval in young and older adults. Psychol. Aging. 1988;3:94–95. doi: 10.1037//0882-7974.3.1.94. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. fifth edition Elsevier Academic Press; 2003. [Google Scholar]
- Porte Y, Buhot MC, Mons NE. Alteration of CREB phosphorylation and spatial memory deficits in aged 129T2/Sv mice. Neurobiol. Aging. 2008a;29:1533–1546. doi: 10.1016/j.neurobiolaging.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Porte Y, Buhot MC, Mons NE. Spatial memory in the Morris water maze and activation of cyclic AMP response element-binding (CREB) protein within the mouse hippocampus. Learn. Mem. 2008b;15:885–894. doi: 10.1101/lm.1094208. [DOI] [PubMed] [Google Scholar]
- Pych JC, Kim M, Gold PE. Effects of injections of glucose into the dorsal striatum on learning of place and response mazes. Behav. Brain Res. 2006;167:373–378. doi: 10.1016/j.bbr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Quartermain D, Judge ME, Jung H. Amphetamine enhances retrieval following diverse sources of forgetting. Physiol. Behav. 1988;43:239–241. doi: 10.1016/0031-9384(88)90245-4. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Gold PE. Glucose injections into the medial septum reverse the effects of intraseptal morphine infusions on hippocampal acetylcholine output and memory. Neuroscience. 1995;68:981–988. doi: 10.1016/0306-4522(95)00204-v. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Pal SN, Unick K, Stefani MR, Gold PE. Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. J. Neurosci. 1998;18:1595–1601. doi: 10.1523/JNEUROSCI.18-04-01595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc. Natl. Acad. Sci. 1996;93:4693–4698. doi: 10.1073/pnas.93.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Bonini JS, Coitinho AS, Vianna MR, Medina JH, Cammarota M, Izquierdo I. Retrograde amnesia induced by drugs acting on different molecular systems. Behav. Neurosci. 2004;118:563–568. doi: 10.1037/0735-7044.118.3.563. [DOI] [PubMed] [Google Scholar]
- Salinas JA, Gold PE. Glucose regulation of memory for reward reduction in young and aged rats. Neurobiol. Aging. 2005;26:45–52. doi: 10.1016/j.neurobiolaging.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Systemic or intra-amygdala injections of glucose facilitate memory consolidation for extinction of drug-induced conditioned reward. Eur. J. Neurosci. 2003;17:1482–1488. doi: 10.1046/j.1460-9568.2003.02578.x. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J. Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu. Rev. Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Radulovic J, Spiess J. Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: relationship to Fos production. Brain Res. Mol. Brain Res. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Gold PE. Intra-septal injections of glucose and glibenclamide attenuate galanin-induced spontaneous alternation performance deficits in the rat. Brain Res. 1998;813:50–56. doi: 10.1016/s0006-8993(98)00876-2. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Gold PE. Intrahippocampal infusions of k-atp channel modulators influence spontaneous alternation performance: relationships to acetylcholine release in the hippocampus. J. Neurosci. 2001;21:609–614. doi: 10.1523/JNEUROSCI.21-02-00609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Nicholson GM, Gold PE. ATP-sensitive potassium channel blockade enhances spontaneous alternation performance in the rat: a potential mechanism for glucose-mediated memory enhancement. Neuroscience. 1999;93:557–563. doi: 10.1016/s0306-4522(99)00128-1. [DOI] [PubMed] [Google Scholar]
- Sternberg DB, Martinez JL, Jr., Gold PE, McGaugh JL. Age-related memory deficits in rats and mice: enhancement with peripheral injections of epinephrine. Behav. Neural Biol. 1985;44:213–220. doi: 10.1016/s0163-1047(85)90212-2. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Bear MF, Alberini CM. A molecular correlate of memory and amnesia in the hippocampus. Nat. Neurosci. 1999;2:309–310. doi: 10.1038/7217. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Monti B, Dolan B, Pollonini G, Alberini CM. Fornix-dependent induction of hippocampal CCAAT enhancer-binding protein [beta] and [delta] co-localizes with phosphorylated cAMP response element-binding protein and accompanies long-term memory consolidation. J. Neurosci. 2001;21:84–91. doi: 10.1523/JNEUROSCI.21-01-00084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietje KR, Anderson DJ, Bitner RS, Blomme EA, Brackemeyer PJ, Briggs CA, Browman KE, Bury D, Curzon P, Drescher KU, Frost JM, Fryer RM, Fox GB, Gronlien JH, Håkerud M, Gubbins EJ, Halm S, Harris R, Helfrich RJ, Kohlhaas KL, Law D, Malysz J, Marsh KC, Martin RL, Meyer MD, Molesky AL, Nikkel AL, Otte S, Pan L, Puttfarcken PS, Radek RJ, Robb HM, Spies E, Thorin-Hagene K, Waring JF, Ween H, Xu H, Gopalakrishnan M, Bunnelle WH. Preclinical characterization of A-582941: a novel alpha7 neuronal nicotinic receptor agonist with broad spectrum cognition-enhancing properties. CNS Neurosci. Ther. 2008;14:65–82. doi: 10.1111/j.1527-3458.2008.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A. The importance of being subtle: small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell. 2007;6:267–273. doi: 10.1111/j.1474-9726.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J. Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn. Mem. 2006;13:349–358. doi: 10.1101/lm.80206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofimiuk E, Holownia A, Braszko JJ. Activation of CREB by St. John's wort may diminish deletorious effects of aging on spatial memory. Arch. Pharm. Res. 2010;33:469–477. doi: 10.1007/s12272-010-0318-y. [DOI] [PubMed] [Google Scholar]
- Vilaró MT, Mengod G, Palacios G, Palacios JM. Receptor distribution in the human and animal hippocampus: focus on muscarinic acetylcholine receptors. Hippocampus. 1993;3:149–156. Spec No. [PubMed] [Google Scholar]
- Viola H, Furman M, Izquierdo LA, Alonso M, Barros DM, de Souza MM, Izquierdo I, Medina JH. Phosphorylated cAMP response element-binding protein as a molecular marker of memory processing in rat hippocampus: effect of novelty. J. Neurosci. 2000;20:RC112. doi: 10.1523/JNEUROSCI.20-23-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KA, Gold PE. Age-related changes in brain catecholamine responses to a single footshock. Neurobiol. Aging. 1984;5:55–59. doi: 10.1016/0197-4580(84)90086-1. [DOI] [PubMed] [Google Scholar]
- Williams CL, McGaugh JL. Reversible lesions of the nucleus of the solitary tract attenuate the memory-modulating effects of posttraining epinephrine. Behav. Neurosci. 1993;107:955–962. [PubMed] [Google Scholar]
- Williams CL, Men D, Clayton EC, Gold PE. Norepinephrine release in the amygdala after systemic injection of epinephrine or escapable footshock: contribution of the nucleus of the solitary tract. Behav. Neurosci. 1998;112:1414–1422. doi: 10.1037//0735-7044.112.6.1414. [DOI] [PubMed] [Google Scholar]
- Winocur G. A neuropsychological analysis of memory loss with age. Neurobiol. Aging. 1988;9:487–494. doi: 10.1016/s0197-4580(88)80102-7. [DOI] [PubMed] [Google Scholar]
- Xu J, Rong S, Xie B, Sun Z, Deng Q, Wu H, Bao W, Wang D, Yao P, Huang F, Liu L. Memory impairment in cognitively impaired aged rats associated with decreased hippocampal CREB phosphorylation: reversal by procyanidins extracted from the lotus seedpod. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:933–940. doi: 10.1093/gerona/glq094. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Lu T, Loerch P. The aging brain. Annu. Rev. Pathol. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- Yin JC, Tully T. CREB and the formation of long-term memory. Curr. Opin. Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Bruce JC, Darby-King A, McLean JH. Isoproterenol increases CREB phosphorylation and olfactory nerve-evoked potentials in normal and 5-HT-depleted olfactory bulbs in rat pups only at doses that produce odor preference learning. Learn. Mem. 2000;7:413–421. doi: 10.1101/lm.35900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornetzer SF, Thompson R, Rogers J. Rapid forgetting in aged rats. Behav. Neural. Biol. 1982;36:49–60. doi: 10.1016/s0163-1047(82)90234-5. [DOI] [PubMed] [Google Scholar]