Abstract
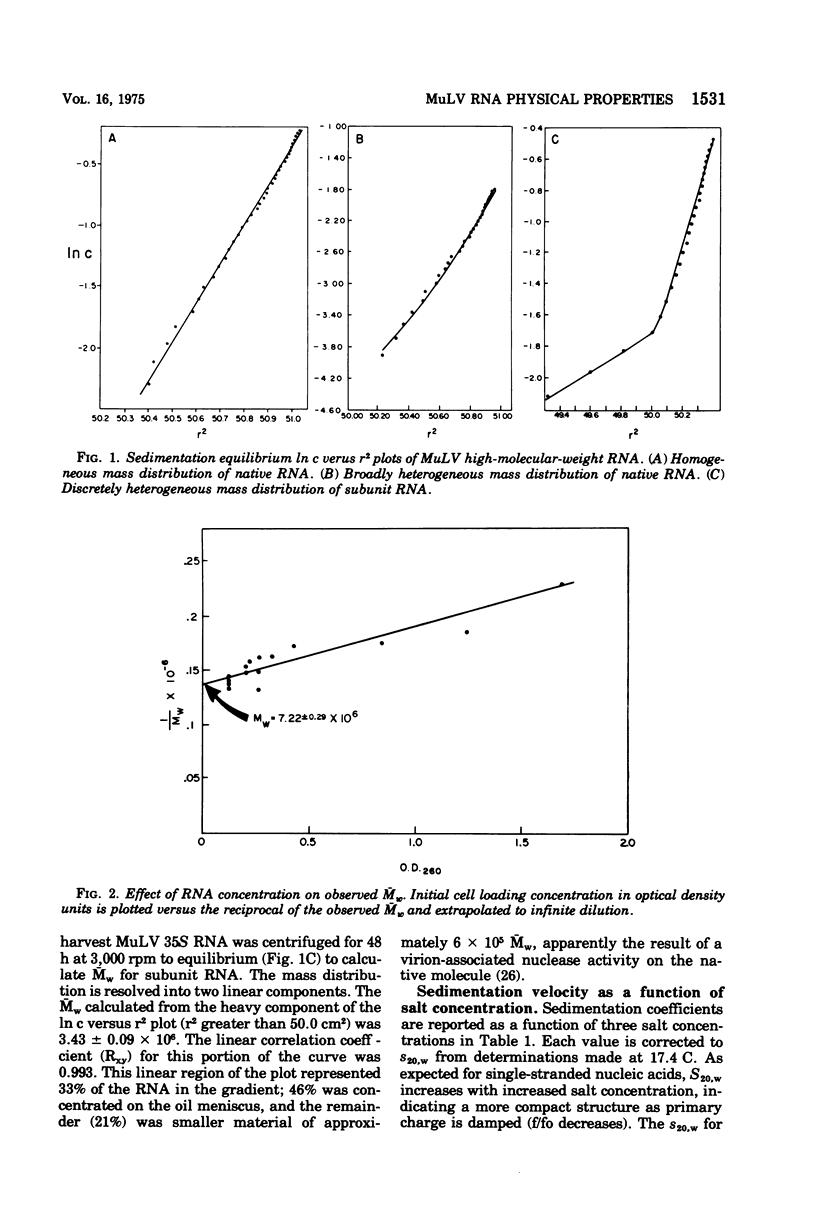
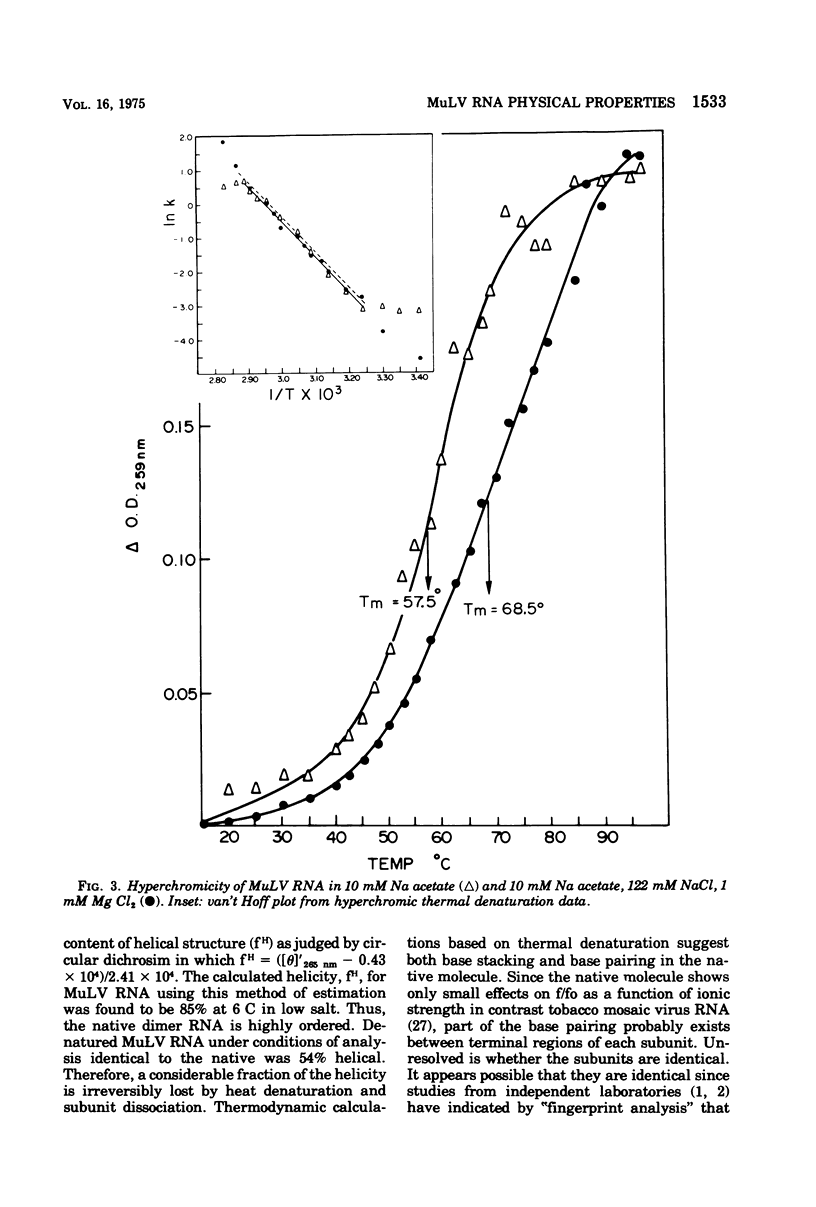
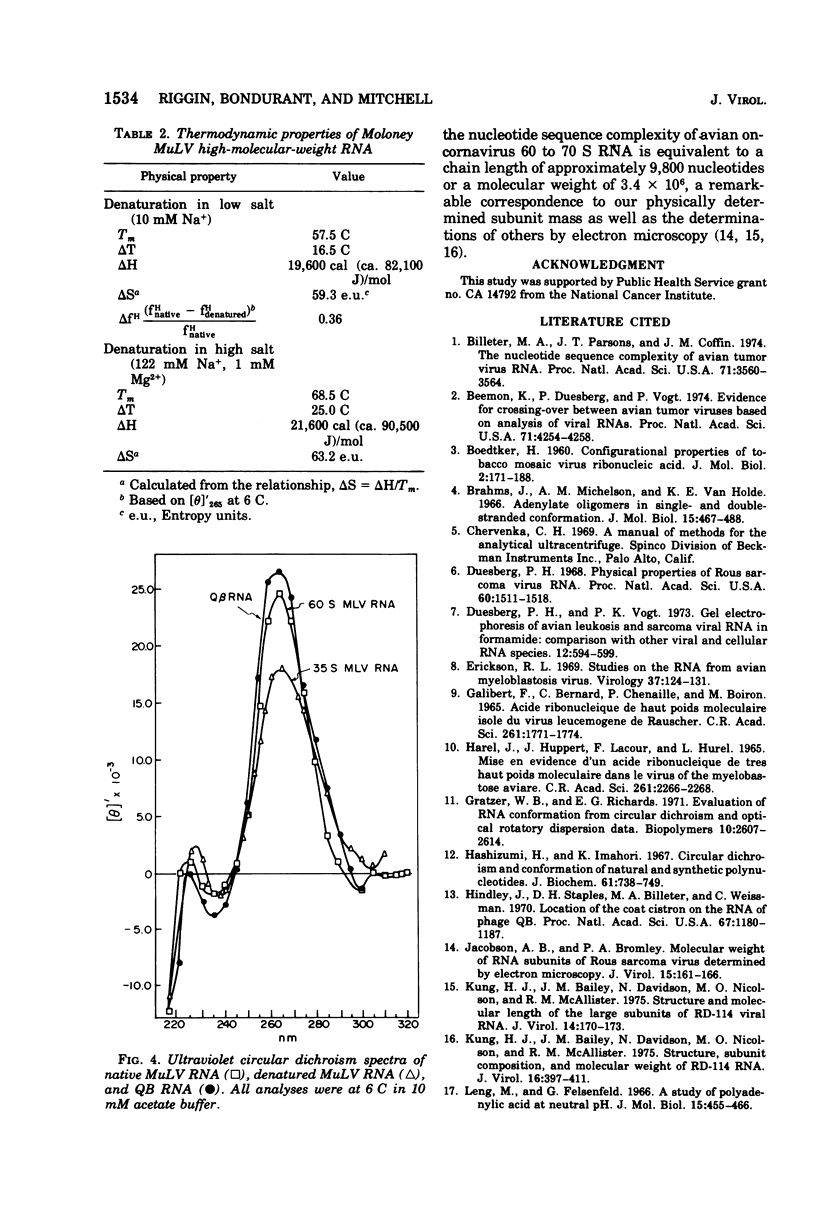
The high-molecular-weight RNA of Moloney murine leukemia virus (MuLV) was analyzed by sedimentation equilibrium ultracentrifugation. Molecular weights of 7.2 x 10(6) and 3.4 x 10(6) were found for the native and subunit forms, respectively, indicating that the native structure is a dimer. S20,w and frictional coefficients were determined for MuLV RNA by analytical velocity centrifugation as a function of ionic strength. The apparent S20,w of native MuLV RNA was 47.3, 57.4, and 66.5 in 0.01, 0.1, and 0.20 M Na+, respectively; the corresponding frictional coefficients were 5.44, 4.48, and 3.87. Native RNA was estimated by circular dichroism to be 85% helical, whereas denatured RNA was 54% helical. Thermal denaturation profiles were obtained from uv absorbance scans. Melting temperatures of 57 and 68 C were obtained for high-molecular-weight RNA in 0.01 M Na+ and 0.122 M Na+, 1mM Mg2+, respectively. van't Hoff plots of the thermal denaturation data gave enthalpies for the helix-coil transition of 21,600 cal (ca. 90,500 J) per mol of cooperatively melting unit in high salt and 19,600 cal (ca. 82,100 J) per mol in low salt, consistent with both base stacking and pairing. The melting of Mu LV RNA occurred over a broad temprange and van't Hoff plots were linear over most of the melting range, indicating a noncooperative process of helix stabilization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahms J., Michelson A. M., Van Holde K. E. Adenylate oligomers in single- and double-strand conformation. J Mol Biol. 1966 Feb;15(2):467–488. doi: 10.1016/s0022-2836(66)80122-5. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Galibert F., Bernard C., Chenaille P., Boiron M. Acide ribonucléique de haut poids moléculaire isolé du virus leucémogène de Rauscher. C R Acad Sci Hebd Seances Acad Sci D. 1965 Aug 18;261(7):1771–1774. [PubMed] [Google Scholar]
- Gratzer W. B., Richards E. G. Evaluation of RNA conformation from circular dichroism and optical rotatory dispersion data. Biopolymers. 1971;10(12):2607–2614. doi: 10.1002/bip.360101220. [DOI] [PubMed] [Google Scholar]
- Harel J., Huppert J., Lacour F., Harel L. Mise en évidence d'un acide ribonucléique de très haut poids moléculaire dans le virus de la myéloblastose aviaire. C R Acad Sci Hebd Seances Acad Sci D. 1965 Sep 13;261(11):2266–2268. [PubMed] [Google Scholar]
- Hashizume H., Imahori K. Circular dichroism and conformation of natural and synthetic polynucleotides. J Biochem. 1967 Jun;61(6):738–749. doi: 10.1093/oxfordjournals.jbchem.a128608. [DOI] [PubMed] [Google Scholar]
- Hindley J., Staples D. H., Billeter M. A., Weissmann C. Location of the coat cistron on the RNA of phage Q-beta. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1180–1187. doi: 10.1073/pnas.67.3.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Bailey J. M., Davidson N., Nicolson M. O., McALLISTER R. M. Structure and molecular length of the large subunits of RD-114 viral RNA. J Virol. 1974 Jul;14(1):170–173. doi: 10.1128/jvi.14.1.170-173.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Bailey J. M., Davidson N., Nicolson M. O., McAllister R. M. Structure, subunit composition, and molecular weight of RD-114 RNA. J Virol. 1975 Aug;16(2):397–411. doi: 10.1128/jvi.16.2.397-411.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng M., Felsenfeld G. A study of polyadenylic acid at neutral pH. J Mol Biol. 1966 Feb;15(2):455–466. doi: 10.1016/s0022-2836(66)80121-3. [DOI] [PubMed] [Google Scholar]
- Luborsky S. W. Sedimentation equilibrium analysis of the molecular weight of a tumor virus RNA. Virology. 1971 Sep;45(3):782–787. doi: 10.1016/0042-6822(71)90195-4. [DOI] [PubMed] [Google Scholar]
- MOELLER W. J. DETERMINATION OF DIFFUSION COEFFICIENTS AND MOLECULAR WEIGHTS OF RIBONUCLEIC ACIDS AND VIRUSES. Proc Natl Acad Sci U S A. 1964 Mar;51:501–509. doi: 10.1073/pnas.51.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel W. F., Delius H., Duesberg P. H. Structure and molecular weight of the 60-70S RNA and the 30-40S RNA of the Rous sarcoma virus. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4541–4545. doi: 10.1073/pnas.71.11.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell W. M., Hash J. H. The N,O-diacetylmuramidase of Chalaropsis species. II. Physical properties. J Biol Chem. 1969 Jan 10;244(1):17–21. [PubMed] [Google Scholar]
- Mitra S., Enger M. D., Kaesberg P. PHYSICAL AND CHEMICAL PROPERTIES OF RNA FROM THE BACTERIAL VIRUS R17. Proc Natl Acad Sci U S A. 1963 Jul;50(1):68–75. doi: 10.1073/pnas.50.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega J. P., Hill W. E. A molecular weight determination of the 16S ribosomal ribonucleic acid from Escherichia coli. Biochemistry. 1973 Aug 14;12(17):3241–3243. doi: 10.1021/bi00741a015. [DOI] [PubMed] [Google Scholar]
- Overby L. R., Barlow G. H., Doi R. H., Jacob M., Spiegelman S. Comparison of two serologically distinct ribonucleic acid bacteriophages. II. Properties of the nucleic acids and coat proteins. J Bacteriol. 1966 Sep;92(3):739–745. doi: 10.1128/jb.92.3.739-745.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijnders L., Sloof P., Sival J., Borst P. Gel electrophoresis of RNA under denaturing conditions. Biochim Biophys Acta. 1973 Oct 26;324(3):320–333. doi: 10.1016/0005-2787(73)90278-5. [DOI] [PubMed] [Google Scholar]
- Riggin C. H., Bondurant M. C., Mitchell W. M. Differences between murine leukemia virus and murine sarcoma virus: effects of virion age and multiplicity of infection on viral RNA. Intervirology. 1974;2(4):209–221. doi: 10.1159/000149426. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Robinson H. L., Duesberg P. H. Tumor virus RNA's. Proc Natl Acad Sci U S A. 1967 Sep;58(3):825–834. doi: 10.1073/pnas.58.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachman H. K., Edelstein S. J. Ultracentrifuge studies with absorption optics. IV. Molecular weight determinations at the microgram level. Biochemistry. 1966 Aug;5(8):2681–2705. doi: 10.1021/bi00872a029. [DOI] [PubMed] [Google Scholar]
- Slegers H., Clauwaert J., Fiers W. Studies on the bacteriophage MS2. XXIV. Hydrodynamic properties of the native and acid MS2 RNA structures. Biopolymers. 1973;12(9):2033–2044. doi: 10.1002/bip.1973.360120910. [DOI] [PubMed] [Google Scholar]
- TASHIRO Y., YPHANTIS D. A. MOLECULAR WEIGHTS OF HEPATIC RIBOSOMES AND THEIR SUBUNITS. J Mol Biol. 1965 Feb;11:174–186. doi: 10.1016/s0022-2836(65)80049-3. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]


