Abstract
Social decisions play a crucial role in the success of individuals and the groups they compose. Group members respond vicariously to benefits obtained by others, and impairments in this capacity contribute to neuropsychiatric disorders like autism and sociopathy. We studied how neurons in three frontal cortical areas encode the outcomes of social decisions as monkeys performed a reward-allocation task. Neurons in the orbitofrontal cortex (OFC) predominantly encoded rewards delivered to oneself. Neurons in the anterior cingulate gyrus (ACCg) encoded reward allocations to the other monkey, reward allocations to oneself, or both. Neurons in the anterior cingulate sulcus (ACCs) signaled reward allocations to the other monkey or no one. Within this network of received (OFC) and foregone (ACCs) reward signaling, ACCg emerges as a key nexus for the computation of shared experience and social reward. Individual and species-specific variations in social decision-making might result from the relative activation and influence of these areas.
Social cohesion depends on vicarious identification with members of one’s group. In social situations, we are aware of our actions and their consequences but also consider those of others, especially those with whom we might interact1. We also estimate the internal states of others, perhaps by simulation2, which in turn shapes our future actions. Social situations can drive observational learning3, and other-regarding preferences influence neural computations that ultimately result in cooperation, altruism, or spite4,5. Disruptions of neural circuits involved in other-regarding processes may underlie social deficits attending neuropsychiatric conditions like autism6. Human imaging and clinical studies have found critical links between social deficits and abnormal brain activity in frontal cortex and its subcortical targets7.
Neural circuits involved in reinforcement learning and decision-making are crucial for normal social interactions8. Critical nodes include the anterior cingulate cortex9–11, the orbitofrontal cortex12–17, and subcortical areas such as the dopaminergic ventral tegmental area and substantia nigra18,19, the striatum20–21, the lateral habenula22, and the amygdala23. Neuroimaging studies in humans report activation of some of these areas by both giving rewards and receiving rewards24–28, and lesions to some of these areas result in impaired social decision-making7. These findings thus suggest a generic circuit for reward-guided learning and decision-making mediates social decisions8. Despite this evidence, and the clear clinical relevance of understanding the neurobiology of social decision-making, precisely how neurons in any of these areas compute social decisions remains unknown, largely due to difficulties in implementing social interactions while simultaneously studying neuronal activity and controlling contextual variables. Single unit recording studies in nonhuman animals, such as macaques, making social decisions of similar complexity to those made by humans would help address this gap.
To address this gap, we implemented a reward-allocation task in pairs of rhesus macaques while at the same time recording from single neurons in three critical nodes in the decision-making network, namely the anterior cingulate gyrus (ACCg), the anterior cingulate sulcus (ACCs), and the orbitofrontal cortex (OFC). Our study capitalized on monkeys’ willingness to engage with a social partner via an interposed computer system while at the same time controlling the sensory and reward environment. We specifically matched choices for the reward outcomes directly received by the actor monkey and controlled for potential secondary acoustic reinforcement effects associated with delivering juice to the recipient monkey (see below). Under these conditions, we found regional biases in the encoding of social decision outcomes with respect to self and another individual. Within this network of received (OFC) and foregone (ACCs) reward signals, ACCg emerges as a key nexus for the computation of shared experience and social reward.
Summary of behavior in the reward-allocation task
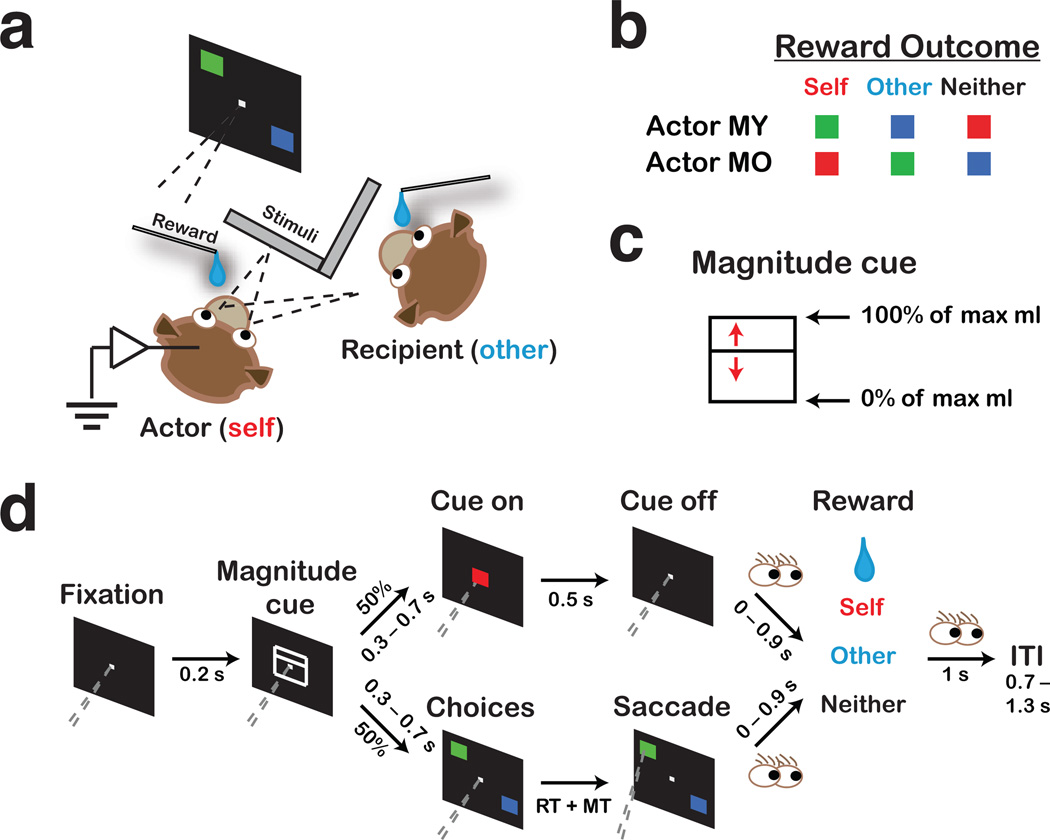
On one half of trials, termed choice trials, actor monkeys chose between visual stimuli that led to juice delivered either to themselves (self reward), to the recipient monkey (other reward), or to neither monkey (neither reward). Offers appeared in pairs of three types, which defined Self:Neither trials, Self:Other trials, and Other:Neither trials (Fig. 1a–d). On the other half, termed cued trials, monkeys observed a single cue that indicated self, other or neither rewards would be delivered by the computer, as defined above.
Figure 1.
Reward-allocation task. (a) Experimental setup for an actor and a recipient monkey. (b) Stimulus-reward outcome mappings for reward delivered to actor (self), recipient (other), or no one (neither), shown separately for each actor. (c) Magnitude cue used to indicate juice amount at stake for each trial (see d). Position of the horizontal bisecting line specified the percentage of maximum reward possible. (d) Task structure (see Online Methods). Top fork, cued trials; bottom fork, choice trials. Dashed gray lines show the angle of the actor’s gaze, converging on the fixation point. Eye cartoons indicate times when the actor could look around. RT, reaction time. MT, movement time. ITI, inter-trial interval.
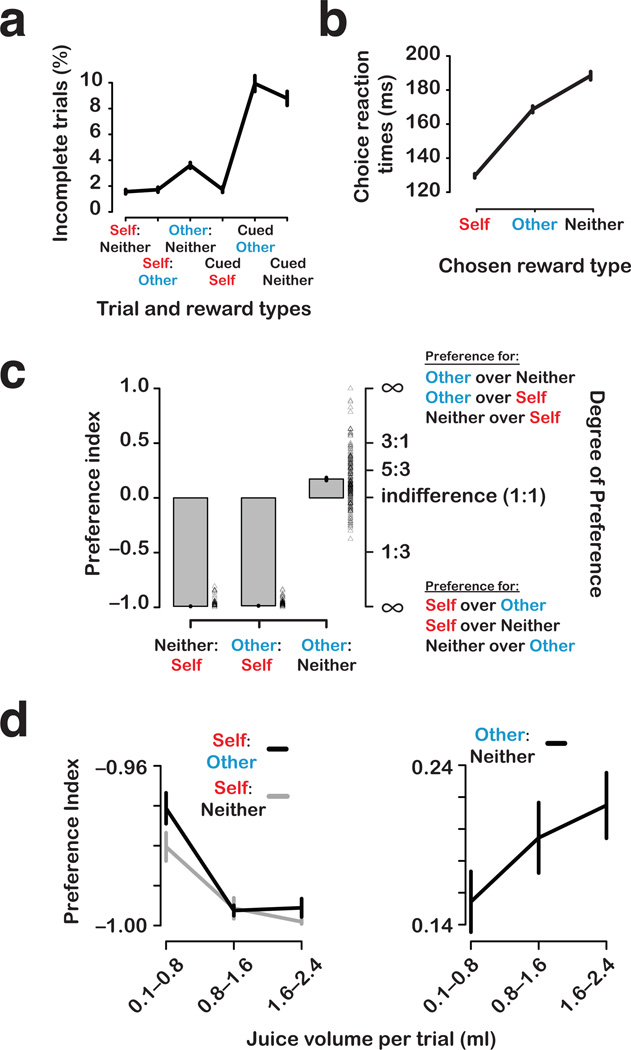
Actors performed the task well (Fig. 2a), as indicated by the low mean number of incomplete trials per session (4.6 ± 0.2% [s.e.m.]) (Online Methods), even when they had no chance of obtaining juice rewards themselves, which was the case for Other:Neither choice trials and for other and neither cued trials (7.4 ± 0.3%). Actors also made significantly fewer errors when they made active decisions (choice trials) then when there was no choice (cued trials), when there was no reward at stake for themselves (P < 0.0001, Welch two sample t-test). These findings suggest monkeys find it rewarding to actively choose what to do, and can be motivated to work without direct reinforcement.
Figure 2.
Behavior in the reward-allocation task. (a) Proportions of incomplete trials (mean ± s.e.m.) (see Online Methods) during the reward-allocation task. (b) Choice reaction times (ms) from trials in which rewards were chosen for self, other, or neither (mean of session medians ± s.e.m). (c) Choice preferences (preference index, mean ± s.e.m.) as a function of reward outcome contrasts. Data points next to each bar show means for individual sessions. The degree of preference axis on the right shows the range of preference indices in ratio terms. (d) Choice preferences (mean ± s.e.m.) as a function of reward magnitude on 219 single-unit sessions collected with the magnitude cue.
Reaction times often serve as a proxy for motivation in incentivized tasks29–33. Reaction times for making different choices demonstrate that actors discriminated the reward types and had orderly preferences amongst them29,33. Actors were fastest to choose self rewards, followed by other rewards and neither rewards (Fig. 2b). Self vs. other reaction times differed by a mean of 39 ms (P < 0.0001; Welch two-sample t-test); other vs. neither differed by a mean of 20 ms (P < 0.0001). The ordered reaction times by monkeys making choices in the reward allocation task suggest that rewarding self is more reinforcing than rewarding the recipient, which is in turn more reinforcing than rewarding no one33.
Finally, actors shifted gaze to the recipients more frequently following juice delivery to them compared to juice delivery to themselves or to neither monkey, consistent with greater interest in the actions of the other monkey when he was rewarded (Supplementary Figure 1). Taken together, these observations support the conclusion that actors were acutely aware of the difference between self, other, and neither reward outcomes33.
We quantified decision preferences by calculating a contrast ratio based on actors’ choices (Online Methods Eq. 1). Consistent with our previous reports33,34, actors preferred self rewards over other or neither rewards, but preferred other over neither rewards (Fig. 2c). On Self:Neither and Self:Other trials, actors almost always chose to reward self (Fig. 2c) (preference index [mean ± s.e.m.]: Self:Neither, −0.99 ± 0.00; Self:Other, −0.99 ± 0.00; significantly different from zero: both P < 0.0001, one sample t-test). By contrast, on Other:Neither trials, actors preferred to allocate rewards to the recipient monkey (Fig. 2c) (0.17 ± 0.01; P < 0.0001, one sample t-test). We observed similar choice preferences for each actor individually (Supplementary Figure 2).
We previously reported that the preference to allocate reward to the other monkey is enhanced by greater familiarity between the two animals, and is abolished if the recipient is replaced with a juice collection bottle33. We also reported that reward withholding is reduced when actor monkeys are dominant toward recipients, and the variability and the degree of preferences often depend on the identity of the recipients33. Furthermore, we reported that actor monkeys prefer to deliver juice to themselves compared to both themselves and the recipient simultaneously, perhaps reflecting the competitive nature of simultaneously drinking juice—a resource controlled outside of experimental sessions in order to motivate performance and often monopolized by dominant monkeys living in pairs with subordinate monkeys in their home cages33 (MLP, personal observation). Finally, exogenously increasing oxytocin levels in the central nervous system amplifies actors’ preference to allocate reward to the other monkey over no one34. Taken together, these patterns of behavior endorse the fundamentally social nature of the reward-allocation task.
We also found that preferences scaled with the magnitude of juice on offer. With larger amounts of juice at stake, actors became more motivated to receive (Self:Neither & Self:Other, slope significantly different from zero: both P < 0.001, type II regression) and also to allocate rewards to the other monkey over no one (Other:Neither, P < 0.05) (Fig. 2d). These findings suggest that both direct and vicarious reinforcement processes that motivate social decisions are magnified by reward magnitude25–27.
Differential encoding of social decision outcomes
We recorded the activity of single neurons in ACCg (n = 81), ACCs (n = 101), and OFC (n = 85) from two actor monkeys (Fig. 3a) during the reward-allocation task. We describe neuronal responses from typical single neurons and the populations below for each region. We analyzed the data for both a choice/cue epoch and a reward epoch (Online Methods). Supplementary Figure 3 shows population data for the individual monkeys. For brevity, we focus on the reward epoch; data for the choice/cue epoch are found in Supplementary Figure 4, as well as in Figures 3 and 4. Overall, we found remarkable resemblances in activity and functional classes (see below) across the choice and reward epochs.
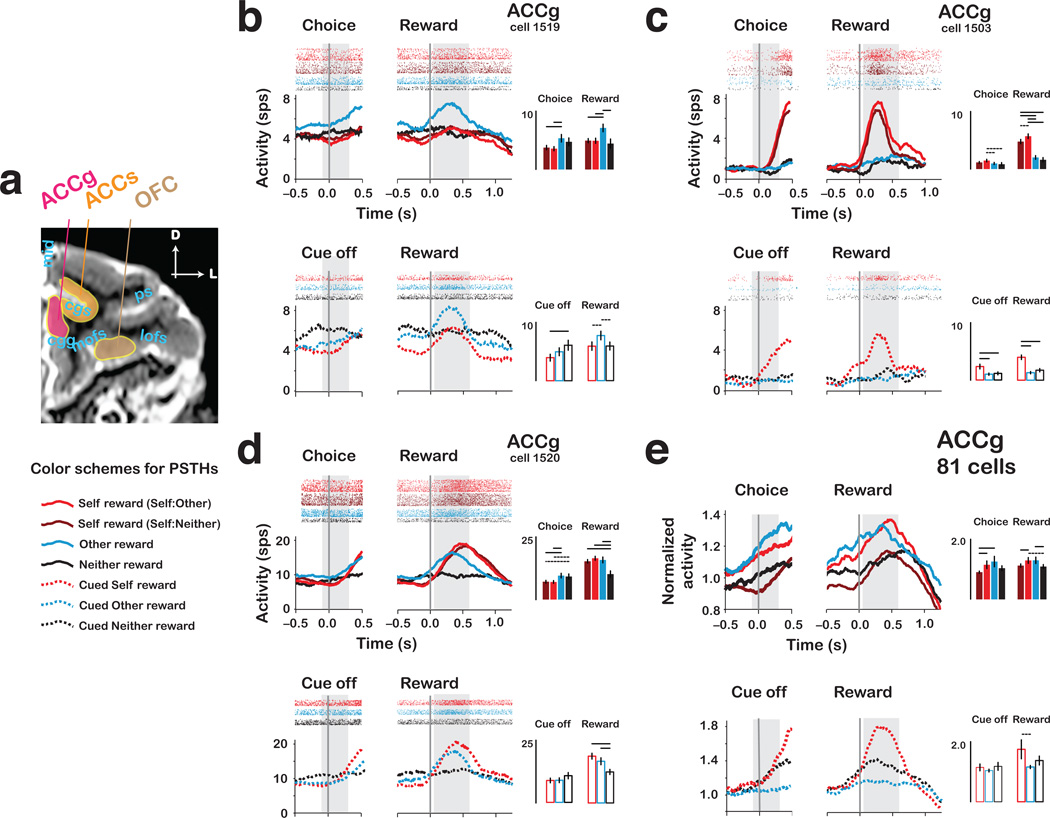
Figure 3.
Single neurons and population responses from ACCg. (a) Structural magnetic resonance image from actor MO, with example electrode paths for ACCg, ACCs and OFC. (Also see Fig. 6.) (b) Mean responses (peri-stimulus time histograms [PSTHs]) and spike rasters for an other-reward preferring ACCg neuron, on choice trials (upper, solid traces) and cued trials (lower, dashed traces). Data are aligned to choice/cue offset (left) and reward onset (right) for each reward outcome. Bar histograms on right show mean ± s.e.m. activity from the two epochs (grey regions). Color codes for PSTH traces and histograms shown below. (c) PSTHs and spike rasters for a self-reward preferring ACCg neuron. (d) PSTHs and spike rasters for a shared self and other reward preferring ACCg neuron. (e) Normalized choice/cue epoch and reward epoch responses for 81 ACCg neurons. c–e, same format as in b. In all bar histogram insets, the horizontal lines above different conditions indicate significance differences (solid, P < 0.05 by paired t-test; dashed, P < 0.05 by bootstrap test).
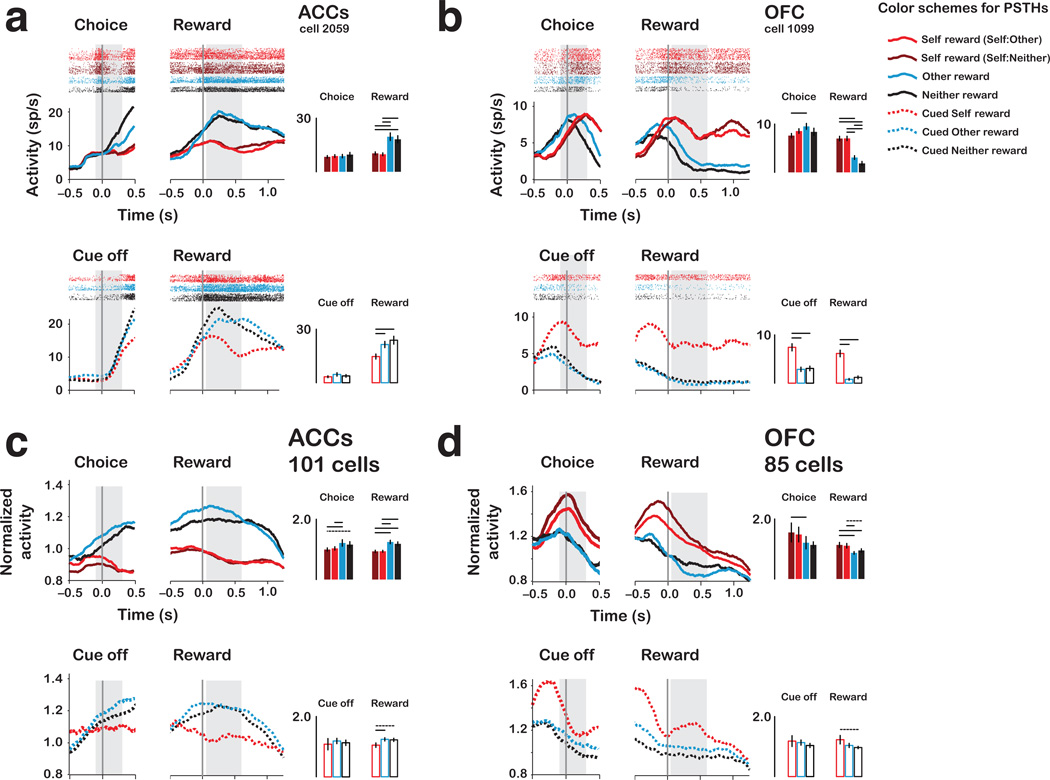
Figure 4.
Single neurons and population responses from ACCs and OFC. (a) PSTHs and spike rasters for a single ACCs neuron preferring forgone rewards. Data are aligned to choice/cue offset (left) and reward onset (right) for each reward outcome. Bar histograms on right show mean ± s.e.m. activity from the two epochs (grey regions). (b) PSTHs and spike rasters for a single OFC neuron preferring self reward. (c) Normalized reward epoch responses of 101 ACCs neurons. (d) Normalized choice/cue epoch and reward epoch responses of 85 OFC neurons. All panels, same format as in Fig. 3.
ACCg
ACCg contained neurons selective for allocating rewards to another individual, receiving rewards, or both. One class of ACCg neuron (Fig. 3b) preferentially responded when actors chose to allocate reward to recipients. On choice trials, this example neuron discharged more strongly when the actor chose other rewards (7.12 ± 0.66 [mean and s.e.m.] spikes/s [sp/s]) compared to self rewards on either Self:Neither or Self:Other trials (4.95 ± 0.36, 4.93 ± 0.45 sp/s, respectively) (both P < 0.01, Welch two sample t-test), and also preferred other rewards over neither rewards (4.44 ± 0.79 sp/s, P < 0.05). This neuron did not differentiate self from neither rewards (P = 0.97, Welch two sample t-test). On cued trials, this neuron only weakly preferred other over self or neither rewards (both P = 0.08, Welch two sample t-test) (Fig. 3b).
By contrast, another class of ACCg neuron (example neuron in Fig. 3c) responded selectively for choosing self rewards. The example neuron in Figure 3c neuron discharged more when the actor chose to reward himself on Self:Neither and Self:Other trials (4.77 ± 0.38, 5.70 ± 0.41 sp/s, respectively) compared to choosing other and neither rewards (2.02 ± 0.32, 1.60 ± 0.39 sp/s, respectively) (all P < 0.0001, Welch two sample t-test). Moreover, it showed stronger responses when the actor received rewards in Self:Other than Self:Neither context, but this effect did not reach statistical significance (P = 0.10, Welch two sample t-test). On cued trials, this neuron preferred self over other or neither rewards (both P < 0.0001, Welch two sample t-test). For both choice and cued trials, the response did not differentiate other and neither rewards (both P > 0.23, Welch two sample t-test).
Finally, a third class of ACCg neuron (example neuron in Fig. 3d) responded equivalently to both received rewards (Self:Neither, 15.28 ± 0.70, Self:Other, 16.47 ± 0.81 sp/s) and allocated rewards to other (15.81 ± 1.16 sp/s) (both P > 0.64, Welch two sample t-test), but responded significantly less to neither rewards (10.17 ± 1.23 sp/s; other vs. neither and self vs. neither: both P < 0.005). Similarly, on cued trials, this neuron preferred other over neither rewards (P < 0.05, Welch two sample t-test), but did not differentiate between self and other rewards (P = 0.27).
Importantly, the fact that the solenoid valves controlling juice delivery (including one for neither rewards that only produced clicks) were placed outside the experimental room, as well as the white noise played inside the room, during sessions rules out a simple explanation that other-reward specific (Fig. 3b) and shared self/other reward responses (Fig. 3d) were merely sensory responses to the sounds of the reward-delivery mechanism.
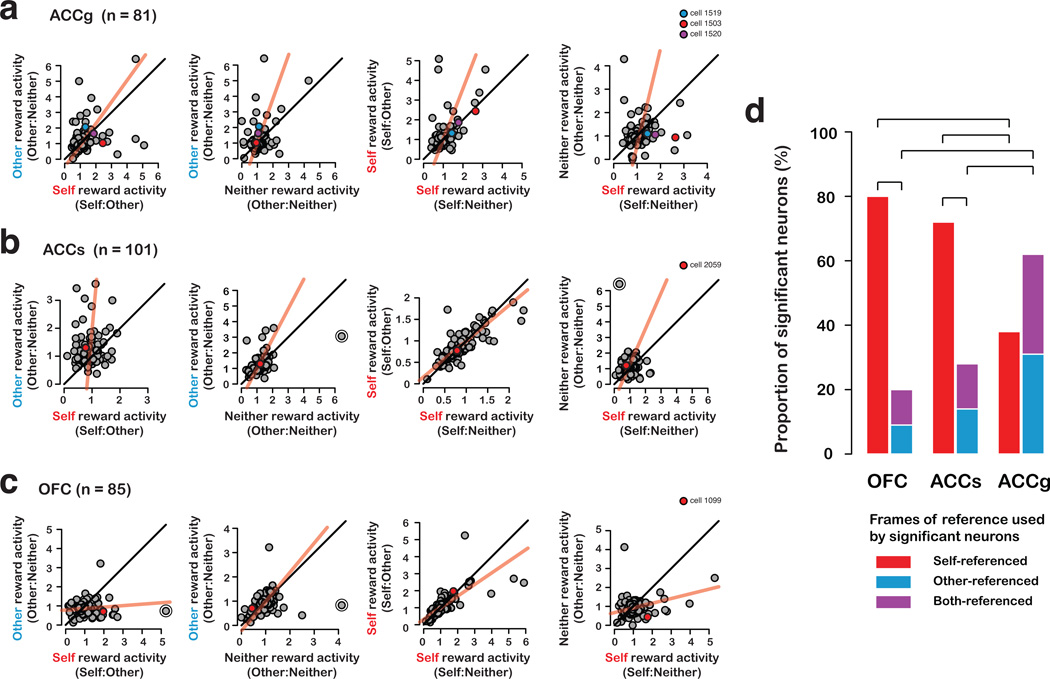
To contrast population coding of decision and reward information in various conditions, we computed a normalized activity bias between each pair of outcomes, expressed as a proportional modulation in mean firing rates normalized by baseline firing rate. In the ACCg population, the mean normalized activity bias for other over neither rewards (other vs. neither) was 0.21 ± 0.10 (s.e.m.), i.e., a 21% difference, which was significant (P < 0.05, paired t-test) (Fig. 3e, 5a). Similarly, the bias for self (from Self:Other) over neither rewards was 0.20 ± 0.12 (P = 0.09, paired t-test). Notably, the population showed equivalent responses for self rewards (Self:Other) and other rewards (0.01 ± 0.12, P = 0.96, paired t-test). On the other hand, it showed a significant bias for self rewards when the actors were presented with a choice between rewarding themselves and recipients compared to when the actors were presented with a choice between rewarding themselves and no one (Self:Other vs. Self:Neither, by 0.17 ± 0.08, P < 0.05, paired t-test), suggesting that ACCg is particularly sensitive to a reward context involving an option to reward another individual. Thus, the ACCg population showed an equivalent preference for other and self rewards, and preferred both over neither rewards.
Figure 5.
Population biases for self, other, and neither rewards. Scatter plots show mean normalized reward epoch responses (proportion of modulation relative to baseline) of individual neurons (from left to right) between self (Self:Other) and other rewards, between other and neither rewards, between self rewards from Self:Neither and Self:Other contexts, and between self (Self:Neither) and neither rewards, for ACCg (a), ACCs (b), and OFC (c) populations. Regression lines (type II) are shown in red (the circled data points are excluded from the regression). Unity lines are shown in black. The example neurons from Fig. 3,4 are indicated on the scatter plots. (d) Proportion of neurons (out of significantly classified neurons) from OFC, ACCs, and ACCg using self-referenced, other-referenced, and both-referenced frames to represent reward outcomes. Inset shows color codes used in the bar graph. Bars indicate significant differences in proportions (P < 0.05, χ2 test).
On cued trials, however, a strikingly different pattern emerged. The population responded strongly to self rewards but barely responded to other rewards (0.59 ± 0.32, P = 0.07, paired t-test) (Fig. 3e). Furthermore, the population now responded no differently to other and neither rewards (0.22 ± 0.14, P = 0.14, paired t-test).
Taken together, these results indicate that ACCg, as a population, encodes both giving and receiving rewards. At the population level, neuronal activity selective for allocating rewards to another individual is specific to active decisions (upper vs. lower: Fig. 3e), similar to what has been reported by fMRI of human ventral striatum during voluntary versus forced charitable donations25. The confluence of neurons selectively responsive to self, other, and both (self and other) rewards in ACCg suggests this area contains the information necessary to mediate the vicarious reinforcement processes that appear to motivate actors to give to recipients.
ACCs
Fig. 4a shows a typical ACCs neuron that fired more strongly preceding other and neither rewards than self rewards. On choice trials, this neuron discharged more strongly when the actor chose not to reward himself (other rewards, 19.64 ± 2.15; neither rewards, 18.19 ± 2.03 sp/s) compared to when he chose to reward himself directly (Self:Neither, 10.31 ± 0.86; Self:Other, 9.79 ± 0.81 sp/s) (all P < 0.001, Welch two sample t-test). This neuron responded equivalently to self rewards in Self:Other and Self:Neither contexts (P = 0.66, Welch two sample t-test), and also responded equivalently to other and neither rewards (P = 0.62), consistent with encoding “foregone” rewards. On cued trials, this neuron responded equivalently to other and neither rewards (P = 0.39, Welch two sample t-test), but less to self rewards (both P < 0.005), resembling the responses to active decisions.
Likewise, the ACCs population showed a strong and equivalent response bias for foregone rewards (self vs. other, activity bias=0.31 ± 0.07; self vs. neither, activity bias=0.25 ± 0.08, both P < 0.005, paired t-test) (Fig. 4c, 5b). The population did not differentiate other from neither rewards (0.06 ± 0.06, P = 0.31, paired t-test). Unlike ACCg, the population did not respond differentially to Self:Other and Self:Neither contexts (differed by 0.003 ± 0.02, P = 0.90, paired t-test). We found similar patterns on cued trials – responses to self rewards were substantially reduced compared to other rewards (0.19 ± 0.09, P < 0.05, paired t-test) and neither rewards (0.18 ± 0.10, P < 0.08) (Fig. 4c). These results indicate that, during social interactions, ACCs neurons predominantly signal foregone rewards.
OFC
Fig. 4b shows a typical OFC neuron that preferentially encoded juice rewards received by the actor. On choice trials, this neuron discharged significantly more for self rewards than for the alternatives on both Self:Neither and Self:Other trials. Activity for self rewards did not differ between the two self reward contexts (7.00 ± 0.47, 7.03 ± 0.46 sp/s, respectively, P = 0.97, Welch two sample t-test), but it exceeded the cell’s activity for other and neither rewards (3.06 ± 0.40, 1.85 ± 0.42 sp/s, respectively; both P < 0.0001). On cued trials, this neuron responded most strongly to self rewards compared to both other and neither rewards (both P < 0.0001, Welch two sample t-test), but it did not respond differently between other and neither rewards (P = 0.25) (Fig. 4b).
The OFC population predominantly encoded self rewards compared to other and neither rewards. The bias for self over other rewards was 30% (0.30 ± 0.09, P < 0.005, paired t-test). For self versus neither rewards, the bias was also significant (0.17 ± 0.08, P < 0.05, paired t-test) (Fig. 4d, 5c). Population activity for other and neither rewards did not differ (0.08 ± 0.06, P = 0.20, paired t-test) (Fig. 4d, 5c). Unlike ACCg, the population did not respond differentially to Self:Other and Self:Neither contexts (differed by 0.06 ± 0.07, P = 0.39, paired t-test). On cued trials, the self reward bias was not present compared to other rewards (0.19 ± 0.16, P = 0.24, paired t-test) and only weakly present over neither rewards (0.26 ± 0.15, P < 0.08). On cued trials, the population did not distinguish other rewards from neither rewards (P = 0.33, paired t-test) (Fig. 4d). These results indicate that OFC neurons predominantly encode rewards received by the actors, and this information was encoded more faithfully during active decision-making.
Neuronal reference frames for social decisions
Neuroimaging and scalp-recording studies in humans can only study neuronal activity at an aggregate level. Our single-unit recording data thus provide a unique opportunity to quantify the frame of reference in which individual neurons within ACCg, ACCs, and OFC encode social decisions. To do this, we classified cells from each area based on an analysis of variance (ANOVA) of neuronal activity of individual neurons with reward outcome (self, other, or neither), trial type (choice or cued), and reward magnitude (small, medium, or large) as factors (Online Methods). Reward epoch responses differed significantly for a large number of neurons from all areas in a manner that depended on reward outcome (ACCg: 57%, ACCs: 72%, OFC: 57%), trial type (ACCg: 36%, ACCs: 52%, OFC: 45%) and reward volume (ACCg: 12%, ACCs: 25%, OFC: 24%) (Supplementary Table 1). Furthermore, we observed remarkable resemblances in reward outcome coding across the choice/cue and reward epochs (Supplementary Figure 4).
Based on the statistical significance of the ANOVA during the choice/cue and reward epochs, we classified individual neurons as self-referenced (i.e., modulation referenced to self rewards, preferring either self or foregone rewards), other-referenced (i.e., modulation referenced to other rewards), both-referenced (i.e., modulation referenced to both self and other rewards, but not neither rewards), or unclassified (Online Methods). Here we consider the proportion of different cell types among the classified neurons based on this scheme. In OFC, 80% (n = 36/45) were self-referenced, whereas only 9% (4/45) were other-referenced and 11% (5/45) were both-referenced (both P < 0.0001, χ2 test). In ACCs, 72% (51/71) were self-referenced, whereas only 14% (10/71) were other-referenced and 14% (10/71) were both-referenced (both P < 0.0001, χ2 test) (Fig. 5d). In contrast, ACCg contained similar proportions that were self-referenced (38%, 12/32), other-referenced (31%, 10/32), and both-referenced (31%, 10/32) (P > 0.79, χ2 test). Critically, ACCg contained a significantly higher proportion of neurons (>60%) that were sensitive to the reward outcome of the recipient monkey (i.e., other-referenced and both-referenced) compared to either OFC or ACCs (both P < 0.005, χ2 test) (Fig. 5d). ACCg also contained a significantly smaller proportion of self-referenced neurons than either OFC or ACCs (both P < 0.005, χ2 test). Finally, we found similar results when we repeated the analysis by including trial-by-trial choice reaction times as covariates (Supplementary Figure 5).
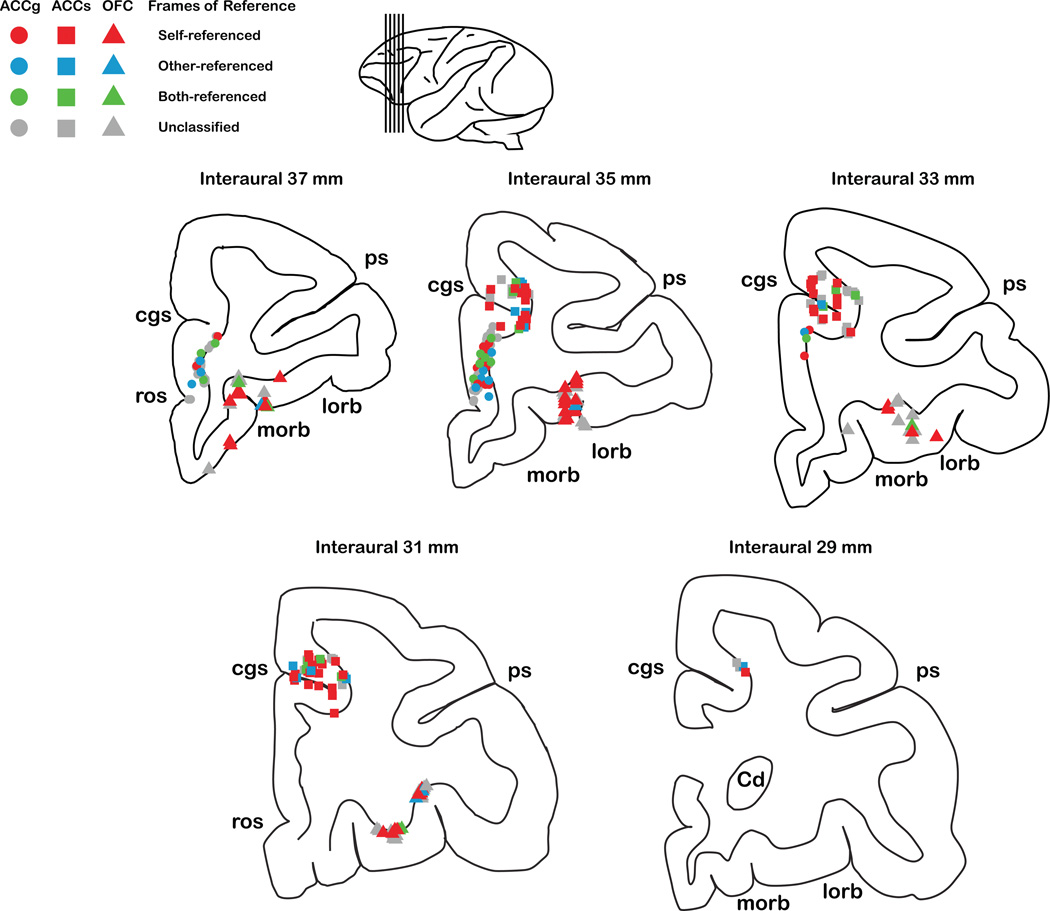
To test whether different neuronal frames of reference (self-, other-, and both-referenced) were anatomically segregated, we used principal component analysis on recording coordinates to identify the major axis with the largest dispersion within three-dimensional space. We then projected neurons to that axis to test differential distributions in individual monkeys separately. Figure 6 shows reconstructed recording locations for each reference frame class for each area. We did not observe any systematic anatomical clustering amongst different frames of reference: self-, other-, and both-referenced neurons within ACCg, ACCs, and OFC were intermingled (all P > 0.56, Wilcoxon rank sum test).
Figure 6.
Anatomical projections of recorded locations of all ACCg, ACCs, and OFC cells. Recording sites were transformed from chamber coordinates into interaural coordinates. The interaural coordinates of individual cells from both monkeys were then projected onto standard stereotaxic maps of rhesus monkeys50, with a 2 mm interaural spacing in the anterior-posterior dimension. Cells are shown on coronal slices and color-coded for the types of frames of reference used, as specified in Supplementary Table 1 (see box). The lateral view of the brain (inset) shows the locations of the coronal sections. cgs, cingulate sulcus; ps, principal sulcus; morb, medial orbitofrontal sulcus; lorb, lateral orbitofrontal sulcus; ros, rostral sulcus; Cd, caudate.
Next we examined whether differential encoding of self, other, and neither rewards was also present prior to making a decision. We found very little evidence for systematic signals early in the trial just, after target onset (from 50ms to 250ms from target onset). In ACCg, only 0, 3, and 1 cells were classified into self-, other-, and both-referenced classes with only 12% of neurons showing significant effect of reward type. In ACCs, only 1, 2, and 3 cells belonged to each category, with only 22% of the neurons with significant reward type effects. Similarly, in OFC, only 2, 2, and 4 cells belonged to each category, with only 28% of the neurons with significant reward type effects. Thus, in our reward allocation task, signals in ACCg, ACCs, and OFC appear to emerge around the time of choice and reward delivery.
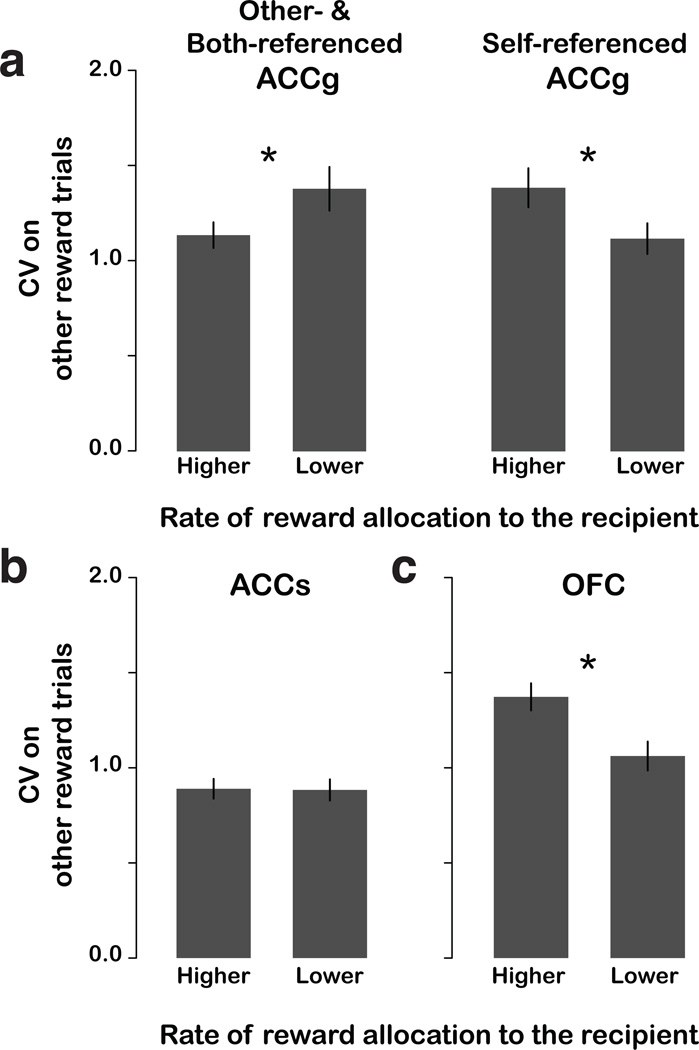
Finally, we examined whether session-to-session variation in prosocial tendencies on Other:Neither trials (Fig. 2c) could be explained by variability in the responses of ACCg neurons—the population most sensitive to other’s rewards. We split recording sessions based on actors’ choices on Other:Neither into two categories: more prosocial (higher other over neither choices relative to the median preference index) and less prosocial (lower other over neither choices relative to the median preference index). Actors tended to be more prosocial on recording sessions when other-referenced and both-referenced ACCg neurons showed less variability in spiking during the reward epoch (P < 0.05, bootstrap test) (Fig. 7a). By contrast, we found that self-referenced ACCg neurons generated more variable responses during the reward epoch when actors were more prosocial (P < 0.05, bootstrap test). ACCs neurons did not show any systematic relationship between response variance and behavior (P = 0.47, bootstrap test; Fig. 7b). Notably, OFC neurons showed a similar pattern to self-referenced ACCg neurons (P < 0.005, bootstrap test; Fig. 7c). These findings reveal suggest a strong link between prosocial behavior and the fidelity of social reward signals carried by those neurons that incorporate the experience of others into their responses. This could be due to enhanced attention to the recipient or other processes known to influence signal to noise in cortical neurons.
Figure 7.
Prosocial behavior and the fidelity of neuronal responses on Other:Neither trials. (a) ACCg; (b) ACCs; (c) OFC. Coefficients of variation in firing rate (CV; Online Methods) during the reward epoch on other reward trials are plotted as a function of whether actors were more or less prosocial on Other:Neither trials based on median split (higher: preference index greater than median; lower: preference index less than median). Asterisks indicate P < 0.05 by bootstrap test.
Discussion
Our findings strongly endorse the hypothesis that distinct frontal regions contribute uniquely to social decisions by differentially processing decision outcomes with respect to actors (self) and their partners (other). The finding that OFC neurons selectively encode self reward is consistent with previous studies implicating this area in representing the subjective value of rewards12,13, but extend those findings by demonstrating that such value signals are encoded egocentrically. Encoding of foregone rewards by ACCs neurons, on the other hand, is consistent with previous data implicating this area in error monitoring and behavioral adjustment35–37. For example, foregone reward signaling by ACCs might be used to learn from observation, rather than direct experience, and adjust ongoing behavior during social interactions. Furthermore, mirroring of self and other rewards by ACCg neurons is consistent with previous studies linking this area to specifically social functions like shared experience and empathy38.
Our findings echo those of a previous study examining the effects of lesions in these same brain regions (Online Methods), which demonstrated that ACCg, but not OFC or ACCs, contributes causally to the use of visual social information to guide behavior9. Specifically, ACCg lesions completely abolished typical hesitation to retrieve food when confronted with social stimuli9. Our findings also agree with previous findings that lesions in ACCs impair the use of reward history to guide decisions adaptively10. Differences between ACCs and ACCg reported here support and extend the finding that learning based on experience is mediated by ACCs, whereas learning from feedback from another individual is mediated by ACCg8. Specifically, in a learning task in which human subjects monitored their history of correct responses as well as the advice given to them by a confederate, BOLD activation in ACCs tracked reward learning rate, whereas BOLD activation in ACCg tracked social learning rate based on advice from the confederate8. In our study, we propose that ACCs tracked foregone rewards relative to self, whereas ACCg tracked reward outcomes of another individual in a more complex manner.
Intriguingly, the ACCg population also responded more strongly when monkeys chose self reward when the alternative was allocating reward to the other monkey compared to the response when monkeys chose self reward when the alternative was rewarding no one. In contrast, neither the OFC neuronal population response nor the ACCs neuronal population response was sensitive to social context when monkeys rewarded themselves. Sensitivity to social context in ACCg endorses a specialized role for this area in computing social decisions – even when one acts selfishly.
It is worthwhile to note that a small number of ACCs and OFC neurons, though much less in proportion compared to ACCg (Fig. 5d, Supplementary Table 1, Supplementary Figure 5), were classified as either other- or both-referenced. This observation supports the idea that a small number of ACCs and OFC neurons do carry information about rewards allocated to another individual. What is striking here is that the majority of OFC and ACCs neurons (80% and 72%, respectively) do not carry such other-regarding information (other- or both-referenced), whereas the majority of ACCg neurons do so (62%). This endorses a fundamentally social role for neurons in ACCg.
A prior study showed that OFC neurons modulate their activity when a monkey receives juice reward together with another individual39, suggesting that value signals in OFC are sensitive to social context. In that study, OFC neurons responded differentially as a function of whether the subject monkey received juice rewards alone or together with another monkey39. Our current study builds upon and extends those findings in three important ways. First, we used a free-choice task that allowed us to infer the subjective value of rewards delivered to self, other, and no one. Remarkably, even in a social context OFC neurons were selective for self reward, the most preferred outcome. Second, we compared the responses of OFC neurons to responses of neurons in ACCg and ACCs recorded in identical task conditions, allowing us to demonstrate regional differences in the encoding of social reward information in primate frontal cortex. Third, when we compared responses of ACCg neurons on free-choice and cued trials we found that responses to rewards delivered to the recipient monkey were largely absent when actors passively observed the event rather than actively choosing it. Taken together, these extensions demonstrate that social context can impact the encoding of reward information in all three areas: OFC appears to be implicated with the evaluation of personally experienced rewards, ACCs evaluates reward information that is not directly experienced, and ACCg multiplexes information about the direct experience of reward and vicarious reinforcement experienced by allocating reward to another individual.
It is noteworthy that ACCs neurons showed much less modulation by actors’ received reward outcomes compared to OFC neurons. This is striking since ACCs neurons often show substantial modulation to received reward in nonsocial settings11. ACCg, on the other hand, contains neurons that compute reward signals in both other and self frames of reference. Together, our findings suggest that, as in sensory and motor systems40, identifying the frames of reference in which reward outcomes are encoded may be important for understanding the neural mechanisms underlying social decision-making8.
Accumulating evidence endorses a special role for the medial-frontal cortex in representing information about another individual8,41–44. For instance, perceived similarity while observing others is correlated with hemodynamic response in the subgenual ACC44. Further, a group of neurons in the primate medial-frontal cortex selectively responds to observing actions performed by other individuals41. Such other-referenced signals, however, are not limited to the medial wall of the frontal cortex. Neurons in the dorsolateral prefrontal cortex (DLPFC) track the behavior of a computer opponent in an interactive game45, and BOLD responses in DLPFC and ventromedial prefrontal cortex (vmPFC) during observational learning track observed action and observed reward prediction errors, respectively46. Furthermore, BOLD activity in anterior frontal areas tracks preferences to donate to charity24. Brain networks involved in mentalizing47, vicarious pain perception48 and empathy49 thus seem to be critical for mediating social interactions, suggesting that other-regarding cognition is orchestrated by a distributed network of frontal cortical areas.
Social and emotional behaviors are highly idiosyncratic among individuals. Understanding the neural mechanisms that drive such individual differences remains one of the most pressing issues in neuroscience. We hypothesize that the differential activation of neurons in ACCg, ACCs, and OFC contribute to individual and, perhaps species, differences in social function.
Online Methods
General and behavioral procedures
All procedures were approved by the Duke University Institutional Animal Care and Use Committee, and were conducted in compliance with the Public Health Service’s Guide for the Care and Use of Laboratory Animals.
Two actor (MY and MO) and five recipient monkeys (Macaca mulatta) participated. For all monkeys, a sterile surgery was performed to implant a head-restraint prosthesis (Crist Instruments) using standard techniques11. Six weeks after surgery, monkeys were trained on a standard, center-out, oculomotor task for liquid rewards. Actor monkeys were then trained on the reward-allocation task (Fig. 1a–d) in the presence of a recipient. Subsequently, a second surgery was performed on actors to implant a recording chamber (Crist) providing access to both the sulcal and gyral regions of the anterior cingulate cortex (ACCs and ACCg, respectively) and the orbitofrontal cortex (OFC). All surgeries were performed under isoflourane anesthesia (1–3%), and the recording chambers were regularly cleaned, treated with antibiotics and sealed with sterile caps.
Horizontal and vertical eye positions were sampled at 1000Hz using an infrared eye monitor camera system (SR Research Eyelink). Stimuli were controlled by PsychToolBox and Matlab (Mathworks). Actors and recipients sat in primate chairs (Crist), 100cm from one another at a 45-degree angle (Fig. 1a). Actors (both males) and recipients (four males, one female) were unrelated and not cagemates. Different pairs were selected depending on the availability of recipient monkeys. Actors were housed in a colony with 12 other male rhesus macaques, some of which were pair-housed. All the male monkeys resided in this colony room, and the one female monkey resided in the adjacent colony room with other females. Out of the total seven actor-recipient pairs tested in the current study, the actor monkey was dominant over the recipient in six cases. Furthermore, three pairs could be classified as “more familiar” with one another because their cages faced each other, as defined previously33. Based on these relationships, we would expect a mixture of prosocial and competitive preferences based on our prior results showing dominant actors are slightly less competitive than subordinates, but also showing that pairs in which the actor is less familiar with the recipient are slightly less prosocial than when they are more familiar.
In the experimental setup, each monkey had his own monitor which displayed identical visual stimuli. Both the actor and recipient monkeys had their own tube from which juice drops were delivered. In order to prevent monkeys from forming secondary associations of solenoid valve clicks or the sound of the recipient drinking the juice reward with respect to different reward types, the solenoid valves that delivered the juice rewards were placed in another room and white noise was also played in the background. Experimenters were unable to hear solenoids anywhere inside the recording room. Our control of the acoustic environment explicitly rules out a simple explanation that both-referenced reward encoding found in ACCg is a product of such secondary sensory associations. Critically, a separate solenoid (also placed in another room) was designated for neither rewards; it only produced clicks but delivered no fluid.
The face region of the recipient, with respect to the gaze angle of the actor (horizontal and vertical eye positions), was determined empirically prior to the experiments. The frequency with which actors looked at recipients was computed from number of gaze shifts to the recipient’s face (±8.5° from the center of the face)33,34. We used a large window to capture gaze shifts that were brief in duration and large in magnitude and often directed at varying depths (e.g., eyes, mouth) (Fig. 1a).
Monkeys performed the task to obtain drops of cherry- or orange-flavored juice. Actors began a trial by shifting gaze (±2.5°) to a central stimulus (0.5° × 0.5°), and maintained fixation (200ms). For 219 single-unit sessions, the reward magnitude at stake (0.1 – 2.4ml) on each trial was cued by the position of a horizontal bisecting line (200ms), indicating the percentage of the maximum possible volume. There were two kinds of trials, termed choice trials and cued trials. Following a variable delay (300, 500, 700ms), choice and cued trials were presented at equal probabilities, randomly interleaved. On choice trials, two visual targets (4° × 4°) appeared at two random locations 7° eccentric in the opposite hemifield. Actors shifted gaze to one target (±2.5°) to indicate a choice within the maximum allowed time of 1.5s (from stimulus onset). The pair of stimuli appearing on a given trial was drawn from the set of three stimuli (Fig. 1b), pseudorandomly selected. On cued trials, actors maintained fixation (±2.5°) while a cue (4° × 4°) appeared centrally (500ms). Cues indicating rewards for the actor, recipient or neither monkey occurred with equal frequency, pseudorandomly determined (Fig. 1b). Reward onset was followed by a 0–900ms delay, from the time of either making a choice or cue offset. Actors were free to look around during this delay and for one second after reward delivery. Reward delivery was followed by an intertrial interval of 700, 1,000, or 1,300ms. Upon making an error (see below), both monkeys received visual feedback (a white rectangle, 10° × 10°) followed by a 5s time out before the next trial.
Recording procedures
All recordings were made using tungsten electrodes (FHC). Single electrodes were lowered using a hydraulic microdrive system (Kopf Instruments, or FHC). Single-unit waveforms were isolated, and action potentials collected, using a 16-channel recording system (Plexon).
In order to guide the placement of recording tracks and localize recording sites, we acquired structural magnetic resonance images (MRI) (3T, 1 mm slices) of each actor’s brain. Detailed localizations were made using Osirix-viewer. In addition to MRI-guidance, we confirmed that electrodes were in ACCg, ACCs, or OFC by listening to grey-matter and white-matter associated sounds while lowering the electrodes. ACCg neurons were recorded from Brodmann areas 24a, 24b and 32; ACCs neurons (dorsal and ventral banks) were recorded from 24c and 24c’; OFC neurons were recorded from 13m and 11 (based on standard anatomical references51,52) (Fig. 3a and Fig. 6).
Single-unit recordings were made from two actor monkeys while each was engaged in a reward-allocation task with a recipient monkey in 267 sessions. A total of 81 ACCg neurons (MY: 45, MO: 36), 101 ACCs neurons (MY: 39, MO: 62), and 85 OFC neurons (MY: 46, MO: 39) were included in the study. Neurons were selected for recording based solely on the quality of isolation. For a small subset of the data (18% of the total) (ACCg: 0%; ACCs: 25%; OFC: 27%), data were collected in a task with a fixed reward size (typically 1.0ml per successful trial) (identical to Fig. 1d except without the magnitude cue). For the majority of the cells (82% [n = 219] of the total), data were either collected in a task with the magnitude cue (Fig. 1d) (ACCg: 100% [n = 81]; ACCs: 60% [n = 61]; OFC: 42% [n = 36]), or both with and without the magnitude cue (i.e., two or more consecutive blocks per cell) (ACCg: 0%; ACCs: 15% [n = 15]; OFC: 31% [n = 26]). We combined the two types of data in our analyses unless otherwise specified.
Data from each cell consisted of firing rates during 440 ± 13 (±217) (median ± s.e.m. (±s.d.)) trials. A trial was considered “incomplete” if the monkey failed to choose a target on choice trials (choice-avoidance error) or to maintain fixation after cue onset on cued trials (forced-choice avoidance error). Such trials were not included in the neural analysis. The monkeys performed the task well, as evidenced by a high percentage of correct trials even on trials in which they did not receive juice reinforcement (Fig. 2a).
Data analysis
Choice preference indices were constructed as contrast ratios (Eq. 1)33,34.
| (1) |
RA and RB were the frequency of making particular choices. For Self:Other trials, RA and RB were number of choices to reward other and self, respectively. For Other:Neither trials, RA and RB were number of choices to reward other and neither, respectively. Finally, for Self:Neither trials, RA and RB were number of choices to reward neither and self, respectively. Indices therefore ranged from –1 to 1, with 1 corresponding to always choosing to allocate reward to other on Other:Neither trials and Self:Other trials, and always choosing not to reward self on Self:Neither trials. An index of –1 corresponds to the opposite, generally stated as choosing not to allocate reward to the other monkey or choosing to reward oneself. Values of 0 indicated indifference. For constructing neuronal preferences, we simply substituted the choice frequency with neuronal firing rates associated with making specific decisions. Response times, the time from the onset of choices to movement onset, were computed using a 20°/sec velocity threshold criterion33,34.
Spike rates were computed during the reward epoch (from 50 to 600ms from reward onset) as well as the choice/cue epoch (from −100 to 300ms from making a choice or cue offset). For the population analyses, we normalized reward firing rates to the average baseline rates for each reward outcome (300ms interval prior to fixation onset). Using marginally different time windows and different normalization methods all resulted in similar conclusions. Coefficients of variation (CV) were calculated for each neuron based on the standard deviation (σ) and mean (μ) using the spike rates (sp/s) from the reward epoch (Eq. 2):
| (2) |
In OFC and ACCs populations, the two self rewards (i.e., self rewards chosen from Self:Neither and Self:Other trials) were largely indifferent (see Fig. 4, 5b, 5c and Results) and thus we combined them by taking means for the CV analysis. In contrast, the population of ACCg neurons responded more strongly to self rewards obtained from a social context (Self:Other) compared to when there was no reward stake for the other monkey (Self:Neither) and thus we consider the two self rewards separately in ACCg (see Fig. 3, 5a and Results).
Analysis of variance (ANOVA) was used to classify the reward response selectivity of individual neurons from each area and performed per individual cells. Two-factor ANOVA was used to classify the selectivity of reward outcome (self, other, or neither) and trial type (choice or cued) for all neurons. Three-factor ANOVA was used to classify the selectivity of reward volume (binned into small, medium, large) for the 82% of cells from all areas that were collected in the task with a magnitude cue. Statistical significance for each reward type was computed by Tukey HSD test. Finally, we excluded three OFC cells when our analyses involved using the data from neither rewards because these cells were recorded on very rare sessions in which the monkeys either never chose the neither reward option or did so fewer than four times. Across all analyses, using slightly different epoch durations for neuronal data analyses led to similar results.
Classification of cell types by significant reward specificity
Based on Tukey HSD tests from the one-way ANOVA on reward outcome (self, other, or neither) for both the choice/cue epoch and reward epoch responses, we classified cells into the following categories: self-referenced, other-referenced, both-referenced, and unclassified. These categories do not imply functional roles but indicate that firing rates were significantly different based on reward outcomes. We refer to a neuron as self-referenced if the responses of the neuron were significantly different (P < 0.05) between self and other rewards as well as between self and neither rewards, but not different between other and neither rewards. We refer to a neuron as other-referenced if the responses of the neuron showed significant differences in firing rates between self and other rewards as well as between other and neither rewards, but not different between self and neither rewards. Finally, we refer to a neuron as both-referenced if the responses of the neuron showed significant differences in responses between self and neither rewards as well as other and neither rewards, but not different between self and other rewards. Neurons that did not fall into one of these categories were considered as unclassified. Applying slightly different criteria or differently configured ANOVA did not change the overall proportional trends of these classes.
Reward magnitude analysis
We examined reward magnitude modulation in 219 neurons (i.e., 82% of all neurons collected with the magnitude cue; 81 ACCg, 76 ACCs, and 62 OFC neurons). We performed a linear regression on the activity (sp/s) of individual neurons across unbinned reward sizes. We fit the data using the reward epoch activity separately for self, other, and neither reward outcomes and obtained fitted slopes (i.e., reward magnitude sensitivity in sp/s/ml) for each reward outcome. For examining the relationship between the reward magnitude sensitivity across actors’ received and foregone reward outcomes, we compared the average signed slopes from all received rewards (self rewards on choice and cued trials) and all foregone rewards (other and neither reward on choice and cued trials) in individual neurons.
Supplementary Material
Acknowledgements
This work was supported by T32 Postdoctoral Training Grant on Fundamental and Translational Neuroscience (S.W.C.C.; 2T32NS051156–06), Ruth K. Broad Biomedical Foundation Postdoctoral Grant (S.W.C.C.), Canadian Institutes of Health Research Doctoral research award (J.F.G.; 84765), National Institute of Mental Health (M.L.P., S.W.C.C.; MH086712), and Department of Defense (M.L.P., S.W.C.C.; AR100035). We thank Jennifer M. Groh, John M. Pearson, David L. Barack, R. Becket Ebitz, Ethan S. Bromberg-Martin, Karli K. Watson, and Benjamin Y. Hayden for helpful discussions. We are very grateful to Steven P. Wise and Camillo Padoa-Schioppa for insightful discussions and comments on the earlier versions of the manuscript. We also thank Monica L. Carlson for general technical assistance.
Footnotes
Supplementary Information
Supplementary Information includes Supplementary Table and Figures.
Author Contributions
S.W.C.C and M.L.P. designed the research and wrote the paper. S.W.C.C. and J.F.G. performed the research and S.W.C.C analyzed the data.
Author Information
The authors declare that they have no competing financial interests.
References
- 1.Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425:785–791. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- 2.Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Berger SM. Conditioning through vicarious instigation. Psychological Review. 1962;5:450–466. [PubMed] [Google Scholar]
- 4.Rilling J, et al. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 5.de Quervain DJ, et al. The neural basis of altruistic punishment. Science. 2004;305:1254–1258. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- 6.Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a "theory of mind"? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 7.Adolphs R. Cognitive neuroscience of human social behaviour. Nature Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 8.Behrens TE, Hunt LT, Rushworth MF. The computation of social behavior. Science. 2009;324:1160–1164. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- 9.Rudebeck PH, Buckley MJ, Walton ME, Rushworth MF. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- 10.Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 11.Hayden BY, Pearson JM, Platt ML. Fictive reward signals in the anterior cingulate cortex. Science. 2009;324:948–950. doi: 10.1126/science.1168488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 13.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennerley SW, Behrens TE, Wallis JD. Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci. 2011;14:1581–1589. doi: 10.1038/nn.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe H, Lee D. Distributed coding of actual and hypothetical outcomes in the orbital and dorsolateral prefrontal cortex. Neuron. 2011;70:731–741. doi: 10.1016/j.neuron.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsujimoto S, Genovesio A, Wise SP. Monkey orbitofrontal cortex encodes response choices near feedback time. J Neurosci. 2009;29:2569–2574. doi: 10.1523/JNEUROSCI.5777-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 19.Sato M, Hikosaka O. Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement. J Neurosci. 2002;22:2363–2373. doi: 10.1523/JNEUROSCI.22-06-02363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shidara M, Aigner TG, Richmond BJ. Neuronal signals in the monkey ventral striatum related to progress through a predictable series of trials. J Neurosci. 1998;18:2613–2625. doi: 10.1523/JNEUROSCI.18-07-02613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos GS, Nagasaka Y, Fujii N, Nakahara H. Encoding of social state information by neuronal activities in the macaque caudate nucleus. Soc Neurosci. 2012;7:42–58. doi: 10.1080/17470919.2011.578465. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moll J, et al. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- 26.Hare TA, Camerer CF, Knoepfle DT, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J Neurosci. 2010;30:583–590. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izuma K, Saito DN, Sadato N. Processing of the incentive for social approval in the ventral striatum during charitable donation. J Cogn Neurosci. 2010;22:621–631. doi: 10.1162/jocn.2009.21228. [DOI] [PubMed] [Google Scholar]
- 28.Kuss K, et al. A reward prediction error for charitable donations reveals outcome orientation of donators. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nsr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 30.Sohn JW, Lee D. Effects of reward expectancy on sequential eye movements in monkeys. Neural Net. 2006;19:1181–1191. doi: 10.1016/j.neunet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J Neurophysol. 1996;75:1061–1073. doi: 10.1152/jn.1996.75.3.1061. [DOI] [PubMed] [Google Scholar]
- 32.O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 33.Chang SW, Winecoff AA, Platt ML. Vicarious reinforcement in rhesus macaques (Macaca mulatta) Front Neurosci. 2011;5:27. doi: 10.3389/fnins.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci U S A. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter CS, et al. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 36.Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- 37.Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 2011;14:1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 39.Azzi JC, Sirigu A, Duhamel JR. Modulation of value representation by social context in the primate orbitofrontal cortex. Proc Natl Acad Sci U S A. 2012;109:2126–2131. doi: 10.1073/pnas.1111715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci. 2002;3:553–562. doi: 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida K, Saito N, Iriki A, Isoda M. Representation of others' action by neurons in monkey medial frontal cortex. Curr biol. 2011;21:249–253. doi: 10.1016/j.cub.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16:235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Waytz A, Zaki J, Mitchell JP. Response of dorsomedial prefrontal cortex predicts altruistic behavior. J Neurosci. 2012;32:7646–7650. doi: 10.1523/JNEUROSCI.6193-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mobbs D, et al. A key role for similarity in vicarious reward. Science. 2009;324:900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seo H, Lee D. Cortical mechanisms for reinforcement learning in competitive games. Philos Trans R Soc Lond B Biol Sci. 2008;363:3845–3857. doi: 10.1098/rstb.2008.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burke CJ, Tobler PN, Baddeley M, Schultz W. Neural mechanisms of observational learning. Proc Natl Acad Sci USA. 2010;107:14431–14436. doi: 10.1073/pnas.1003111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hampton AN, Bossaerts P, O'Doherty JP. Neural correlates of mentalizing-related computations during strategic interactions in humans. Proc Natl Acad Sci USA. 2008;105:6741–6746. doi: 10.1073/pnas.0711099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon D, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singer T, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 50.Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Academic Press; 2000. [Google Scholar]
- 51.Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- 52.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.