Abstract
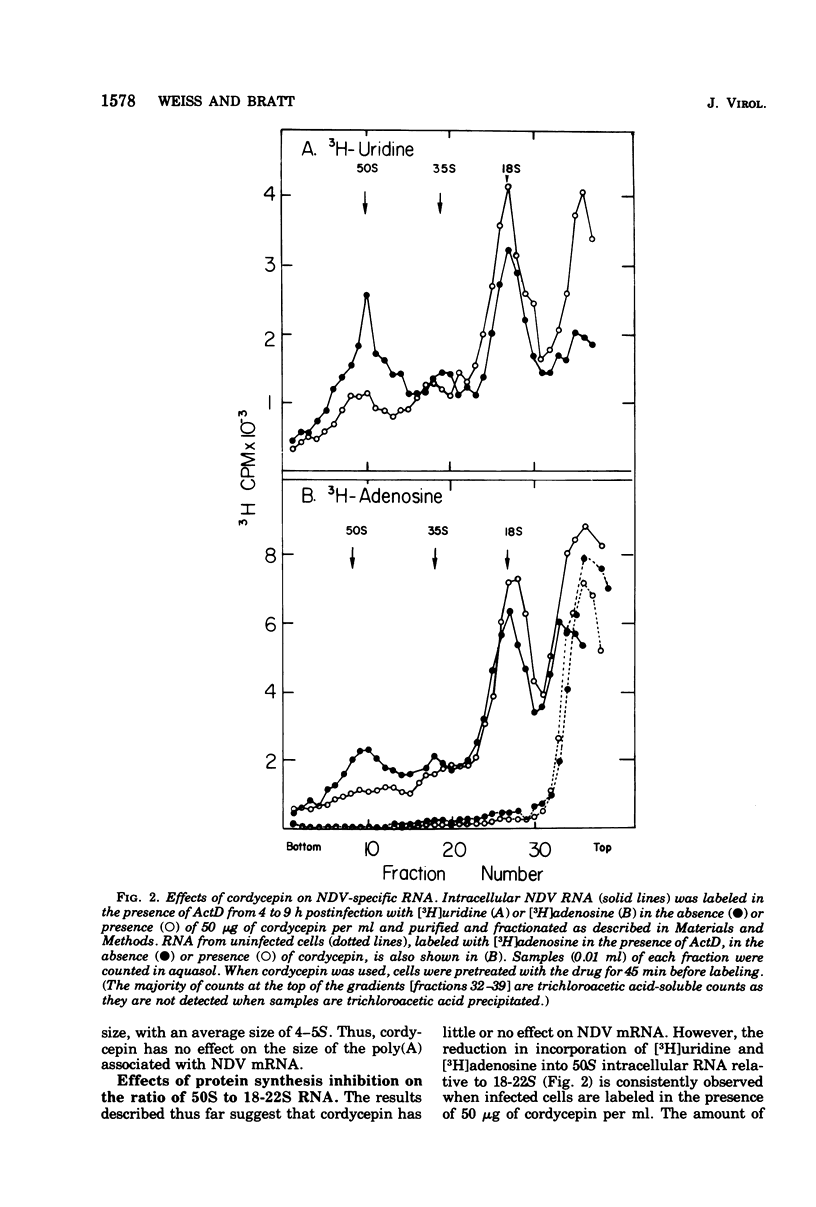
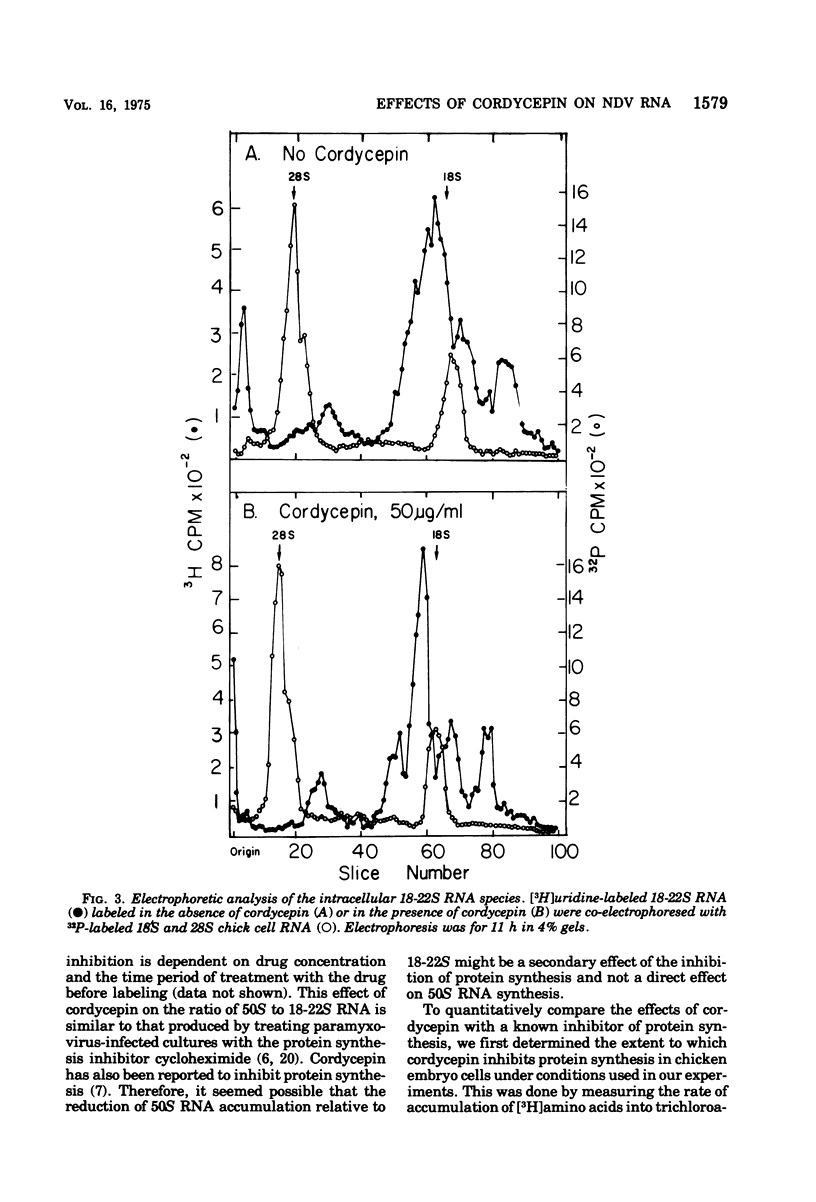
Cordycepin (3'-deoxyadenosine) has no effect on the size or relative proportions of Newcastle disease virus-specific 18-22S mRNA species nor on the amount or size of the polyadenylic acid associated with them. Cordycepin does, however, cause an inhibition of incorporation of [3H]uridine into 50S virus-specific RNA relative to 18-22S RNA. This inhibition is probably not a direct effect of the drug on the synthesis of 50S viral RNA. Like cycloheximide, another drug which inhibits 50S RNA accumulation in paramyxovirus-infected cells, cordycepin inhibits protein synthesis as measured by amino acid incorporation. It is likely that the inhibition of 50S RNA accumulation is a secondary effect of protein synthesis inhibition. This is supported by the finding that concentrations of cordycepin and cycloheximide, which inhibit protein synthesis to the same extent, have the same effect on the ratio of 50 to 18-22S virus-specific RNA.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavell L. A., Bratt M. A. Relationship between the ribonucleic acid synthesizing capacity of ultraviolet-irradiated Newcastle disease virus and its ability to induce interferon. J Virol. 1971 Oct;8(4):500–508. doi: 10.1128/jvi.8.4.500-508.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby D. S., Finnerty V., Lucas-Lenard J. Fate of mRNA of L-cells infected with mengovirus. J Virol. 1974 Apr;13(4):858–869. doi: 10.1128/jvi.13.4.858-869.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B. S., Bratt M. A. Separation of the messenger RNAs of Newcastle disease virus by gel electrophoresis. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2544–2548. doi: 10.1073/pnas.70.9.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E. Polyadenylation of vesicular stomatitis virus mRNA. J Virol. 1974 May;13(5):1055–1060. doi: 10.1128/jvi.13.5.1055-1060.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R., Bratt M. A. Conditional dependence of fusion from within and other cell membrane alterations by Newcastle disease virus. J Virol. 1974 Oct;14(4):813–820. doi: 10.1128/jvi.14.4.813-820.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Bratt M. A. Ribonucleic acid polymerase in virions of Newcastle disease virus: comparison with the vesicular stomatitis virus polymerase. J Virol. 1971 Mar;7(3):389–394. doi: 10.1128/jvi.7.3.389-394.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Adesnik M., Salditt M., Sheiness D., Wall R., Molloy G., Philipson L., Darnell J. E. Further evidence on the nuclear origin and transfer to the cytoplasm of polyadenylic acid sequences in mammalian cell RNA. J Mol Biol. 1973 Apr 15;75(3):515–532. doi: 10.1016/0022-2836(73)90458-0. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. Cell-free translation of paramyxovirus messenger RNA. J Virol. 1973 Nov;12(5):1020–1027. doi: 10.1128/jvi.12.5.1020-1027.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy B. W., Cox N. J., Armstrong S. J., Barry R. D. Multiplication of influenza virus in the presence of cordycepin, an inhibitor of cellular RNA synthesis. Nat New Biol. 1973 Jun 6;243(127):172–174. doi: 10.1038/newbio243172a0. [DOI] [PubMed] [Google Scholar]
- Mendecki J., Lee S. Y., Brawerman G. Characteristics of the polyadenylic acid segment associated with messenger ribonucleic acid in mouse sarcoma 180 ascites cells. Biochemistry. 1972 Feb 29;11(5):792–798. doi: 10.1021/bi00755a018. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Roth M. F. Permeation as the rate-limiting step in the phosphorylation of uridine and choline and their incorporation into macromolecules by Novikoff hepatoma cells. Competitive inhibition by phenethyl alcohol, persantin, and adenosine. Biochemistry. 1969 Dec;8(12):4782–4789. doi: 10.1021/bi00840a020. [DOI] [PubMed] [Google Scholar]
- Robinson W. S. Sendai virus RNA synthesis and nucleocapsid formation in the presence of cycloheximide. Virology. 1971 Jun;44(3):494–502. doi: 10.1016/0042-6822(71)90362-x. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Bratt M. A. Polyadenylate sequences on Newcastle disease virus mRNA synthesized in vivo and in vitro. J Virol. 1974 Jun;13(6):1220–1230. doi: 10.1128/jvi.13.6.1220-1230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


