Abstract
Neuroimaging techniques are starting to reveal significant overlap in the brain circuitry underlying addiction and disorders of dyscontrol over rewarding behaviors (such as binge eating disorder and obesity). Positron emission tomography (PET) has demonstrated impaired striatal dopamine (DA) signaling (decreased D2 receptors) in drug addiction and obesity that is associated with reduced baseline glucose metabolism in medial and ventral prefrontal brain regions. Functional magnetic resonance imaging (fMRI) has documented brain activation abnormalities that also implicate DA-modulated striato-cortical pathways. In this review we map findings from recent neuroimaging studies that differentiate brain activation in drug/food addiction from those in controls within brain networks functionally connected with ventral and dorsal striatum. We show that regions found to be abnormal in addiction and obesity frequently emerge at the overlap of the dorsal and the ventral striatal networks. Medial temporal and superior frontal regions functionally connected with dorsal striatum display greater vulnerability in obesity and eating disorders than in drug addictions, indicating more widespread abnormalities for obesity and eating disorders than for addictions. This corroborates involvement of both ventral striatal (predominantly associated with reward and motivation) and dorsal striatal networks (associated with habits or stimulus response learning) in addiction and obesity but also identify distinct patterns between these two disorders.
Dopamine (DA) encodes prediction signals for natural and drug reinforcers and facilitates conditioning (learning of reward associations) by modulating brain activity in subcortical and cortical regions (Ikemoto, 2010). Drugs of abuse are compulsively consumed by humans or self-administered by laboratory animals because they are inherently rewarding (Di Chiara and Imperato, 1988;Volkow and Li, 2005). Drugs of abuse have been shown to cause abrupt increases in extracellular DA in the striatum (Volkow et al., 1997a) that parallel the time course of the subjective “high” (Volkow et al., 1997b). However, other neurotransmitters such as cannabinoids and opioids, and neuropeptides also play important roles in reward and addiction and are intimately involved in triggering the neuroplastic changes that follow repeated drug use and involve changes in glutamatergic signaling in striatocortical pathways (Kalivas, 2009;Koob and Le Moal, 2008;Lüscher and Malenka, 2011). Preclinical and clinical studies assessing the response to drug/food cues have shown increases in extracellular DA in striatum that were associated with enhanced motivation to consume drugs/foods. This evidences the involvement of DA in cue-induced overeating, just as it has been shown to underlie its involvement in cue-induced relapse in drug addiction (Avena et al., 2008;Volkow et al., 2008a). Thus, it has been postulated that DA-modulated circuits showing drug-related impairments in drug addiction could also be implicated in pathologic, compulsive eating behaviors (Volkow et al., 2012b;Volkow et al., 2008a).
During the last two decades positron emission tomography (PET) studies have evaluated the role of DA in association with glucose metabolism in reward and addiction (Volkow et al., 2011c;Volkow et al., 2012b;Volkow et al., 2012a). The role of striatal DA on baseline brain activity, on the responses to drugs and on the responses to drug cues, has been studied with PET technology using multiple tracer approaches in both addicted and non addicted individuals (Fig 1). The combined use of D2 receptor (ie., [11C]raclopride, [18F]n-methylspiroperidol) and DA transporter (such as [11C]cocaine, [11C]d threo-methylphenidate) radioligands with fludeoxyglucose ([18F]FDG, ligand used to measure brain glucose metabolism) demonstrated that the availability of DA D2 receptors (D2R) and transporters (DAT) in striatum is associated with metabolic activity in frontal and temporal cortices (Volkow et al., 1993;Volkow et al., 2008b;Volkow et al., 2006;Volkow et al., 1996c) (Fig 2). These studies have consistently demonstrated impaired DA function in striatum (decreases in D2R, reduced DA release) and its association with reduced baseline glucose metabolism (marker of brain function) in frontal (orbitofrontal cortex, anterior cingulate, dorsolateral prefrontal) and temporal cortices (most notable in insula) (Volkow et al., 1993).
Fig 1. Striatal DA neurotransmission abnormalities in addiction and obesity.
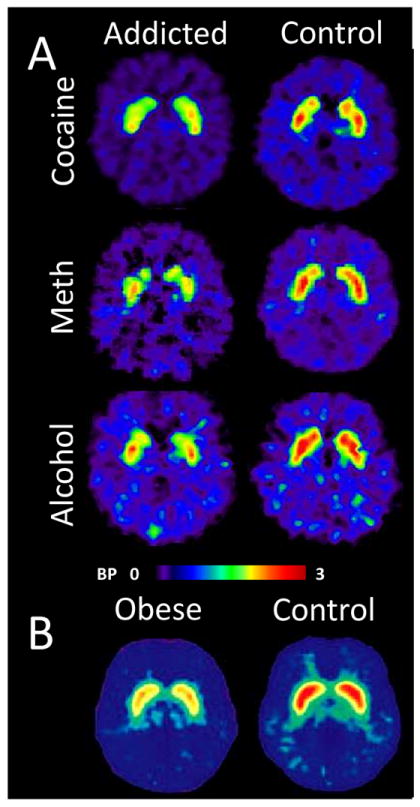
Positron emission tomography (PET) studies using [11C]raclopride, a 20 min half-life radiotracer that binds to DA D2/D3 receptors, have demonstrated reduced striatal binding potential (BP) in addicts to cocaine (Volkow et al., 1990;Volkow et al., 1997a;Volkow et al., 2002b;Martinez et al., 2009;Martinez et al., 2011), methamphetamine (Volkow et al., 2001a;Volkow et al., 2001c), alcohol (Volkow et al., 1996c;Martinez et al., 2005), heroin (Martinez et al., 2012), and nicotine (Brody et al., 2009) and also in morbidly obese subjects (Wang et al., 2001) when compared to healthy controls. Reduced striatal DA release has been found in alcoholics (Martinez et al., 2007 and cocaine (Martinez et al., 2007) addicts whereas higher striatal DA release was found in non-obese individuals suffering from a binge eating disorder (Wang et al., 2011a). Loss of striatal DA transporters has been reported in methamphetamine addicts (Volkow et al., 1999a;Volkow et al., 2001b;Volkow et al., 2001c).
Fig 2.
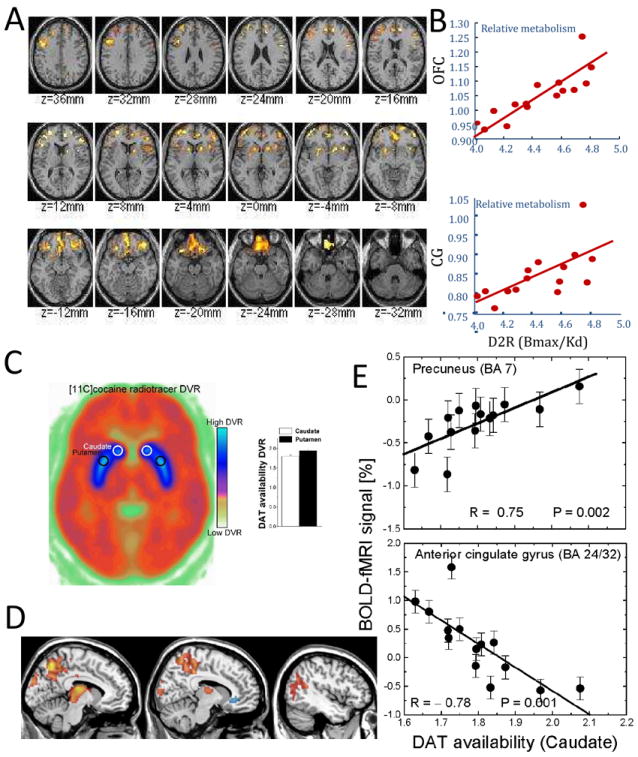
Association between brain metabolism and DA neurotransmission: (A) Statistical axial maps of correlations between relative glucose metabolism and DA D2 receptors (D2R) in the striatum for subjects with a family history of alcoholism and (B) scatter plots exemplifying the linear association between relative metabolism in orbitofrontal cortex (OFC) and cingulate gyrus (CG) (vertical axis) and D2R in the striatum [data from (Volkow et al., 2006)]. Association between brain activation and DA neurotransmission: (C) Axial PET image at the level of the striatum (blue) showing the average availability DA transporters (DAT); (D) the correlation between DAT in striatum and fMRI activation during a visual attention task in 14 healthy humans; and (E) scatter plots exemplifying the correlation of DAT in striatum with the fMRI activation in precuneus and deactivation in ACC [data from (Tomasi et al., 2009a)].
In parallel functional magnetic resonance imaging (fMRI) studies have assessed changes in brain function and connectivity in addicted subjects (Goldstein and Volkow, 2011). The role of brain activation has been studies with fMRI using the endogenous blood-oxygenation-level-dependent (BOLD) contrast (Ogawa et al., 1990) and a plethora of task activation paradigms. These studies have shown that addiction affects not only the reward circuit, but also brain regions involved in attention, memory, motivation, executive function, mood and interoception (Volkow et al., 2011c).
More recently, PET and fMRI multimodality studies have documented an association between DA neurotransmission in striatum and fMRI responses in the default mode network (DMN; including ventral prefrontal cortex and precuneus) (Tomasi et al., 2009a;Braskie et al., 2011) that deactivates during task performance in healthy controls (Tomasi et al., 2006;Fox et al., 2005) (Fig 2). Pharmacological fMRI studies using stimulant drugs with DA enhancing effects such as modafinil and methylphenidate have also suggested an association between DA signaling and DMN function (Minzenberg et al., 2011;Tomasi et al., 2011). Other pharmacological PET and fMRI studies demonstrated that stimulants (methylphenidate) can attenuate limbic brain responses to cocaine cues (Volkow et al., 2010b) and normalize fMRI responses during a cognitive task (Goldstein et al., 2010;Li et al., 2010) in cocaine addicts. However, the association between impaired DA neurotransmission and abnormal activation in addiction and obesity is still poorly understood.
Dopaminergic responses to drugs and food
All addictive drugs display ability to increase DA in striatum, particularly in the nucleus accumbens (ventral striatum), which underlies their rewarding effects (Koob, 1992). DA neurons located in the ventral tegmental area (VTA) and substantia nigra (SN) in the midbrain project to the striatum via the mesolimbic and nigrostriatal pathways. The rewarding and conditioning effects of drugs (and very likely to food too) seem to be predominantly driven by transient and pronounced increases in DA cell firing (Wise, 2009) that result in high DA concentrations that are necessary to stimulate the low affinity D1 receptors (Zweifel et al., 2009). In humans, PET studies have shown that several drugs increase DA in dorsal and ventral striatum and that these increases are associated with the subjective rewarding effects of the drugs [stimulants (Drevets et al., 2001;Volkow et al., 1999), nicotine (Brody et al., 2009), alcohol (Boileau et al., 2003) and cannabis (Bossong et al., 2009)]. Dopaminergic responses might also play a role in the rewarding effects of foods and contribute to excessive consumption and obesity (Volkow et al., 2011b). Certain foods, particularly those rich in sugars and fat, are potently rewarding and can promote over-eating (Lenoir et al., 2007) because like drugs they increase striatal DA release (Norgren et al., 2006). Moreover, food can increase DA in ventral striatum solely on the basis of its caloric content and independent of palatability (de Araujo et al., 2008). Whereas food-reward associations were advantageous in environments where food sources were scarce and/or unreliable, this mechanism is now a liability in our modern societies where food is plentiful and constantly available.
Other neurotransmitters than dopamine (cannabinoids, opioids, and serotonin) as well as neuropeptide hormones (insulin, leptin, ghrelin, orexin, glucagon like peptide, agouti related protein, PYY) have been implicated in the rewarding effects of food and in the regulation of food intake (Atkinson, 2008;Cason et al., 2010;Cota et al., 2006). Furthermore, food-related striatal DA increases alone cannot explain the difference between normal food intake and excessive compulsive food consumption since these also occur in healthy individuals who do not overeat. Therefore, as for addiction, downstream adaptations are likely to be involved in the loss of control over food intake. These neuroadaptations might lead to decreases in tonic DA cell firing, enhanced phasic DA cell firing in response to drug or food cues and reduced executive function including impairments in self-control (Grace, 2000;Wanat et al., 2009).
Striatocortical connectivity
Cortical correlates of striatal dopaminergic deficits in addition are not unexpected. Anatomical studies in non-human primates and in rodents documented that motor, somatosensory and dorsolateral prefrontal cortices project to dorsal striatum (Künzle, 1975;Künzle, 1977;Selemon and Goldman-Rakic, 1985;Middleton and Strick, 2002;Kelly and Strick, 2004;Künzle and Akert, 1977), and that anterior cingulate (ACC) and orbitofrontal (OFC) cortices project to ventral striatum (Ilinsky et al., 1985;Selemon and Goldman-Rakic, 1985;Ferry et al., 2000;Haber et al., 2006;Powell and Leman, 1976;Yeterian and Van Hoesen, 1978).
Recently, Di Martino and colleagues were able recapitulate these striatocortical circuits using brief (< 7 min) MRI scanning sessions at rest in 35 human subjects (Di Martino et al., 2008) and supported a meta-analysis of PET and fMRI studies that identified functional connectivity between the anterior dorsal striatum and the insula (Postuma and Dagher, 2006). Resting state functional connectivity (RSFC) is advantageous when studying patients with functional deficits because the data is collected at rest avoiding performance confounds (task stimulation paradigms require the subjects’ cooperation and motivation), and has potential as a biomarker for diseases that affect the brain DA system.
Recent studies have documented impairments in functional connectivity both in drug addiction and in obesity. Specifically lower functional connectivity has been reported between dopaminergic midbrain nuclei (VTA and SN) with striatum and with the thalamus (Gu et al., 2010;Tomasi et al., 2010), between the hemispheres (Kelly et al., 2011), and between the striatum and cortex (Hanlon et al., 2011) in cocaine addicts. Abnormal striato-cortical connectivity was also documented in social drinkers (Rzepecki-Smith et al., 2010), opioid abusers (Ma et al., 2010;Liu et al., 2009;Ma et al., 2011;Upadhyay et al., 2010) and obese subjects (Nummenmaa et al., 2012;Kullmann et al., 2012;García-García et al., 2012). Overall, these studies suggest that abnormal connectivity between cortical and subcortical regions might underlie the pathological states in drug addiction and obesity. Open access to large RSFC databases integrating datasets from multiple studies promises increased statistical power and sensitivity to characterize the connectivity of the human brain (Biswal et al., 2010;Tomasi and Volkow, 2011). Here we reproduce the RSFC patterns from dorsal and ventral striatal seeds documented by Di Martino and colleagues (Di Martino et al., 2008) in a large sample of healthy subjects. The coordinates of the abnormal clusters documented by previous neuroimaging studies on food/drug addiction were projected into these striatal networks to assess their implication in addiction and obesity. Other striatal seed regions (i.e. dorsal caudate) were unnecessary because their functional connectivity patterns were largely included within the union of the ventral and dorsal RSFC patterns.
The RSFC patterns were computed using the three largest datasets (Beijing: N=198; Cambridge: N=198; Oulu: N=103) of the public image repository “1000 Functional Connectomes Project” (http://www.nitrc.org/projects/fcon_1000/), which included a total of 499 healthy subjects (188 males and 311 females; age: 18-30 years). We used the approach by Di Martino et al. to map dorsal and ventral striatal networks. The standard image post processing (realignment and spatial normalization to the MNI space) was carried with the statistical parametric mapping package (SPM5; Wellcome Trust Centre for Neuroimaging, London, UK). Then, seed-voxel correlation analysis with Gram-Schmidt orthogonalization (Margulies et al., 2007;Di Martino et al., 2008) was used to compute the functional connectivity of the bilateral dorsal (x = ±28 mm, y = 1 mm, z = 3 mm) and ventral (x = ±9 mm, y = 9 mm, z = -8 mm) striatal seed regions (0.73ml cubic volumes). In addition, the functional connectivity of a bilateral primary visual cortex seed (x = ±6 mm, y = -81 mm, z = 10 mm; calcarine cortex, BA 17) was computed as a control network. These RSFC maps were spatially smoothed (8-mm) and included in a voxel-wise one-way analysis of variance (ANOVA) SPM5 model, independently for dorsal and ventral striatal seeds. Voxels with T-score > 3 (p-value < 0.001, uncorrected) were considered to be significantly connected to the seed regions and were included as part of the networks.
The RSFC pattern of the dorsal striatal seeds (Fig 3) was bilateral and included dorsolateral prefrontal (BAs: 6, 8, 9, 44-46), inferior (BA: 47) and superior frontal (BAs: 8-10), temporal (BAs: 20, 22, 27, 28, 34, 36-38, 41-43), inferior and superior parietal (BAs: 2, 3, 4, 5, 7, 39, 40), occipital (BA: 19), and cingulate (BAs: 23, 24, 32), occipital (BA 19) and limbic (BA: 30) cortices, thalamus, putamen, globus pallidus, caudate, midbrain, pons, and cerebellum. The RSFC pattern of the ventral striatal seeds was also bilateral and included ventral orbitofrontal (BA: 11), superior frontal (BAs: 8-10), temporal (BAs: 20, 21, 27-29, 34, 36, 38), inferior parietal (BA: 39), and cingulate (BAs: 23-26, 32) and limbic (BA: 30) cortices, thalamus, putamen, globus pallidus, caudate, midbrain, pons, and cerebellum. These ventral and dorsal patterns overlapped in inferior (BA: 47) and superior frontal (BAs: 9), temporal (BAs: 20, 27, 28, 34, 36, 38), cingulate (BAs: 23, 24, 32) and limbic (BA: 30) cortices, thalamus, putamen, globus pallidus, caudate, midbrain, pons, and cerebellum. Thus, there was significant overlap as well as significant differences between these dorsal and ventral network patterns that corroborate those from Di Martino et al (Di Martino et al., 2008) and are consistent with the patterns reported by anatomical studies (Haber et al., 2000). The RSFC pattern of the primary visual cortex (V1) was also bilateral and included occipital (BAs 17-19), temporal (BA 37), superior parietal (BA 7), auditory (BAs 22 and 42) and premotor (BA 6) cortices and bilateral posterior superior cerebellum (Fig 3). Thus, the V1 connectivity pattern was smaller (volume of the V1 network = 16% gray matter volume) and partially overlapped the dorsal striatal network (6% gray matter volume in BAs 6, 7, 19 and 37) but not the ventral striatal network.
Fig 3. RSFC networks from dorsal and ventral striatum.
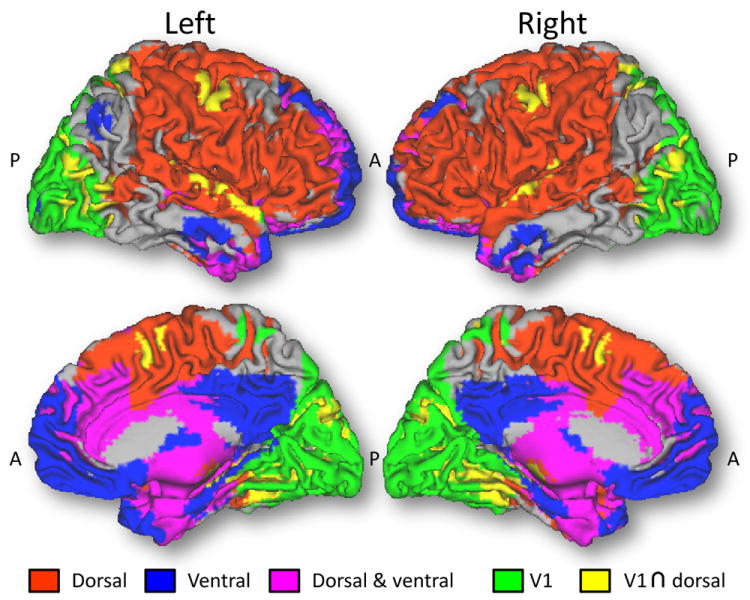
Functional connectivity patterns from seed regions in dorsal (putamen; xyz = ±28, 1, 3 mm) and ventral (nucleus accumbens; xyz = ±9, 9, -8 mm) striatum. Red: brain regions functionally connected to the dorsal seed but not to the ventral seed; Blue: brain regions functionally connected to the ventral seed but not to the dorsal seed. Magenta: brain regions functionally connected to both ventral and dorsal seeds. The seed-voxel correlation approach with Gram-Schmidt orthogonalization proposed by Di Martino et al. was used to compute orthogonal functional connectivity patterns for the dorsal and ventral seeds in 499 healthy young adult subjects (188 males and 311 females) using resting-state datasets from the “1000 Functional Connectomes Project”.
Meta-analysis
In what follows we review functional neuroimaging studies on alcohol, cocaine, methamphetamine, and marijuana (Tables 1-4), as well as obesity and eating disorders (Tables 5 and 6) that were published between January 1, 2001 and December 31, 2011; nicotine addiction was not included because there were only five fMRI studies on nicotine addiction and none assessed brain activation differences between smokers and non-smokers. The words “activation”, “connectivity”, “dopamine”, “cocaine”, “marijuana”, “cannabis”, “methamphetamine”, “alcohol”, “PET”, and “MRI” were included in a search of peer-reviewed publications in PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) to identify relevant brain imaging studies. Only studies that reported the spatial coordinates of the clusters (in the Montreal Neurological Institute (MNI) or Talairach stereotactic frames of references) showing significant activation/ metabolic differences between drug users/obese patients and controls (P < 0.05, corrected for multiple comparisons) were included in the analysis.
Table 1.
Summary of functional magnetic resonance imaging studies (conducted between 2001 and 2011) on effects of alcohol addiction on brain function that were included in Figs 4 and 5. Studies are grouped by stimulation paradigms into four major categories. Number of alcoholic (S) and control (C) subjects included and tasks are provided in addition to main results.
| Subjects | Task | Results | |
|---|---|---|---|
| Memory | |||
| Gundersen et. al; 2008 | 12S & 13C | Working memory | dACC; cerebellum ↓ |
| Yoon et.al; 2009 | 12S & 12C | Word/face memory encoding | Temporal, parietal and cingulate cortices ↑ |
| Vollstädt-Klein; 2010 | 11S & 19C | Spatial working memory | ACC ↑ |
| Cue-reactivity | |||
| George et. al; 2001 | 10S & 10C | Alcohol sip & cues | Left DLPFC and anterior thalamus ↑ |
| Grusser et. al; 2004 | 10S & 10C | Visual alcohol cues | Striatum, ACC and medial PFC ↑ |
| Filbey et. al; 2008 | 37S | Alcoholic taste | OFC, inf frontal, temporal, occipital, IPC, PCC, caudate and thalamus ↑ |
| Vollstädt-Klein et. al; 2010 | 21S & 10C | Visual alcohol cues | Dorsal striatum ↑; ventral striatum and PFC ↓ |
| Gilman et.al; 2012 | 14S & 14C | Emotional cues & IV ethanol | Nacc ↑ in social but not in heavy drinkers |
| Inhibition | |||
| Heitzeg et.al; 2010 | 41S & 20C | Go/NoGo | OFC, VPFC and dmPFC ↑ |
| Silveri et. al; 2011 | 18S & 14C | Stroop interference | AC C and DLPFC ↑ |
| Emotion | |||
| Wrase et. al;2007 | 16S & 16C | Emotion and monetary reward | Ventral and dorsal striatum ↓ |
| Heitzeg et.al; 2008 | 22S & 6C | Emotion | OFC & insula/putamen ↑; Ventral striatum amygdala and dmPFC ↓ |
Arrows indicate activation increases (↑) or decreases (↓).
Table 4.
Summary of functional magnetic resonance imaging studies (conducted between 2001 and 2011) on effects of marijuana addiction on brain function included in Figs 4 and 5. Studies are grouped by stimulation paradigm into four major categories. Number of marijuana (S) and control (C) subjects and tasks are provided in addition to main results.
| Subjects | Task | Results | |
|---|---|---|---|
| Memory & attention | |||
| Chang et. al; 2006 | 24S & 19C | Visual attention | PFC, parietal and vermis↓; frontal, parietal & occipital ↑ |
| Padula et.al; 2007 | 17S & 17C | Spatial working memory | Striatum, insula, precuneus and superior parietal ↑ |
| Cue-reactivity | |||
| Filbey et. al; 2009 | 31S & 21C | Tactile drug cues | VTA, thalamus, ACC, insula and amygdala ↑ |
| Inhibition | |||
| Tapert et. al; 2007 | 16S & 17C | Go/NoGo | DLPFC, parietal, occipital ↑ |
| Roberts et. al; 2010 | 20S & 20C | Go/NoGo | Prefrontal, parietal, temporal and cingulate ↑ |
| visual & motor | |||
| King et. al; 2011 | 30S & 30C | Finger tapping & checkerboard | Premotor and visual cortex↓ |
Arrows indicate activation increases (↑) or decreases (↓).
Table 5.
Summary of functional magnetic resonance imaging studies (conducted between 2001 and 2012) on effects of obesity on brain function included in Figs 4 and 6. Studies are grouped by stimulation paradigm into two major categories. Number of obese (S) and control (C) subjects and tasks are provided in addition to main results.
| Subjects | Task | Results | |
|---|---|---|---|
| Cue reactivity | |||
| Rothemund et. al; 2007 | 13S & 13C | High/low calorie visual cues | striatum, HIPP, INS↑ (high calorie) |
| Stoeckel et. at; 2008 | 12S & 12C | High/low calorie visual cues | OFC, AMY, Nacc, VPFC, INS, ACC, GP, striatum, HIPP↑ |
| Wallner-Liebmann et al; 2010 | 12S & 12C | High/low calorie visual cues | Insulin plasma level was correlated with HIPP activation |
| Dimitropoulos et. al; 2012 | 22S & 16C | High/low calorie visual cues | temporal and limbic ↑ after eating |
| Other (Intake, reward, gastric distention) | |||
| Stice et. al; 2008 | 76S | Food intake & TaqIA A1 gene | TaqIA: no A1 > A1: BMI correlate with striatal activation |
| Tomasi et. al; 2009b | 24S | Gastric distention | Cerebellum and insula↑; midbrain, hypothalamus, amygdala↓ |
| Stice et. al; 2011 | 35S & 25C | Reward | Adolecents with high risk for obesity: caudate↑ |
Arrows indicate activation increases (↑) or decreases (↓).
Table 6.
Summary of functional magnetic resonance imaging studies (conducted between 2001 and 2011) on effects of eating and eating disorders on brain function included in Figs 4 and 6. Number of patients (S) and control (C) subjects and tasks are provided in addition to main results.
| Subjects | Task; modality | Results | |
|---|---|---|---|
| Eating | |||
| Batterham et. al; 2007 | 8C | PYY/saline infusion; fMRI | Low PYY: HyTHA↑; high PYY: OFC↑ |
| Haase et. al; 2009 | 18C | Taste paradigm; fMRI | Hunger: INS, THA, SN, pHipp, Hipp, AMY, ACC ↑ |
| Wang et. al; 2009 | 23C | Inhibition of food stimulation; PET | Males > females: AMY, HIPP, INS, OFC and striatum ↓ |
| Haase et. al; 2011 | 21C | Taste paradigm; fMRI | Males > females: Limbic regions, insula, OFC, and cerebellum↑ |
| Eating Disorders | |||
| Schienle et. al; 2009 | 31S & 36C | Food picture paradigm; fMRI | OFC, ACC and Insula↑ |
| Lock et. all; 2011 | 27S & 13C | Go/NoGo; fMRI | Binge –eaters > control & anorexia: ACC, temporal, HyTHA and DLPFC ↑ |
Arrows indicate activation increases (↑) or decreases (↓).
A coordinate-based meta-analysis was used to evaluate the degree of agreement between studies. We used an activation likelihood estimation approach (Wager et al., 2004) to build likelihood functions for each reported cluster. Specifically, a 3D Gaussian density (15-mm full-width-half-maximum) was centered at the MNI coordinates of each cluster that reported significant activation differences with respect to controls for drug users, obese individuals and eating disorder patients, regardless if they were increases or decreases. The SPM5 one-way ANOVA was used to analyze the statistical significance of the likelihood maps (3-mm isotropic resolution) corresponding to the 44 studies on drug addiction (Tables 1-4), and the 13 studies on obesity and eating disorders (Tables 5 and 6). The meta-analysis showed that the anterior and middle cingulate cortices frequently demonstrate activation abnormalities in neuroimaging studies on drug addiction, and that the putamen/posterior insula, hippocampus, superior prefrontal cortex (PFC), middle and inferior temporal cortices and cerebellum frequently demonstrate activation abnormalities in studies on obesity and eating disorders (PFWE < 0.05, corrected for multiple comparisons in the whole brain using the random field theory with family-wise error correction; Fig 4; Table 7). This meta-analysis also showed that the likelihood of abnormal activation findings in putamen/posterior insula, hippocampus, parahippocampus and temporal cortices is usually higher for studies on obesity and eating disorders than for studies on drug addiction (PFWE < 0.05; Fig 4; Table 7). In ACC (BA 24 and 32), PFC (BA 8), putamen/posterior insula, hippocampus (BA 20), cerebellum, middle and superior temporal (BAs 21, 41 and 42) and supramarginal gyri the strength of the functional connectivity was stronger for the dorsal than for the ventral striatum and in the anterior medial frontal cortex (BAs 10 and 11) was stronger for the ventral than for the dorsal striatum (PFWE < 0.05; Table 7).
Fig 4. Coordinate-based meta-analysis of neuroimaging studies on drug addiction, obesity and eating disorders.
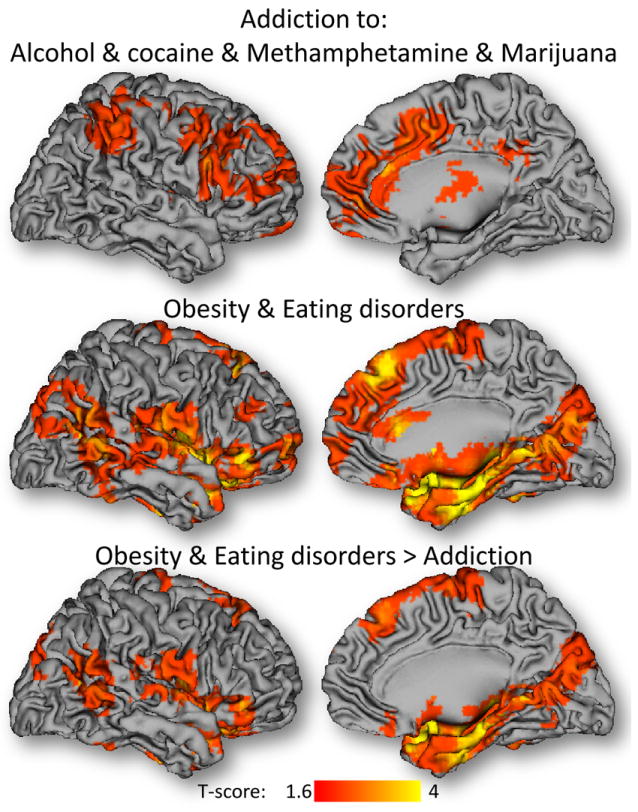
Statistical significance of the activation likelihood estimation maps corresponding to the 44 studies on drug addiction (Tables 2-5), and the 13 studies on obesity and eating disorders (Tables 6-7). The activation likelihood estimation maps were computed using the coordinates of activation clusters reporting statistically significant increases or decreases in these populations with respect to matched controls (PFWE < 0.05, corrected at the cluster level).
Table 7.
Coordinate-base meta-analysis of neuroimaging studies on drug addiction, obesity and eating disorders published between 2001 and 2011 (Tables 2-7). MNI coordinates (x, y, z) and statistical significance (T-score) for clusters that demonstrated significant abnormalities (increases or decreases with respect to matched controls) across subjects (PFWE < 0.05, corrected for multiple comparisons at the cluster level with the random field theory using a family-wise error) and for their functional connectivity with dorsal (D) and ventral (V) striatum.
| Region | BA/nucleus | x[mm] | y[mm] | z[mm] | k | T | Functional Connectivity | ||
|---|---|---|---|---|---|---|---|---|---|
| V[T] | D[T] | D>V[T] | |||||||
| Addiction (Alcohol & Cocaine & Methamphetamine & Marijuana)
| |||||||||
| Anterior cingulum | 32 | -12 | 30 | 24 | 841 | 3.52 | 14 | 26 | 5 |
| Anterior cingulum | 24 | -3 | 30 | 21 | 3.45 | 20 | 16 | -2 | |
| Middle cingulum | 24 | -9 | 12 | 36 | 3.37 | NS | 42 | 30 | |
|
| |||||||||
| Eating disorders & Obesity
| |||||||||
| Putamen/posterior insula | -30 | -15 | 12 | 7440 | 6.87 | NS | 56 | 42 | |
| Hippocampus | 20 | -30 | -9 | -15 | 6.47 | 16 | 37 | 15 | |
| Putamen/posterior insula | 27 | 3 | -6 | 6.02 | 14 | 76 | 55 | ||
| Superior medial frontal | 9 | 0 | 36 | 42 | 334 | 3.91 | NS | 2 | NS |
| Superior medial frontal | 8 | 12 | 27 | 57 | 3.87 | NS | 14 | 4 | |
| Supplementary motor area | 8 | -3 | 24 | 60 | 3.8 | NS | 6 | 4 | |
| Superior frontal | 11 | 24 | 57 | 3 | 114 | 3.81 | 6 | NS | -10 |
| Middle frontal | 10 | 36 | 60 | 6 | 3.15 | NS | NS | -6 | |
| Superior medial frontal | 10 | 9 | 60 | 3 | 3 | 17 | -9 | -23 | |
| Inferior temporal | 37 | 48 | -48 | -24 | 131 | 3.53 | -9 | -13 | -6 |
| Cerebellum | 36 | -57 | -39 | 3.43 | 7 | 6 | 3 | ||
| Cerebellum | 27 | -60 | -33 | 3.18 | 4 | 13 | 8 | ||
| Middle Temporal | 21 | 45 | -48 | 6 | 245 | 3.16 | NS | 10 | 16 |
|
| |||||||||
| Eating disorders &Obesity > Addiction
| |||||||||
| Putamen/posterior insula | -30 | -15 | 12 | 513 | 6.03 | 0 | 56 | 42 | |
| Hippocampus | 20 | -33 | -6 | -18 | 0 | 5.32 | 12 | 31 | 11 |
| Fusiform | 20 | -36 | -15 | -24 | 0 | 4.74 | 9 | 13 | NS |
| Putamen | 27 | 3 | -6 | 582 | 4.92 | 14 | 76 | 55 | |
| Hippocampus | 20 | 39 | -3 | -18 | 0 | 4.87 | 3 | 21 | 11 |
| Parahippocampus | 30 | 21 | -33 | -9 | 0 | 4.39 | 4 | 5 | NS |
| Superior temporal | 41 | -39 | -39 | 6 | 217 | 4.34 | NS | 17 | 15 |
| Supramarginal | 48 | -54 | -39 | 27 | 0 | 4.34 | -10 | 28 | 25 |
| Middle temporal | 42 | -54 | -42 | 12 | 0 | 4.08 | -5 | 22 | 17 |
k: cluster size (3-mm isotropic voxels). Statistical model one-way analysis of variance.
Alcohol
In alcoholics, postmortem studies and brain imaging studies have reported reductions in D2R in striatum, including NAc (Freund and Ballinger, 1989). fMRI studies on alcoholics have reported abnormal responses to cue-reactivity, working memory, inhibition, and emotional paradigms in cortical and subcortical brain regions (Table 1). During cue-reactivity or with exposure to alcohol, more than 67% of the activation clusters that differentiated alcoholics from controls were included in the striatal networks (Fig 5). For instance, intravenous ethanol increased activation in ventral striatum and other limbic areas in social drinkers but not in heavy drinkers (Gilman et al., 2012) and alcohol taste cues activated PFC, striatum and midbrain in heavy drinkers (Filbey et al., 2008). Alcohol sips increased fMRI activation in dorsolateral PFC (DLPFC) and anterior thalamus when alcoholics were exposed to alcohol cues (George et al., 2001). Alcoholics also demonstrated higher fMRI activation than controls in putamen, ACC and medial PFC and decreases in ventral striatum and PFC when viewing alcohol/control cues (Grüsser et al., 2004;Vollstädt-Klein et al., 2010b). Clusters reporting alcohol-related activation abnormalities during cue-reactivity tasks were more frequently located in the “overlapping” network defined by the intersection of the dorsal and ventral networks (Fig 3, magenta; 21% of the gray matter volume) than in regions that were functionally connected to V1, regardless if they overlapped (yellow) or not (green) with the striatal networks. These data suggests that exposure to alcohol-associated cues engage the intersection of the ventral and dorsal striatal networks in agreement with PET findings showing deficits in ventral and dorsal striatal D2R and in DA signaling in alcoholics (Volkow et al., 2007).
Fig 5. Relative number of abnormal clusters per network: Drug addiction.
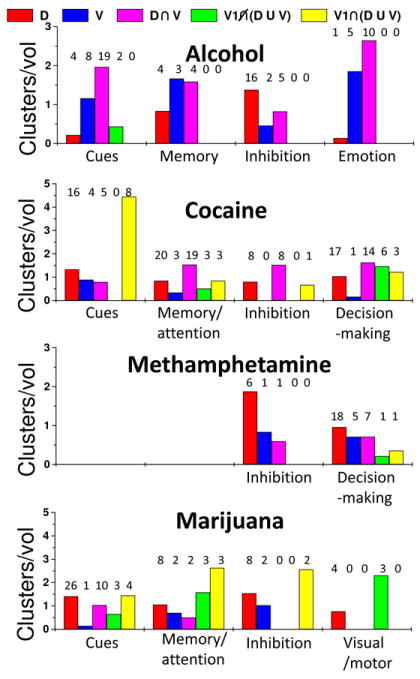
Cluster fractions normalized by network volume fractions were used as a metric to quantify how well the RSFC networks of the dorsal and ventral striatum included abnormal activation clusters reported in neuroimaging studies on drug addiction. Studies that used similar brain activation paradigms (horizontal axis) were grouped according to Tables 2-5. Numeric labels represent the number of clusters that overlapped the dorsal (D; red), ventral (V; blue) patterns and their overlap (D U V; magenta), as well as those included in regions functionally connected with the primary visual cortex (V1) that did (gray) and did not (black) overlap the striatal networks.
The striatal networks also included a large fraction of alcohol-related findings for working memory and memory encoding tasks. To assess the effect of alcohol intoxication on cognitive function, Gundersen et al. evaluated fMRI activation during n-back working memory when subjects drank alcohol versus when they drank soft drinks. They found that acute alcohol intake decreased activation in dorsal ACC and cerebellum, and that these decreases varied with cognitive load and blood alcohol concentrations (Gundersen et al., 2008). Alcoholics assessed with a working memory task demonstrated weaker lateralization of fMRI activation in parahippocampal regions, supporting the hypothesis that the right hemisphere is more vulnerable to alcohol-related damage than the left one (Yoon et al., 2009), and increased ACC activation compared to controls (Vollstädt-Klein et al., 2010a). More than 90% of the alcohol-related activation findings occurred in the striatal networks. These findings strongly support an association between activation abnormalities during working memory and striatal dysfunction in alcoholics.
The striatal networks also included a significant fraction of alcohol-related findings in studies on emotion and inhibitory control. During anticipation of monetary gain detoxified alcoholics exhibited lower activation in ventral striatum than controls but showed higher striatal activation during alcohol-cue exposure, which were correlated with alcohol craving in alcoholics but not in controls (Wrase et al., 2007). Studies on adolescents at risk for alcoholism (children of alcoholics, or COA) reported higher activation in dorsomedial PFC and less activation in ventral striatum and amygdala for alcohol-vulnerable subjects than for alcohol-resilient controls (Heitzeg et al., 2008). Studies on impulsivity reported greater fMRI activation in DLPFC and ACC during the Stroop interference test (Silveri et al., 2011), and lower deactivation in ventral striatum, ventral PFC, and OFC during an inhibitory go/no-go task (Heitzeg et al., 2010) for COA than for control adolescents. The high prevalence of findings within the striatal networks during these studies (> 83%) strongly suggests that alcohol vulnerability and related impairments in inhibitory capacity and control mechanisms are associated with striatal dysfunction. Indeed, we documented higher than normal availability of D2R in dorsal and ventral striatum associated with normal function in prefrontal brain regions (OFC, ACC, DLPFC) and anterior insula in COA who were not alcoholics as adults (Figure 2) (Volkow et al., 2006). We postulated that the striatal increases in D2R enabled them to maintain normal function in prefrontal brain regions, protecting them against alcoholism.
Cocaine
The striatal networks captured 83% of the abnormal activation clusters in cocaine subjects suggesting cortico-striatal dysfunction in cocaine addiction. Drug cues (words) showed lower fMRI activation in rostral ventral and caudal dorsal ACC than neutral words in cocaine addicts (Goldstein et al., 2007b) who showed lower activation than controls in these ACC regions (Goldstein et al., 2009a) but higher activation in midbrain (Goldstein et al., 2009b). Administration of the DA enhancing medication methylphenidate (20 mg oral) normalized the hypo ACC activation in cocaine addicts (Goldstein et al., 2010). During a cocaine-cue video, brain activation in left DLPFC and bilateral occipital cortex was stronger for cocaine subjects than for healthy controls (Wilcox et al., 2011). However, glucose metabolism in left insula, OFC and NAc, and right parahippocampus was lower when cocaine subjects watched a cocaine-cue video than when they watched a neutral-cue video and methylphenidate (20 mg, oral) reduced the abnormal response to the cocaine-cues (Volkow et al., 2010b). When instructed to inhibit their craving prior to exposure to cocaine-cues, cocaine abusers were able to reduce metabolism of the OFC and NAc (compared to the condition when they did not aim to control their craving), an effect that was predicted by baseline metabolism in the right inferior frontal cortex (BA 44) (Volkow et al., 2010a). In cocaine addicted women, but not in men, exposure to cocaine-cues (video and measured with PET and FDG) was associated with a significant reduction in metabolism in cortical brain regions that are located within the striatal networks and are also part of the control networks (Volkow et al., 2011a). Inasmuch as DA modulates control networks through striatal cortical pathways these findings support the involvement of control networks in addiction. Upon exposure to the stimulant drug itself (intravenous methylphenidate, which cocaine abusers reported to have similar effects to those of intravenous cocaine) cocaine abusers showed increased metabolic activation in OFC and ventral cingulate whereas control subjects decreased metabolic activity in these regions (Volkow et al., 1995).
The striatal networks also captured 71% of cocaine-related abnormal activation clusters during working memory and visual attention tasks and the control regions (functionally connected to V1) that overlapped the dorsal striatal network (Fig 3, yellow) had much higher likelihood of abnormalities than those that did not overlap the striatal networks (green). During verbal n-back working memory cocaine subjects demonstrated lower activation in thalamus and midbrain, dorsal striatum, ACC, and limbic regions (amygdala and parahippocampus) and hyper activation in PFC and parietal cortices (Tomasi et al., 2007a). Some of these abnormalities were accentuated in the cocaine abusers with positive urines for cocaine at time of study suggesting that the deficits may reflect in part early cocaine abstinence (Tomasi et al., 2007a). Indeed, during early abstinence treatment-seeking cocaine-dependent individuals exhibited hypo activation in striatum, ACC, inferior PFC, precentral gyrus, and thalamus compared to controls (Moeller et al., 2010). Other studies on working memory reveled that cocaine cues can increase brain activation in occipital cortex (Hester and Garavan, 2009). During visual attention tasks, cocaine abusers had lower thalamic activation and higher occipital cortex and PFC activation than controls (Tomasi et al., 2007b). The association between cortico-striatal dysfunction and abnormal fMRI activation during memory and attention tasks occurred predominantly at the intersection of the dorsal and ventral networks, which had 3 times higher likelihood (relative number of cluster normalized by network volume) than regions not functionally connected to the striatum (Fig 5).
During decision-making with the Iowa gambling task cocaine abusers demonstrated higher regional cerebral blood flow (rCBF; measured with 15O-water PET) in right OFC and lower rCBF in DLPFC and medial PFC compared to controls (Bolla et al., 2003). During a forced-choice task under three monetary value conditions cocaine subjects showed lower fMRI responses to monetary reward in OFC, PFC and occipital cortex, midbrain, thalamus, insula and cerebellum (Goldstein et al., 2007a). Lower than normal D2R availability in dorsal striatum was associated with decreased thalamic activation responses whereas in ventral striatum it was associated with increased medial PFC activation in cocaine addicted individuals (Asensio et al., 2010). Similarly to cognitive tasks, findings at the intersection of the dorsal and ventral networks exhibited higher likelihood than those in regions not functionally connected to the striatum.
Sixty-four % of the brain clusters reported by fMRI studies on inhibitory tasks were included in the striatal networks. During go/no-go inhibition cocaine addicts demonstrated lower activation than controls in OFC, supplementary motor area and ACC, regions that might be critical for cognitive control (Hester and Garavan, 2004). Short- and long-term abstinent cocaine users exhibited differential activation in PFC, temporal cortex, cingulum, thalamus and cerebellum (Connolly et al., 2011). During different inhibitory tasks (Stroop interference) cocaine addicts showed lower rCBF in left ACC and right PFC, and higher rCBF in right ACC than controls (Bolla et al., 2004). Striatal functional connectivity failed to explain brain activation differences from studies that used stop-signal tasks (Li et al., 2008). These studies showed lower activation in ACC, parietal and occipital cortices in cocaine abusers. PET studies measuring mu opioid receptors (using [11C]carfentanil) showed higher specific binding in frontal and temporal cortices for one-day abstinent cocaine dependent subjects than for controls, and these abnormalities decreased with abstinence and correlated with cocaine use (Gorelick et al., 2005;Ghitza et al., 2010).
Methamphetamine
Compared to control subjects, metamphetamine abusers tested during early detoxification demonstrated decreased glucose metabolism in striatum and thalamus whereas they showed increased activity in parietal cortex (Volkow et al., 2001a). This suggested that both DA as well as non DA modulated brain regions are affected by chronic metamphetamine consumption (Volkow et al., 2001a). Moreover decreased striatal DA activity was associated with a greater likelihood of relapse during treatment (Wang et al., 2011b), protracted abstinence was associated with partial recovery of striatal DAT (Volkow et al., 2001b) and of regional brain metabolism (Wang et al., 2004), and reductions in striatal D2R were also associated with reduction in metabolism in OFC in recently detoxified methamphetamine abusers (Volkow et al., 2001a).
A large fraction (70%) of the methamphetamine-related fMRI findings was encompassed by the striatal networks (Fig 5). When compared to controls, methamphetamine dependent individuals exhibited higher ACC activation during go/no-go response inhibition (Leland et al., 2008), and lower right PFC activation during Stroop interference (Salo et al., 2009). Most of these abnormal activation clusters (88%) occurred within the dorsal network (including its overlap with the ventral network). During decision-making, however, a lower fraction (64%) of the clusters was encompassed by the striatal networks. Using a two-choice prediction task, Paulus and colleagues found that fMRI activation was lower in PFC (Paulus et al., 2002), OFC, ACC and parietal cortex for methamphetamine-dependent subjects than for controls (Paulus et al., 2003). Moreover, a combination of activation responses in these regions best predicted the time to relapse and showed different activation patterns as a function of error rate in left insula and DLPFC (Paulus et al., 2005).
Marijuana
The involvement of striatal dysfunction in marijuana addiction is less clear because neither baseline striatal D2R nor striatal DA release (after amphetamine challenge) abnormalities were observed in recent PET studies with [11C]raclopride (Urban et al., 2012;Stokes et al., 2012). An FDG study showed that when given tetrahydrocannabinol (THC) chronic marijuana abusers showed increases in OFC and medial PFC and in striatum whereas controls did not, but it increased cerebellar metabolism in both abusers and controls suggesting that striatal networks are involved in marijuana addiction (Volkow et al., 1996b). Tactile marijuana-related cues versus neutral cues were shown to increase fMRI activation in VTA, thalamus, ACC, insula, and amygdala, supporting the involvement of striatal networks, as well as in other prefrontal, parietal and occipital cortices and cerebellum in recently abstinent marijuana users (Filbey et al., 2009). During a visual attention task, marijuana abusers had lower fMRI activation in right PFC, parietal cortex and cerebellum (normalized with duration of abstinence) and higher activation in frontal, parietal and occipital cortices than controls (Chang et al., 2006). During working memory, however, marijuana abusers exhibited decreased activation in temporal lobes, ACC, parahippocampus and thalamus with increased task performance, a group × performance interaction effect that was opposite in controls (Padula et al., 2007). During go/no-go inhibition, adolescents with histories of marijuana use showed higher fMRI activation in DLPFC, parietal and occipital cortices, and insula than adolescents without histories of marijuana use (Tapert et al., 2007). During visuomotor integration with a visually paced finger sequencing task cued by a flashing checkerboard, marijuana users had higher PFC activation and lower visual cortex activation than controls (King et al., 2011). Sixty-nine % of the abnormal activation clusters in studies on effects of marijuana on brain function were located in regions functionally connected to the striatum.
Obesity
Compulsive-like feeding behavior in obese rats has been associated with downregulation of striatal D2R (Johnson and Kenny, 2010) and obesity has been linked to lower striatal D2R in humans (Wang et al., 2001), suggesting that common neuroadaptations in DA striatal pathway may underlie obesity and drug addiction. Baseline PET studies of brain glucose metabolism in obese individuals reported reductions in metabolic activity in OFC and ACC that were associated with lower than normal striatal D2R availability (Volkow et al., 2008b).
Brain activation in dorsal and ventral striatum, insula, hippocampus, OFC, amygdala, medial PFC and ACC elicited by visual exposures to high caloric foods was higher for obese than for control women (Rothemund et al., 2007;Stoeckel et al., 2008). Similarly, visual food cues elicited increased fMRI activation responses in frontal, temporal, and limbic regions for obese adults than for controls (Dimitropoulos et al., 2012), and hippocampal activation showed a correlation with fasting plasma levels of insulin and waist circumference in adolescents (Wallner-Liebmann et al., 2010). Striatal activation in response to chocolate milkshake intake was associated with gains in body weight and with the presence of the A1 allele of the TaqIA restriction fragment length polymorphism, which is associated with D2R gene binding in the striatum and compromised striatal DA signaling (Stice et al., 2008). Adolescents with high risk for obesity showed higher activation in caudate and operculum in response to chocolate milkshake intake than those with low risk for obesity (Stice et al., 2011). During gastric distention, as it occurs during meal ingestion, obese subjects had increased fMRI activation than normal weight subjects in cerebellum and posterior insula and decreased activation in amygdala, midbrain, hypothalamus, thalamus, pons, and anterior insula (Tomasi et al., 2009b). Eighty-two % of the activation clusters from these studies on cue-reactivity occurred in regions functionally connected to the striatum (Fig 6). Consistent with these activation response PET studies measuring D2R with [18F]fallypride in obese subject showed an inverse correlation between ghrelin and D2R in dorsal and ventral striatum and in inferior temporal cortex, temporal pole, insula and amygdala (Dunn et al., 2012).
Fig 6. Relative number of abnormal clusters per network: Obesity and eating disorders.
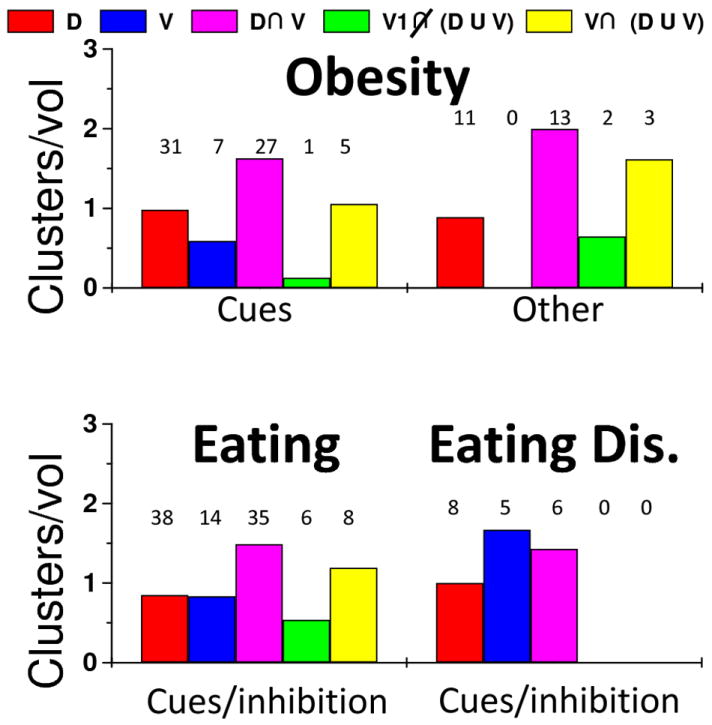
Cluster fractions normalized by network volume fractions were used as a metric to quantify how well the RSFC networks of the dorsal and ventral striatum included abnormal activation clusters reported in neuroimaging studies on obesity and eating disorders. Studies that used similar brain activation paradigms (horizontal axis) were grouped according to Tables 6-7. Numeric labels represent the number of clusters that overlapped the dorsal (D; red), ventral (V; blue) patterns and their overlap (D U V; magenta), as well as those included in regions functionally connected to the primary visual cortex (V1) that did (gray) and did not (black) overlap the striatal networks.
Food perception and control of food intake
In normal conditions food intake is thought to be determined by both homeostatic (balance of energy and nutrients in the body) and non-homeostatic (pleasure of eating) factors, and brain DA has been associated with eating behaviors (Volkow et al., 2003b). Pharmacological fMRI studies have shown that hypothalamic activation predicts food intake when the plasma level concentration of PYY, a peptide hormone that provides a physiological gut-derived satiety signal to the brain, is low and that activation in OFC striatum, VTA, SN, cerebellum, PFC, insula and cingulum can predict feeding behavior when PYY plasma level concentration is high (Batterham et al., 2007).
Event-related studies contrasting brain responses to sucrose taste and tasteless water showed that hunger was associated with fMRI activation in insula, thalamus, cerebellum, cingulum, SN as well as cortical brain regions whereas satiety was associated with deactivation in parahippocampus, hippocampus, amygdala and ACC (Haase et al., 2009). In this study the differential effect of hunger versus satiety on brain activation to taste stimuli (salty, sour, bitter, sweet) was stronger for males than for females, particularly in dorsal striatum, amygdala, parahippocampus and posterior cingulum (Haase et al., 2011). PET studies on inhibitory control in hunger conditions that used real food stimulation revealed that purposeful inhibition of the desire for food decreased glucose metabolism in amygdala, hippocampus, insula, striatum and OFC in men but not in women (Wang et al., 2009). A large fraction (> 31%) of the activation clusters occurred in regions functionally connected to both dorsal and ventral striatum (Fig 6, magenta).
Eating disorders
Pharmacological studies have shown that disruption of DA signaling in the striatum can inhibit normal feeding in rodents (Sotak et al., 2005;Cannon et al., 2004) and that DA signaling modulates reactivity to food cues in humans (Volkow et al., 2002). PET studies of patients suffering from anorexia (over control of eating habits) showed higher than normal striatal D2R availability (Frank et al., 2005). In contrast, a recent study in non-obese patients with binge eating disorder showed that while they did not differ in D2R availability from controls they showed enhanced striatal DA release during food stimulation (Wang et al., 2011a). fMRI studies showed that when exposed to pleasant food pictures patients with binge eating disorder had stronger medial OFC responses that controls whereas patients with bulimia nervosa had stronger ACC and insula responses than controls (Schienle et al., 2009). During go/no-go inhibition, binge eating/purging female adolescents showed higher activation in temporal cortex, PFC and ACC than controls, and anorexia nervosa patients exhibited higher activation in hypothalamus and lateral PFC (Lock et al., 2011). Since only one of these clusters was located outside the striatal networks, these data also corroborates a role of cortico-striatal networks in eating disorders.
Prefrontal regions
The prefrontal cortex and the striatum are inter modulated via cortico-striatal networks modulated by DA (Haber, 2003). The frontal cortex plays a complex role in cognition, including inhibitory control, decision making, emotional regulation, purposefulness, motivation and salience attribution among others. It has been hypothesized that dysfunctions in frontal regions might impair the control over compulsive drug intake (Kalivas, 2004;Volkow and Fowler, 2000), and that frontal cortex disruptions can have serious consequences in drug addiction (Volkow et al., 2006).
The frontal abnormalities revealed by our meta-analysis are consistent with the correlations between striatal D2R reductions and decreased metabolic activity in ACC, OFC and DLPFC previously reported for cocaine and methamphetamine abusers and alcoholics (Volkow et al., 1993;Volkow et al., 2001a;Volkow et al., 2007). Since ACC, lateral OFC and DLPFC are involved with inhibitory control and decision making (Goldstein and Volkow, 2002;Phan et al., 2002), this association suggests that loss of control over drug intake (Volkow et al., 1996a) could reflect improper DA-regulation in these frontal regions. This hypothesis is supported by studies that associated striatal D2R reductions and impulsivity scores in methamphetamine abusers (Lee et al., 2009) and rodents (Everitt et al., 2008) and by those that linked ACC impairments with obsessive compulsive behaviors and impulsivity (Rolls, 2000). However, another possibility is that early abnormalities in frontal regions trigger repeated drug use and neuroadaptations that decrease striatal D2R. For instance, non-alcoholic individuals with a family history of alcoholism had higher than normal striatal D2R that was associated with normal metabolism in ACC, OFC and DLPFC, suggesting that that normal activity in prefrontal regions promoting inhibitory control and emotional regulation could be the mechanism that protected these subjects against alcohol abuse (Volkow et al., 2006). Interestingly, a recent study that compared sibling discordant for stimulant addiction showed significant differences in volume of medial OFC (Ersche et al., 2012), suggesting that these differences reflected the exposure to the drug rather than genetic vulnerability (Volkow and Baler, 2012).
Temporal regions
The striatum is also connected with medial temporal lobe structures (hippocampus parahippocampal gyrus) that are essential for explicit memory but also for conditioning (Haber et al., 2006). Brain activation studies on reward-motivated learning have documented the involvement of medial temporal lobe structures in subsequent memory enhancements (Adcock et al., 2006;Wittmann et al., 2005). Thus drug cues could trigger craving memory activating learning circuits in the medial temporal cortex, and this enhanced activation of memory circuits could contribute to overcome the inhibitory control exerted by the prefrontal cortex in food and drug addiction (Volkow et al., 2003a). Our meta-analysis revealed that drug addiction, obesity and eating disorders are characterized by common brain activation abnormalities in medial temporal cortex (hippocampus, parahippocampal gyrus and amygdala), superior and inferior temporal cortices and posterior insula (PFWE< 0.05). The pattern of brain activation abnormalities partially overlapped the dorsal (40%), ventral (10%) and overlapping (48%) networks; only 2% of the abnormalities did not show overlap with the striatal networks. Our meta-analysis also revealed stronger abnormalities in medial temporal lobe structures in obesity and eating disorders compared to drug addiction (Fig 4). This suggests that these temporal regions are involved in the regulation of eating behaviors to a greater extent than in the regulation of drug intake. Specifically food intake is regulated both by homeostatic and reward pathways and while the homeostatic system modulates the reward pathway, it also modulates other brain regions through the various peripheral hormones and neuropeptides that regulate hunger and satiety. Indeed medial temporal regions (hippocampus, parahippocampus) express leptin receptors (Williams et al., 1999) and insulin-like growth factor receptors (Wilczak et al., 2000) as well as mRNA for the ghrelin receptor gene (Guan et al., 1997). Thus greater involvement of medial temporal cortices in obesity than in addiction is consistent with the involvement of hormones and neuropeptides that regulate food intake via the homeostatic pathway.
Reward and habits
For both drug and food intake reward processes in ventral striatum initially drive the motivation to repeat the behavior. However with repeated exposure conditioned responses and learned associations shift the incentive motivation to the conditioned stimulus that predicts the reward. This transition, along with the associated enhanced motivation to do the behaviors necessary to consume the reward (drug or food), requires involvement of the dorsal striatum (Belin and Everitt, 2008). In addition, repeated exposure to associated pairing results in habits that can further drive behavior (including eating or taking drugs or alcohol) also involve dorsal striatal regions. However in reviewing the significant overlap between ventral and dorsal striatal connectivity it is therefore not surprising that studies show activation of ventral and dorsal striatum both with reward and conditioning. Similarly while the dorsal striatum is predominantly associated with habits their formation may also require a progression from ventral to dorsal striatal regions (Everitt et al., 2008).
Vulnerable networks in addiction and obesity
An important finding from this study is that functional abnormalities in food or drug addiction tend to occur in brain regions functionally connected to both dorsal and ventral striatum. These vulnerable regions are essential for cognitive control (anterior cingulum and supplementary motor area), reward and motivation (striatum and medial OFC) and reward-motivated learning (hippocampus and parahippocampal gyrus). The overlap of striatal connectivity patterns suggests that dopaminergic modulation from both dorsal and ventral striatum is essential in these regions, and their higher vulnerability suggests that food/drug addiction might alter delicate striatal modulation balance and brain activation in these regions.
Limitations
Our meta-analysis includes studies on acute effects of drugs and food (cues), as well as studies on cognition (memory, attention, inhibition, decision-making) and emotion when drugs or food are not present. Since the direct and the long-term effects of food/drug addiction are different the participants in the former studies may or may not be the most vulnerable to brain changes. These could have increased the variability, limiting the interpretation of the results. The over expression of the medial temporal lobe abnormalities in obesity and eating disorders compared to those in drug addiction may reflect the severity of the disorders as it is not easy to equate intensity, duration or age of initiation of the disorder.
In summary this analysis of recent brain imaging studies on different types of drug addiction and disorders characterized by behavioral dyscontrol over a rewarding behaviors (eating) shows that there is an over representation of abnormal activation (both to cues and during cognitive tasks) that frequently occur in areas where there is overlap between the ventral and the dorsal striatal pathways. This corroborates in humans that both the ventral striatum (predominantly associated with rewards processing) and the dorsal striatum (predominantly associated with habits and rituals in addiction) are disrupted in addictive disorders (Wise, 2009) and that these abnormalities influence processing of rewards (drugs and food) reward-associated stimuli (cues) and cognitive processes necessary for self-control (executive function). However medial temporal cortical regions that are part of the dorsal striatal pathway showed greater vulnerability to obesity and eating disorders than to drug addiction (Fig 4), indicating that there are also distinct pattern of abnormalities between these set of disorders.
Table 2.
Summary of functional neuroimaging studies (conducted between 2001 and 2011) on effects of cocaine addiction on brain function that were included in Figs 4 and 5. Studies are grouped by stimulation paradigm into five major categories. Number of cocaine (S) and control (C) subjects, tasks and imaging modality are provided in addition to main results.
| Subjects | Task; modality | Results | |
|---|---|---|---|
| Memory & attention | |||
| Tomasi et al; 2007a | 16S & 16C | Working memory; fMRI | Midbrain, thalamus, VPFC & limbic ↓; prefrontal & parietal ↑ |
| Tomasi et al; 2007b | 14S & 14C | Visual attention; fMRI | Thalamus, parietal and VPFC↓; occipital & prefrontal ↑ |
| Hester et al; 2009 | 16S | Working memory; fMRI | Cocaine cues -> Inf frontal, precuneus, PCC, occipital and cerebellum ↑ |
| Moeller et al; 2010 | 19S & 14C | Working memory; fMRI | Striatum, Thalamus, PFC ↓ |
| Cue-reactivity | |||
| Goldstein et al; 2007b | 14S | Drug/neutral word cues; fMRI | cdACC ↑; VPFC ↓ |
| Goldstein et al; 2009a | 17S & 17C | Drug/neutral word cues; fMRI | ACC ↓ |
| Goldstein et al; 2009b | 15S & 15C | Drug/neutral word cues; fMRI | Midbrain ↑ |
| Volkow et al; 2010b | 24S | Visual cocaine cues; PET MPH | Insula, OFC, Nacc and parahippocampus ↓ MPH attenuates this effect |
| Goldstein et al; 2010 | 13S & 14C | Drug/neutral word cues; fMRI | MPH normalizes ACC hypo-activation |
| Wilcox et al; 2011 | 14S & 16C | Visual cocaine cues; fMRI | ACC and PFC ↑ |
| Decision making | |||
| Bolla et at; 2003 | 13S & 13C | Gambling task; PET | OFC↑; VPFC and DLPFC ↓ |
| Goldstein et al; 2007a | 16S & 13C | Monetary reward; fMRI | Cortical and subcortical regions ↓ |
| Asensio et al; 2010 | 7S | M reward & D2R;PET & fMRI | Low striatal D2R: Thal↓; ACC↑ |
| Inhibition | |||
| Hester et al; 2004 | 15S & 15C | Go/no go task; fMRI | ACC and right PFC ↓ |
| Bolla et al; 2004 | 13S & 13C | Stroop interference; PET | PFC↑; ACC ↓↑ |
| Connolly et al; 2011 | 18S & 9C | Go/NoGo; fMRI | PFC, inferior frontal, cingulate and cerebellum ↑ |
| Other (Stop signal & molecular targets) | |||
| Gorelick et al; 2005 | 17S & 16C | mu-opioid receptor; PET | ACC and anterior frontal ↑ (elevated after 12 weeks abstinence) |
| Li et al; 2008 | 15S & 15C | Stop signal task; fMRI | VPFC ↓ |
| Ghitza et al; 2010 | 25S | mu-opioid receptor; PET & CBT | VPFC and PFC ↑mu-opioid receptor predict treatment outcome |
Arrows indicate activation increases (↑) or decreases (↓).
Table 3.
Summary of fMRI studies (conducted between 2001 and 2011) on effects of methamphetamine addiction on brain function that were included in Figs 4 and 5. Studies are grouped by stimulation paradigm into two major categories. Number of methamphetamine (S) and control (C) subjects and tasks are provided in addition to main results.
| Subjects | Task | Results | |
|---|---|---|---|
| Decision-making | |||
| Paulus et. al; 2002 | 10S & 10C | Two-choice prediction | DLPFC, VPFC↓ |
| Paulus et. al; 2003 | 14S & 14C | Two-choice prediction | OFC, DLPFC, ACC and superior parietal ↓ |
| Paulus et. al; 2005 | 18S & 22C | Two-choice prediction | Relapsers: Insula, PCC↓ |
| Monterosso et.al; 2007 | 12S & 17C | Delay discounting | DLPFC, IPS↓ |
| Paulus et. al; 2008 | 12S & 12C | Two-choice prediction | Insula (correlations with performance) |
| Hoffman et. al; 2008 | 19S & 17C | Delay discounting | Precuneus, caudate, DLPFC, ACC ↓ |
| Inhibition | |||
| Leland et. al; 2008 | 19S & 19C | Go/NoGo | ACC (correlations with false alarm rate) |
| Salo et. al; 2009 | 12S & 16C | Stroop interference | Right PFC↓ |
Arrows indicate activation increases (↑) or decreases (↓).
Acknowledgments
This work was accomplished with support from the National Institutes of Alcohol Abuse and Alcoholism (2RO1AA09481).
Footnotes
Declaration of interest
The authors report no declarations of interest.
References
- Adcock R, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli J. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Asensio S, Romero M, Romero F, Wong C, Alia-Klein N, Tomasi D, Wang G, Telang F, Volkow N, Goldstein R. Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse. 2010;64:397–402. doi: 10.1002/syn.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson T. Central and peripheral neuroendocrine peptides and signalling in appetite regulation: considerations for obesity pharmacotherapy. Obes Rev. 2008;9:108–120. doi: 10.1111/j.1467-789X.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- Avena N, Rada P, Hoebel B. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham R, ffytche D, Rosenthal J, Zelaya F, Barker G, Withers D, Williams S. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt B. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Biswal B, Mennes M, Zuo X, Gohel S, Kelly C, Smith S, Beckmann C, Adelstein J, Buckner R, Colcombe S, Dogonowski A, Ernst M, Fair D, Hampson M, Hoptman M, Hyde J, Kiviniemi V, Kötter R, Li S, Lin C, Lowe M, Mackay C, Madden D, Madsen K, Margulies D, Mayberg H, McMahon K, Monk C, Mostofsky S, Nagel B, Pekar J, Peltier S, Petersen S, Riedl V, Rombouts S, Rypma B, Schlaggar B, Schmidt S, Seidler R, Siegle GJ, Sorg C, Teng G, Veijola J, Villringer A, Walter M, Wang L, Weng X, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang Y, Zhang H, Castellanos F, Milham M. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad J, Pihl R, Benkelfat C, Leyton M, Diksic M, Tremblay R, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Bolla K, Eldreth D, London E, Kiehl K, Mouratidis M, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong M, van Berckel B, Boellaard R, Zuurman L, Schuit R, Windhorst A, van Gerven J, Ramsey N, Lammertsma A, Kahn R. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34:759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Braskie M, Landau S, Wilcox C, Taylor S, O’Neil J, Baker S, Madison C, Jagust W. Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults. Hum Brain Mapp. 2011;32:947–961. doi: 10.1002/hbm.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody A, Mandelkern M, Olmstead R, Allen-Martinez Z, Scheibal D, Abrams A, Costello M, Farahi J, Saxena S, Monterosso J, London E. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology. 2009;32:282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon C, Abdallah L, Tecott L, During M, Palmiter R. Dysregulation of striatal dopamine signaling by amphetamine inhibits feeding by hungry mice. Neuron. 2004;44:509–520. doi: 10.1016/j.neuron.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Cason A, Smith R, Tahsili-Fahadan P, Moorman D, Sartor G, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Connolly C, Foxe J, Nierenberg J, Shpaner M, Garavan H. The neurobiology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2011.08.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Tschop M, Horvath T, Levine A. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- de Araujo I, Oliveira-Maia A, Sotnikova T, Gainetdinov R, Caron M, Nicolelis M, Simon S. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies D, Kelly A, Uddin L, Shehzad Z, Biswal B, Walters J, Castellanos F, Milham M. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite. 2012;58:303–312. doi: 10.1016/j.appet.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W, Gautier C, Price J, Kupfer D, Kinahan P, Grace A, Price J, Mathis C. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Dunn J, Kessler R, Feurer I, Volkow N, Patterson B, Ansari M, Li R, Marks-Shulman P, Abumrad N. Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity. Diabetes Care. 2012;35:1105–1111. doi: 10.2337/dc11-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche K, Jones P, Williams G, Turton A, Robbins T, Bullmore E. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Everitt B, Belin D, Economidou D, Pelloux Y, Dalley J, Robbins T. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry A, Ongür D, An X, Price J. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425:447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Filbey F, Claus E, Audette A, Niculescu M, Banich M, Tanabe J, Du Y, Hutchison K. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F, Schacht J, Myers U, Chavez R, Hutchison K. Marijuana craving in the brain. Proc Natl Acad Sci U S A. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Snyder A, Vincent J, Corbetta M, Van Essen D, Raichle M. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G, Bailer U, Henry S, Drevets W, Meltzer C, Price J, Mathis C, Wagner A, Hoge J, Ziolko S, Barbarich-Marsteller N, Weissfeld L, Kaye W. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry. 2005;58:908–912. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Freund G, Ballinger WJ. Neuroreceptor changes in the putamen of alcohol abusers. Alcohol Clin Exp Res. 1989;13:213–218. doi: 10.1111/j.1530-0277.1989.tb00314.x. [DOI] [PubMed] [Google Scholar]
- García-García I, Jurado M, Garolera M, Segura B, Sala-Llonch R, Marqués-Iturria I, Pueyo R, Sender-Palacios M, Vernet-Vernet M, Narberhaus A, Ariza M, Junqué C. Alterations of the salience network in obesity: A resting-state fMRI study. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M, Anton R, Bloomer C, Teneback C, Drobes D, Lorberbaum J, Nahas Z, Vincent D. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Ghitza U, Preston K, Epstein D, Kuwabara H, Endres C, Bencherif B, Boyd S, Copersino M, Frost J, Gorelick D. Brain mu-opioid receptor binding predicts treatment outcome in cocaine-abusing outpatients. Biol Psychiatry. 2010;68:697–703. doi: 10.1016/j.biopsych.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J, Ramchandani V, Crouss T, Hommer D. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37:467–477. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Alia-Klein N, Tomasi D, Carrillo J, Maloney T, Woicik P, Wang R, Telang F, Volkow N. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci U S A. 2009a;106:9453–9458. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Alia-Klein N, Tomasi D, Zhang L, Cottone L, Maloney T, Telang F, Caparelli E, Chang L, Ernst T, Samaras D, Squires N, Volkow N. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007a;164:1–9. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Tomasi D, Alia-Klein N, Carrillo J, Maloney T, Woicik P, Wang R, Telang F, Volkow N. Dopaminergic response to drug words in cocaine addiction. J Neurosci. 2009b;29:6001–6006. doi: 10.1523/JNEUROSCI.4247-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Tomasi D, Rajaram S, Cottone L, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow N. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007b;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Volkow N. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Volkow N. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Woicik P, Maloney T, Tomasi D, Alia-Klein N, Shan J, Honorio J, Samaras D, Wang R, Telang F, Wang G, Volkow N. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Natl Acad Sci U S A. 2010;107:16667–16672. doi: 10.1073/pnas.1011455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick D, Kim Y, Bencherif B, Boyd S, Nelson R, Copersino M, Endres C, Dannals R, Frost J. Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biol Psychiatry. 2005;57:1573–1582. doi: 10.1016/j.biopsych.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Grace A. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Supp 2):S119–S128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Grüsser S, Wrase J, Klein S, Hermann D, Smolka M, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus D, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Gu H, Salmeron B, Ross T, Geng X, Zhan W, Stein E, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Yu H, Palyha O, McKee K, Feighner S, Sirinathsinghji D, Smith R, Van der Ploeg L, Howard A. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Gundersen H, Grüner R, Specht K, Hugdahl K. The effects of alcohol intoxication on neuronal activation at different levels of cognitive load. Open Neuroimag J. 2008;2:65–72. doi: 10.2174/1874440000802010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage. 2009;44:1008–1021. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase L, Green E, Murphy C. Males and females show differential brain activation to taste when hungry and sated in gustatory and reward areas. Appetite. 2011;57:421–434. doi: 10.1016/j.appet.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber S, Fudge J, McFarland N. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S, Kim K, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon C, Wesley M, Stapleton J, Laurienti P, Porrino L. The association between frontal-striatal connectivity and sensorimotor control in cocaine users. Drug Alcohol Depend. 2011;115:240–243. doi: 10.1016/j.drugalcdep.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg M, Nigg J, Yau W, Zubieta J, Zucker R. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcohol Clin Exp Res. 2008;32:414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg M, Nigg J, Yau W, Zucker R, Zubieta J. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol Psychiatry. 2010;68:287–295. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacol Biochem Behav. 2009;93:270–277. doi: 10.1016/j.pbb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilinsky I, Jouandet M, Goldman-Rakic P. Organization of the nigrothalamocortical system in the rhesus monkey. J Comp Neurol. 1985;236:315–330. doi: 10.1002/cne.902360304. [DOI] [PubMed] [Google Scholar]
- Johnson P, Kenny P. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kalivas P. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kelly C, Zuo X, Gotimer K, Cox C, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos F, Milham M. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69:684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R, Strick P. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143 doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- King G, Ernst T, Deng W, Stenger A, Gonzales R, Nakama H, Chang L. Altered brain activation during visuomotor integration in chronic active cannabis users: relationship to cortisol levels. J Neurosci. 2011;31:17923–17931. doi: 10.1523/JNEUROSCI.4148-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob G, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Häring H, Fritsche A, Preissl H. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2012;33:1052–1061. doi: 10.1002/hbm.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975;88:195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Künzle H. Projections from the primary somatosensory cortex to basal ganglia and thalamus in the monkey. Exp Brain Res. 1977;30:481–492. doi: 10.1007/BF00237639. [DOI] [PubMed] [Google Scholar]
- Künzle H, Akert K. Efferent connections of cortical, area 8 (frontal eye field) in Macaca fascicularis. A reinvestigation using the autoradiographic technique. J Comp Neurol. 1977;173:147–164. doi: 10.1002/cne.901730108. [DOI] [PubMed] [Google Scholar]
- Lee B, London E, Poldrack R, Farahi J, Nacca A, Monterosso J, Mumford J, Bokarius A, Dahlbom M, Mukherjee J, Bilder R, Brody A, Mandelkern M. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leland D, Arce E, Miller D, Paulus M. Anterior cingulate cortex and benefit of predictive cueing on response inhibition in stimulant dependent individuals. Biol Psychiatry. 2008;63:184–190. doi: 10.1016/j.biopsych.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed S. Intense sweetness surpasses cocaine reward. Plos One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Morgan P, Matuskey D, Abdelghany O, Luo X, Chang J, Rounsaville B, Ding Y, Malison R. Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients. Proc Natl Acad Sci U S A. 2010;107:14455–14459. doi: 10.1073/pnas.1002467107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liang J, Qin W, Tian J, Yuan K, Bai L, Zhang Y, Wang W, Wang Y, Li Q, Zhao L, Lu L, von Deneen K, Liu Y, Gold M. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci Lett. 2009;460:72–77. doi: 10.1016/j.neulet.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Lock J, Garrett A, Beenhakker J, Reiss A. Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. Am J Psychiatry. 2011;168:55–64. doi: 10.1176/appi.ajp.2010.10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka R. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Fu X, Li N, Wang C, Zhang H, Qian R, Xu H, Hu X, Zhang D. Abnormal brain default-mode network functional connectivity in drug addicts. Plos One. 2011;6:e16560. doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang C, Zhang H, Jiang X, Xu H, Fu X, Hu X, Zhang D. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D, Kelly A, Uddin L, Biswal B, Castellanos F, Milham M. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Middleton F, Strick P. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cereb Cortex. 2002;12:926–935. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- Minzenberg M, Yoon J, Carter C. Modafinil modulation of the default mode network. Psychopharmacology (Berl) 2011;215:23–31. doi: 10.1007/s00213-010-2111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F, Steinberg J, Schmitz J, Ma L, Liu S, Kjome K, Rathnayaka N, Kramer L, Narayana P. Working memory fMRI activation in cocaine dependent subjects: Association with treatment response. Psych Res Neuroimaging. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren R, Hajnal A, Mungarndee S. Gustatory reward and the nucleus accumbens. Physiol Behav. 2006;89:531–535. doi: 10.1016/j.physbeh.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Hirvonen J, Hannukainen J, Immonen H, Lindroos M, Salminen P, Nuutila P. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. Plos One. 2012;7:e31089. doi: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Nat Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula C, Schweinsburg A, Tapert S. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychol Addict Behav. 2007;21:478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M, Hozack N, Frank L, Brown G, Schuckit M. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Paulus M, Hozack N, Zauscher B, Frank L, Brown G, Braff D, Schuckit M. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;20:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paulus M, Tapert S, Schuckit M. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Phan K, Wager T, Taylor S, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Postuma R, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Powell E, Leman R. Connections of the nucleus accumbens. Brain Res. 1976;105:389–403. doi: 10.1016/0006-8993(76)90589-8. [DOI] [PubMed] [Google Scholar]
- Rolls E. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht H, Klingebiel R, Flor H, Klapp B. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Rzepecki-Smith C, Meda S, Calhoun V, Stevens M, Jafri M, Astur R, Pearlson G. Disruptions in functional network connectivity during alcohol intoxicated driving. Alcohol Clin Exp Res. 2010;34:479–487. doi: 10.1111/j.1530-0277.2009.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Ursu S, Buonocore M, Leamon M, Carter C. Impaired Prefrontal Cortical Function and Disrupted Adaptive Cognitive Control in Methamphetamine Abusers: A Functional Magnetic Resonance Imaging Study. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry. 2009;65:654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Selemon L, Goldman-Rakic P. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri M, Rogowska J, McCaffrey A, Yurgelun-Todd D. Adolescents At Risk for Alcohol Abuse Demonstrate Altered Frontal Lobe Activation During Stroop Performance. Alcohol Clin Exp Res. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotak B, Hnasko T, Robinson S, Kremer E, Palmiter R. Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 2005;1061:88–96. doi: 10.1016/j.brainres.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small D. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger K, Epstein L, Small D. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2011;31:4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel L, Weller R, Cook Er, Twieg D, Knowlton R, Cox J. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Stokes P, Egerton A, Watson B, Reid A, Lappin J, Howes O, Nutt D, Lingford-Hughes A. History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. J Psychopharmacol. 2012;26:144–149. doi: 10.1177/0269881111414090. [DOI] [PubMed] [Google Scholar]
- Tapert S, Schweinsburg A, Drummond S, Paulus M, Brown S, Yang T, Frank L. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli E, Chang L. Common deactivation patterns during working memory and visual attention tasks: An intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27:694–705. doi: 10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein R, Telang F, Maloney T, Alia-Klein N, Caparelli E, Volkow N. Cocaine abusers have widespread disruption in brain activation patterns to a working memory task. Brain Res. 2007a;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein R, Telang F, Maloney T, Alia-Klein N, Caparelli E, Volkow N. Thalamocortical dysfunction in cocaine abusers: implications in attention and perception. Psych Res Neuroimaging. 2007b;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N. Association between Functional Connectivity Hubs and Brain Networks. Cereb Cortex. 2011;21:2003–2013. doi: 10.1093/cercor/bhq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N, Wang G, Wang R, Telang F, Caparelli E, Wong C, Jayne M, Fowler J. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage. 2011;54:3101–3110. doi: 10.1016/j.neuroimage.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N, Wang R, Carrillo J, Maloney T, Alia-Klein N, Woicik P, Telang F, Goldstein R. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. Plos One. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N, Wang R, Telang F, Wang G, Chang L, Ernst T, Fowler J. Dopamine Transporters in Striatum Correlate with Deactivation in the Default Mode Network during Visuospatial Attention. PLoS ONE. 2009a;4:e6102. doi: 10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang G, Wang R, Backus W, Geliebter A, Telang F, Jayne M, Wong C, Fowler J, Volkow N. Association of body mass and brain activation during gastric distention: implications for obesity. PLoS ONE. 2009b;4:e6847. doi: 10.1371/journal.pone.0006847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban N, Slifstein M, Thompson J, Xu X, Girgis R, Raheja S, Haney M, Abi-Dargham A. Dopamine release in chronic cannabis users: a [(11)c]raclopride positron emission tomography study. Biol Psychiatry. 2012;71:677–683. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Baler R. Neuroscience. To stop or not to stop? Science. 2012;335:546–548. doi: 10.1126/science.1218170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Chang L, Wang G, Fowler J, Ding Y, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine d(2) receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow N, Chang L, Wang G-J, Fowler J, Franceschi D, Sedler M, Gatley S, Miller E, Hitzemann R, Ding Y-S, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001b;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Ding Y, Fowler J, Wang G. Cocaine addiction: hypothesis derived from imaging studies with PET. J Addict Dis. 1996a;15:55–71. doi: 10.1300/J069v15n04_04. [DOI] [PubMed] [Google Scholar]
- Volkow N, Fowler J. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow N, Fowler J, Wang G. The addicted human brain: insights from imaging studies. J Clin Invest. 2003a;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Fowler J, Wang G, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson J. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010a;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res. 1996b;67:29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- Volkow N, Li T. The neuroscience of addiction. Nat Neurosci. 2005;8:1429–1430. doi: 10.1038/nn1105-1429. [DOI] [PubMed] [Google Scholar]
- Volkow N, Tomasi D, Wang G, Fowler J, Telang F, Goldstein R, Alia-Klein N, Wong C. Reduced metabolism in brain “control networks” following cocaine-cues exposure in female cocaine abusers. PLoS One. 2011a;6:e16573. doi: 10.1371/journal.pone.0016573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Baler R. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011b;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Begleiter H, Porjesz B, Fowler J, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos P. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Gatley S, Hitzemann R, Chen A, Dewey S, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997a;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Gatley S, MacGregor R, Schlyer D, Hitzemann R, Wolf A. Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18F-N-methylspiroperidol. Psychiatry Res. 1996c;67:11–16. doi: 10.1016/0925-4927(96)02809-0. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Gatley S, Wong C, Hitzemann R, Pappas N. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther. 1999;291:409–415. [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Jayne M, Franceschi D, Wong C, Gatley S, Gifford A, Ding Y, Pappas N. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008a;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012a;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Tomasi D, Baler R. Food and Drug Reward: Overlapping Circuits in Human Obesity and Addiction. Curr Top Behav Neurosci. 2012b doi: 10.1007/7854_2011_169. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011c;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Ma Y, Fowler J, Wong C, Ding Y, Hitzemann R, Swanson J, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 1995;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Maynard L, Jayne M, Fowler J, Zhu W, Logan J, Gatley S, Ding Y, Wong C, Pappas N. Brain dopamine is associated with eating behaviors in humans. Int J Eat Disord. 2003b;33:136–142. doi: 10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Telang F, Fowler J, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Telang F, Fowler J, Thanos P, Logan J, Alexoff D, Ding Y, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008b;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Tomasi D, Telang F, Fowler J, Pradhan K, Jayne M, Logan J, Goldstein R, Alia-Klein N, Wong C. Methylphenidate attenuates limbic brain inhibition after cocaine-cues exposure in cocaine abusers. PLoS ONE. 2010b;5:e11509. doi: 10.1371/journal.pone.0011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997b;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Hermann D, Rabinstein J, Wichert S, Klein O, Ende G, Mann K. Increased activation of the ACC during a spatial working memory task in alcohol-dependence versus heavy social drinking. Alcohol Clin Exp Res. 2010a;34:771–776. doi: 10.1111/j.1530-0277.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010b;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Wager T, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wallner-Liebmann S, Koschutnig K, Reishofer G, Sorantin E, Blaschitz B, Kruschitz R, Unterrainer H, Gasser R, Freytag F, Bauer-Denk C, Schienle A, Schäfer A, Mangge H. Insulin and hippocampus activation in response to images of high-calorie food in normal weight and obese adolescents. Obesity. 2010;18:1552–1557. doi: 10.1038/oby.2010.26. [DOI] [PubMed] [Google Scholar]
- Wanat M, Willuhn I, Clark J, Phillips P. Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev. 2009;2:195–213. doi: 10.2174/1874473710902020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Geliebter A, Volkow N, Telang F, Logan J, Jayne M, Galanti K, Selig P, Han H, Zhu W, Wong C, Fowler J. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity. 2011a;19:1601–1608. doi: 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Smith L, Volkow N, Telang F, Logan J, Tomasi D, Wong C, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler J. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2011b doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Volkow N, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler J. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Wang G, Volkow N, Logan J, Pappas N, Wong C, Zhu W, Netusil N, Fowler J. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang G, Volkow N, Telang F, Jayne M, Ma Y, Pradhan K, Zhu W, Wong C, Thanos P, Geliebter A, Biegon A, Fowler J. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci U S A. 2009;106:1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C, Teshiba T, Merideth F, Ling J, Mayer A. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczak N, De Bleser P, Luiten P, Geerts A, Teelken A, De Keyser J. Insulin-like growth factor II receptors in human brain and their absence in astrogliotic plaques in multiple sclerosis. Brain Res. 2000;863:282–288. doi: 10.1016/s0006-8993(00)02153-3. [DOI] [PubMed] [Google Scholar]
- Williams L, Adam C, Mercer J, Moar K, Slater D, Hunter L, Findlay P, Hoggard N. Leptin receptor and neuropeptide Y gene expression in the sheep brain. J Neuroendocrinol. 1999;11:165–169. doi: 10.1046/j.1365-2826.1999.00293.x. [DOI] [PubMed] [Google Scholar]
- Wise R. Roles for nigrostriatal–not just mesocorticolimbic–dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann B, Schott B, Guderian S, Frey J, Heinze H, Düzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wüstenberg T, Bermpohl F, Kahnt T, Beck A, Ströhle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yeterian E, Van Hoesen G. Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res. 1978;139:43–63. doi: 10.1016/0006-8993(78)90059-8. [DOI] [PubMed] [Google Scholar]
- Yoon H, Chung J, Oh J, Min H, Kim D, Cheon Y, Joe K, Kim Y, Cho Z. Differential activation of face memory encoding tasks in alcohol-dependent patients compared to healthy subjects: an fMRI study. Neurosci Lett. 2009;450:311–316. doi: 10.1016/j.neulet.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Zweifel L, Parker J, Lobb C, Rainwater A, Wall V, Fadok J, Darvas M, Kim M, Mizumori S, Paladini C, Phillips P, Palmiter R. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]


