Abstract
Objective
Generalized social phobia (gSP), also known as generalized social anxiety disorder, is characterized by excessive fear of scrutiny by others and pervasive avoidance of social interactions. Pathophysiological models of gSP implicate exaggerated reactivity of the amygdala and insula in response to social evaluative threat, making them plausible targets for treatment. Although selective serotonin reuptake inhibitor (SSRI) treatment is known to be an effective treatment, little is known about the mechanism by which these agents exert their anxiolytic effects at a brain level in gSP.
Method
We acquired functional magnetic resonance imaging (fMRI) data of brain response to social signals of threat (fearful/angry faces) in twenty-one GSAD patients before and after they completed 12 weeks of open label treatment with the SSRI sertraline. For comparison, nineteen healthy control (HC) subjects also underwent two fMRI scans, 12 weeks apart.
Results
Whole-brain voxel-wise analysis of variance revealed significant Group×Time interactions in the amygdala and the ventral medial prefrontal cortex (vmPFC). Follow up analyses showed that treatment in gSP subjects: 1) reduced amygdala reactivity to fearful faces (which was exaggerated relative to HCs prior to treatment); and 2) enhanced vmPFC activation to angry faces (which was attenuated relative to HCs prior to treatment). However, these brain changes were not significantly related to social anxiety symptom improvement.
Conclusions
SSRI treatment response in gSP is associated with changes in a discrete limbic-paralimbic brain network, representing a neural mechanism by which SSRIs may exert their actions.
Keywords: treatment, fMRI, amygdala, ventromedial prefrontal cortex, SSRI, anxiety
Introduction
Generalized social phobia (herein ‘gSP’), also known as generalized social anxiety disorder, is characterized by an exaggerated and pervasive fear and avoidance of scrutiny by others. Social phobia is very common (1), typically originates prior to adolescence, foretells significant functional impairment and psychiatric comorbidity including anxiety, mood and substance use disorders, and does not remit unless adequately treated (2–4). Patients with gSP exhibit an enhanced bias for social signals that convey threat such as faces of anger, contempt, and/or fear (5), which may arise from dysfunction of discrete brain regions that appraise these signals.
A recent meta-analysis revealed that in most studies, gSP patients exhibit exaggerated reactivity particularly in amygdala and insula to social cues that signal threat or situations that evoke anxiety (6), regions involved in processing danger signals and generating negative affective experiences, including fear (7, 8). Given its central role in the pathophysiology of anxiety disorders (9), the amygdala in particular is thought to be a key target of anxiolytic interventions (10). Abnormalities in prefrontal areas functionally and anatomically connected to the amygdala/insula such as the anterior cingulate (ACC) and medial prefrontal (mPFC) cortices have also been implicated in gSP, albeit less consistently (11–18). As such, dysfunction of these regions may also serve as plausible targets for therapeutic intervention in gSP.
Selective-serotonin reuptake-inhibitors (SSRIs) are an evidence-based treatment for gSP (2). Consistent with other ‘activation’ neuroimaging studies in clinically depressed subjects (19, 20), there is some evidence that effective treatment with anti-anxiety pharmacotherapy (SSRIs, nefazodone, tiagabine) reduces the heightened amygdala, insula and ACC responses to social evaluative threat in gSP patients (16, 21–23), while increasing activity in the ventral mPFC (vmPFC) (14, 16). Interestingly, some evidence suggests that acute administration of SSRIs appears to down-regulate amygdala hyper-responsivity to threat-relevant stimuli in healthy participants (24, 25), supporting the effects observed in animal models (26); however, acute SSRIs have also been shown to up-regulate amygdala reactivity to emotional faces (27) so the evidence is mixed and could be attributed a number of factors (e.g., healthy vs. ill, acute vs. subacute vs. chronic; anxiety vs. depression). Findings of treatment enhancing vmPFC function are of particular interest and relevance as this region has been implicated in implicit and explicit emotion regulation of anxious states (28, 29). Besides the studies measuring regional cerebral blood flow using positron emission tomography (PET) that have highlighted pharmacotherapy effects on brain activity in gSP (14, 16, 21–23), there is surprisingly little corroborating empirical evidence from studies that use fMRI to examine if SSRI treatment similarly resolves the amygdala, insula, and/or medial frontal dysfunction in relation to processing social signals of threat commonly observed in gSP.
The goal of the present study was to address this gap in evidence. In the context of an open-label 12 week clinical trial of the SSRI sertraline in gSP patients, we used fMRI coupled with a validated facial expression (fearful, angry, happy) processing task to examine the change in amygdala-insula-medial frontal (e.g., ACC, vmPFC) function during perception of social threat cues before and after SSRI treatment (pre-treatment [PreTx] scan and post-treatment [PostTx] scan, respectively). For comparison and to control for effects of re-exposure to threat stimuli with repeated scanning, we enrolled a group on healthy control (HC) volunteers who were also scanned twice, 12 weeks apart. We predicted that SSRI pharmacotherapy (PostTx versus PreTx) would ‘normalize’ aberrant brain responses in gSP subjects observed at baseline – specifically, by attenuating amygdala, insula and ACC and enhancing vmPFC responses to social signals of threat. Moreover, we hypothesized that these changes would parallel clinical response to treatment such that PostTx versus PreTx change in brain activation would correlate with PostTx versus PreTx change in generalized social anxiety symptom severity. Although this hypothesis was confined to a priori areas of interest (amygdala, insula, ACC, mPFC), we also conducted an exploratory hypothesis to examine the neural correlates of treatment response (i.e., improvement in social anxiety symptoms from pre- to post-treatment) across the entire brain.
METHOD
Subjects
Twenty-one untreated (e.g., unmedicated and not in psychotherapy) gSP and nineteen healthy control (HC) volunteers participated in this study. This study was conducted at the University of Chicago (gSP n=12; HC n=14) and at the University of Michigan (gSP n=9; HC n=5). Each subject underwent a screening evaluation involving structured clinical interviews and assessments by trained clinicians and semi-structured medical and psychiatric interviews with the study psychiatrist (KLP). All subjects were characterized with the: 1) Structured Clinical Interview for DSM-IV (SCID); 2) Liebowitz Social Anxiety Scale (LSAS); 3) Hamilton Depression Rating Scale (HAM-D); 4) Hamilton Anxiety Rating Scale (HAM-A); 5) Beck Depression Inventory (BDI); and 6) Spielberger Trait-State Anxiety Inventory (STAI). Table 1 details the demographic and clinical characteristics of the subjects. Additional inclusion/exclusion criteria and subject characteristics can be found Supplemental Methods. All subjects provided written informed consent, and the study was approved by both local university hospital institutional review boards.
Table 1.
Demographic and Clinical Characteristics of Patients and Control Subjects
| gSP (N=21) | HC (N=19) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | |
| Age (years) | 25.91 | 5.50 | 26.95 | 8.11 | −0.48 | 0.634 |
| Education (years) | 15.14 | 1.62 | 15.42 | 1.43 | −0.57 | 0.570 |
| Liebowitz Social Anxiety Scale | 82.29 | 13.02 | 9.17a | 7.40 | 17.80 | <0.0001 |
| Spielberger State Anxiety | 41.33 | 8.65 | 24.46b | 4.91 | 5.96 | <0.0001 |
| Spielberger Trait Anxiety | 49.10 | 9.00 | 26.55b | 5.50 | 7.23 | <0.0001 |
| Beck Depression Inventory | 10.57 | 7.26 | 1.33 | 1.97 | 5.23 | <0.0001 |
| Hamilton Depression | 4.52 | 3.59 | 0.63 | 1.09 | 4.19 | <0.001 |
| Hamilton Anxiety | 6.81 | 6.00 | 1.12 | 1.80 | 3.77 | <0.001 |
| N | % | N | % | χ2 | p | |
| Gender | 0.37 | 0.545 | ||||
| Male | 8 | 38.10 | 10 | 52.63 | ||
| Female | 13 | 61.90 | 9 | 47.37 | ||
| Race | 2.70 | 0.259 | ||||
| Caucasian | 15 | 71.43 | 17 | 89.47 | ||
| Asian | 4 | 19.05 | 2 | 10.53 | ||
| African American | 2 | 9.52 | ||||
gSP=generalized social phobia; HC=healthy control. Liebowitz Social Anxiety Scale and Spielberger Anxiety Inventory measures were not obtained in some HC subjects
(HC n = 12;
HC n = 11).
SSRI Sertraline Treatment
Treatment consisted of the SSRI sertraline hydrochloride in an open-label, fixed-dosing design over 12 weeks. Patients were evaluated at weeks 1, 2, 4, 8, and 12 by the study psychiatrist (KLP) in medication management sessions to assess symptom change and adverse events, with the target dose of 100mg/day reached after 2 weeks; clinical response was measured with the Clinical Global Impression-Improvement (CGI-I) scale. Although we did not measure sertraline blood levels, the study psychiatrist and staff inquired about missed doses and conducted a pill count to confirm the subject report. No subject in the study ever missed more than 2 consecutive daily doses over the course of the 12 week study, and no subject regularly (>3 times) missed the dose. After eight weeks, the dose of sertraline was increased from 100 to 150mg/day (maximum dose) based on clinical response according to CGI-I scores (if there is no or minimal improvement [CGI-I score>2]). At study completion, all participants were on stable doses of sertraline for at least 4 weeks before the post-treatment (PostTx) fMRI scan, which occurred 12 weeks after starting and while on medication.
fMRI Task
Brain activation was assessed using a modified version of the Emotional Face Matching Task [EFMT] (30), which has been previously validated and described in our pharmacological fMRI studies in healthy (31) and gSP (32) subjects. This task was designed to isolate brain (e.g., amygdala, insula) response to signals of threat (angry and fearful faces) against those that do not convey any perceived threat (happy faces); the contrast of angry/fearful expression against happy expressions (herein referred to as ‘AvH’ and ‘FvH’) allows specificity for the threat signal while matching the non-emotional face element. Also, prior evidence in our laboratory (12, 13) has specifically shown that gSP subjects would differ in their limbic-frontal reactivity to threat (angry/fearful) but not non-threat (happy) signals, and that this ‘activation’ difference is less evident in healthy controls (33); in other words, in order to maximize the activation ‘signal’ for the SSRI treatment to target, we chose to contrast threatening against happy faces which yields the most robust and consistent finding of exaggerated amygdala reactivity in gSP based on our (12, 13) and others’ previous work (6) and given that contrasts between threat against neutral faces or against fixation/shapes were less powered to detect gSP versus HC differences (12, 13). Moreover, prior work had also suggested that different face expressions may convey different messages about the ‘source’ of threat (e.g., direct threat from angry faces, indirect threat from fearful faces) and may differentially engage amygdala, insula, ACC and mPFC (34). Moreover, findings from prior studies have suggested that patients with gSP exhibit different brain patterns of response relative to controls depending on processing fearful versus angry faces (35), including evidence previously reported on this cohort showing the EFMT task effects (18). Collectively, this evidence prompted us to examine effects of SSRI treatment of brain responses to angry and fearful faces separately.
In brief, this task involved photographs from a validated set of face stimuli (36) presented in a block-design during which participants view a trio of faces and select one of two faces (bottom) that expressed the same emotion as the target face (top). The target and congruent probe faces displayed one of three expressions (fearful, angry or happy), and the other (incongruent) probe face always displayed a neutral/non-emotional expression. The paradigm consisted of 18 blocks total (9 blocks of matching emotional faces with each target expression of fearful, angry or happy interleaved with 9 blocks of matching shapes [a non-specific/"baseline" condition]). Participants used right handed button press to record response.
fMRI Data Acquisition
This study was conducted on two separate 3 Tesla GE Signa System (General Electric; Milwaukee, WI) scanners using the same standard radiofrequency coil – one at the University of Chicago (gSP n=12; HC n=14) and another the University of Michigan (gSP n=9; HC n=5). However, all scanning was performed with blood oxygen-level dependent (BOLD)-sensitive whole-brain fMRI using the same GE software (LX 8.3, Neuro-optimized gradients) and acquired using the exact same T2*-weighted reverse spiral gradient-recall echo sequence (echo time=25ms, repetition time=2000ms, 64x64 matrix, flip angle=77°, field of view=24cm, 3.75mm2 inplane voxels, 30 contiguous 5mm axial slices/volume) optimized to minimize susceptibility artifacts in the regions of interest such as the amygdala. A high-resolution T1 scan was also acquired for anatomical localization.
fMRI Data Preprocessing
All the participants included in this analysis met inclusion criteria for minimal head movement (>2mm or >2degrees of displacement) during both scans. The first four volumes from each run were discarded to allow for T1 equilibration effects. Data were preprocessed and analyzed using statistical parametric mapping (SPM5; Wellcome Department of Cognitive Neurology, London; www.fil.ion.ucl.ac.uk/spm). The scans were analyzed using conventional steps: 1) temporally/slice-time, motion corrected; 2) warped (non-linear) to a canonical brain in Montreal Neurologic Institute (MNI) space; 3) resampled to 2 mm3 voxels; 4) smoothed with an 8 mm3 kernel. The time series was processed with: 1) canonical hemodynamic response function; 2) a 128-second high-pass filter; 3) corrections for serial correlations (e.g., autoregressive model of the order 1); and 4) global normalization. Realignment (i.e., movement) parameters were included in the model to correct for motion artifacts.
Using a box-car model, a priori defined linear contrasts of interest (AvH and FvH) were generated for each subject, and then entered into a second-level general linear model treating subject as a random effect (i.e., a random effects analysis). An analysis of variance (ANOVA) using Group (gSP, HC) and Time (PreTx/scan 1, PostTx/scan 2) as between- and within-subjects factors, respectively, was conducted to test for main effect of Group, main effect of Time, and Group × Time interactions. Significance testing for the a priori hypothesis that a Group × Time interaction would emerge in the amygdala, insula, ACC and/or mPFC was set at p<0.05, family-wise-error corrected for multiple comparisons within these predetermined anatomical regions of interest (ROIs) (37). To clarify significant Group × Time interactions in these areas, parameter estimates (β weights, arbitrary units [a.u.]) of brain activation, an index of BOLD signal change from these ROIs was extracted from each subject and plotted for each group at each scan/time point, followed by independent and paired t-tests. For a whole-brain exploratory analysis to examine effects outside of ROIs, we set the significance at p<0 .005 (uncorrected) with a cluster extent threshold of greater than 20 contiguous voxels (volume>160mm3) to balance between type I and type II errors (38), consistent with prior fMRI studies of gSP (39, 40). Anatomical localization was determined using stereotaxic atlases using the MNI coordinate system (37). In order to test the secondary hypothesis that brain activation changes would parallel clinical response to treatment, a Pearson correlation coefficient analysis was conducted between the extracted BOLD signal treatment change (ΔPosTx-PreTx) from significant ROIs and the treatment change (ΔPosTx-PreTx) in LSAS social anxiety severity scores; significance set at p<0.05, with a Bonferroni correction for the number of correlations performed.
To obviate bias towards a limited set of a priori brain regions, an exploratory whole-brain voxel-wise analysis was conducted to examine the relationship between changes in social anxiety symptom severity (LSASΔPosTx-PreTx) and changes in brain response (FvHΔPosTx-PreTx; AvHΔPosTx-PreTx) using the user-specific regression analysis within SPM. For this exploratory analysis, we set the significance at p < 0 .005 (uncorrected) with a cluster extent threshold of greater than 20 contiguous voxels (volume > 160mm3).
RESULTS
Treatment Effects on Social Anxiety Severity
After 12 weeks of sertraline treatment, social anxiety severity, as indexed by the LSAS score, dropped significantly from a Mean (SD) of 82.29 (13.02) to 44.71 (25.44) (t=7.24, p < 0.001), nearly a 50% reduction and similar to prior SSRI trials in gSP (2). The large effect size observed here may in part be due to the entry criteria which excluded prior failure of response to sertraline or another SSRI. At PostTx, two-thirds of the gSP group (14 of 21) were considered to be ‘Responders’ as rated to be ‘very much improved’ or ‘much improved’ (CGI-I score of 1 or 2), and 7 of 21 patients had a CGI-I score of >2 treatment and considered ‘Non-Responders’.
Behavioral Performance and Treatment Effects
Participants performed the on-line EFMT very well, averaging > 90% correct responses within two seconds of the trial duration. Repeated-measures analysis of variance for measures of accuracy and response times showed no significant main effect of Group, main effect of Time, or Group × Time interactions to any emotion (fearful, angry, happy) (all ps > 0.05).
fMRI Activation Results
Whole-brain voxel-wise ANOVA revealed a significant Group × Time interaction for left amygdala reactivity to fearful (vs. happy) faces and for left orbital frontal gyrus/vmPFC reactivity to angry (vs. happy) faces (Table 2, Figure 1).
Table 2.
Brain Activation to Social Signals of Threat: Whole-brain Voxel-wise ANOVAa
| MNI Coordinates | Volume | |||||
|---|---|---|---|---|---|---|
| Region | x | y | z | (mm3) | F | |
|
Fearful > Happy |
||||||
| Main Effect of Group | Cuneus | 6 | −80 | 24 | 328 | 14.01 |
| Middle Frontal Gyrus | −44 | 28 | 52 | 328 | 10.94 | |
| Main Effect of Time | Mid Cingulate | 12 | 36 | 32 | 3720 | 17.65 |
| Inferior Frontal Gyrus | −60 | 18 | 22 | 856 | 15.22 | |
| 58 | 42 | 6 | 592 | 15.20 | ||
| 36 | 6 | 26 | 360 | 14.06 | ||
| 42 | 28 | 18 | 1280 | 13.42 | ||
| Middle Frontal Gyrus | 28 | 18 | 42 | 496 | 14.84 | |
| Caudate | −4 | 20 | −6 | 416 | 12.63 | |
| Paracentral Lobule | −10 | −24 | 66 | 168 | 12.22 | |
| Superior Parietal Gyrus | 34 | −72 | 62 | 168 | 11.92 | |
| Group × Time Interaction | Superior Parietal Gyrus | −18 | −60 | 58 | 2536 | 21.66 |
| 22 | −62 | 54 | 1296 | 14.62 | ||
| Inferior Parietal Gyrus | −54 | −26 | 36 | 3888 | 18.02 | |
| Middle Frontal Gyrus | 32 | 32 | 58 | 1856 | 15.71 | |
| −30 | 46 | 36 | 560 | 14.32 | ||
| 50 | 0 | 54 | 520 | 13.64 | ||
| −34 | 50 | 20 | 488 | 13.24 | ||
| Precentral Gyrus | 60 | 8 | 30 | 976 | 14.06 | |
| −34 | 0 | 64 | 416 | 11.29 | ||
| Supramarginal Gyrus | 60 | −24 | 42 | 1904 | 13.70 | |
| 68 | −20 | 26 | 1040 | 12.63 | ||
| Parahippocampal Gyrus | 22 | −26 | −22 | 256 | 13.47 | |
| Inferior Frontal Gyrus | 58 | 42 | 12 | 376 | 13.14 | |
| −20 | 24 | −24 | 160 | 11.60 | ||
| 60 | 24 | 20 | 216 | 9.91 | ||
| Supplementary Motor Area | 4 | 4 | 58 | 496 | 12.67 | |
| Postcentral Gyrus | −34 | −38 | 72 | 912 | 11.94 | |
| Amygdala | −18 | 2 | −22 | 216 | 11.83 | |
| Putamen | −24 | −8 | 8 | 176 | 11.12 | |
|
Angry > Happy |
||||||
| Main Effect of Group | Insula | −32 | −24 | 22 | 9600 | 32.34 |
| Inferior Frontal Gyrus | −24 | 36 | −12 | 2048 | 25.11 | |
| −22 | 18 | −14 | 384 | 11.61 | ||
| −54 | 14 | 14 | 240 | 11.50 | ||
| Rolandic Operculum | 34 | −16 | 16 | 11128 | 18.97 | |
| Middle Temporal Gyrus | −52 | −10 | −12 | 2432 | 18.90 | |
| 62 | −40 | 6 | 1768 | 18.15 | ||
| −38 | 24 | −36 | 280 | 12.31 | ||
| −66 | −32 | −4 | 2672 | 11.74 | ||
| Angular Gyrus | 46 | −66 | 24 | 1368 | 15.65 | |
| Inferior Temporal Gyrus | 50 | −16 | −24 | 1968 | 14.84 | |
| Amygdala | −22 | −4 | −24 | 800 | 14.52 | |
| Lingual Gyrus | 18 | −48 | −4 | 456 | 13.27 | |
| Middle Frontal Gyrus | −46 | 56 | 18 | 504 | 13.14 | |
| −32 | 26 | 52 | 216 | 11.43 | ||
| Cerebellum | −14 | −50 | −38 | 440 | 13.06 | |
| Cuneus | 6 | −78 | 24 | 624 | 12.78 | |
| Superior Parietal Gyrus | −22 | −48 | 66 | 408 | 12.76 | |
| Superior Temporal Gyrus | 66 | −24 | 10 | 320 | 11.81 | |
| Anterior Cingulate | −8 | 30 | −6 | 272 | 11.62 | |
| Caudate | 4 | 10 | −6 | 840 | 11.54 | |
| Superior Occipital Gyrus | 20 | −88 | 22 | 176 | 11.28 | |
| Main Effect of Time | No significant clusters | |||||
| Group × Time Interaction | Inferior Parietal Gyrus | −40 | −30 | 32 | 2296 | 20.59 |
| Supramarginal Gyrus | 56 | −28 | 36 | 1720 | 15.15 | |
| Superior Parietal Gyrus | −16 | −56 | 56 | 376 | 13.74 | |
| Middle Temporal Gyrus | −66 | −8 | −26 | 232 | 12.91 | |
| Orbital Frontal Gyrusb | 4 | 50 | −14 | 240 | 11.17 | |
All listed clusters significant at p<0 .005 (uncorrected) with a cluster extent threshold of greater than 20 contiguous voxels. Areas showing a priori hypothesized treatment-related changes are bolded and italicized.
Medial Orbital Gyrus is referred to as ventral medial prefrontal cortex in the text. MNI, Montreal Neurological Institute; F, F-score.
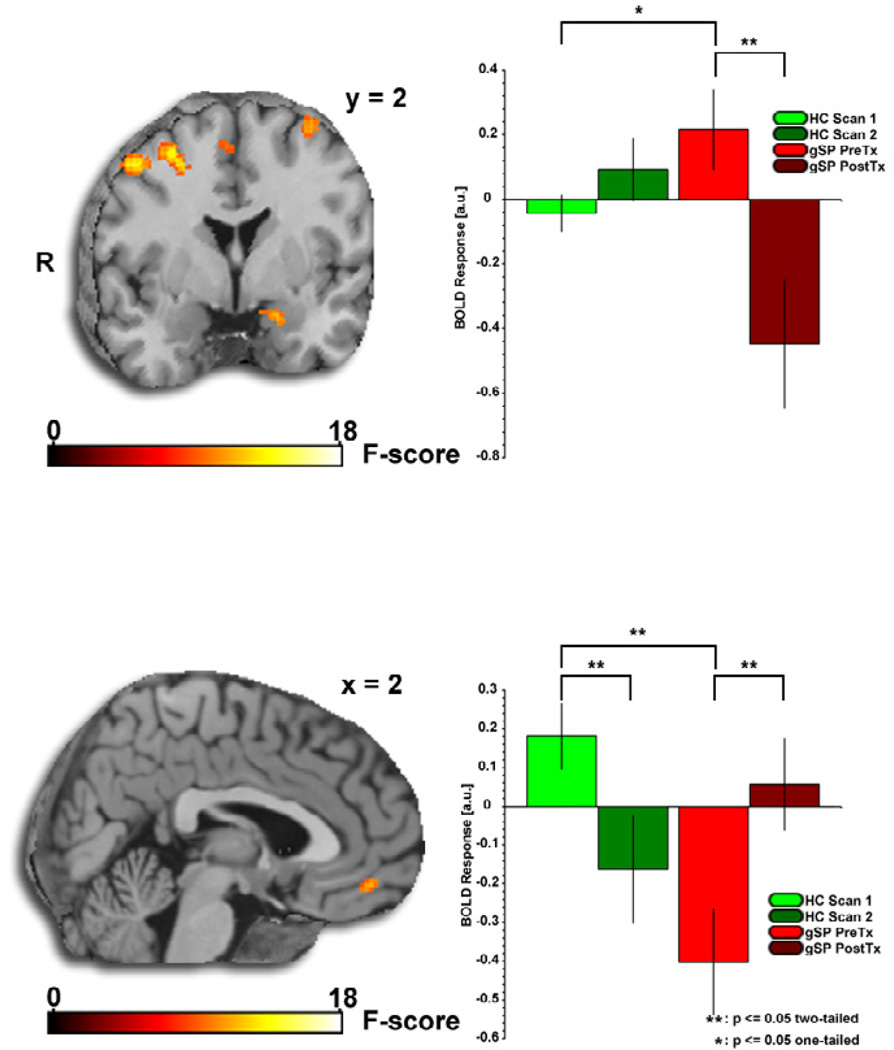
Figure 1.
Brain changes after 12 weeks of sertraline treatment in patients with generalized social phobia. Brain maps depict whole-brain voxel-wise ANOVA F-map showing significant Group×Time interactions in the amygdala and ventral medial prefrontal cortex (vmPFC) in response to fearful and angry faces, respectively. Bar graphs depict extracted BOLD signal change from amygdala and vmPFC clusters showing: 1) Amygdala reactivity to fearful faces is greater in the generalized social phobia (gSP) group than the healthy control (HC) group at pre-treatment (gSPPre-Tx > HCScan 1, p < 0.05) and is attenuated by treatment (gSPPre-Tx > gSPPost- Tx, p < 0.05); and 2) vmPFC reactivity to angry faces is less in the gSP group than the HC group at pre-treatment (gSPPre-Tx < HCScan 1, p < 0.05) and is enhanced by treatment (gSPPre-Tx < gSPPost- Tx, p < 0.05).
Follow-up analysis within the amygdala ROI revealed that its reactivity to fearful faces is greater in the gSP group than the healthy control (HC) group at pretreatment (gSPPreTx > HCScan1, p < 0.05) and is attenuated by treatment (gSPPreTx > gSPPostTx, p < 0.05). At PostTx/Scan 2, the amygdala response to fearful (vs. happy) faces in gSP was no longer greater than that in HCs. Of note (as shown in Figure 1), within the gSP group, the left amygdala exhibited a robust activation to fearful faces at the PreTx scan (MNI coordinates, [−16, −2, −14], Z-score = 3.28, volume = 712mm3, pcorrected < 0.05), which was no longer evident at PostTx. Also we confirmed that the SSRI effect observed from pre- to post-treatment was driven by a significant attenuation of amygdala reactivity to fearful faces and not simply by an enhancement of amygdala reactivity to happy faces (see Supplementary Figure S1).
Follow-up analysis within the vmPFC ROI revealed that its reactivity to angry faces is attenuated in the gSP group compared to the HC group at pre-treatment (gSPPre-Tx < HCScan 1, p < 0.05) and is enhance by treatment (gSPPre-Tx < gSPPost-Tx, p < 0.05). At PostTx/Scan 2, no group differences were observed in vmPFC response (gSPPostTx < HCScan2, p > 0.1). Of note (as shown in Figure 1), within the gSP group, the vmPFC exhibited a robust deactivation to angry faces at the PreTx scan ([0, 44, −8], Z-score = 3.22, volume = 712mm3, pcorrected < 0.05), which was no longer evident at PostTx.
In addition, we conducted post hoc analyses to confirm that the observed effects in the amygdala and vmPFC were not driven by differences between the two sites of data collection. First, whole-brain voxel-wise comparison between the two scanners did not yield a significant difference in activation in the amygdala reactivity to fearful faces or vmPFC response to angry faces. Second, using extracted parameter estimates of activation from the amygdala and vmPFC ROIs, we conducted a repeated measures ANOVA on Group, Time, and Site as factors and did not observe significant main effect of Site or interactions with Site (all ps > 0.1).
Pearson correlational analyses of treatment change (ΔPosTx - PreTx) in LSAS social anxiety severity scores and BOLD signal treatment change (ΔPosTx-PreTx) in amygdala and in vmPFC did not yield any significant relationships (ps>0.005, uncorrected). The exploratory whole-brain voxel-wise regression analysis between social anxiety symptom severity (LSASΔPosTx-PreTx) and brain activation change (FvHΔPosTx-PreTx; AvHΔPosTx-PreTx) showed that decreasing social anxiety was primarily associated with decreases in visual and parietal cortical areas to angry and fearful faces, and with increases in superior temporal gyrus response to angry faces and increases in postcentral and mid cingulate gyrus response to fearful faces (Table 3). This analysis did not reveal that Pre-Tx to Post-Tx change in LSAS was related to change in amygdala, insula, ACC or mPFC.
Table 3.
Pre-Treament to Post-Treatment Decrease Social Anxiety Severity and Change in Brain Activation: Whole-brain Voxel-wise Regressiona
| MINI Coordinates | Volume | |||||
|---|---|---|---|---|---|---|
| Region | x | y | z | (mm3) | Z | |
|
Fearful > Happy |
||||||
| Positive Correlation | Middle Occipital Gyrus | −34 | −92 | 22 | 1208 | 3.11 |
| (decreasing activation) | ||||||
| Negative Correlation | Postcentral Gyrus | 44 | −30 | 54 | 1224 | 3.28 |
| (increasing activation) | Superior Frontal Gyrus | −14 | 32 | 32 | 200 | 2.92 |
| Mid Cingulate | −4 | −28 | 46 | 216 | 2.67 | |
|
Angry > Happy |
||||||
| Positive Correlation | Precuneus | −10 | −78 | 52 | 7904 | 4.46 |
| (decreasing activation) | Superior Occipital Gyrus | 24 | −80 | 44 | 2512 | 3.80 |
| Middle Occipital Gyrus | 36 | −76 | 12 | 1336 | 3.79 | |
| Fusiform Gyrus | −26 | −2 | −48 | 448 | 3.62 | |
| Superior Frontal Gyrus | −20 | 14 | 64 | 216 | 3.34 | |
| Superior Parietal Gyrus | −22 | −52 | 50 | 672 | 3.28 | |
| Lingual Gyrus | 16 | −76 | −14 | 192 | 2.94 | |
| Negative Correlation | Superior Frontal Gyrus | −10 | 30 | 64 | 272 | 3.02 |
| (increasing activation) | ||||||
All listed clusters significant at p<0 .005 (uncorrected) with a cluster extent threshold of greater than 20 contiguous voxels. Areas showing a priori hypothesized treatment-related changes are bolded and italicized, significant at p<0 .05 (SVC-corrected for multiple comparisons).
Medial Orbital Gyrus is referred to as ventral medial prefrontal cortex in the text. MNI, Montreal Neurological Institute; Z, Z-score.
Additional results from post hoc analysis separating treatment ‘Responders’ and ‘Non-Responders’ in amygdala and vmPFC reactivity, correlations between amygdala and vmPFC reactivity at after treatment, and correlational and regression analysis between change in brain activity with change in depressive symptoms can be found in the Supplemental Results.
DISCUSSION
The goal of the present study was to examine the effect of treatment on brain responses to social signals of threat (angry, fearful faces) in patients with gSP in the context of an open-label 12 week clinical trial of the SSRI sertraline, an FDA-approved, evidence-based treatment for gSP (2). As predicted, we observed that SSRI treatment in gSP subjects reduced left amygdala reactivity to fearful faces, which had been exaggerated relative to HCs prior to treatment. Second, we observed that SSRI treatment in gSP subjects enhanced left vmPFC response to angry faces, which had been attenuated relative to HCs prior to treatment. However, these brain changes were not directly associated with the extent of social anxiety symptom improvement.
Interestingly, exaggerated amygdala reactivity to negative emotion processing (e.g., threat / ‘harsh’ faces, symptom provocation, aversive images) has been frequently observed in a number of prior functional imaging studies in social phobia and other anxiety disorders (6). Moreover, exaggerated reactivity in the amygdala is consistent with its role in fear expression (41) making it a most plausible brain target for SSRI intervention in gSP (2). SSRIs (e.g., citalopram) and other pharmacological interventions (e.g., neurokinin-1 antagonist GR205171) that are effective at reducing social anxiety symptoms have been shown to reduce amygdala reactivity in patients with gSP (21, 22). Together, these results support the hypothesis that SSRI medications exert their effects on the extent to which amygdala responds to threatening stimuli in gSP. However, it should be noted that these effects on brain activity, particularly amygdala reactivity to emotional faces, may not be related to clinical change (e.g., social anxiety symptom improvement) given that prior studies have shown that SSRIs even when administered acutely (one dose) down-regulate amygdala hyper-responsivity to fearful face stimuli even in healthy volunteers, without psychiatric illness (24, 25, 42), and observation supported by animal studies (26). Collectively, these data suggest that SSRIs modify the extent to which the amygdala responds to social cues that signal danger in the environment (43).
Although prior brain based models of gSP have primarily focused on exaggerated reactivity of amygdala, the vmPFC has recently gained increased attention in relation to its role in anxiety psychopathology (28). Amongst prefrontal regions, the vmPFC has been posited to play a unique role in regulation of emotion (44, 45), particularly in the regulation of exaggerated anxiety states (28, 29). Here we observed that SSRI treatment enhanced vmPFC response to angry faces, and that at post-treatment, vmPFC response was negatively correlated with amygdala reactivity to fearful faces. The vmPFC has been previously shown to be hypo-active during symptom provocation in gSP (46, 47). Prior imaging studies of gSP have similarly shown effects on vmPFC following tiagabine and nefazodone treatments (14, 16). Because less is known about vmPFC as a plausible target for treatment, these findings require replication and further dissection in future studies.
The observation that SSRI treatment attenuates amygdala reactivity to social signals of threat in gSP is consistent with prior evidence from studies of patients with major depression, who also show exaggerated amygdala reactivity to negative faces before treatment (19, 20, 48) suggesting the effects may be common across these disorders. In studies of depressed subjects, SSRI effects on prefrontal cortex and ACC have also been reported. For example, SSRI antidepressant treatment has been shown to increase dorsolateral prefrontal cortex (DLPFC) response to fearful faces (49) in depressed adults and decreased orbitofrontal frontal and subgenual ACC response to fearful faces in depressed adolescents (50). Using sad faces as probes, Fu and colleagues showed that symptomatic improvement following SSRI treatment was associated with a reduction in pregenual ACC response (48). Although insufficient evidence exists to differentiate SSRI’s brain mechanism of action in anxiety disorders from that in depression, available data point to a common node of action in the amygdala in terms of attenuation of exaggerated reactivity, whereas its effects on ACC reactivity to socio-emotional information may be more related to depression, consonant with prior evidence of increased resting ACC metabolism at baseline that is reversed by SSRI treatment (51). Future studies are much needed to investigate the commonalities and differences in brain sites of action of SSRI pharmacotherapy in anxiety and depression using the same socio-emotional probes.
These findings should be considered in the context of notable limitations of the study. First, the study design lacked a placebo or wait-list control, and therefore, the neural and clinical findings cannot be causally attributed to SSRI treatment and could be related to a number of plausible factors not related to treatment such as natural course of the illness over the 3 month period, differential regression to the mean in patients and controls and/or to placebo/expectancy effects (23, 52), though clinical effects of this magnitude are highly unlikely due to placebo. Second, although similar in size to recent functional neuroimaging studies on SSRI treatment effects in depression, our small sample size may have increased risk for false negatives, and may have contributed to not finding treatment effects in the insula and ACC. Moreover because the fMRI studies occurred in two different sites, unknown and unestimated variance in imaging data not accounted for from two different scanners may have contributed to an increased risk of false negatives. In addition, because most of the gSP group was considered to have a positive treatment response, the study may not have had sufficient power to detect a significant correlation between changes in brain activation with that in symptom severity or to examine differences in brain changes between treatment responders (n=14) versus non-responders (n=7). The exploratory whole-brain symptom change regression analysis (Table 3) suggests that brain areas correlating with SSRI treatment response may not be localized within the amygdala, insula, ACC or mPFC. Although these observations fit with a broader model incorporating a larger set of brain targets relevant to the pathophysiology of gSP (9, 35), the exploratory nature of these findings warrant replication and further investigation. Because the neural changes amygdala and vmPFC were unrelated to social anxiety symptom improvement, we caution against interpreting those changes as being directly related to treatment response. Third, our findings cannot be generalized to other anxiety disorders or to other pharmacologic treatments or psychosocial interventions such as cognitive behavioral therapy (CBT) (21), also proven to be effective in treating gSP. Future studies are needed to determine if the brain effects observed here are specific to SSRIs as a treatment or shared across any therapeutic modality as long as the treatment is effective.
In conclusion, our findings provide evidence that treatment with the SSRI sertraline attenuates amygdala and enhances vmPFC reactivity to social signals of threat in patients with generalized social anxiety disorder. Future studies with randomized placebo-controlled and/or comparative active treatment designs and larger samples are needed to determine whether SSRI treatment effects are mediated by these specific patterns of brain changes, so that we can better delineate mechanisms of therapeutic actions of SSRIs and other effective treatments and predictors of treatment response.
Supplementary Material
Acknowledgements
Supported by a grant from the National Institutes Health, National Institute of Mental Health Patient-Oriented Career Development Award K23MH076198 (to KLP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr. Phan, Dr. Coccaro, Dr. Liberzon, Dr. Stein, Mr. Angstadt and Ms. Kreger report no biomedical financial interests or potential conflicts of interest. Dr. Mayberg reports that she is Consultant to and licensor of intellectual property to St Jude Medical, Inc., unrelated to the content of this manuscript.
REFERENCES
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Stein MB, Stein DJ. Social anxiety disorder. Lancet. 2008;371:1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 3.Ruscio AM, Brown TA, Chiu WT, Sareen J, Stein MB, Kessler RC. Social fears and social phobia in the USA: results from the National Comorbidity Survey Replication. Psychol Med. 2008;38:15–28. doi: 10.1017/S0033291707001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneier FR. Clinical practice. Social anxiety disorder. N Engl J Med. 2006;355:1029–1036. doi: 10.1056/NEJMcp060145. [DOI] [PubMed] [Google Scholar]
- 5.Clark DM, McManus F. Information processing in social phobia. Biological Psychiatry. 2002;51:92–100. doi: 10.1016/s0006-3223(01)01296-3. [DOI] [PubMed] [Google Scholar]
- 6.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 8.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathew SJ, Price RB, Charney DS. Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genet C Semin Med Genet. 2008;148C:89–98. doi: 10.1002/ajmg.c.30172. [DOI] [PubMed] [Google Scholar]
- 11.Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry. 2005;57:975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 12.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 13.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between Amygdala Hyperactivity to Harsh Faces and Severity of Social Anxiety in Generalized Social Phobia. Biological Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Evans KC, Simon NM, Dougherty DD, Hoge EA, Worthington JJ, Chow C, et al. A PET study of tiagabine treatment implicates ventral medial prefrontal cortex in generalized social anxiety disorder. Neuropsychopharmacology. 2009;34:390–398. doi: 10.1038/npp.2008.69. [DOI] [PubMed] [Google Scholar]
- 15.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, Gross RE, et al. The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006;31:2243–2253. doi: 10.1038/sj.npp.1301053. [DOI] [PubMed] [Google Scholar]
- 17.Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 2008;165:1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol. 2012;89:273–276. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 20.Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- 22.Furmark T, Appel L, Michelgard A, Wahlstedt K, Ahs F, Zancan S, et al. Cerebral blood flow changes after treatment of social phobia with the neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol Psychiatry. 2005;58:132–142. doi: 10.1016/j.biopsych.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Faria V, Appel L, Ahs F, Linnman C, Pissiota A, Frans O, et al. Amygdala Subregions Tied to SSRI and Placebo Response in Patients with Social Anxiety Disorder. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Murphy SE, Norbury R, O'Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry. 2009;194:535–540. doi: 10.1192/bjp.bp.108.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izumi T, Inoue T, Kitaichi Y, Nakagawa S, Koyama T. Target brain sites of the anxiolytic effect of citalopram, a selective serotonin reuptake inhibitor. Eur J Pharmacol. 2006;534:129–132. doi: 10.1016/j.ejphar.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 27.Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33:3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci. 2007;1121:546–561. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- 29.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 31.Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. Journal of Neuroscience. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: Amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 35.Freitas-Ferrari MC, Hallak JE, Trzesniak C, Filho AS, Machado-de-Sousa JP, Chagas MH, et al. Neuroimaging in social anxiety disorder: a systematic review of the literature. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:565–580. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 36.Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. Journal of Neuroscience Methods. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 37.Walter B, Blecker C, Kirsch P, Sammer G, Schienle A, Stark R, et al. MARINA: An easy to use tool for the creation of MAsks for Interest Analyses. Neuroimage. 2003;19:S47. [Google Scholar]
- 38.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phan KL, Klumpp H. Neuroendocrinology and Neuroimaging Studies of Social Anxiety Disorder. In: Hofmann S, DiBartolo PM, editors. Social Anxiety: Clinical, Developmental, and Social Perspectives. Second Edition. London: Elsevier; 2010. pp. 273–312. [Google Scholar]
- 41.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 42.Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berl) 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harmer CJ. Emotional Processing and Antidepressant Action. Curr Top Behav Neurosci. 2012 doi: 10.1007/7854_2012_210. [DOI] [PubMed] [Google Scholar]
- 44.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 47.Van Ameringen M, Mancini C, Szechtman H, Nahmias C, Oakman JM, Hall GB, et al. A PET provocation study of generalized social phobia. Psychiatry Res. 2004;132:13–18. doi: 10.1016/j.pscychresns.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 49.Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112:206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao R, Calley CS, Hart J, Mayes TL, Nakonezny PA, Lu H, et al. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. Am J Psychiatry. 2012;169:381–388. doi: 10.1176/appi.ajp.2011.11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 52.Furmark T, Appel L, Henningsson S, Ahs F, Faria V, Linnman C, et al. A link between serotonin-related gene polymorphisms, amygdala activity, and placeboinduced relief from social anxiety. J Neurosci. 2008;28:13066–13074. doi: 10.1523/JNEUROSCI.2534-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.