Abstract
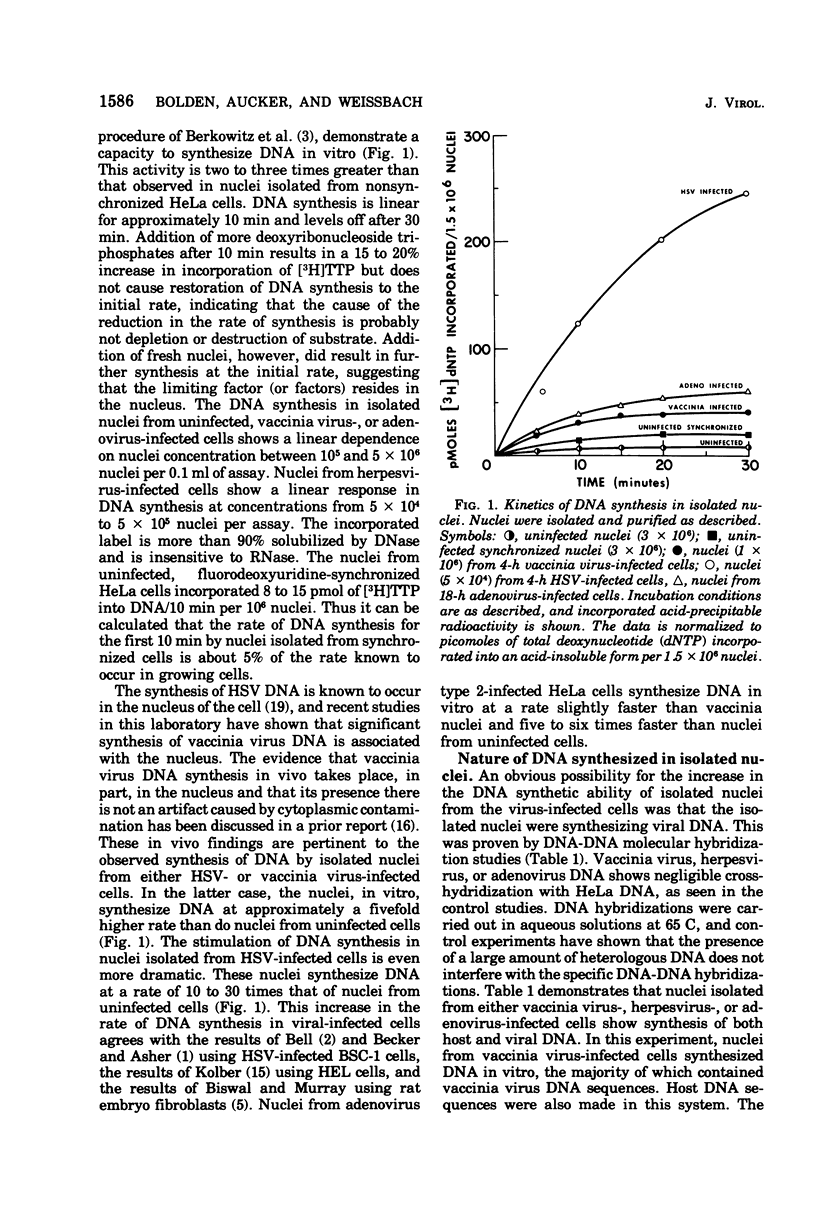
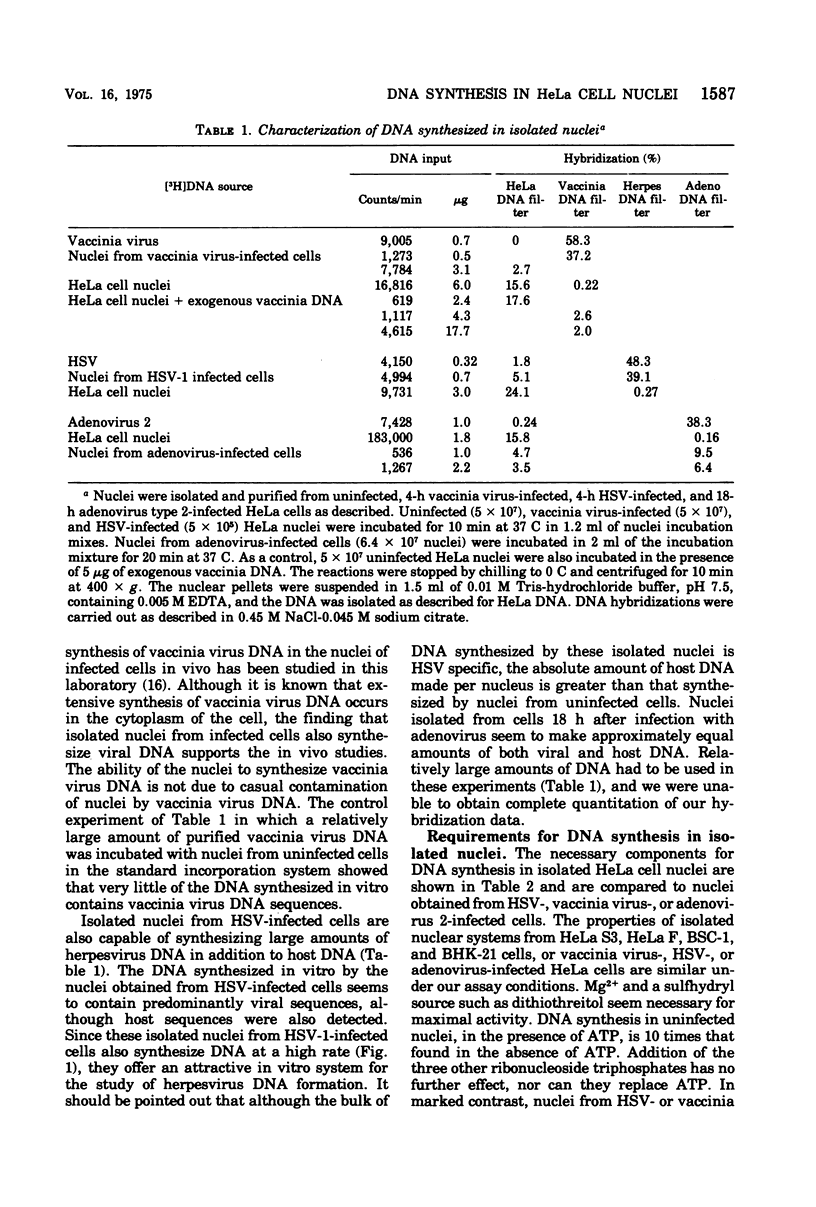
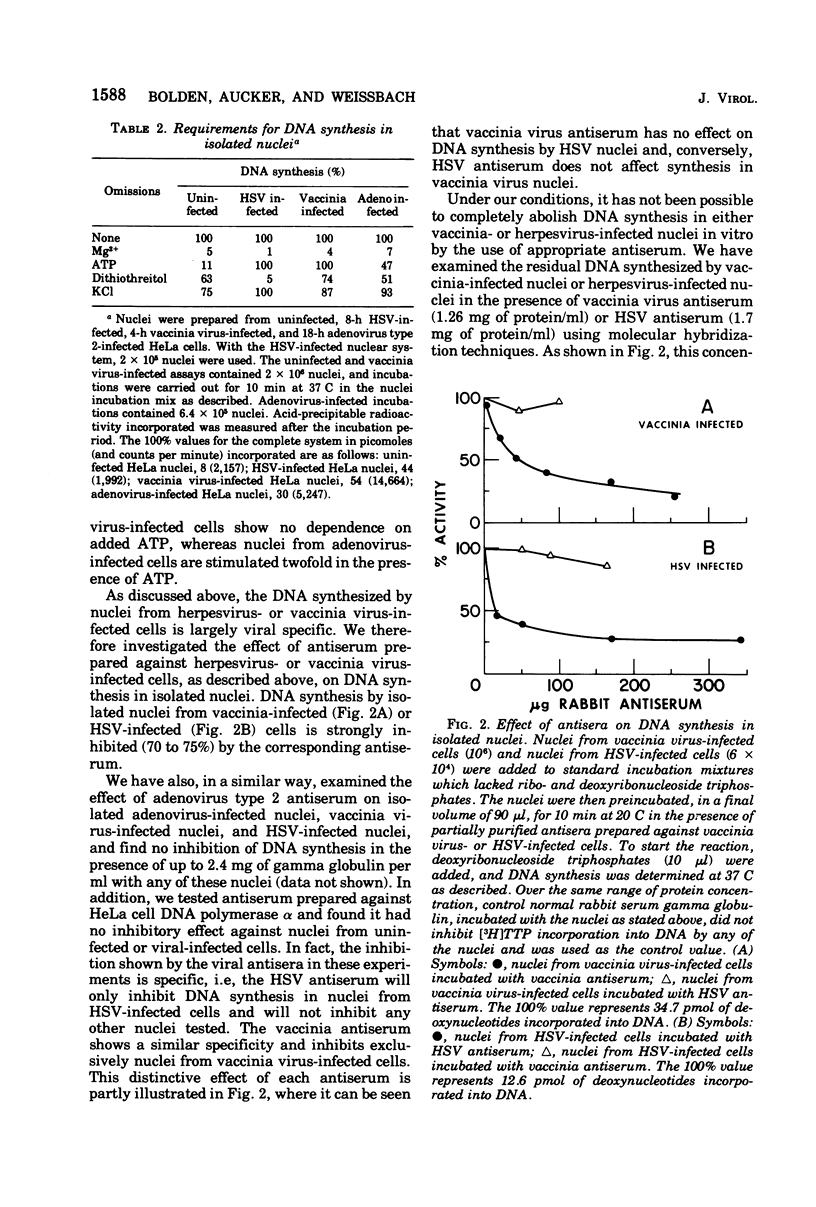
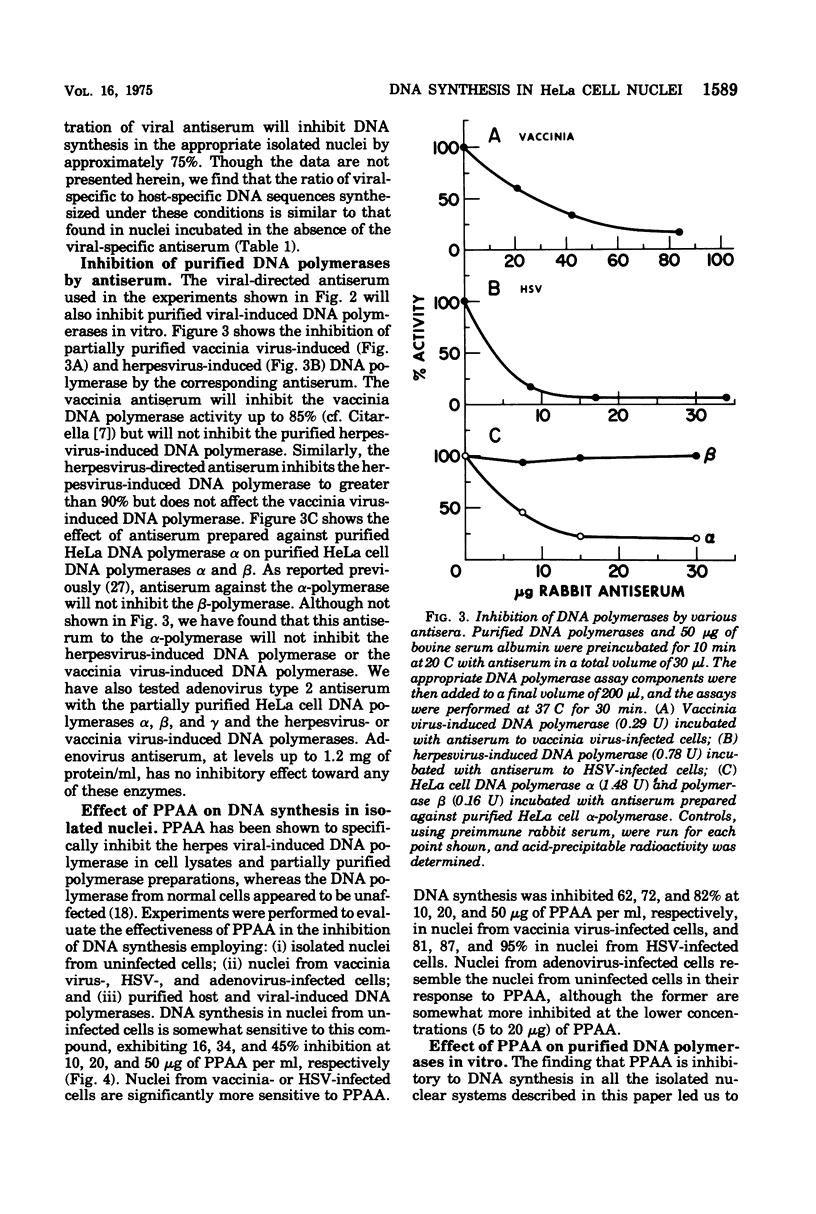
Purified nuclei, isolated from appropriately infected HeLa cells, are shown to synthesize large amounts of either herpes simplex virus (HSV) or vaccinia virus DNA in vitro. The rate of synthesis of DNA by nuclei from infected cells is up to 30 times higher than the synthesis of host DNA in vitro by nuclei isolated from uninfected HeLa cells. Thus HSV nuclei obtained from HSV-infected cells make DNA in vitro at a rate comparable to that seen in the intact, infected cell. Molecular hybridization studies showed that 80% of the DNA sequences synthesized in vitro by nuclei from herpesvirus-infected cells are herpesvirus specific. Vaccinia virus nuclei from vaccinia virus-infected cells, also produce comparable percentages of vaccinia virus-specific DNA sequences. Adenovirus nuclei from adenovirus 2-infected HeLa cells, which also synthesize viral DNA in vitro, have been included in this study. Synthesis of DNA by HSV or vaccinia virus nuclei is markedly inhibited by the corresponding viral-specific antisera. These antisera inhibit in a similar fashion the purified herpesvirus-induced or vaccinia virus-induced DNA polymerase isolated from infected cells. Phosphonoacetic acid, reported to be a specific inhibitor of herpesvirus formation and the herpesvirus-induced DNA polymerase, is equally effective as an inhibitor of HSV DNA synthesis in isolated nuclei in vitro. However, we also find phosphonoacetic acid to be an effective inhibitor of vaccinia virus nuclear DNA synthesis and the purified vaccinia virus-induced DNA polymerase. In addition, this compound shows significant inhibition of DNA synthesis in isolated nuclei obtained from adenovirus-infected or uninfected cells and is a potent inhibitor of HeLa cell DNA polymerase alpha.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker Y., Asher Y. In vitro synthesis of DNA in nuclei isolated from herpes simplex virus-infected cells, untreated and treated with metabolic inhibitors. Virology. 1975 Jan;63(1):209–220. doi: 10.1016/0042-6822(75)90386-4. [DOI] [PubMed] [Google Scholar]
- Bell D. DNA synthesis in "nuclear monolayers" from BSC-1 cells infected with herpes virus. Nature. 1974 Apr 5;248(448):505–508. doi: 10.1038/248505a0. [DOI] [PubMed] [Google Scholar]
- Berkowitz D. M., Kakefuda T., Sporn M. A simple and rapid method for the isolation of enzymatically active HeLa cell nuclei. J Cell Biol. 1969 Sep;42(3):851–854. doi: 10.1083/jcb.42.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Brent T. P. DNA replication in isolated HeLa cell nuclei. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1213–1219. doi: 10.1016/0006-291x(73)90594-9. [DOI] [PubMed] [Google Scholar]
- Biswal N., Murray B. K. Synthesis of herpes simplex virus type 1 (HSV-1) DNA in isolated nuclei. I. Conditions for isolation of nuclei capable of viral DNA synthesis. Intervirology. 1974;4(1):1–13. doi: 10.1159/000149838. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Brown M., Bollum F. J. Induction of DNA polymerase in mouse L cells. J Mol Biol. 1973 Feb 15;74(1):1–8. doi: 10.1016/0022-2836(73)90349-5. [DOI] [PubMed] [Google Scholar]
- Citarella R. V., Muller R., Schlabach A., Weissbach A. Studies on vaccinia virus-directed deoxyribonucleic acid polymerase. J Virol. 1972 Oct;10(4):721–729. doi: 10.1128/jvi.10.4.721-729.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Friedman D. L., Mueller G. C. A nuclear system for DNA replication from synchronized HeLa cells. Biochim Biophys Acta. 1968 Jul 23;161(2):455–468. doi: 10.1016/0005-2787(68)90122-6. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hershey H. V., Stieber J. F., Mueller G. C. Dna synthesis in isolated HeLa nuclei. A system for continuation of replication in vivo. Eur J Biochem. 1973 Apr;34(2):383–394. doi: 10.1111/j.1432-1033.1973.tb02770.x. [DOI] [PubMed] [Google Scholar]
- Ito K., Arens M., Green M. Isolation of DNA polymerase gamma from an adenovirus 2 DNA replication complex. J Virol. 1975 Jun;15(6):1507–1510. doi: 10.1128/jvi.15.6.1507-1510.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber A. R. In vitro synthesis of DNA in nuclei isolated from human lung cells infected with herpes simplex type II virus. J Virol. 1975 Feb;15(2):322–331. doi: 10.1128/jvi.15.2.322-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaColla P., Weissbach A. Vaccinia virus infection of HeLa cells. I. Synthesis of vaccinia DNA in host cell nuclei. J Virol. 1975 Feb;15(2):305–315. doi: 10.1128/jvi.15.2.305-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus L. H. A novel system for DNA synthesis in isolated nuclei. FEBS Lett. 1973 Sep 1;35(1):166–168. doi: 10.1016/0014-5793(73)80602-7. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ELLISON S. A., ROSE H. M., MOORE D. H. Structure and development of viruses as observed in the electron microscope. I. Herpes simplex virus. J Exp Med. 1954 Aug 1;100(2):195–202. doi: 10.1084/jem.100.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Winnacker E. L., Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. II. Evidence for semi-conservative replication. J Mol Biol. 1972 Dec 30;72(3):539–552. doi: 10.1016/0022-2836(72)90173-8. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E., Overby L. R. Inhibition of DNA polymerase from herpes simplex virus-infected wi-38 cells by phosphonoacetic Acid. J Virol. 1975 May;15(5):1281–1283. doi: 10.1128/jvi.15.5.1281-1283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Probst G. S., Bikoff E., Keller S. J., Meyer R. R. DNA biosynthesis in nuclei. I. Characterization of DNA synthesis by isolated rat liver nuclei using endogenous DNA as primer. Biochim Biophys Acta. 1972 Oct 11;281(2):216–227. [PubMed] [Google Scholar]
- Qasba P. K. Synthesis of simian virus 40 DNA in isolated nuclei. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1045–1049. doi: 10.1073/pnas.71.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radsak K. DNA synthesis in isolated nuclei and isolated mitochondria of herpes simplex virus infected HeLa cells. FEBS Lett. 1973 Apr 1;31(1):42–46. doi: 10.1016/0014-5793(73)80070-5. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J., SALZMAN N. P. Deoxyribonucleic acid synthesis in vaccinia virus-infected HeLa cells. Virology. 1963 Apr;19:551–560. doi: 10.1016/0042-6822(63)90050-3. [DOI] [PubMed] [Google Scholar]
- Shimada H., Terayama H. DNA synthesis in isolated nuclei from the brains of rats at different post-partal stages and the infant rat brain cytosol factor stimulating the DNA synthesis in infant rat brain nuclei. Biochim Biophys Acta. 1972 Dec 22;287(3):415–426. doi: 10.1016/0005-2787(72)90285-7. [DOI] [PubMed] [Google Scholar]
- Spadari S., Muller R., Weissbach A. The dissimilitude of the low and high molecular weight deoxyribonucleic acid-dependent deoxyribonucleic acid polymerases of HeLa cells. J Biol Chem. 1974 May 10;249(9):2991–2992. [PubMed] [Google Scholar]
- Spadari S., Weissbach A. HeLa cell R-deoxyribonucleic acid polymerases. Separation and characterization of two enzymatic activities. J Biol Chem. 1974 Sep 25;249(18):5809–5815. [PubMed] [Google Scholar]
- Spadari S., Weissbach A. RNA-primed DNA synthesis: specific catalysis by HeLa cell DNA polymerase alpha. Proc Natl Acad Sci U S A. 1975 Feb;72(2):503–507. doi: 10.1073/pnas.72.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadari S., Weissbach A. The interrelation between DNA synthesis and various DNA polymerase activities in synchronized HeLa cells. J Mol Biol. 1974 Jun 15;86(1):11–20. doi: 10.1016/s0022-2836(74)80003-3. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]
- Weissbach A., Schlabach A., Fridlender B., Bolden A. DNA polymerases from human cells. Nat New Biol. 1971 Jun 9;231(23):167–170. doi: 10.1038/newbio231167a0. [DOI] [PubMed] [Google Scholar]
- Weissbach A. Vertebrate DNA polymerases. Cell. 1975 Jun;5(2):101–108. doi: 10.1016/0092-8674(75)90017-3. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Synthesis of polyoma DNA by isolated nuclei. Biochem Biophys Res Commun. 1971 Aug 20;44(4):952–957. doi: 10.1016/0006-291x(71)90804-7. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Sussenbach J. S. The mechanism of adenovirus-DNA synthesis in isolated nuclei. Eur J Biochem. 1972 Nov 7;30(3):584–592. doi: 10.1111/j.1432-1033.1972.tb02130.x. [DOI] [PubMed] [Google Scholar]



