Abstract
Background
Multidrug-resistant Escherichia coli (MDR E. coli) has become a major public health concern in Sudan and many countries, causing failure in treatment with consequent huge health burden.
Objectives
To determine the prevalence and susceptibility of MDR E. coli isolated from patients in hospitals at Khartoum State.
Methods
Between May to August 2011, E. coli (n = 232) isolated from clinical specimens, identified, tested their antimicrobials susceptibility and screened for extend spectrum â-lactamase production as per standard methods.
Results
Of the 232 E. coli isolates, the majority were from urine (65.1%). MDR E. coli were present in 214 (92.2%). Of these, the resistance rates were recorded to: amoxicillin 97.7%, cefuroxime 92.5%, trimethoprim-sulfamethoxazole 88.3%, tetracycline 77.1%, nalidixic acid 72%, ceftriaxone 64%, ciprofloxacin 58.4%, ofloxacin 55.1%, amoxicillin-clavulanate 50.4%, ceftazidime, gentamicin 35% each, nitrofurantoin 22.4%, chloramphenicol, tobramicin 18.2% each and amikacin 1.9%. Overall MDR E. coli, 53.3% were resistant to > 7 antimicrobial agents and ESBL was detected in 32.7%. Isolates from males were more resistant than those from females (p < 0.05).
Conclusions
Drug-resistance surveillance and epidemiological analysis of patient data is need periodically and can be informative for appropriate management of antimicrobial resistance.
Keywords: Multi-drug resistance, E. coli, Sudan
Introduction
Escherichia coli (E. coli) is a common pathogen linked with community-associated as well as nosocomial infections1,2. In the last few years, the emergence and wide dissemination of E. coli strains showing resistance to broad-spectrum of antimicrobial agents has been reported1,3,6. Emergence of resistance to multiple antimicrobial agents in pathogenic bacteria has become a significant public health threat as there are fewer, or even sometimes no, effective antimicrobial agents available for infections caused by these bacteria3–7. According to the European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC), multi-drug resistant (MDR) is defined as non-susceptibility to at least one agent in three or more antimicrobial categories4. MDR bacteria are the principal cause of failure in the treatment of infectious diseases, resulting in increases in the term and magnitude of morbidity, higher rates of mortality, and a greater health cost burden9,10. However, recently researchers have been investigated the activity of non-antimicrobial agents such as proanthocyanidin to prevent MDR bacterial infections29.
Multi-drug resistant E. coli are widely distributed in hospitals and are increasingly being isolated from community. Thus, it is urgent need to find out new antimicrobial agents8,10,11. However, new families of antimicrobial agents have a short life expectancy11. Several monitoring programmes have been initiated to generate baseline data about the prevalence of MDR in different bacterial species, including E. coli8,12. Many studies from Europe7 and USA6,13 have investigated MDR among E. coli isolates. Most bacterial isolates from Asian and African countries have shown high MDR rates14–18. In Sudan, limited data are currently available on the prevalence of MDR amongst significant bacterial isolates19,20. The aims of this study were to determine the prevalence and antimicrobials susceptibility of MDR E. coli collected from clinical specimens of patients in different hospitals in Khartoum State. It also, investigates the possible differences in antimicrobial resistance of E. coli isolated from a various type of clinical specimens in relation to the patient sex, age and settings
Methods
Isolates
Between May to August 2011, E. coli (n = 232) were isolated from clinical specimens of patients at different teaching hospitals in Khartoum State, Sudan. The participating hospitals were: Khartoum Teaching Hospital, Khartoum North Educational Hospital, National Health Laboratory, Omdurman Teaching Hospital, Soba University Hospital and Turkish Teaching Hospital.
E. coli were isolated from patient clinical specimens including urine, wound pus, high vaginal swabs, Ear discharges, blood, stool, semen and miscellaneous body fluids after following conventional procedures21. Significant bacterial growth was included in this study and they were identified on the basis of cultural characteristics, gram stain and conventional biochemical tests22 then confirmed by API 20E identification system (Biomerieux MarcyI'Etoile, France). When confirmed as E. coli, the isolates were preserved at −70 °C in tryptic soy broth containing 20% sterile glycerol. The database of each specimen source and patient sex, age and setting were recorded.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing of E. coli isolates was performed on Muller-Hinton agar plate (Oxoid, Basingstoke England) by the Kirby-Bauer disk diffusion method following the Clinical and Laboratory Standards Institute-(CLSI)23. The antimicrobial agents which were tested from different categories (Table 1) including: amikacin (30µg), amoxicillin (10µg), amoxicillin-clavulanate (30µg), ceftazidime (30µg), ceftriaxone (30ìg) cefuroxime (30µg), chloramphenicol (30µg), ciprofloxacin (5µg), gentamicin (10µµg), nalidixic acid (30µg), nitrofurantoin (50µg), ofloxacin (5µg), tetracycline (30µg), tobramicin (10µg) and trimethoprim-sulfamethoxazole (25µg) (Oxoid, England). E. coli ATCC 25922 was used as control strains and was tested each time when susceptibility testing was performed. Test results were only validated in the cases where inhibition zone diameters of the control strains were within performance ranges in accordance to guidelines23. Resistant and intermediate results were considered as nonsusceptible4. MDR E. coli was defined as non-susceptibility to at least one agent in three or more antimicrobial categories4.
Table 1.
Antimicrobial resistance pattern among E. coli (n = 214) isolated from different hospitals in Khartoum State, Sudan
| Antimicrobial and category* |
Hospital | Overall agent antimicrobial resistance (%) |
|||||
| Khartoum (n= 68) |
KNH (n=32) |
NHL (n=11) |
Omdur man (n= 35) |
Soba (n= 42) |
Turkish (n=26) |
||
| Penicillins | |||||||
| Amoxicillin | 98.5 | 96.9 | 100 | 97.1 | 97.6 | 96.2 | 97.7 |
| Penicillins + â-lactamase inhibitors | |||||||
| Amoxicillin-CA | 47.1 | 56.3 | 36.4 | 57.1 | 52.4 | 53.8 | 51.4 |
| Nom-extend spectrum cephalosporins; 1st and 2nd generation | |||||||
| Cefuroxime | 92.6 | 93.8 | 90.9 | 100 | 88.1 | 88.5 | 92.5 |
| Extend-spectrum cephalosporins; 3rd generation | |||||||
| Ceftriaxone | 73.5 | 59.4 | 45.5 | 71.4 | 59.5 | 50 | 64 |
| Ceftazidime | 51.5 | 18.8 | 18.2 | 40 | 23.8 | 30.8 | 35 |
| Quinolones | |||||||
| Ciprofloxacin | 57.4 | 68.8 | 81.8 | 51.4 | 50 | 61.5 | 58.4 |
| Nalidixic acid | 70.6 | 81.3 | 90.9 | 62.9 | 69.0 | 73.1 | 72 |
| Ofloxacin | 55.9 | 62.5 | 63.6 | 51.4 | 45.2 | 61.5 | 55.1 |
| Folate pathway inhibitors | |||||||
| SXT | 92.6 | 81.3 | 90.9 | 94.3 | 83.3 | 84.6 | 88.3 |
| Tetracyclines | |||||||
| Tetracycline | 82.4 | 68.8 | 72.7 | 91.4 | 64.3 | 76.9 | 77.1 |
| Aminoglycosides | |||||||
| Amikacin | 1.5 | 3.1 | 0 | 0 | 4.8 | 0 | 1.9 |
| Gentamicin | 35.3 | 31.3 | 9.1 | 37.1 | 40.5 | 38.5 | 35 |
| Tobramicin | 19.1 | 9.4 | 36.4 | 17.1 | 11.9 | 30.8 | 18.2 |
| Phenicols | |||||||
| Chloramphenicol | 32.4 | 18.8 | 0 | 17.1 | 4.8 | 11.5 | 18.2 |
| Nitrofurans | |||||||
| Nitrofurantoin | 26.5 | 18.8 | 27.3 | 28.6 | 16.7 | 15.4 | 22.4 |
Adapted from Magiorakos et al. (2011).
KNH = Khartoum North Hospital; NHL = National Health Laboratory; CA = Clavulanate; SXT = Trimethoprim-sulfamethoxazole
Extend spectrum â-lactamase production
All E. coli isolates recognizing reduced susceptibility to ceftazidime and ceftriaxone they were screened for ESBLs production by the double-disk synergy test according to CLSI recommendations23.
In this test, the organism is swabbed onto a Mueller-Hinton agar plate. A susceptibility disk containing amoxicillin-clavulanate is placed in the center of the plate, and disks containing ceftazidime and cefotaxime are placed 30 mm (center to center) from the amoxicillin-clavulanate disk. A clear extension of the edge of the inhibition zone of cephalosporin towards amoxicillin-clavulanate disk is interpreted as positive for ESBL production24.
Statistical analysis
All outcome data were analyzed using Statistical Package for Social Sciences (SPSS; Version10.0). The differences between resistance patterns of E. coli strains were determined using Independent samples T-Test and One-Way Analysis of Variance (ANOVA). All p-values were based on 2-tailed tests of significance where p < 0.05 is considered statistically significant.
Results
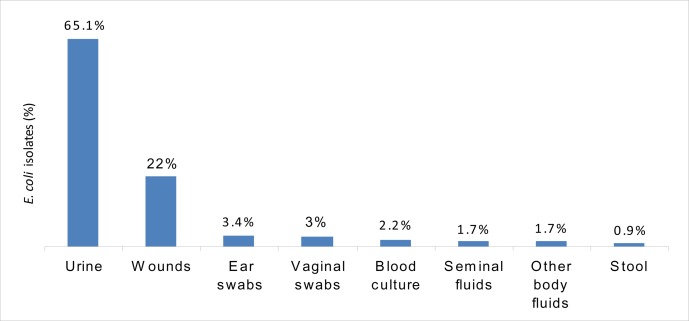
The prevalence of E. coli recovered from various clinical specimens (232) collected from different hospitals in Khartoum State is shown in Figure 1. Urine (65.1%) and wound (22%) specimens represented the majority of specimens. The isolates were obtained from all aged groups: 138 (59.5%) were females and 94 (40.5%) were males. Of the total number of isolates, 180 (77.6 %) were obtained from adult patients.
Figure 1.
Prevalence of E. coli (n = 232) recovered from various clinical specimens collected from hospitals in Khartoum State
Antimicrobial resistance rates and multidrug-resistant phenotypes
Of the 232 isolates tested, MDR E. coli were present in 214 (92.2%). Of these 32.7 % were found to be ESBL producer. Antimicrobial resistance patterns of the 214 MDR E. coli isolates against 15 antimicrobial agents is shown in table 1. High resistance rates were observed to amoxicillin (97.7%), cefuroxime (92.5%), trimethoprim-sulfamethoxazole (88.3%), tetracycline (77.1%), nalidixic acid (72%) and ceftriaxone (64%). Moderate resistance rates were observed to ciprofloxacin (58.4%), ofloxacin (55.1%), amoxicillin-clavulanate (51.4%), ceftazidime and gentamicin (35% each). Lower resistance rates were observed for nitrofurantoin (22.4%), chloramphenicol and tobramicin (18.2% each). Resistance to amikacin was uncommon (1.9%).
Frequency of antimicrobial resistance pattern of E. coli by hospitals
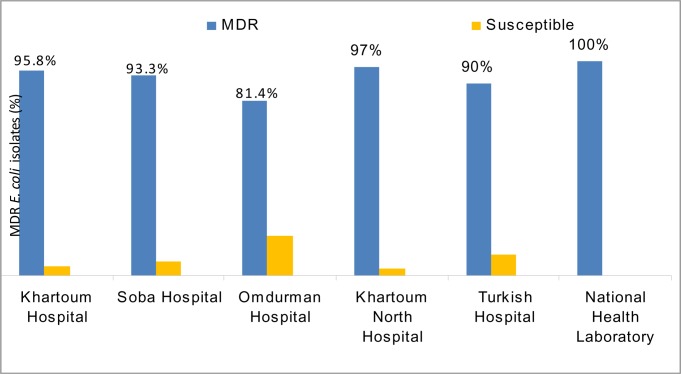
The frequencies of MDR E. coli isolated from different hospitals in Sudan are shown in Figure 2. The resistance rates of isolates to ceftazidime, ceftriaxone and chloramphenicol were significantly higher (p < 0.05) in Khartoum hospital than other hospitals. The resistance rates of isolates to ciprofloxacin and ofloxacin were found significantly higher in Khartoum and Khartoum North than Soba and Omdurman hospitals (p < 0.05) (table 1). But the resistance rates for nitrofurantoin, tetracycline, trimethoprim-sulfamethoxazole, were found significantly higher in isolates from Khartoum and Omdurman hospitals than isolates from Soba and Khartoum North hospitals (p < 0.05). The resistance rates of isolates to gentamicin were found high in Soba hospital but low resistance rates were observed to chloramphenicol (table 1).
Figure 2.
Distribution of MDR E. coli (n = 214) isolated from different hospitals in Khartoum state
Frequency of MDR E. coli according to the number of drug resistance
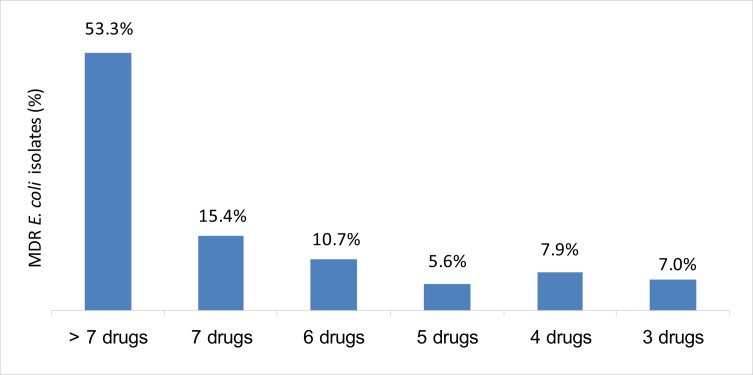
The frequency of MDR to 3, 4, 5, 6, 7 and more than 7 of total 15 antimicrobial agents were 15 (7.0%), 17 (7.9%), 13 (5.6%), 23 (10.7%), 33 (15.4%) and 114 (53.3%), respectively. Of the 92.2% MDR isolates, the most prevalent patterns were resistance to more than 7 (114; 53.3%), followed by 7 (33; 15.4%) and 6 (23; 10.7%) of antimicrobial agents (figure. 3).
Figure 3.
Frequency of MDR E. coli (n = 214) according to its resistance to three or more antimicrobial agents
Frequency of antimicrobial resistance pattern of E. coli by sex and age
Table 2 shows the resistant pattern of the 214 MDR E. coli isolated from males and females. Isolates from males were showed higher resistance rates than those from females to ceftazidime (p = 0.023), ceftriaxone (p = 0.004), chloramphenicol (p = 0.005), nalidixic acid (p = 0.002), ofloxacin (p = 0.024), tetracycline (p =0.006) and trimethoprim-sulfamethoxazole (p = 0.02). In contrast, resistance rates to nalidixic acid and ofloxacin were higher in females than males isolates (table 2).
Table 2.
Antimicrobial resistance (%) among E. coli (n = 214) isolated from different hospitals in Khartoum state in relation to patient's sex and age
| Antimicrobial agent | Sex | Age | ||||
| Male (n= 89) |
Female (n= 125) |
p. value | Adult (n= 168) |
Child (n=46) |
p. value | |
| Amikacin | 1.1 | 2.4 | 0.499 | 1.8 | 2.2 | 0.864 |
| Amoxicillin | 96.6 | 98.4 | 0.400 | 97 | 100 | 0.238 |
| Amoxicillin-clavulanate | 48.3 | 53.6 | 0.448 | 51.8 | 50 | 0.831 |
| Ceftazidime | 43.8 | 28.8 | 0.023 | 35.7 | 32.6 | 0.697 |
| Ceftriaxone | 75.3 | 56 | 0.004 | 63.7 | 65.5 | 0.849 |
| Cefuroxime | 95.5 | 90.4 | 0.163 | 93.5 | 89.1 | 0.326 |
| Chloramphenicol | 27 | 12 | 0.005 | 15.5 | 28.3 | 0.047 |
| Ciprofloxacin | 50.6 | 64 | 0.050 | 62.5 | 43.5 | 0.020 |
| Gentamicin | 38.2 | 32.8 | 0.417 | 34.5 | 37 | 0.761 |
| Nalidixic acid | 60.7 | 80 | 0.002 | 75 | 60.9 | 0.059 |
| Nitrofurantoin | 24.7 | 20.8 | 0.500 | 22.6 | 21.7 | 0.900 |
| Ofloxacin | 46.1 | 61.6 | 0.024 | 58.3 | 43.5 | 0.073 |
| Tetracycline | 86.5 | 70.4 | 0.006 | 77.4 | 76.1 | 0.854 |
| Tobramicin | 21.3 | 16 | 0.320 | 19 | 15.2 | 0.553 |
| Trimethoprim-sulfame-thoxazole | 94.4 | 84 | 0.020 | 87.5 | 91.3 | 0.479 |
Distribution of MDR E. coli according to age showed no statistical differences between adults and children were observed to the most antimicrobial agents. However, there were significant differences observed in two antimicrobial agents ciprofloxacin (p = 0.02) was higher in adults and chloramphenicol (p = 0.047) was higher in children (table 2).
Discussion
The present study showed that the prevalence of MDR among E. coli isolates was higher (92.2%) in comparison to previous study carried out in Sudan19. These authors recorded 58% MDR E. coli isolated from urinary tract infections in the year 2000. In this study, the prevalence of MDR E. coli isolates is similar to those reported in south America3, but relatively higher than those reported in neighboring countries such as 87% in Egypt15 and 74.6% in Ethiopia14 and other countries as 83.9% in Nigeria16 and 74% in Saudi Arabia17. Furthermore, our findings are much higher compared to those reported in Europe1 and USA6.
In this study, there are high resistance rates of MDR E. coli isolates to the first-line oral antimicrobial agents such as amoxicillin, cefuroxime, trimethoprimsulfamethoxazole, tetracycline nalidixic acid and amoxicillin-clavulanate These findings represent alarming increased rates in resistant E. coli are comparable to other studies in Sudan19 and elsewhere3,6,25.
Resistance to fluoroquinolones varies geographically and is an emerging problem in both developed and developing countries.13,28. In the present study, MDR E. coli isolates showed relatively high resistance rates to ofloxacin and ciprofloxacin. This has been hypothesized to be related to the inappropriate use of fluoroquinolones for humans2. Also, prolonged use of low dose of the more potent fluoroquinolones such as ciprofloxacin has been shown to be the most significant risk factor for acquisition of resistance27.
Whilst the third-generation cephalosporins such as ceftriaxone and ceftazidime have been used to treat gram-negative bacterial infections of various body sites5,25, the current study showed high levels of resistance to second and third generation cephalosporins. A possible explanation for the high resistance found might be the presence of ESBL in these strains 18. Since ESBL mediated resistance to wide range of antimicrobial classes 8,18, it is important that routine screening of ESBL in clinical isolates is carried out to prevent widespread of resistant isolates in our hospitals.
Like studies elsewhere 7,15,25, our MDR E. coli isolates were found to be effective against aminoglycoside agents. Amikacin appears to have wider range of activity than tobramicin, gentamicin and other tested antimicrobial agents. The explanation for amikacin is probably the fact that these are very powerful drugs used only in hospital settings and not as first-line therapy. Therefore, they have lower selective pressure due to their restricted use25.
The emergence of E. coli isolates with different MDR phenotypes, involving co-resistance to three or more unrelated families of antimicrobial agents, has been previously reported by others and is considered a serious health concern1,3,6. Similar to reports by others 3,7,16, the current study expressed high resistance rates to different classes of antimicrobial agents. Almost our MDR E. coli isolates were found to be multi-resistant to the commonly used antimicrobials agents of amoxicillin, cefuroxime, trimethoprim-sulfamethoxazole tetracycline, and nalidixic acid. These resistance profiles were common and could be accounted for by a number of known acquired resistance genes3. Since proanthocyanidin is a non-antimicrobial agents used for the prevention of urinary tract infection caused by susceptible E. coli strains via inhibiting Pfimbriated adhesion to uroepithelial cells30. Recently, Gupta et al.29 have been observed that proanthocyanidin prevent also adhesion of most MDR E. coli strains to the uroepithelial cells.
In the present study, MDR E. coli from Khartoum hospital had high resistance rates to most of the antimicrobial agents. Also, our report showed a variable difference in antimicrobial resistance of MDR E. coli between each hospital in Khartoum State. MDR may vary according to geographical regions. Although previous use of antimicrobials may be involved in the regional differences of antimicrobial resistance, consider other factors such as the patients' characteristics or sampling bias and the social and geological factors that attribute to this variation of MDR as previously described26. Large-scale, local studies, hospital and departmental data, therefore, are required to understand drug resistance in each setting.
In the current study, MDR E. coli isolates from males were more likely to have resistance to antimicrobial agents than those from females. Similar findings have been described worldwide6,7,13,25. The resistance pattern was somewhat affected in gender, but that definitely depended on the site of infection25 and clinical parameters of the population investigated26.
No statistical differences (p = 0.249) were found in antimicrobial resistance between adults and children. Similar results were reported by Boyd et al. (2008). However, it is important to emphasize on the high prevalence of fluoroquinolones resistance in isolates from children which is not recommended for treatment of pediatric patients.
Conclusion
This study concluded that MDR E. coli is escalating in Khartoum State (92.2%) compared to previous surveys (58%). Multiple resistances to antimicrobial drugs among E. coli isolates complicate therapeutic management of infections. Drug-resistance surveillance and epidemiological analysis of patient data is needed periodically and can be informative for appropriate management of antimicrobial resistance. Understanding the molecular basis of resistance acquisition and transmission can contribute to the development of new strategies to combat this phenomenon.
Acknowledgments
The authors express gratitude to all staff of the Departments of Microbiology Laboratories of participating hospitals namely: Khartoum, Soba, Omdurman, Khartoum North, National Health Laboratory and Turkish hospitals, for help and support.
References
- 1.Oteo J, Lázaro E, de Abajo FJ, Baquero F, Campos J. Spanish members of EARSS. Antimicrobial-resistant invasive Escherichia coli, Spain. Emerg Infect Dis. 2005;11:546–553. doi: 10.3201/eid1104.040699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drago L, Nicola L, Mattina R, Vecchi ED. In vitro lselection of resistance in Escherichia coli and Klebsiella spp. at in vivo fluoroquinolone concentrations. BMC Microbiol. 2010;10:119. doi: 10.1186/1471-2180-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartoloni A, Pallecchi L, Benedetti M, et al. Multidrug-resistant commensal Escherichia coli in children, Peru and Bolivia. Emerg Infect Dis. 2006;12:907–913. doi: 10.3201/eid1206.051258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2011 May 7; doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 5.Bilal NE, Gedebou M. Clinical and community strains of Klebsiella pneumoniae: multiple and increasing rates of antibiotic resistance in Abha, Saudi Arabia. Br J Biomed Sci. 2000;57:185–191. [PubMed] [Google Scholar]
- 6.Sahm DF, Thornsberry C, Mayfield DC, Jones ME, Karlowsky JA. Multidrug-resistant urinary tract isolates of Escherichia coli: prevalence and patient demographics in the United States. Antimicrob Agents Chemother. 2001;45:1402–14026. doi: 10.1128/AAC.45.5.1402-1406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oteo J, Campos J, Baquero F. Spanish members of the European Antimicrobial Resistance Surveillance System. Antibiotic resistance in 1962 invasive isolates of Escherichia coli in 27 Spanish hospitals participating in the European Antimicrobial Resistance Surveillance System (2001) J Antimicrob Chemother. 2002;50:945–952. doi: 10.1093/jac/dkf255. [DOI] [PubMed] [Google Scholar]
- 8.Peralta G, Sánchez MB, Garrido JC. Impact of antibiotic resistance and of adequate empirical antibiotic treatment in the prognosis of patients with Escherichia coli bacteraemia. J Antimicrob Chemother. 2007;60:855–863. doi: 10.1093/jac/dkm279. [DOI] [PubMed] [Google Scholar]
- 9.Howard DH, Scott RD, 2nd, Packard R, Jones D. The Global Impact of Drug Resistance. Clin Infect Dis. 2003;36(Suppl 1):S4-1. doi: 10.1086/344656. [DOI] [PubMed] [Google Scholar]
- 10.Coates A, Hu Y, Bax R, Page C. The future challenges facing the development of new antimicrobial drugs. Nat Rev Drug Discov. 2002;1:895–910. doi: 10.1038/nrd940. [DOI] [PubMed] [Google Scholar]
- 11.Rahman S, Parvez AK, Islam R, Khan MH. Antibacterial activity of natural spices on multiple drug resistant Escherichia coli isolated from drinking water, Bangladesh. Ann Clin Microbiol Antimicrob. 2011;10:10. doi: 10.1186/1476-0711-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerra B, Junker E, Schroeter A, Helmuth R, Guth BEC, Beutin L. Phenotypic and genotypic characterization of antimicrobial resistance in Escherichia coli O111 isolates. J Antimicrob Chemother. 2006;57:1210–1214. doi: 10.1093/jac/dkl127. [DOI] [PubMed] [Google Scholar]
- 13.Boyd LB, Atmar R, Randall GL, Hamill RJ, Steffen D, Zechiedrich L. Increased fluoroquinolone resistance with time in Escherichia coli from >17,000 patients at a large county hospital as function of culture site, age, sex, and location. BMC Infect Dis. 2008;8:4. doi: 10.1186/1471-2334-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kibret M, Abera B. Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. Afr Health Sci. 2011;11(S1):S40–S45. doi: 10.4314/ahs.v11i3.70069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salem MM, Muharram M, Alhosiny IM. Distribution of Classes 1 and 2 Integrons among Multi Drug Resistant E. coli Isolated from Hospitalized Patients with Urinary Tract Infection in Cairo, Egypt. Australian Journal of Basic and Applied Sciences. 2010;4:398–407. [Google Scholar]
- 16.Ngwai YB, Akpotu MO, Obidake RE, Sounyo AA, Onanuga A, Origbo SO. Antimicrobial susceptibility of Escherichia coli and other coliforms isolated from urine of asymptomatic students in Bayelsa State, Nigeria. African Journal of Microbiology Research. 2010;5:184–191. [Google Scholar]
- 17.Bilal NE, Gedebou M, Al-Mohayia MH. Gram-negative bacilli from hospital and non-hospital personnel: pharyngeal carriage, multi-drug resistance and extended-spectrum â lactamase in Abha, Saudi Arabia. Biomedical Research. 2001;12:251–258. [Google Scholar]
- 18.Kader AA, Kumar AK. Prevalence of extended-spectrum â-lactamase among multidrug resistant Gram-negative isolates from a general hospital in Saudi Arabia. Saudi Med J. 2004;25:570–574. [PubMed] [Google Scholar]
- 19.Ahmed AA, Osman H, Mansour AM, et al. Antimicrobial agents' resistance in bacterial isolates from patients with diarrhea and urinary tract infection in the Sudan. Am J Trop Med Hyg. 2000;63:259–263. [PubMed] [Google Scholar]
- 20.Hamdan HZ, Ziad AHZ, Ali SK, Adam I. Epidemiology of urinary tract infections and antibiotics sensitivity among pregnant women at Khartoum North Hospital. Ann Clin Microbiol Antimicrob. 2011;10:2. doi: 10.1186/1476-0711-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomson RB, Jr, Miller JM. Specimen collection, transport, and processing: bacteriology. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of Clinical Microbiology. 8th edition. Washington, DC: American Society for Microbiology; 2003. pp. 286–330. [Google Scholar]
- 22.Farmer JJ., 3rd . Enterobacteriaceae: Introduction and Identification. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of Clinical Microbiology. 8th edition. Washington, DC: American Society for Microbiology; 2003. pp. 636–653. [Google Scholar]
- 23.Clinical and Laboratory Standards Institute, author. CLSI document M100-S21. 1. Vol. 31. Wayne, Pa: Clinical and Laboratory Standards Institute; 2011. Performance standards for antimicrobial disk susceptibility tests; twenty first Informational supplement. [Google Scholar]
- 24.Jarlier V, Nicolas MH, Fournier G, Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer betalactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 25.Sahuquillo-Arce JM, Selva M, Perpinã'n H, et al. Antimicrobial Resistance in More than 100,000 Escherichi coli Isolates According to Culture Site and Patient Age, Gender, and Location. Antimicrob Agents Chemother. 2011;55:1222–1228. doi: 10.1128/AAC.00765-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee G, Cho YH, Shim BS, Lee SD. Risk Factors for Antimicrobial Resistance among the Escherichia coli Strains Isolated from Korean Patients with Acute Uncomplicated Cystitis: A Prospective and Nationwide Study. J Korean Med Sci. 2010;25:1205–1209. doi: 10.3346/jkms.2010.25.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chenia HY, Pillay B, Pillay D. Analysis of the mechanisms of fluoroquinolone resistance in urinary tract pathogens. J Antimicrob Chemother. 2006;58:1274–1278. doi: 10.1093/jac/dkl404. [DOI] [PubMed] [Google Scholar]
- 28.Namboodiri SS, Opintan JA, Lijek RS, Newman MJ, Okeke IN. Quinolone resistance in Escherichia coli from Accra, Ghana. BMC Microbiol. 2011;11:44. doi: 10.1186/1471-2180-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta A, Dwivedi M, Mahdi AA, Nagana Gowda GA, Khetrapal CL, Bhandari M. Inhibition of adherence of multi-drug resistant E. coli by proanthocyanidin. Urol Res. 2012;402:143–150. doi: 10.1007/s00240-011-0398-2. [DOI] [PubMed] [Google Scholar]
- 30.Howell AB, Botto H, Combescure C, et al. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect Dis. 2010;10:94. doi: 10.1186/1471-2334-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]