Abstract
Background
Nodding syndrome (repetitive nodding and progressive generalized seizures) is assuming epidemic proportions in South Sudan, Tanzania and Uganda.
Objective
To describe clinical and epidemiological features of nodding syndrome in southern Sudan based on preliminary investigations conducted in 2001 and 2002.
Method
Household surveys, clinical, electrophysiological (EEG) assessments, informant interviews and case-control studies were conducted in the town of Lui and the village of Amadi in southern Sudan.
Results
Nodding syndrome is characterized by involuntary repetitive nodding of the head, progressing to generalized seizures; mental and physical deterioration. The EEGs were consistent with progressive epileptic encephalopathy. Prevalence of Nodding syndrome in Lui and Amadi was 2.3% and 6.7% respectively. All case control studies showed a positive association between cases and Onchocerca volvulus. A history of measles was negatively associated with being a case: 2/13 of cases and 11/19 of controls had had measles: odds ratio 0.13 (95% CI 0.02, 0.76). Environmental assessment did not reveal any naturally occurring or manmade neurotoxic factors to explain Nodding Syndrome, although fungal contamination of food could not be ruled out.
Conclusion
Nodding Syndrome was strongly associated with Onchocerca volvulus. There was no evidence to suggest an environmental pollutant, chemical agent, or other toxic factor
Keywords: nodding, syndrome, South Sudan, clinical, epidemiology
Introduction
In 2001, the World Health Organization was invited to investigate an epidemic of an illness among children characterized by episodes of repetitive nodding of the head. These episodes would occur several times each day, often during meals, and in some cases the illness progressed to generalized tonic-clonic seizures, and to physical and mental deterioration. Similar head-nodding episodes, associated with physical and mental retardation and seizures, have been previously described in children in Uganda 1–2, Tanzania3–4 and Liberia5, and an association with onchocercias infection has been suggested. Our studies were the first to characterize Nodding Syndrome in southern Sudan. This paper describes preliminary investigations of this unusual neurological syndrome. The investigations focused on Lui town and Amadi village, which are located in Mundri county, Equatoria region. This is a poor, rural area of herders and subsistence farmers. Most people live in family compounds of mud huts with thatched roofs, and these compounds are connected by foot paths. There are no paved roads and few motorized vehicles. The Yei river is located about 2 km from Lui and less than 1 km from Amadi. There is one hospital in Lui. Because of armed conflict in the former southern Sudan, inhabitants of Lui and Amadi have been displaced several times, most recently during 1994–1997.
Methods
A case of Nodding Syndrome was defined as the occurrence of episodes of involuntary head nodding in a resident of Lui or Amadi. Cases were identified through a review of Lui hospital records, interviews with key informants, and house to house surveys. The local name for this illness is ‘Adravu Legnaro’, and it is well known, and feared, by the inhabitants of these communities. Clinical histories, complete physical examinations, and limited laboratory tests were performed on 66 cases, and electroencephalograms (EEG) were recorded on 39 cases including 32 with a history of nodding. A household census of Lui and of four of five parishes of Amadi was carried out to estimate the prevalence of nodding syndrome. Key informants provided background on diet, displacements, population exposures, prophylactic medication, and beliefs about the disease and its social and psychological impact. Three case-control studies were undertaken to explore possible risk factors for Nodding Syndrome. The first two studies, carried out in November 2001 by the Carter Center and Health Net International, included cases with nodding only, cases with both nodding and seizures, and cases with seizures only.
In the first study, conducted in Lui, 39 cases were identified and 31 controls were recruited from the local primary school. In the second study, conducted in Amadi, 30 cases were identified and 34 controls were recruited by the village chief. Participants in both studies were given clinical examinations and tested for the presence of Onchocerca volvulus, Trypanosoma brucei gambiense, Loa loa, Wuchereria bancrofti, and Mansonella perstans. The third study, conducted by WHO in Lui in January 2002, compared 13 cases of nodding syndrome without seizures to 19 children with no history of nodding or seizures. The controls were matched to the cases by age and neighborhood of residence.
A physical examination and skin-snip examination were done. Blood samples were taken from cases and controls and cerebro-spinal fluid from cases only. Caretakers were questioned about exposure to dietary risk factors, and family displacements. Woolf's formula was used for calculating confidence limits for the odds ratios.
Ethics considerations
The studies described in this paper were part of an outbreak investigation which is a public health intervention, not a research project. As the WHO-led outbreak investigation took place, before the latest version of the International ethical guidelines for epidemiological studies was issued by WHO in 2009, no prior approval was sought from an ethical review committee.
Retrospective advice from the Research Ethics Review Committee of WHO, which came to the following conclusions: ‘Given the current concerns over the nodding disease situation elsewhere in the world, it would be unethical to further retain potentially useful data and to defer publication of the 2002 investigation in Mundri County, on the ground that ethical issues were not properly addressed at that time.’
The current paper describes an outbreak investigation during a complex emergency comprising civil warfare, community displacement and drought. The investigation was requested by local communities and orally approved by Lui Hospital and by the local administrator (a civilian representative of the rebel force in control of the area). No institutional committee's approval had been obtained prior to the investigation, since it was primarily a public health response and not research. There was no pre-defined protocol before entering southern Sudan, as the nature of the illness was unclear and it was also unclear what type of investigation would be possible given the security situation and periodic aerial bombardments.
Because of high illiteracy, informed oral consent was obtained from adult caretakers of children and from adult subjects aged 18 years and above, using local translators and hospital staff.
The decision to undertake a small case-control study, which involved some invasive procedures, was taken at the end of the week after discussion within the team and consultation with community leaders and hospital staff. The team found it unethical not to test some hypotheses that might help identify the cause of the outbreak. It was not clear if there would be another opportunity to do this given the security situation.
Skin snips (to test for association with onchocerciasis) and blood were taken from all subjects. There were no refusals among cases, and one skin snip refusal by a control. Two other controls had no skin snip taken, nor blood drawn, with no reason mentioned. There were no medications given as part of the investigations, but children presenting with injuries and other diseases were provided care by Lui hospital. Those with positive skin-snips were treated and those with seizures given anti-epileptic drugs and caretakers were given advice on preventing harm during seizures.
Given the neurological nature of the syndrome, it was believed that the CSF may contain clues as to the origin of the disease. Although invasive, it was felt ethically justified because of the severity of illness, and potential benefits to cases should the analysis of CSF provide further insight. Keeping to the principle of collecting only samples that could help control the outbreak, CSF was not taken from controls. There were no refusals.
While the WHO team was in the field, they discovered two previous case-control studies testing the association between Onchocera volvulus and nodding syndrome. These studies used skin snips collected by the Carter Center and Health Net International, as part of an ongoing evaluation of the onchocerciasis control programme and were conducted by one of the members of the WHO led team in 2002. These studies are included in the current report, as they have not been reported elsewhere, with agreement of the investigator and co-author of this paper.
Results
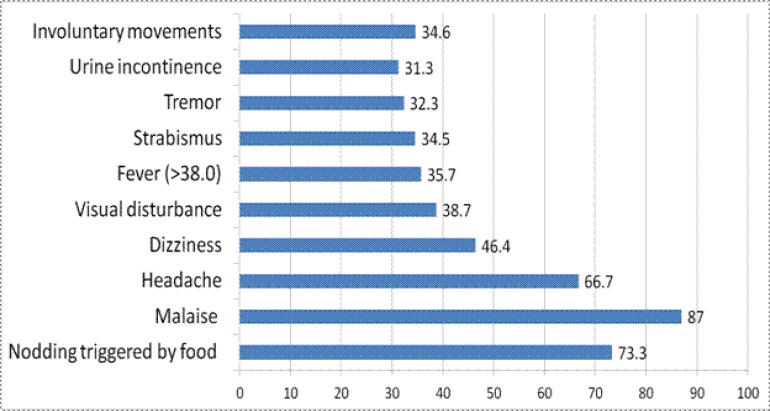
Typically, episodes of nodding syndrome consisted of repetitive, involuntary dropping of the head, repeated 10 to 20 times per minute, and continued for 2 to 5 minutes. Nodding was reported to occur especially upon waking in the morning or while eating. For some children, head nodding did not disrupt eating or the ability to follow commands, including instructions to rise, walk, and turn, whereas other children were unresponsive to commands and stared absently during nodding episodes.
In addition to headnodding and sometimes directly following the involuntary movement, patients presented a range of other signs that suggested either generalized tonic-clonic seizure without evidence of partial seizures, or psychomotor seizures. Seizures were sometimes accompanied by urinary incontinence. Some caretakers reported episodes of sudden shouting or screaming and at times jumping up and running in circles, and several children were reported to have burned themselves during seizures. Agitation, weakness, general body pain, sleepiness during the day, changes in mental ability, itching, and very cold extremities were also reported.
In some cases physical signs included mental retardation, developmental stunting, dwarfism, and poor development of secondary sexual characteristics. Polymerasechain reaction testing revealed no evidence of Onchocerca volvulus in cerebrospinal fluid obtained from the cases. Case histories indicated that cases often evolved from head-nodding to nodding and tonic-clonic seizures, or to tonic-clonic seizures alone, with episodes increasing in frequency and severity. Community leaders described the condition as beginning with head-nodding and progressing over months to years to include seizures, severe wasting, stunting and mental retardation. Affected children appeared quiet and listless, and did not play with other children. The community believed the condition to be invariably fatal. EEGs recorded from 32 subjects with Nodding Syndrome revealed universal recurrent paroxysmal pathological discharges. Nodding episodes were recorded in 3 cases, and nodding activity took the form of an isolated, diffuse delta-theta slow wave increasingly polymorphous as the disease progressed, followed by a brief and small fast discharge. This paroxysmal activity was observed to recur in a pseudo-periodical manner for up to several minutes. The act of eating local food triggered head-nodding or even a grand mal seizure. Study of one female subject who reliably began to nod within minutes commencing to eat local food prepared from maize (ugali) was able to follow commands to stand, walk, turn and sit, while continuing to eat with head nodding. Strangely, head nodding was not induced when eating a western candy bar but the movement disorder promptly re-appeared after she switched back to ugali.
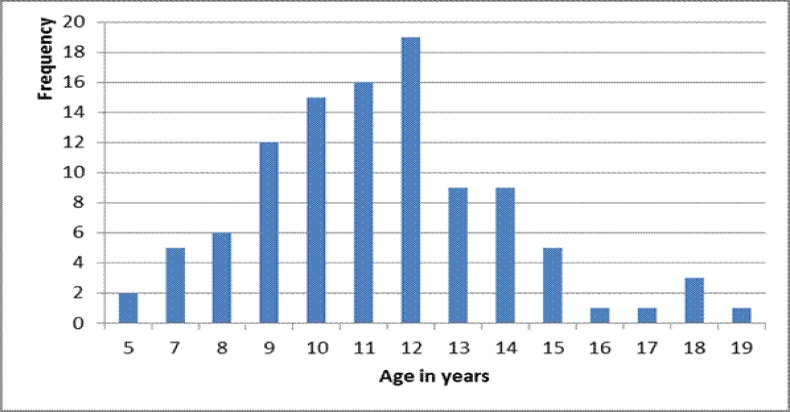
The first recorded case from Mundri County appeared in 1991. Key informants from Lui who date the introduction of nodding syndrome to just after the beginning of the last displacement (1994–1997) have no recollection of children with nodding from their own childhood. The prevalence of Nodding Syndrome in Lui and Amadi was estimated at 2.3% (41/1783) and 6.7% (57/854) respectively. There were 13 families with more than one affected child. Cases ranged in age from 5 to 21 years with a median of 12 years. The ratio of male to female cases was 1.2. The age at onset of suspected cases ranged from two to 18 years, with a median of 12 years, and the mean duration of illness was 3.0 years. Two deaths of children who had Nodding Syndrome were reported in the Lui household survey. Key informants in the 39 villages surrounding Lui and Amadi reported a total of 37 deaths of children with nodding syndrome over the years.
All three case-control studies showed a positive association between case status and a positive skin snip for Onchocerca volvulus microfilaria (table 1). In the last case-control study, there was no significant association with a history of ivermectin use (based on care-giver reports to investigators) (61.5% of cases did receive Ivermectine at least once versus 36.8% of the controls OR 2.79 (0.64–11.75). A history of measles was negatively associated with being a case: 15.4% (2/13) of cases and 61.1% (11/19) of controls gave a history of having had measles: odds ratio 0.12 (95% CI 0.05, 0.015). Possible differences in measles vaccination history among cases and controls were not determined.
Table 1.
Relationship of Nodding syndrome and Onchocerca volvulus skin-snip results in 3 case-control studies
| Study | Cases | Controls | Odds ratio | 95% CI |
| % positive (+/total) | % positive (+/total) | |||
| Amadi(2001) | 96.7% (29/30) | 50% (17/34) | 29.0 | (3.5, 237.7) |
| Lui (2001) | 89.7% (35/39) | 48.4% (15/31) | 9.3 | (2.7, 32.6) |
| Lui (2002) | 92.3% (12/13) | 43.8% (7/16) | 15.4 | (1.6, 148.8) |
CI Confidence Intervals
Discussion
Several conclusions can be drawn from these studies. Nodding syndrome is a progressive, epileptic encephalopathy, affecting children and teenagers and is sometimes associated with mental deterioration, stunted development of physique, and/or secondary sexual features. Three case-control studies indicated an epidemiologic association with evidence of infestation with Onchocerca volvulus, but there was no evidence of Onchocerca volvulus in the cerebral spinal fluid (CSF). The absence of evidence of Onchocerca volvulus in the CSF confirms our lack of understanding of the role (if any) of Onchocerca volvulus in the pathogenesis of nodding syndrome. Work from Tanzania had similar findings: patients had evidence of Onchocerca volvulus infection in the skin without evidence of Onchocerca volvulus in the CSF6. The same authors, using magnetic imaging, demonstrated pathologies within the hippocampus and gliosis elsewhere in the brain6.
It is curious to note that none of our nodding syndrome patients came from the Dinka tribe. Recent reports of Nodding Syndrome from Western Equatoria did not mention any ethnic distribution of the condition7, 8. Noteworthy is that the Dinka are itinerant and thus spent much less time in the proximity of the black-fly infested riverine villages where Nodding Syndrome is endemic. This needs to be investigated further.
The etiology of nodding syndrome is unknown. The case-control study assessed a limited panel of parasites; the strongest association with Nodding Syndrome was the presence of parasitic infestation with Onchocerca volvulus, a combination that has been noted on previous occasions1, 2, 4. There was no evidence to indicate the operation of an environmental pollutant, chemical agent, or other toxic factor.
The clinical manifestations of nodding syndrome did not appear consistent with any known dietary toxin although head nodding was commonly triggered by the act of eating the local diet and a tremorgenic mycotoxin cannot be ruled out.
There is urgent need for larger studies to elucidate the etiology and optimal treatment for these children. Additional consideration of dietary and non-infectious environmental factors associated with Nodding Syndrome will be reported elsewhere.
Table 2.
Percentage of children with positive test results from November 2001 case-control study
| Lui | Amadi | Odds ratio | P value | |||
| cases | controls | cases | controls | |||
| N=39 | N=31 | N=30 | N=34 | |||
| Loa loa | 0.0% | 0.0% | 0.0% | 0.0% | Un-defined | |
| M perstans | 41.0 | 9.6 | 66.6 | 50.0 | 3.22 | .005 |
| Lymphatic filariasisa | 0.0 | 9.0 | 0.0 | 7.6 | Un-defined | 0.47 |
| Trypanosomiasis | 12.8 | 9.6 | 0.0 | 5.8 | 0.84 | 0.94 |
| Onchocerciasis | 89.7 | 48.3 | 96.6 | 76.4 | 9.20 | 0.00003 |
For lymphatic filariasis the sample sizes were 8, 11, 18 and 13 for Lui and Amadi cases and controls respectively.
Table 3.
Percent of cases and controls exposed to possible risk factors
| Percent of | Percent of | Odds ratio | Lower OR | Upper | Probability (Fisher's | |
| cases | controls | (OR) | confidence | confidence | exact test) | |
| limit OR | limit OR | |||||
| Exposures to illnes | ||||||
| Previous Meningitis | 0.0 | 5.56 | undefined | 1.00 | ||
| Previous Measles | 15.38 | 58% | 0.13 | 0.02 | 0.76 | 0.025* |
| Positive skin snip for | 92.31 | 43.75 | 15.43 | 1.60 | 148.82 | 0.008* |
| Onchocerciasis | ||||||
| Treatment with | 61.54 | 36.84 | 2.79 | 0.64 | 11.75 | 1.00 |
| ivermectin | ||||||
| Other exposures | ||||||
| Internal displacement | 92.31 | 73.68 | 4.29 | 0.44 | 41.95 | 0.36 |
| Male sex | 23.08 | 31.58 | 0.65 | 0.13 | 3.26 | 0.70 |
| Dietary exposures | ||||||
| Cultivation of crops | ||||||
| Narango | 92.31 | 88.89 | 1.50 | 0.12 | 0.19 | 1.00 |
| Serena | 41.67 | 16.67 | 3.57 | 0.66 | 19.34 | 0.21 |
| Bari | 69.23 | 66.67 | 1.13 | 0.24 | 5.21 | 1.00 |
| Diri | 84.62 | 88.89 | 0.69 | 0.08 | 5.64 | 1.00 |
| Sorghum types eaten by child | ||||||
| Narango | 84.62 | 100.00 | undefined | 0.16 | ||
| Serena | 53.85 | 15.79 | 6.22 | 1.20 | 32.3 | 0.049* |
| Bari | 76.92 | 68.42 | 1.54 | 0.31 | 7.72 | 0.70 |
| Diri | 92.31 | 89.47 | 1.41 | 0.11 | 17.4 | 1.00 |
| Serena major food | 23.08 | 11.11 | 2.40 | 0.33 | 16.97 | 0.63 |
| Other dietary elements | ||||||
| Baboon meat | 69.23 | 33.33 | 4.50 | 0.97 | 20.83 | 0.07 |
| Baboon brain | 46.15 | 22.22 | 3.00 | 0.63 | 14.23 | 0.25 |
| Colored seeds | 83.33 | 50.00 | 5.00 | 0.82 | 30.46 | 0.11 |
| Cawa | 84.62 | 94.44 | 0.32 | 0.03 | 4.01 | 0.56 |
| Unripe sorghum | 92.3 | 83.33 | 2.40 | 0.22 | 26.12 | 0.62 |
Significant at the .05 level.
Figure 1.
Age distribution of cases examined (n=139) with ever had nodding (n=134)
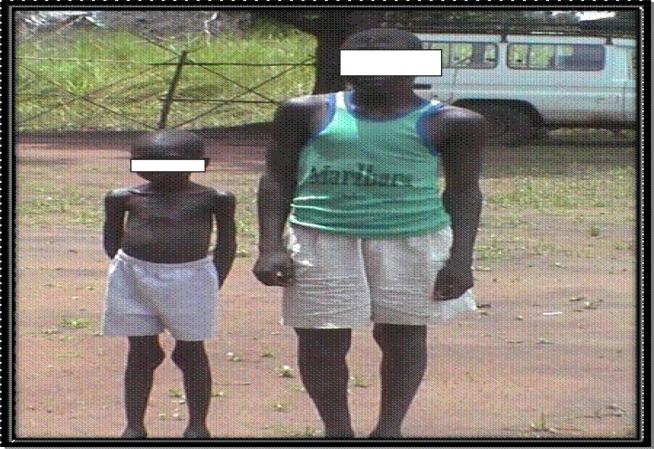
Figure 2.
The smaller patient has Nodding syndrome and is the same age as the taller adolescent who is of the same age, but does not have nodding syndrome. (Photograph by courtesy of Stella Chungong)
Figure 3a.
Distribution on Nodding syndrome patients by clinical finding, Lui, Mundri county, south Sudan
Figure 3b.
More symptoms of children with Nodding syndrome, Lui, Mundri county, south Sudan
Acknowledgements
The authors are grateful to Prof. Bernardo Dalla Bernardina Dipartimento di Neuropsichiatria and Dr. Piero Amodio for their contribution. We also acknowledge Micromed, Mogliano Veneto, Italy, who provided the EEG equipment and software and to SO.KO, Agrate Brianza, Italy who provided the photovoltaic cells. The contribution of the doctors and other staff in Lui and other areas are gratefully acknowledged.
Conflict of interest
None declared
References
- 1.Raper A R L. Endemic dwarfism in Uganda. East African Medical Journal. 1950;9:339–359. [PubMed] [Google Scholar]
- 2.Kaiser C, Bennibger C, Asaba G, Mugisa C, Kabagambe G, Kipp W, et al. Clinical and electro-clinical classification of epileptic seizures in West Uganda. Bull Soc Athol Exot. 2000;4:255–259. [PubMed] [Google Scholar]
- 3.Jilek-Aaall L, Jilek W, Miller JR. Clinical and genetic aspects of siezure disorders prevalent in an isolated African population. Epilepsia. 1979;20:613–622. doi: 10.1111/j.1528-1157.1979.tb04845.x. [DOI] [PubMed] [Google Scholar]
- 4.Matuja WPB, Kilonzo G, Mbena P, Mwango'mbola RL, Wong P, Goodfellow P, et al. Risk factors for epilepsy in a rural area in Tanzania: a community-based case-control study. Neuroepidemiology. 2001;20:241–247. doi: 10.1159/000054797. [DOI] [PubMed] [Google Scholar]
- 5.Van der Waals FW, Goudsmit J, Gajdusek C, See-se Clinical characteristics of highly prevalent seizure disorders in Gbawein and Wroughbarh clan region of Grand Bassa County, Liberia. Neuroepidemiology. 1983;2:35–44. [Google Scholar]
- 6.Winkler AS, Friedrich K, Konig R, Meindl M, Helbok R I U, et al. The head nodding Syndrome: clinical manifestations and possible causes. Epilepsia. 2008;49(12):2008. doi: 10.1111/j.1528-1167.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 7.Nyungura JL, Akim T, Lako A, Abe G, Lejeng L, William G. Investigation into nodding Syndrome in Witto Payam, Western Equatoria state, 201. Southern Sudan Medical Journal. 2011;4(1):3. [Google Scholar]
- 8.Reik L, Abubakar A, Opoka M, Mindra G, Sejvar J, Dowell SF, et al. Nodding syndrome South Sudan 2011. MMWR. 2012;61(3):52–54. [PubMed] [Google Scholar]