Abstract
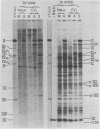
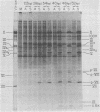
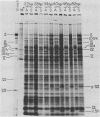
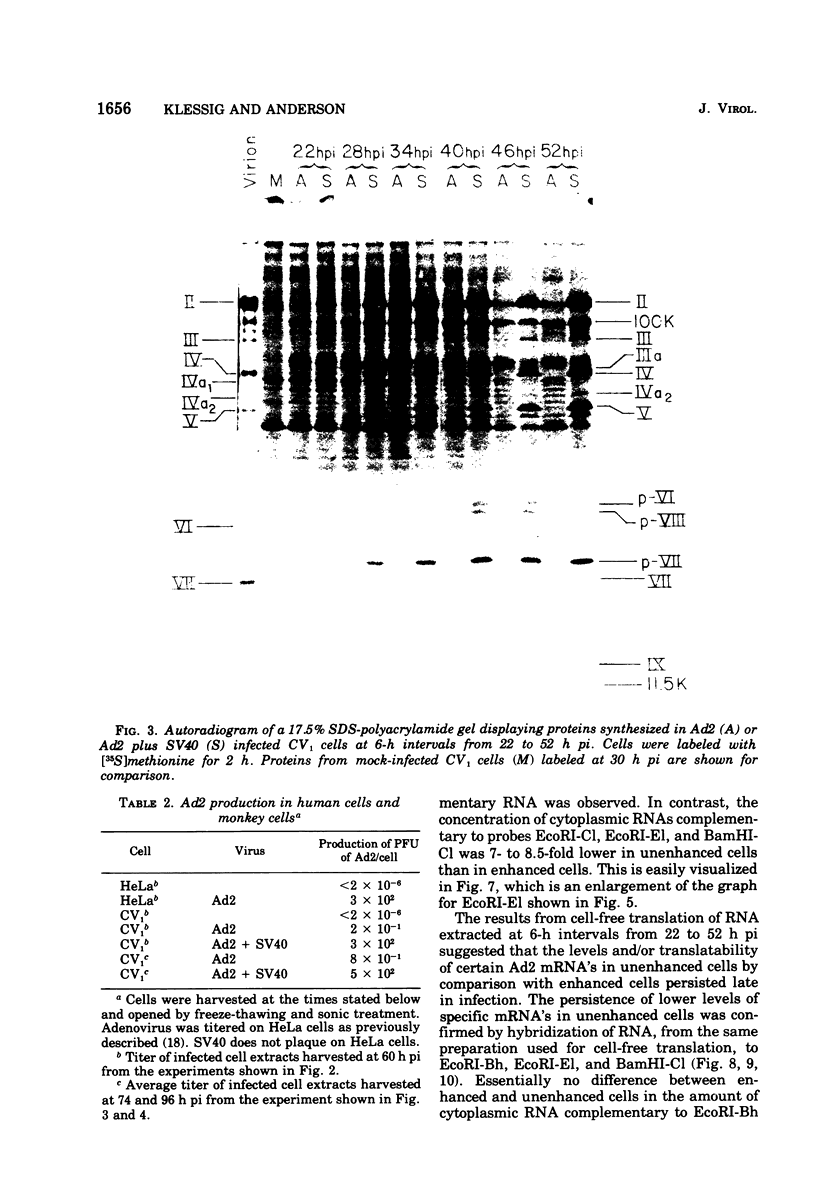
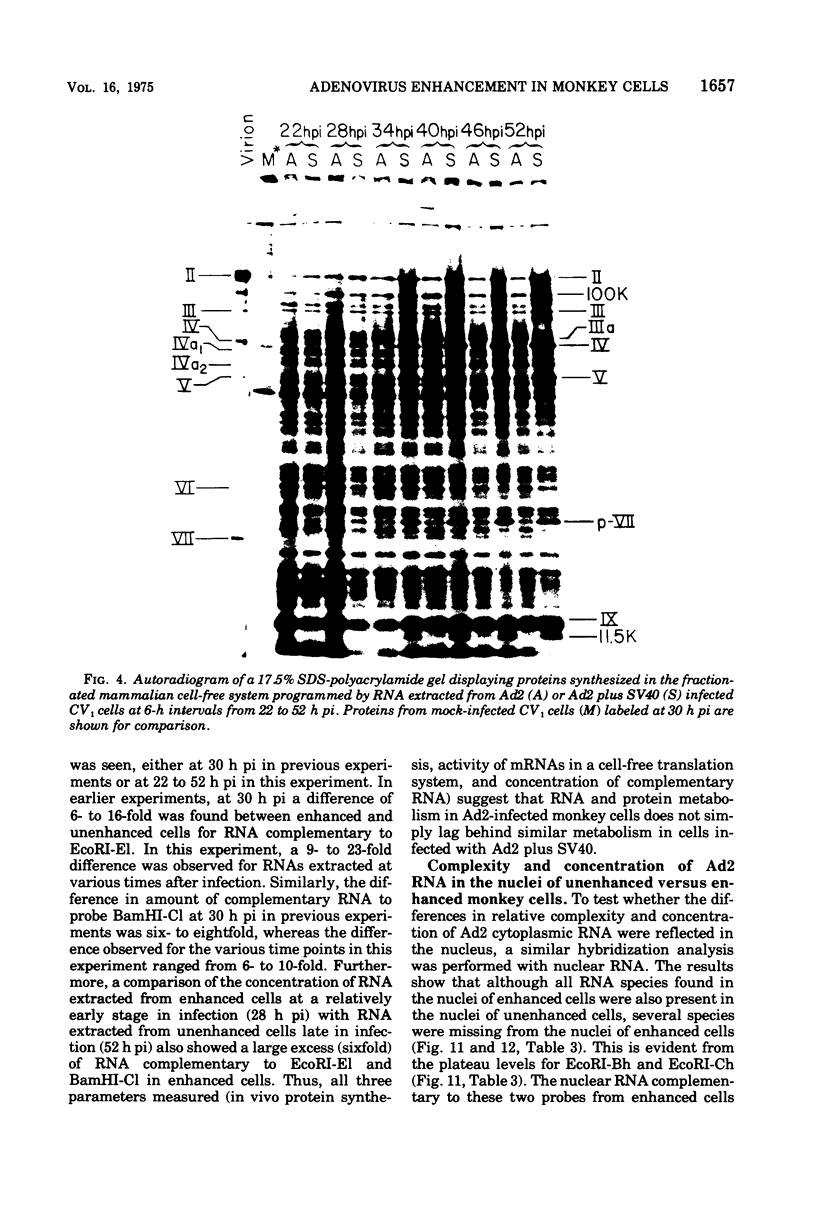
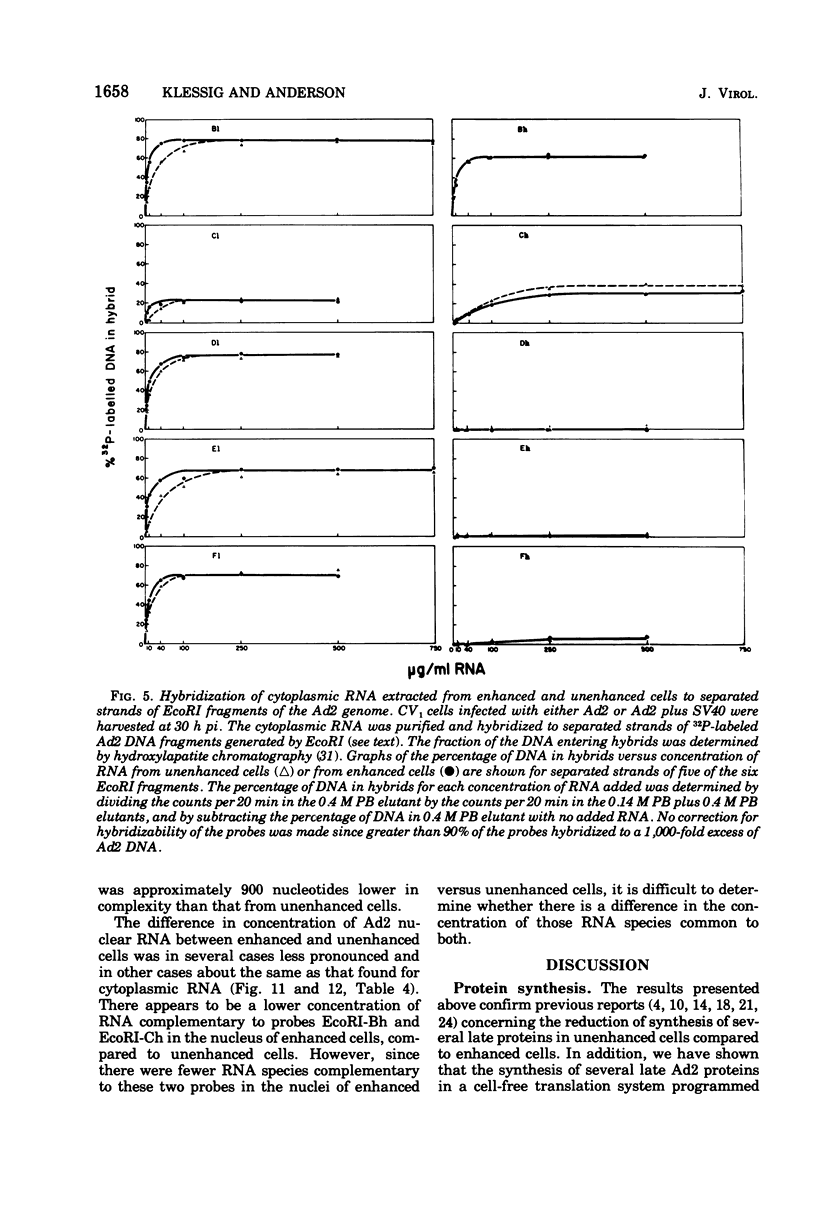
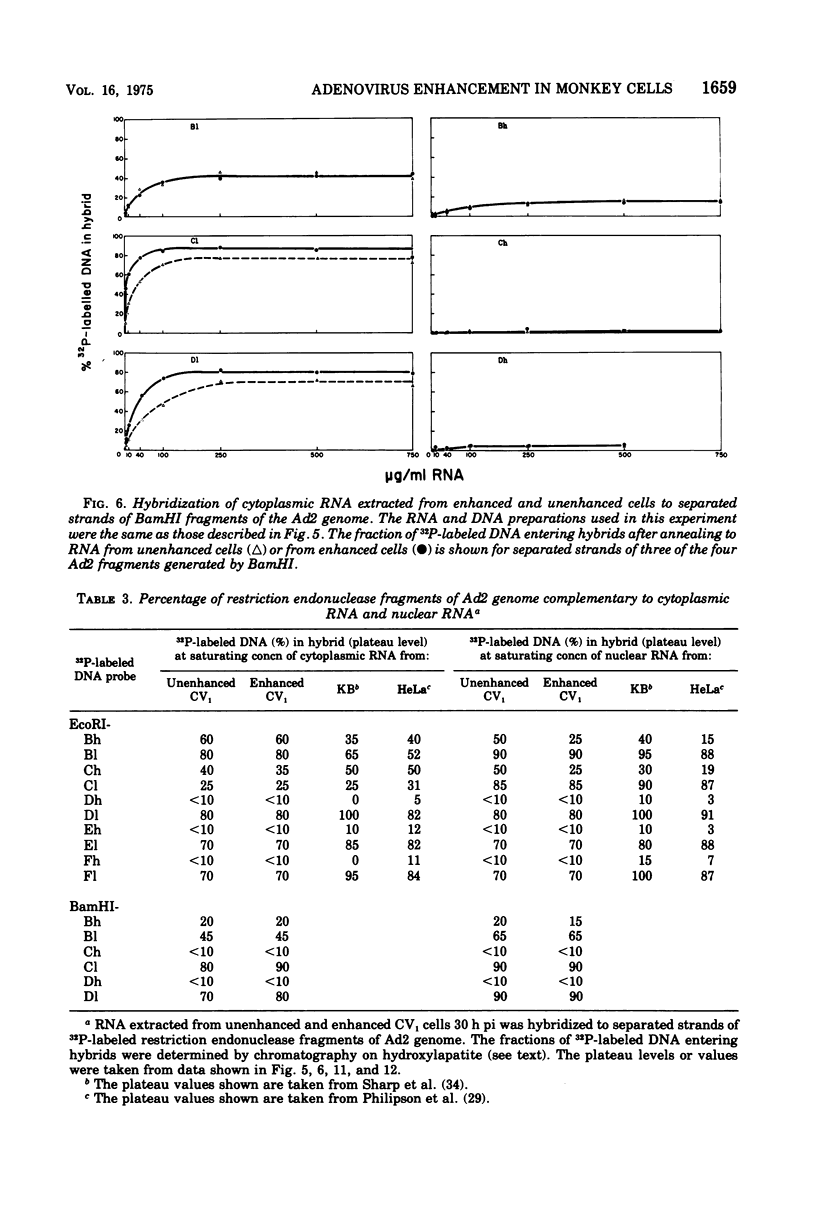
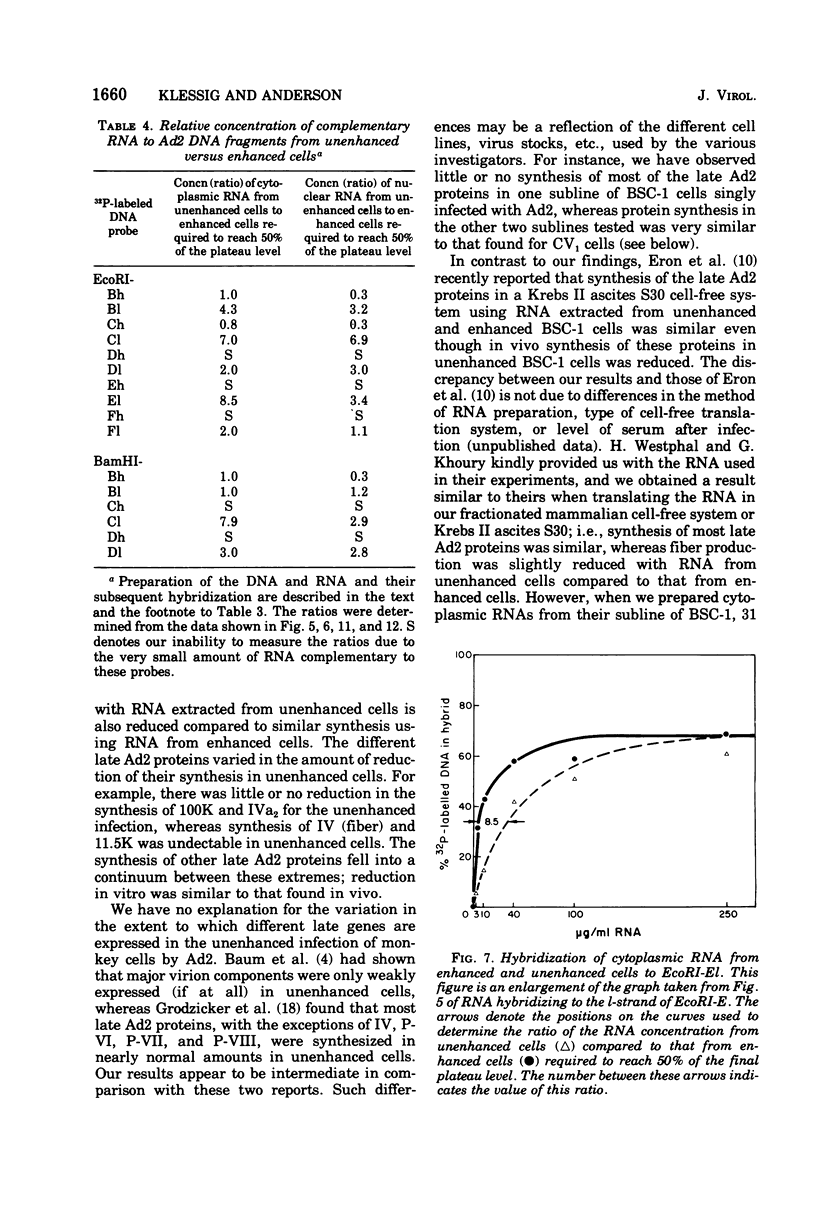
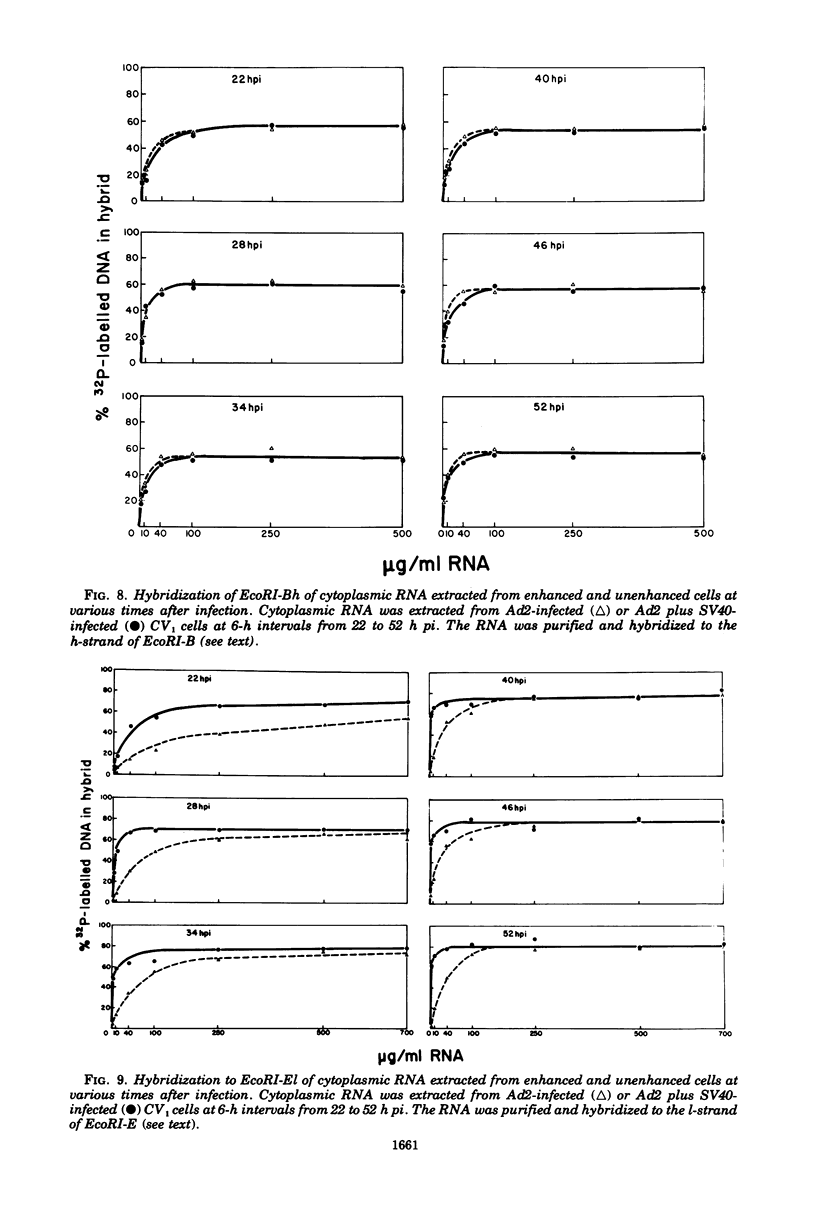
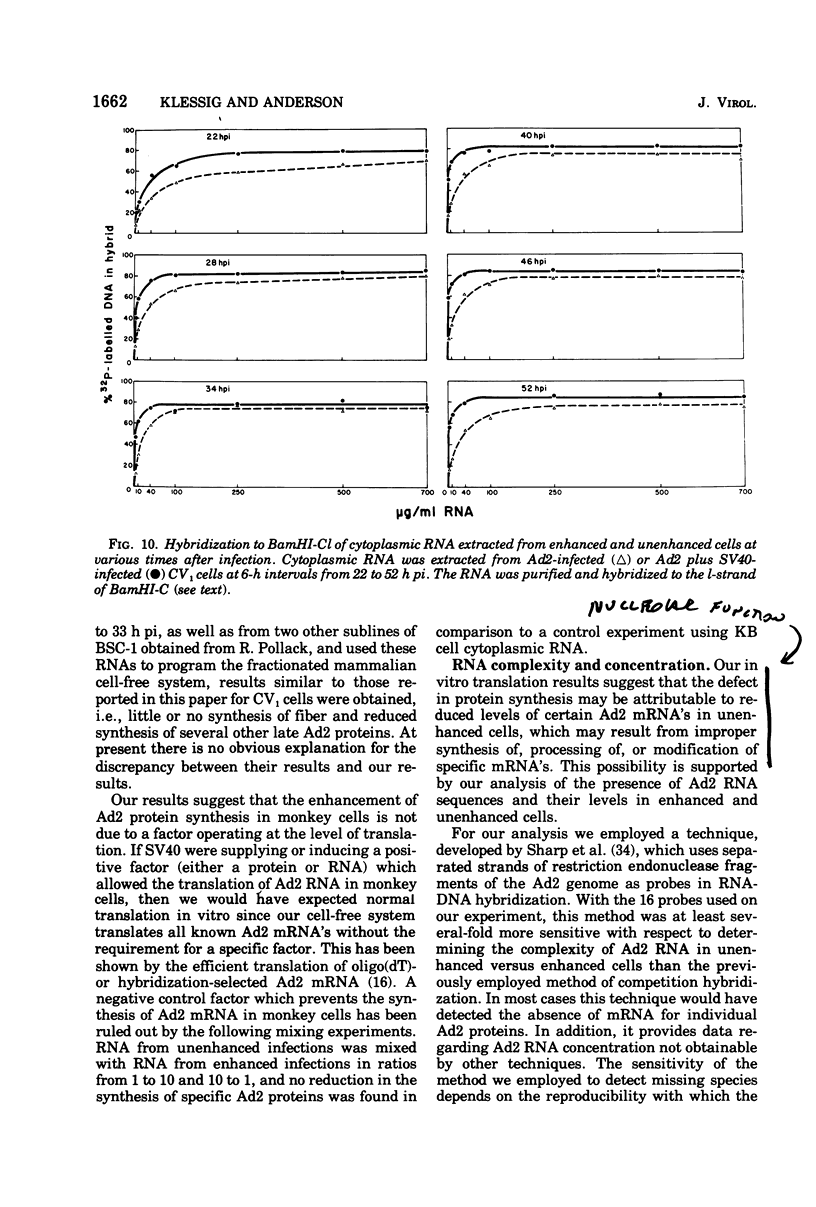
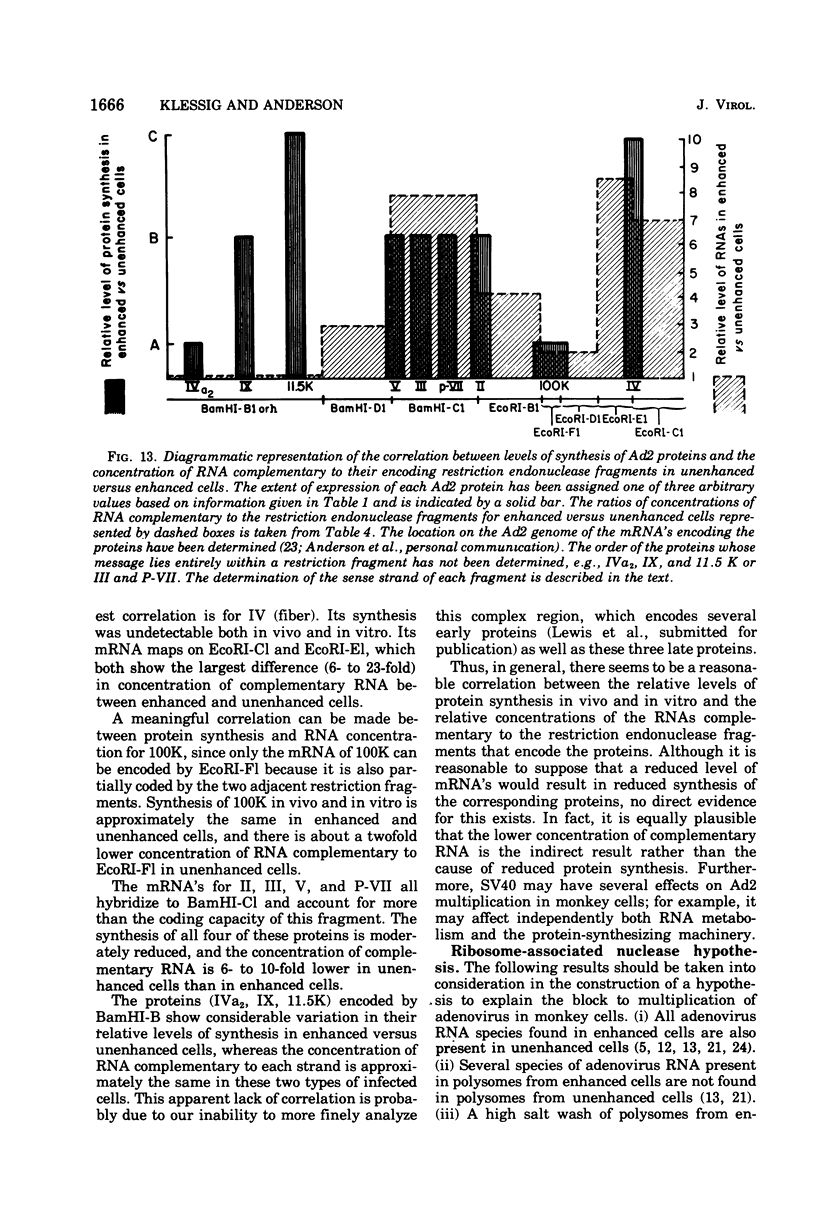
The block to adenovirus 2 (Ad2) multiplication in monkey cells can be overcome by coinfection with simian virus 40 (SV40). To identify this block we have compared the synthesis of Ad2 proteins in monkey cells infected with Ad2 alone (unenhanced) or with Ad2 plus SV40 (enhanced). Synthesis of viral proteins in enhanced cells was virtually identical to that found for permissive infection of human cells by Ad2 alone. In contrast, the unenhanced cells were strikingly deficient in the production of the IV (fiber) and 11.5K proteins whereas the synthesis of 100K and IVa2 was normal. Synthesis of a number of other proteins such as II, V, and P-VII was partially reduced. A similar specific reduction in synthesis of these proteins was found when their messages were assayed by cell-free translation. This result suggests that the block to Ad2 protein synthesis is at the RNA level rather than with the translational machinery of monkey cells. Analysis of the complexity and the concentration of Ak2-specific RNAs, using hybridization of restriction endonuclease fragments of the Ad2 genome to increasing concentrations of RNA, shows that although all species of late Ad2 mRNA are present, the concentration of several species is reduced sevenfold or more in unenhanced monkey cells as compared with enhanced cells. These species come from regions of the genome known to encode the deficient proteins. A model for the failure of adenovirus to multiply in monkey cells, based on abnormal processing of specific adenovirus messages, is presented.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. G., Horwitz M. S., Maizel J. V., Jr Studies of the mechanism of enhancement of human adenovirus infection in monkey cells by simian virus 40. J Virol. 1972 Aug;10(2):211–219. doi: 10.1128/jvi.10.2.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. G., Wiese W. H., Reich P. R. Studies on the mechanism of enhancement of adenovirus 7 infection in African green monkey cells by simian virus 40: formation of adenovirus-specific RNA. Virology. 1968 Feb;34(2):373–376. doi: 10.1016/0042-6822(68)90253-5. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Altman S. Characterization of ribonuclease NU cleavage sites in a bacteriophage phi80-induced ribonucleic acid. J Biol Chem. 1975 Feb 25;250(4):1460–1463. [PubMed] [Google Scholar]
- Bothwell A. L., Altman S. Partial purification and properties of an endoribonuclease isolated from human KB cells. J Biol Chem. 1975 Feb 25;250(4):1451–1459. [PubMed] [Google Scholar]
- Craig E. A. Analysis of early adenovirus 2 RNA using Eco R-R1 viral DNA fragments. J Virol. 1975 May;15(5):1202–1213. doi: 10.1128/jvi.15.5.1202-1213.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmyter J., Melnick J. L., Rawls W. E. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J Virol. 1968 Oct;2(10):955–961. doi: 10.1128/jvi.2.10.955-961.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L. Post-transcriptional restriction of human adenovirus expression in monkey cells. J Virol. 1975 May;15(5):1256–1261. doi: 10.1128/jvi.15.5.1256-1261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. A., Butel J. S., Rapp F. Interaction of a simian papovavirus and adenoviruses. I. Induction of adenovirus tumor antigen during abortive infection of simian cells. J Bacteriol. 1966 Feb;91(2):813–818. doi: 10.1128/jb.91.2.813-818.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. I., Baum S. G. Posttranscriptional block to adenovirus replication in nonpermissive monkey cells. Virology. 1974 Jul;60(1):45–53. doi: 10.1016/0042-6822(74)90364-x. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Baum S. G. Synthesis of viral ribonucleic acid during restricted adenovirus infection. J Virol. 1972 Aug;10(2):220–227. doi: 10.1128/jvi.10.2.220-227.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. P., Lyons M. J., Ginsberg H. S. Biochemical consequences of type 2 adenovirus and Simian virus 40 double infections of African green monkey kidney cells. J Virol. 1970 May;5(5):586–597. doi: 10.1128/jvi.5.5.586-597.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Metz D. H., Esteban R. M., Tovell D. R., Ball L. A., Kerr I. M. Mechanism of interferon action: inhibition of viral messenger ribonucleic acid translation in L-cell extracts. J Virol. 1972 Dec;10(6):1184–1198. doi: 10.1128/jvi.10.6.1184-1198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuke M., Thomas C. A., Jr Isolation of open-circular DNA molecules by retention in agar gels. J Mol Biol. 1970 Sep 14;52(2):395–397. doi: 10.1016/0022-2836(70)90040-9. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Anderson C., Sharp P. A., Sambrook J. Conditional lethal mutants of adenovirus 2-simian virus 40 hybrids. I. Host range mutants of Ad2+ND1. J Virol. 1974 Jun;13(6):1237–1244. doi: 10.1128/jvi.13.6.1237-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. L., Sopori M. L., Lengyel P. Inhibition of protein synthesis directed by added viral and cellular messenger RNAs in extracts of interferon-treated Ehrlich ascites tumor cells. Location and dominance of the inhibitor(s). Biochem Biophys Res Commun. 1973 Sep 18;54(2):777–783. doi: 10.1016/0006-291x(73)91491-5. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Sopori M. L., Lengyel P. Release of the inhibition of messenger RNA translation in extracts of interferon-treated Ehrlich ascites tumor cells by added transfer RNA. Biochem Biophys Res Commun. 1974 Apr 8;57(3):763–770. doi: 10.1016/0006-291x(74)90612-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Nakajima K., Oda K., Shimojo H. Complementation of translational defect for growth of human adenovirus type 2 in Simian cells by a Simian virus 40-induced factor. J Mol Biol. 1973 Dec 5;81(2):207–223. doi: 10.1016/0022-2836(73)90190-3. [DOI] [PubMed] [Google Scholar]
- Kumar A., Lindberg U. Characterization of messenger ribonucleoprotein and messenger RNA from KB cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):681–685. doi: 10.1073/pnas.69.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Transcription and transport of virus-specific ribonucleic acids in African green monkey kidney cells abortively infected with type 2 adenovirus. J Virol. 1972 Dec;10(6):1109–1117. doi: 10.1128/jvi.10.6.1109-1117.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Arrand J. R., Delius H., Keller W., Pettersson U., Roberts R. J., Sharp P. A. Cleavage maps of DNA from adenovirus types 2 and 5 by restriction endonucleases EcoRI and HpaI. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):397–400. doi: 10.1101/sqb.1974.039.01.051. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Ishitsuka H., Oda K. An SV40-induced initiation factor for protein synthesis concerned with the regulation of permissiveness. Nature. 1974 Dec 20;252(5485):649–653. doi: 10.1038/252649a0. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U., Tibbetts C., Vennström B., Persson T. RNA synthesis and processing in adenovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):447–456. doi: 10.1101/sqb.1974.039.01.057. [DOI] [PubMed] [Google Scholar]
- RABSON A. S., O'CONOR G. T., BEREZESKY I. K., PAUL F. J. ENHANCEMENT OF ADENOVIRUS GROWTH IN AFRICAN GREEN MONKEY KIDNEY CELL CULTURES BY SV40. Proc Soc Exp Biol Med. 1964 May;116:187–190. doi: 10.3181/00379727-116-29197. [DOI] [PubMed] [Google Scholar]
- Reich P. R., Baum S. G., Rose J. A., Rowe W. P., Weissman S. M. Nucleic acid homology studies of adenovirus type 7-SV40 interactions. Proc Natl Acad Sci U S A. 1966 Feb;55(2):336–341. doi: 10.1073/pnas.55.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Sharp P. A., Keller W. Transcription of Simian virus 40. I. Separation of the strands of SV40 DNA and hybridization of the separated strands to RNA extracted from lytically infected and transformed cells. J Mol Biol. 1972 Sep 14;70(1):57–71. doi: 10.1016/0022-2836(72)90163-5. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]