Abstract
We hypothesized that atomoxetine (ATMX) would produce similar brain effects in attention-deficit/hyperactivity disorder (ADHD) as those of methylphenidate (MPH). Eleven ADHD adults performed the Multi-Source Interference Task (MSIT) during fMRI at baseline and after 6 weeks of ATMX treatment. ATMX was associated with increased fMRI activation of dorsolateral prefrontal cortex, parietal cortex and cerebellum; but not dorsal anterior midcingulate cortex (daMCC). These results suggest that ATMX and MPH have similar but non-identical brain effects.
Keywords: Neuroimaging, Psychopharmacology, Neurobiology
1. Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is among the most common neurobehavioral disorders in children and adolescents, and frequently persists into adulthood. Atomoxetine (ATMX), a non-stimulant norepinephrine transporter (NET) specific reuptake inhibitor, is a safe and effective treatment for ADHD (Adler et al., 2005). While a few studies have provided insights (Arnsten, 2006; Seneca et al., 2006; Logan et al., 2007; Pliszka, 2007; Chamberlain et al., 2009; Takano et al., 2009), the neural mechanisms by which ATMX exerts therapeutic effects remain unresolved.
Convergent data implicate dysfunction of many brain regions in ADHD pathophysiology, including the dorsal anterior midcingulate (daMCC), dorsolateral prefrontal (DLPFC), and parietal cortices (together, the cingulo-frontal-parietal [CFP] cognitive-attention network), along with striatum and cerebellum (Paloyelis et al., 2007; Bush, 2009; Bush, 2011). Thus, it was expected that ATMX treatment would affect the CFP network. We used the Multi-Source Interference Task (MSIT), a cognitive fMRI paradigm with demonstrated ability to activate daMCC and the CFP network (Bush and Shin, 2006). Since clinical response may not maximally differentiate from placebo until 6 weeks with ATMX (Montoya et al., 2009) or MPH (Spencer et al., 2005; Biederman et al., 2006), we scanned subjects at baseline and after 6 weeks treatment.
Specifically, we hypothesized ATMX would increase fMRI activation in CFP regions that subserve attention and cognition. We also predicted that compared to our previously published sample of 21 adults with ADHD-childhood onset (ADHD-CO) (Bush et al., 2008) ATMX fMRI responses would be similar to those produced by MPH and differentiable from placebo. To facilitate cross-study comparability and explore the degree of neurobiological overlap of ADHD-NOS with ADHD-CO, we compared baseline MSIT fMRI data of the 11 adults with ADHD-NOS to that from the previously published sample.
2. Methods
Methods were identical to those used in the previously published sample (Bush et al., 2008), except as indicated here, and are included in the Supplemental Material. Written informed consent was obtained per Massachusetts General Hospital Subcommittee on Human Subjects guidelines. Eleven unmedicated adults (6M/5F) with ADHD, Not Otherwise Specified (ADHD-NOS) completed the study. The previously published MPH (N = 11) and placebo (N = 10) samples (Bush et al., 2008) were diagnosed with ADHD-childhood onset (ADHD-CO). Inclusion and exclusion criteria for all subjects (N = 32) were otherwise identical. MSIT procedures (Bush and Shin, 2006; Bush et al., 2008) are summarized in Supplemental Figure 1. The ATMX group displayed the expected performance effects and did not differ in severity or demographically from the prior published sample (see Supplemental, Table 1).
Functional MRI analyses were as follows. The primary contrast examined which brain regions displayed an effect of 6 weeks of ATMX. A multi-step random effects repeated measures ANOVA GLM analysis was used. First, a mask representing all voxels MSITInterference > MSITControl (P < .05 uncorrected) for all 32 subjects (11 ATMX, 11 MPH, 10 placebo) during Scan 2 was applied to restrict analysis to brain areas specifically involved in cognition. Within masked voxels for the ATMX group, a random effects repeated measures ANOVA GLM identified regions with MSITInterference fMRI activation higher at 6 weeks of ATMX treatment than at baseline. A stringent cluster constraint requiring ≥ 7 contiguous P < .05 voxels (Forman et al., 1995) produced a regional false positive probability of P < 1 × 10−4 corrected for multiple comparisons and matched prior conservative α thresholds.
Secondary analyses compared ATMX fMRI responses to published MPH and placebo data (Bush et al., 2008). For ATMX versus placebo and MPH analyses, a mask representing all MSITInterference > MSITControl voxels (P < .05 uncorrected) for all 32 subjects during Scans 1 and 2 restricted analyses to brain areas specifically involved in cognitive task performance. Within masked voxels, random effects repeated measures ANOVA GLMs identified brain regions with higher Scan 2 MSITInterference activation in the ATMX group than the MPH and Placebo groups. An additional step tested for interactions (i.e., regions had to show both a treatment group (ATMX versus placebo or MPH, respectively) × scan (Scan 1 baseline versus Scan 2 six weeks) interaction, and a confirmatory two-tailed t-test had to indicate significantly different Scan 2 activation in ATMX than placebo or MPH, respectively.
Exploratory analysis compared baseline fMRI responses of the ADHD-CO (N = 21) and ADHD-NOS (N = 11) groups during the MSIT. A GLM compared the MSITInterference activation levels at baseline.
3. Results
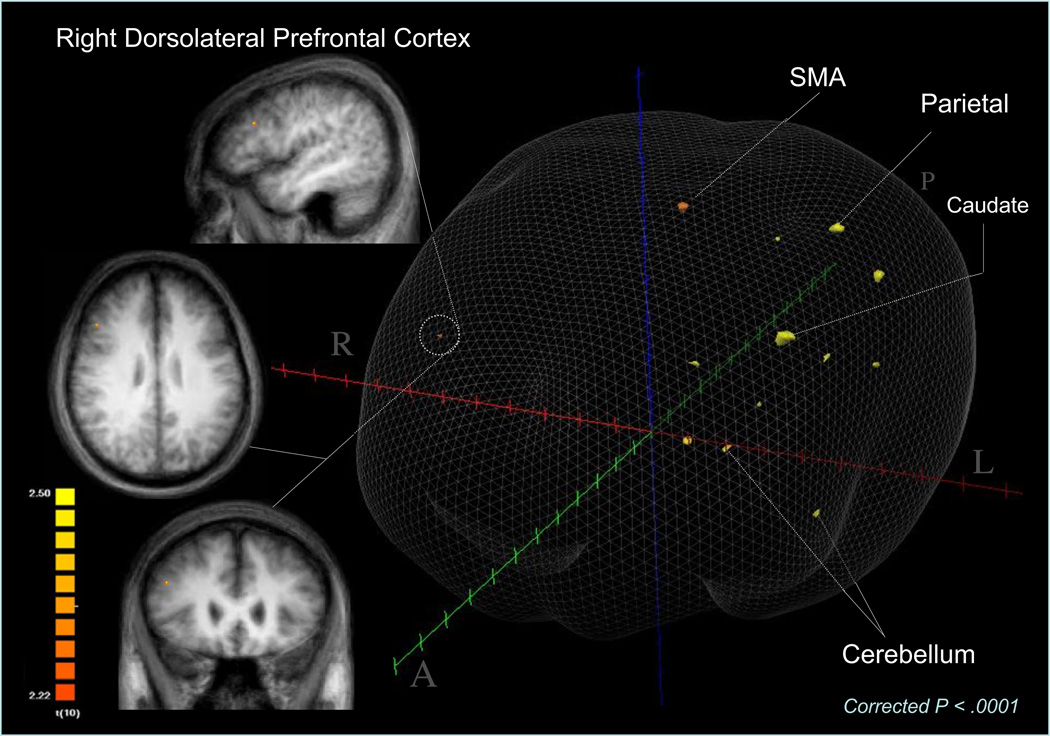
Primary within subject analysis showed six weeks of ATMX treatment increased activation of many elements of the CFP cognitive-attention network and other brain regions that MPH was shown to activate previously (Bush et al., 2008). Specifically, Figure 1 and Supplemental Table 2 show ATMX increased activation of right DLPFC, inferior parietal lobule, supplementary motor area, insular cortex, caudate, bilateral cerebellum and fusiform gyrus. Notably, ATMX did not produce the predicted increase of daMCC activation.
Figure 1.
Atomoxetine Produces Higher fMRI Activation at 6 Weeks
Although the ATMX versus Placebo contrast did not show differences at 6 weeks in a priori predicted areas of the CFP attention network, seven post hoc clusters showed ATMX>Placebo at six weeks (Supplemental Table 2). Similarly, ATMX versus MPH analysis produced no differences in a priori areas, but two post hoc clusters in MCC showed ATMX>MPH at 6 weeks. No region showed MPH > ATMX group at six weeks.
Baseline GLM comparisons of MSITInterference activation between the ADHD-CO (N = 21) and ADHD-NOS (N = 11) samples showed no significant differences between the two groups.
4. Discussion
Six weeks of ATMX treatment was associated with increased activation of the CFP cognitive-attention network and other brain regions that were previously documented to activate by MPH treatment (Bush et al., 2008). Notably ATMX did not increase daMCC activation from baseline. Secondarily, while ATMX did not differentiate versus MPH or Placebo in a priori predicted areas, post hoc analyses identified ATMX>Placebo at 6 weeks in multiple MCC clusters and caudate; and ATMX>MPH in two MCC clusters. Neither of these comparisons showed a significant treatment group × scan interaction. Also, no differences were found in baseline fMRI activation between ADHD-CO and ADHD-NOS groups. Together, the data indicate ATMX and MPH have similar but non-identical brain effects.
To our knowledge this is the first fMRI study of ATMX in ADHD. In fact, there have been few imaging studies of ATMX, and all have used a single dose in healthy humans or were done in patients with schizophrenia (Friedman et al., 2008; Chamberlain et al., 2009; Graf et al., 2011; Marquand et al., 2011; Hester et al., 2012; Marquand et al., 2012). Our findings complement and extend such work by showing ATMX effects in adults with ADHD over a longer, clinically relevant trial period, and using a different task.
The data show that ATMX was associated with increased activation of DLPFC, parietal cortex, caudate, and cerebellum—brain regions implicated in attention, motor control and the pathophysiology of ADHD (Bush, 2010). Further, the brain responses to ATMX and MPH were generally similar, but notable differences existed. ATMX did not activate daMCC as MPH has been shown to do (Bush et al., 2008). Also, ATMX did not produce significant activation of other regions activated by MPH, such as premotor cortex and thalamus. Together, the data indicate that while drug effects may overlap, differences exist in neurobiological actions.
Together with reported findings of ATMX NET binding changes (Logan et al., 2007), our data suggest ATMX may exert primary effects by NET receptor binding in the locus coeruleus, thalamus, midbrain, cingulate cortex and supplementary motor area, along with possible secondary downstream modulatory effects of DLPFC, parietal cortex and cerebellum. ATMX may initiate effects by blocking NET and increasing synaptic availability of norepinephrine, which may boost “downstream” signal-to-noise in fronto-parietal and cerebellar regions. This could improve attention, filtering of distracting information, and possibly regulate motor inhibition (thereby reducing hyperactivity and impulsivity). Differential actions of ATMX and MPH are broadly in line with the conceptualization of Arnsten (Arnsten, 2009) who concluded ATMX works mainly through postsynaptic alpha(2A)-receptors on the dendritic spines of PFC pyramidal cells and partially through D1-dopamine receptor actions. Some of the overlap of ATMX and MPH effects could be explained by findings that both inhibit NET (Hannestad et al., 2010), though complex differences between the drugs remain to be characterized.
Limitations include results being only valid for ADHD adults and that, aside from predicted fronto-parietal and cerebellar activations, other identified regions are post hoc and must be replicated. Moreover, the sample was small and there was no direct comparison group. Future work seeking to compare ATMX, MPH and/or placebo should consider a randomized or crossover design. Specifically, a crossover design would serve to eliminate anatomical variability between different subject groups in case the ATMX effect is subtle. Also, while identifying regions by post-medication activation is justifiable and valid for this pilot study, it does introduce the potential for circularity. Future work building on this pilot study should consider employing a matched healthy control group whose data will allow independent definition of regions of interest, avoiding circularity (Kriegeskorte et al., 2010). Finally, ADHD-NOS and ADHD-CO comparisons in this pilot study are preliminary pending replication.
In this pilot study, six weeks of treatment with ATMX was associated with increased activation of brain regions implicated in the pathophysiology of ADHD including DLPFC, parietal cortex, caudate and cerebellum. Many of these regions had been activated by MPH in our prior fMRI study, and here the observed differences between ATMX and MPH activation patterns were minimal. Notably, ATMX did not significantly activate daMCC. The data indicate that differences in cortical effects of ATMX and MPH may underlie their differential clinical effects.
Supplementary Material
Table 1.
MSIT Performance Data
| Group | Scan 1 (Baseline, t = 0) Interference |
Scan 1 (Baseline, t = 0) Control |
Scan 2 (t = 6 weeks) Interference |
Scan 2 (t = 6 weeks) Control |
|---|---|---|---|---|
| REACTION TIMES (ms) | ||||
| ATMX (All) | 1026 ± 77 | 702 ± 104 | 922 ± 145 | 634 ± 121 |
| ACCURACY (%) | ||||
| ATMX (All) | 95.6 ± 3.1 | 95.4 ± 5.8 | 92.1 ± 9.0 | 90.0 ± 8.4 |
Means and standard deviations are presented for RT (ms) and accuracy (% correct). RT and accuracy data were lost/corrupted for two subjects, both of whom were ATMX treatment responders. Thus the term ‘all’ refers to full remaining group data (N = 9 of 11), divided between ATMX Responders (N = 5 of 7 responders) and ATMX Failures (N = 4). Repeated measures ANOVAs showed that the ATMX group displayed the expected interference effects (RTinterference > RTcontrol with significant main effects of condition [F1,8 = 130.2, P < .001]) and scan (F1,8 = 9.5, P < .05), but no scan × condition interaction (P = .12, N.S.) for RT. For accuracy, the ATMX group did not show a significant main effect of condition [F1,8 = 1.1, P = .32, N.S.]) or scan (F1,8 = 2.9, P = 1.3, N.S.), and did not display a significant scan × condition interaction (P = .53, N.S.). Separate 3 (Group: ATMX vs. MPH vs. Placebo) by 2 (Condition: MSITinterference vs. MSITcontrol) by 2 (Scan [Scan 1 (baseline, t = 0) vs. Scan 2 (t = 6 weeks)] repeated measures ANOVAs were performed for RT and accuracy. For RT, there were main effects of group (F2,27 = 11.2, P < .001), scan (F2,27 = 68.3, P < .001) and condition (F2,27 = 503.5, P < .0001), but no scan × group (P = .98), condition × group (P = .53), nor scan × condition × group (P = .72) interactions for RT. For accuracy, there were main effects of group (F2,27 = 8.3, P < .01) and condition (F2,27 = 4.7, P < .05), but not of scan (F2,27 = 1.0, P = .33). There were scan × group (F2,27 = 3.7, P < .05) and condition × group (F2,27 = 5.6, P < .01) interactions, but not a scan × condition × group (F2,27 = 0.1, P = .92, N.S.) interaction.
Table 2.
Brain Regions Showing Higher MSIT Activation After a 6-Weeks Trial of Atomoxetine than at Baseline (time = 0) in ADHD Adults
| Brain Region | x | y | z | Region Size (Voxels) |
|---|---|---|---|---|
| R. DLPFC - Middle Frontal Gyrus (9) | 45 | 22 | 28 | 7 |
| L. Supplementary Motor Area (6) | −8 | −4 | 58 | 23 |
| L. Insula | −30 | 2 | 13 | 7 |
| L. Substantia nigra | −3 | −14 | −8 | 25 |
| L. Postcentral Gyrus (1) | −51 | −23 | 40 | 29 |
| L. Inferior Parietal Lobule (40) | −36 | −33 | 46 | 35 |
| L. Caudate Tail | −22 | −34 | 15 | 56 |
| L. Posterior Cingulate Cortex (31) | −12 | −55 | 34 | 9 |
| L. Cerebellum | −21 | −60 | −47 | 17 |
| R. Cerebellum | 4 | −54 | −29 | 16 |
| L. Fusiform Gyrus (19) | −24 | −58 | 1 | 13 |
| L. Fusiform Gyrus (19) | −36 | −64 | −2 | 15 |
Group-averaged activation locations and extents are shown for brain regions that showed higher fMRI activation in ADHD adults (N = 11) during MSIT Interference trials after 6 weeks of ATMX. The activation locations shown are the result of a random effects GLM analysis, with a voxel-wise threshold of P < .05 and to which an additional rigorous 7 contiguous voxel cluster constraint was applied to correct for multiple comparisons (cluster corrected P < .0001). Stereotactic coordinates are presented for local maxima according to the convention of Talairach and Tournoux (1988). Coordinates are expressed in millimeter units. The origin (0,0,0) is the anterior commissure at the midsagittal plane, with x > 0 corresponding to right of midsagittal, y > 0 corresponding to anterior, and z > 0 corresponding to superior. Cytoarchitectural areas are indicated in parentheses after the named structure.
Acknowledgments
The authors thank the subjects for their patience and willingness to follow through with multiple testing sessions. We also thank Paul Hammerness MD, Carter Petty, Robert Doyle MD, Courtney Ziefle, Megan Aleardi, Meghan Kotarski and our research assistants for their assistance with subject recruitment and screening; Mary Foley, Larry White, Jill Clark and Julie Bates for their assistance with scanning; the staff of Brain Innovation (Armin Heinecke) for consultation and guidance on data analytic software solutions, and the editor and the anonymous reviewers for their helpful comments.
Financial Disclosures
Grant support was provided by Eli Lilly & Company, the Benson-Henry Institute at MGH for Mind-Body Medicine, the Pediatric Psychopharmacology Council Fund, the David Judah Fund, and the Mental Illness and Neuroscience Discovery (MIND) Institute. General support for the investigators and/or for the clinical studies from which subjects were ascertained was also provided by the Centers for Disease Control (5 R01 DP000339), the McIngvale Fund, and McNeil Pharmaceuticals (GB, JH); NIMH MH/HD 62152, (LJS); the National Alliance for Research on Schizophrenia and Depression, the Johnson and Johnson Center for the Study of Psychopathology (JB); and the Center for Functional Neuroimaging Technologies (P41RR14075). This study was initiated by the investigators. Eli Lilly & Company and McNeil Consumer & Specialty Pharmaceuticals provided funding and assisted in study design, but did not assist in the conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Dr. Bush has, or has had in the past, a relationship with one or more organizations listed below as follows: research support, former advisory board member and/or speaker’s honoraria from Eli Lilly and Company, McNeil Pharmaceuticals and Novartis Pharmaceuticals; fellowship support from Pfizer, Inc.; and has received speaker’s honoraria from Shire U.S. Inc., Janssen Pharmaceuticals, Johnson & Johnson and McNeil Pharmaceuticals. Dr. Bush has served as a judge for Intel Corporation Science Talent Search for which he received honoraria. Dr. Bush does not now and has not at any time had a financial interest in any of these entities.
Ms. Holmes has received research coordinator support from Eli Lilly, McNeil, the Centers for Disease Control (CDC) and the Benson-Henry Institute for Mind-Body Medicine.
Dr. Surman has received research support from Abbott, Alza, Cephalon, Eli Lilly, the Hilda and Preston Davis Foundation, McNeil, Merck, New River, National Institutes of Health, Organon, Pamlab, Pfizer, Shire, and Takeda; has been a speaker for Janssen-Ortho, McNeil, Novartis, and Shire; and has been a consultant/advisor to McNeil, Shire, Somaxon and Takeda.
Dr. Eric Mick receives research support from the following sources: Ortho-McNeil Janssen Scientific Affairs, Pfizer, Shire Pharmaceuticals, and has been an advisory board member for Shire Pharmaceuticals.
Dr. Seidman has received investigator initiated research funding support from multiple not-for profit entities including the National Institute of Mental Health, the National Institute on Aging, the Commonwealth of Massachusetts Department of Mental Health, the National Alliance for Research on Schizophrenia and Depression (NARSAD), and the Sidney R. Baer Jr. Foundation. He has not received funding from for-profit entities in the past 24 months. In the past he received unrestricted educational support from Janssen Pharmaceuticals and has served as a consultant for Shire.
Dr. Joseph Biederman is currently receiving research support from the following sources: Elminda, Janssen, McNeil, and Shire. In 2012, Dr. Joseph Biederman received an honorarium from the MGH Psychiatry Academy for a tuition-funded CME course. In 2011, Dr. Joseph Biederman gave a single unpaid talk for Juste Pharmaceutical Spain, received honoraria from the MGH Psychiatry Academy for a tuition-funded CME course, and received an honorarium for presenting at an international scientific conference on ADHD. He also received an honorarium from Cambridge University Press for a chapter publication. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Eli Lilly, Shire and AstraZeneca; these royalties are paid to the Department of Psychiatry at MGH. In 2010, Dr. Joseph Biederman received a speaker’s fee from a single talk given at Fundación Dr.Manuel Camelo A.C. in Monterrey Mexico. Dr. Biederman provided single consultations for Shionogi Pharma Inc. and Cipher Pharmaceuticals Inc.; the honoraria for these consultations were paid to the Department of Psychiatry at the MGH. Dr. Biederman received honoraria from the MGH Psychiatry Academy for a tuition-funded CME course. In previous years, Dr. Joseph Biederman received research support, consultation fees, or speaker’s fees for/from the following additional sources: Abbott, Alza, AstraZeneca, Boston University, Bristol Myers Squibb, Celltech, Cephalon, Eli Lilly and Co., Esai, Fundacion Areces (Spain), Forest, Glaxo, Gliatech, Hastings Center, Janssen, McNeil, Medice Pharmaceuticals (Germany), Merck, MMC Pediatric, NARSAD, NIDA, New River, NICHD, NIMH, Novartis, Noven, Neurosearch, Organon, Otsuka, Pfizer, Pharmacia, Phase V Communications, Physicians Academy, The Prechter Foundation, Quantia Communications, Reed Exhibitions, Shire, the Spanish Child Psychiatry Association, The Stanley Foundation, UCB Pharma Inc., Veritas, and Wyeth.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Shin and Dr. Makris have no relevant financial disclosures.
References
- Adler LA, Spencer TJ, et al. Long-term, open-label study of the safety and efficacy of atomoxetine in adults with attention-deficit/hyperactivity disorder: an interim analysis. J Clin Psychiatry. 2005;66(3):294–299. doi: 10.4088/jcp.v66n0304. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stimulants: Therapeutic Actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, et al. A randomized, placebo-controlled trial of OROS-methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;59(9):829–835. doi: 10.1016/j.biopsych.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Bush G. Cingulate Neurobiology and Disease. B.A. Vogt: Oxford University Press; 2009. Dorsal anterior midcingulate cortex: Roles in normal cognition and disruption in attention-deficit/hyperactivity disorder. [Google Scholar]
- Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35(1):278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):1160–1167. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Shin LM. The Multi-Source Interference Task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat Protoc. 2006;1(1):308–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- Bush G, Spencer TJ, et al. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry. 2008;65(1):102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65(7):550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Carpenter D, et al. A pilot study of adjunctive atomoxetine treatment to second-generation antipsychotics for cognitive impairment in schizophrenia. J Clin Psychopharmacol. 2008;28(1):59–63. doi: 10.1097/jcp.0b013e318161318f. [DOI] [PubMed] [Google Scholar]
- Graf H, Abler B, et al. Neural correlates of error monitoring modulated by atomoxetine in healthy volunteers. Biol Psychiatry. 2011;69(9):890–897. doi: 10.1016/j.biopsych.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, et al. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry. 2010;68(9):854–860. doi: 10.1016/j.biopsych.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nandam LS, et al. Neurochemical Enhancement of Conscious Error Awareness. J Neurosci. 2012;32(8):2619–2627. doi: 10.1523/JNEUROSCI.4052-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Lindquist MA, et al. Everything you never wanted to know about circular analysis, but were afraid to ask. J Cereb Blood Flow Metab. 2010;30(9):1551–1557. doi: 10.1038/jcbfm.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Wang GJ, et al. Imaging the norepinephrine transporter in humans with (S,S)-[11C]O-methyl reboxetine and PET: problems and progress. Nucl Med Biol. 2007;34(6):667–679. doi: 10.1016/j.nucmedbio.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Marquand AF, De Simoni S, et al. Pattern classification of working memory networks reveals differential effects of methylphenidate, atomoxetine, and placebo in healthy volunteers. Neuropsychopharmacology. 2011;36(6):1237–1247. doi: 10.1038/npp.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquand AF, O'Daly OG, et al. Dissociable effects of methylphenidate, atomoxetine and placebo on regional cerebral blood flow in healthy volunteers at rest: A multi-class pattern recognition approach. Neuroimage. 2012;60(2):1015–1024. doi: 10.1016/j.neuroimage.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya A, Hervas A, et al. Evaluation of atomoxetine for first-line treatment of newly diagnosed, treatment-naive children and adolescents with attention deficit/hyperactivity disorder. Curr Med Res Opin. 2009;25(11):2745–2754. doi: 10.1185/03007990903316152. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, et al. Functional MRI in ADHD: a systematic literature review. Expert Rev Neurother. 2007;7(10):1337–1356. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka SR. Pharmacologic treatment of attention-deficit/hyperactivity disorder: efficacy, safety and mechanisms of action. Neuropsychol Rev. 2007;17(1):61–72. doi: 10.1007/s11065-006-9017-3. [DOI] [PubMed] [Google Scholar]
- Seneca N, Gulyas B, et al. Atomoxetine occupies the norepinephrine transporter in a dose-dependent fashion: a PET study in nonhuman primate brain using (S,S)-[18F]FMeNER-D2. Psychopharmacology (Berl) 2006;188(1):119–127. doi: 10.1007/s00213-006-0483-3. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(5):456–463. doi: 10.1016/j.biopsych.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Takano A, Gulyas B, et al. Saturated norepinephrine transporter occupancy by atomoxetine relevant to clinical doses: a rhesus monkey study with (S,S)-[(18)F]FMeNER-D (2) Eur J Nucl Med Mol Imaging. 2009;36(8):1308–1314. doi: 10.1007/s00259-009-1118-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.