Abstract
We tested the hypothesis that curcumin supplementation would reverse arterial dysfunction and vascular oxidative stress with aging. Young (Y, 4–6 mo) and old (O, 26–28 mo) male C57BL6/N mice were given normal or curcumin supplemented (0.2%) chow for 4 weeks (n = 5–10/group/measure). Large elastic artery stiffness, assessed by aortic pulse wave velocity (aPWV), was greater in O (448 ± 15 vs. 349 ± 15 cm/s) and associated with greater collagen I and advanced glycation end-products and less elastin (all P < 0.05). In O, curcumin restored aPWV (386 ± 15 cm/s), collagen I and AGEs to levels not different vs. Y. Ex vivo carotid artery acetylcholine (ACh)-induced endothelial-dependent dilation (EDD, 79 ± 3 vs. 94 ± 2%), nitric oxide (NO) bioavailability and protein expression of endothelial NO synthase (eNOS) were lower in O (all P < 0.05). In O, curcumin restored NO-mediated EDD (92 ± 2%) to levels of Y. Acute ex vivo administration of the superoxide dismutase (SOD) mimetic TEMPOL normalized EDD in O control mice (93 ± 3%), but had no effect in Y control or O curcumin treated animals. O had greater arterial nitrotyrosine abundance, superoxide production and NADPH oxidase p67 subunit expression, and lower manganese SOD (all P < 0.05), all of which were reversed with curcumin. Curcumin had no effects on Y. Curcumin supplementation ameliorates age-associated large elastic artery stiffening, NO-mediated vascular endothelial dysfunction, oxidative stress and increases in collagen and AGEs in mice. Curcumin may be a novel therapy for treating arterial aging in humans.
Keywords: AGEs, arterial stiffness, endothelial function, collagen
Introduction
Cardiovascular diseases (CVD) are the leading cause of mortality in the United States and advancing age is the primary risk factor for CVD (Roger and others, 2012). Arterial dysfunction with aging, characterized by large elastic artery stiffening and endothelial dysfunction, contributes importantly to the increase in CVD risk with aging (Lakatta and Levy, 2003). Increases in large elastic artery stiffness with aging, as shown by the gold standard clinical measure of aortic pulse wave velocity (aPWV) (Laurent and others, 2006; Vlachopoulos and others, 2010), is believed to be mediated in part by increases in collagen I deposition, reductions in elastin and modifications of these and other proteins by advanced glycation end-products (AGEs) (Lakatta and Levy, 2003; Zieman and others, 2005). Age-related endothelial dysfunction, as indicated by impaired endothelium-dependent dilation (EDD), is a consequence of reduced nitric oxide (NO) bioavailability (Celermajer and others, 1994; d'Uscio and others, 1997; Taddei and others, 1995) that may or may not be associated with changes in the NO-synthesizing enzyme endothelial nitric oxide synthase (eNOS) (Brandes and others, 2005; Muller-Delp, 2006; Seals and others, 2012).
Oxidative stress, as indicated by increased oxidant modification of biomolecules such as nitration of tyrosine residues on proteins (i.e., nitrotyrosine) (Radi, 2004), plays an important role in arterial aging (Brandes and others, 2005; Donato and others, 2007; Seals and others, 2012). Vascular oxidative stress with aging is mediated by excessive bioavailability of superoxide, which is associated with both increased expression of the p67 subunit of the oxidant enzyme NADPH oxidase (NOX) and reduced expression of the mitochondrial antioxidant enzyme manganese superoxide dismutase (MnSOD) (Donato and others, 2007; Fleenor and others, 2012; Pierce and others, 2011; Rippe and others, 2010; Sindler and others, 2011). Recently, we have shown excessive arterial superoxide production with aging in mice measured directly with electron paramagnetic resonance spectroscopy (Fleenor and others, 2012; Rippe and others, 2010; Sindler and others, 2011), and that both acute (Durrant and others, 2009; Rippe and others, 2010; Sindler and others, 2011) and chronic (Fleenor and others, 2012) treatment with the SOD mimetic and superoxide scavenger, TEMPOL, reverses large elastic artery stiffening and/or endothelial dysfunction in old mice. Therefore, interventions that normalize superoxide-dependent oxidative stress and can be safely administered to humans have the translational potential to improve large elastic artery stiffening and endothelial dysfunction with aging in humans.
Curcumin, from the plant curcuma longa, is a safe, naturally occurring polyphenol found in the Indian spice turmeric that has long been used in traditional Indian medicine (Miriyala and others, 2007). In some models of disease, supplementation with curcumin lowers superoxide/oxidative stress (Nakmareong and others, 2011b) and improves vascular dysfunction in young animals (Majithiya and Balaraman, 2005; Nakmareong and others, 2011b; Rungseesantivanon and others, 2010). Curcumin increases MnSOD expression in cultured esophageal cells (Schiffman and others, 2012) and extends lifespan (Kitani and others, 2004; Kitani and others, 2007) in non-disease mouse models. Moreover, curcumin is a readily accessible and has a good safety record in clinical trials (Cheng and others, 2001). It is unknown, however, if dietary curcumin supplementation can reverse age-related arterial dysfunction and oxidative stress.
In the present study, we tested the hypothesis that 4 weeks of dietary supplementation with curcumin would reduce large elastic artery stiffness, reverse age-associated changes in arterial collagen I, elastin and/or AGEs, and improve endothelial function by increasing NO bioavailability in old mice. We also tested the hypothesis that curcumin would normalize arterial oxidative stress and that this would be associated with reduced superoxide production and NOX expression, as well as increased expression of MnSOD.
Methods
Animals
Young (4–6 months) and old (26–28 months) male C57BL/6N mice at ages corresponding to ~25 and 75 year old humans, respectively (Flurkey and others, 2007), were purchased from the National Institute of Aging rodent colony. All mice were housed at the University of Colorado at Boulder in a 12:12 light:dark cycle vivarium and had ad libitum access to water and rodent chow. For 4 weeks, control mice were given normal rodent chow and treated mice were given curcumin-supplemented food at a dose (0.2%, Harlan) shown to increase survival in C57BL/6 mice (Kitani and others, 2004; Kitani and others, 2007). The number of animals studied per group was: Young control, 5; Old control, 10; Young curcumin, 7; Old curcumin, 10. Mortality was similar between old control and old curcumin treated mice. No young mice in either the control or curcumin group died. All protocols have been approved by the University of Colorado at Boulder Animal Care and Use Committee and abide to the Guide to the Care and Use of Laboratory Animals as stated in the National Institutes of Health guidelines (8th Edition, Revised 2011).
Aortic Pulse Wave Velocity
aPWV was assessed non-invasively as previously described by our laboratory and others (Fleenor and others, 2012; Kim and others, 2009; Reddy and others, 2003; Sindler and others, 2011). Briefly, isoflurane (2%) was used to anesthetize mice that were placed supine with legs secured to ECG electrodes on a heated board. Doppler probes were placed on the skin at the transverse aortic arch and abdominal aorta ~4 cm apart. For each site, the pre-ejection time, or time between the R-wave of the ECG to the foot of the Doppler signal was determined. To calculate aPWV, the distance between the probes was divided by the difference in the thoracic and abdominal pre-ejection times and is presented as centimeters/second (cm/s). Following aPWV measures, mice were euthanized by exsanguination via cardiac puncture while anesthetized with isoflurane.
Immunohistochemistry
Standard immunohistochemistry procedures and quantification were performed as previously described by our laboratory (Fleenor and others, 2010; Fleenor and others, 2012). Liquid nitrogen cooled isopentane was used to freeze aortic segments in optimal cutting temperature compound (Fisher Scientific). Acetone fixed sections (7 µm) were stained in a single batch with the Dako EnVision+ System-HRP-DAB kit as recommended by the manufacturer (Dako) with primary antibodies incubated at 4° C for 1 hour. A labeled polymer secondary was applied for 30 minutes, and 2-minute exposure to diaminobenzidine was used to visualize the staining. Slides were dehydrated in alcohols (50%, 75%, 80%, 95%, 100%), cleared in xylenes and cover-slipped. A Nikon Eclipse TS100 photomicroscope captured digital images at a 4X magnification that were analyzed with Image-Pro Plus software (Media Cybernetics). Primary antibodies used were specific for collagen I (1:4000, Millipore), alpha elastin (1:25, Abcam) and AGEs (1:200, GeneTex). A negative control was performed for each antibody.
Carotid artery vasodilatory responses
Ex vivo carotid artery endothelium-dependent (EDD) and endothelium-independent dilations were assessed as previously described in detail (Durrant and others, 2009; Fleenor and others, 2012; Rippe and others, 2010; Sindler and others, 2011). To determine EDD, increases in inner luminal diameter were assessed in response to acetycholine (ACh, 1 X 10−9 – 1 X 10−4 M) with and without the co-administration of the NO synthase inhibitor N-G-nitro-L-arginie (L-NAME, 0.1 mM, 30 minute incubation), or the SOD mimetic, TEMPOL (1mM, 60 minute incubation) after a submaximal preconstriction with phenylephrine (2 µm). Sodium nitroprusside (SNP: 1 X 10−10 – 1 X 10−4 M) was used to assess endotheliumin-dependent dilation.
All dose response data are presented on a percent basis. Preconstriction was calculated as a percentage of maximal diameter as previously described (Durrant and others, 2009; Fleenor and others, 2012; Rippe and others, 2010; Sindler and others, 2011). NO-dependent dilation was determined from the maximal EDD in the absence or presence of L-NAME according to the following formula:
NO-dependent dilation (%) = Maximal dilationACh − Maximal dilationACh + L-NAME
Aortic superoxide production
Electron paramagnetic resonance (EPR) spectrometry was used to measure superoxide production as described previously (Fleenor and others, 2012; Rippe and others, 2010; Sindler and others, 2011). Briefly, 2-millimeter aortic rings cleaned of perivascular fat were incubated at 37° C in 200 µl of Krebs-HEPES buffer with the 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (Alexis Biochemicals) spin trap for 60 minutes. After incubation the samples were analyzed immediately on an MS300 X-band EPR spectrometer (Magnettech).
Western blotting
Protein expression was determined in aortic samples by western blotting as previously described (Durrant and others, 2009; Fleenor and others, 2012; Rippe and others, 2010; Sindler and others, 2011). Aortas were cleaned of the surrounding perivascular fat, snap frozen in liquid nitrogen and stored at −80° C. Samples were homogenized in RIPA lysis buffer that had protease and phosphatase inhibitors (Roche) as well as a 0.01% phosphatase inhibitor cocktail (Sigma). Protein (10 micrograms/lane) was loaded in a 4–12% polyacrylamide gradient gel that was separated by electrophoresis and transferred to a nitrocellulose membrane. Membranes were probed with primary antibodies specific for collagen I (1:1000, Millipore), alpha elastin (1:100, abcam), AGEs (1:1000, GeneTex), nitrotyrosine (1:100, Abcam), NOX subunit, p67 (1:1000, Cell Signaling), MnSOD (1:1000, Stressgen), ecSOD (1:500, Sigma), CuZnSOD (1:2000, Stressgen), and endothelial NO synthase (1:500, BD Biosciences). ImageJ software (NIH) was used to analyze the bands that were normalized to β-tubulin and expressed relative to the control group.
Statistical analysis
Analyses were performed with SPSS version 19 software (IBM) and all data are presented as mean ± S.E.M. A two-way ANOVA was used to analyze aPWV, immunohistochemistry, superoxide and western blots. For vessel dilation studies, a two-way repeated measures ANOVA was used to analyze the data. Least square differences post hoc tests were used where appropriate. Significance was set at P < 0.05.
Results
Animal characteristics
Animal characteristics are presented by group (n = 5–10/group) in Table 1. Body mass, heart mass and maximal carotid diameter was greater in old compared with young control mice (P < 0.05). Food intake and heart rate did not differ with age. Young curcumin supplemented animals had greater body mass compared with young controls (P < 0.05), and greater food intake compared with all other groups (P < 0.05). Curcumin consumption was greater in young compared with old treated animals (P < 0.05). Heart mass, heart:body weight ratio, heart rate and maximal carotid diameter were not significantly different in curcumin treated mice compared with controls.
Table I.
Animal Characteristics
| YC | OC | YCUR | OCUR | |
|---|---|---|---|---|
| Body mass (g) | 24.4 ± 0.3 | 33.0 ± 0.6* | 29.7 ± 0.7* | 31.6 ± 1.0* |
| Heart mass (mg) | 141 ± 7 | 190 ± 7† | 154 ± 5 | 181 ± 6† |
| Heart:Body Ratio | 5.8 ± 0.3 | 5.8 ± 0.2 | 5.2 ± 0.2 | 5.8 ± 0.4 |
| Heart rate (bpm) | 340 ± 10 | 353 ± 9 | 335 ± 13 | 342 ± 11 |
| Maximal Carotid Diameter (µm) | 406 ± 4.3 | 450 ± 5.9† | 405 ± 8.0 | 458 ± 6.5† |
| Food intake (g/day) | 4.0 ± 0.1 | 4.1 ± 0.1 | 4.8 ± 0.2# | 4.2 ± 0.2 |
| Curcumin consumption (mg/kg/day) | 323 ± 21 | 225 ± 16** |
Values are mean ± SEM.
P < 0.05 vs. YC;
P < 0.05 vs. YC and YCUR;
P < 0.05 vs. YC, OC, OCUR;
P < 0.05 vs. YCUR.
YC, Young control; OC, Old control; YCUR, Young curcumin; OCUR, Old curcumin
aPWV and aortic collagen I, elastin and AGEs
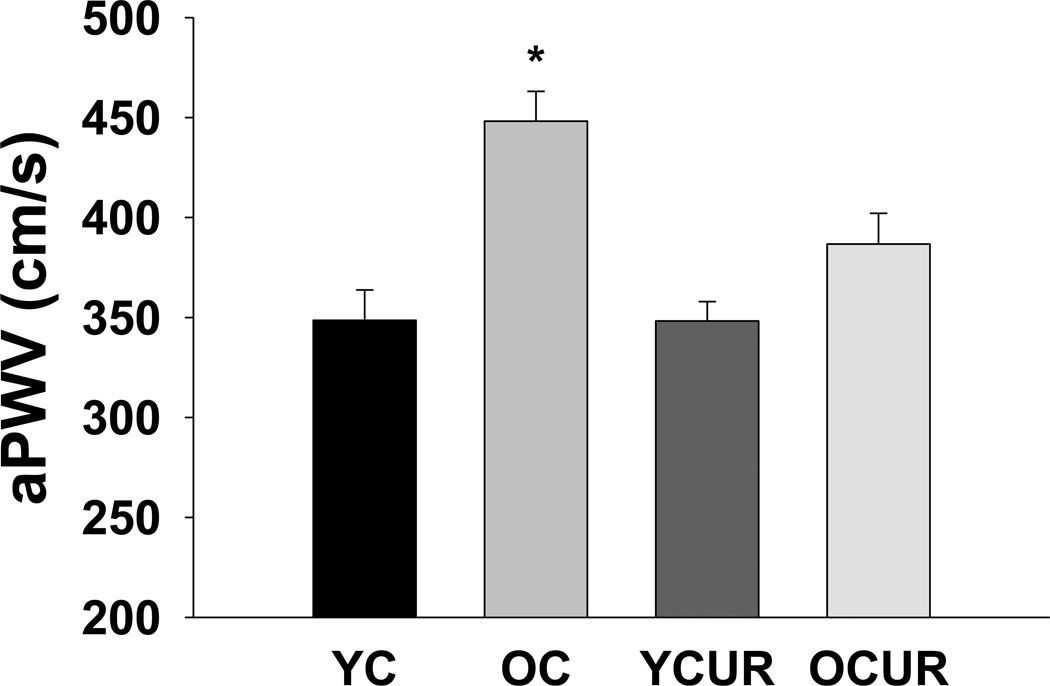
Old control mice had greater aPWV compared with young controls (P < 0.05, Figure 1). Curcumin reduced aPWV in old mice to levels not significantly different from young controls, but had no effect in young animals.
Figure 1. Aortic pulse wave velocity (aPWV).
aPWV in young and old control (YC and OC) and curcumin supplemented (YCUR and OCUR) mice (n = 5–10/group). Values are mean ± S.E.M. * P < 0.05 vs. YC, YCUR, and OCUR.
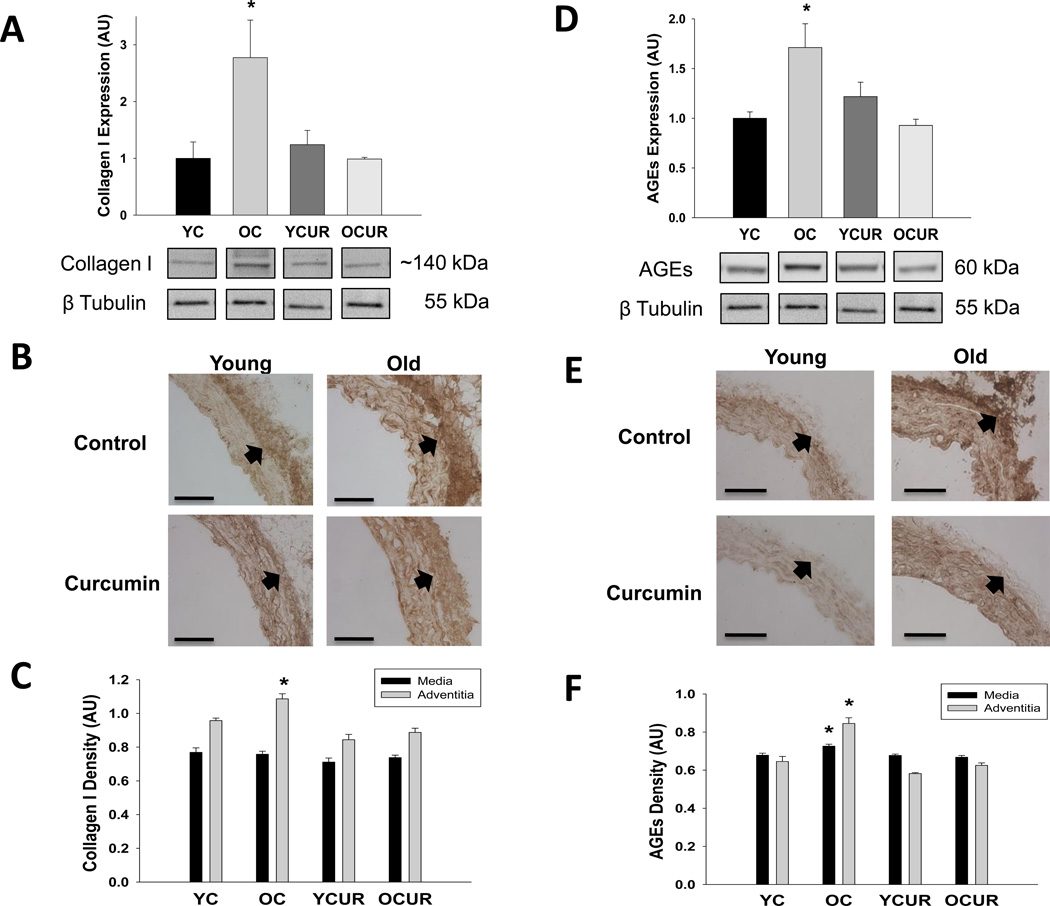
Collagen I was greater in the whole aorta (Figure 2A) and in the adventitial layer (Figure 2B and 2C) of old compared with young control mice (P < 0.05). Curcumin normalized collagen in the whole aorta and adventitial layer of old animals (P < 0.05) without affecting expression of young treated mice. Elastin was lower in the whole aorta and medial layer of old compared with young control mice (P < 0.05, Table 2). Curcumin treatment did not affect elastin expression in either age group.
Figure 2. Collagen I and AGEs protein expressions.
Aortic expression of collagen type I (A, B, C) and AGEs (D, E, F) assessed by western blot of whole artery lysates (A, D; n = 3–4/group) and histological cross-sections (B, E; n = 5–10/group) with quantificiation (C, F) from young and old control (YC and OC) and curcumin supplemented (YCUR and OCUR) mice. Values are mean ± S.E.M. * P < 0.05 vs. YC, YCUR, and OCUR. Arrows demarcate the medial-adventitial border; Bar = 100 µm.
Table II.
Elastin Protein Expression
| YC | OC | YCUR | OCUR | |
|---|---|---|---|---|
| Elastin | ||||
| Whole aorta (WB, AU) | 1.0 ± 0.1 | 0.6 ± 0.1* | 0.9 ± 0.1 | 0.5 ± 0.1* |
| Media (IHC, AU) | 0.67 ± 0.03 | 0.60 ± 0.01* | 0.63 ± 0.01 | 0.59 ± 0.01* |
| Adventitia (IHC, AU) | 0.55 ± 0.01 | 0.56 ± 0.01 | 0.53 ± 0.01 | 0.55 ± 0.01 |
Values are mean ± SEM.
P < 0.05 Main effect of age.
YC, Young control; OC, Old control; YCUR, Young curcumin; OCUR, Old curcumin; WB, western blot; IHC, Immunohistochemistry; AU, arbitrary units.
AGEs were greater in the whole aorta (Figure 2D) and in the medial and adventitial layers (Figure 2E and 2F) of old compared with young control animals (P < 0.05). Curcumin supplementation completely normalized AGEs in the whole aorta, media and adventitial layers in old mice (P < 0.05), without affecting levels in young mice.
Vascular endothelial function: modulation by NO bioavailability and oxidative stress
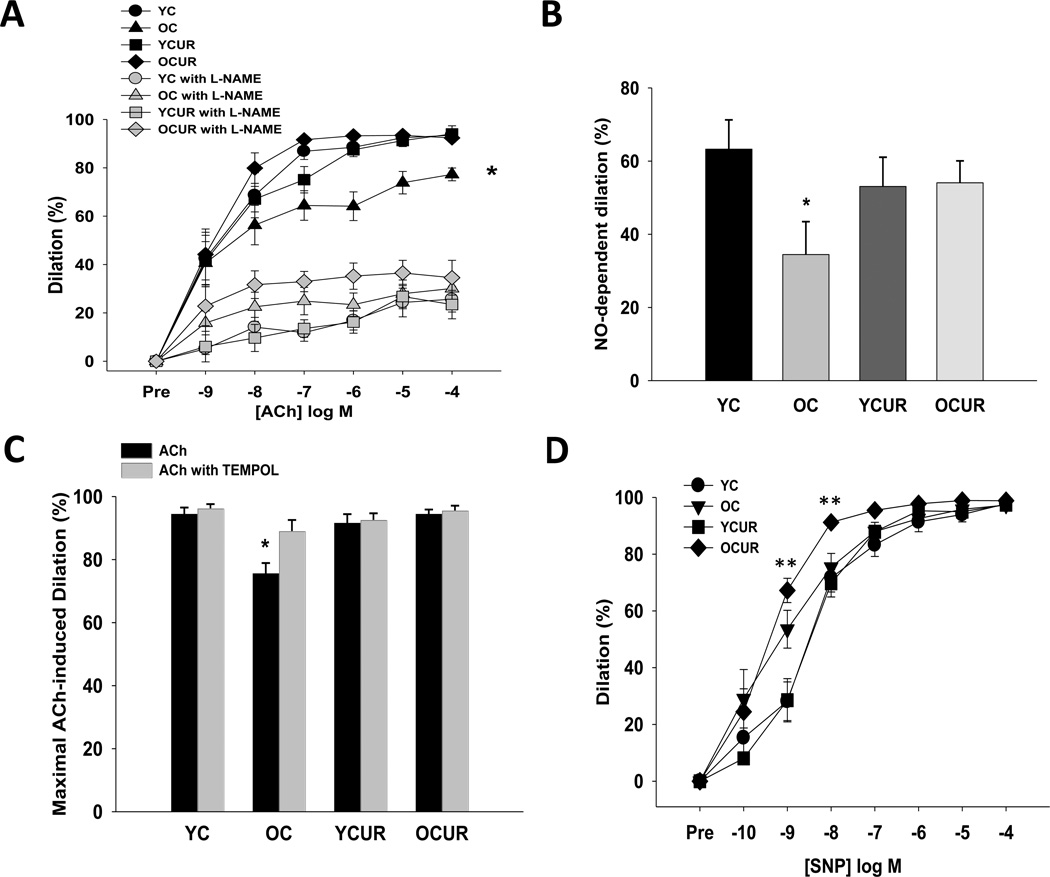
ACh-induced EDD was reduced in old compared with young control mice (P < 0.05, Figure 3A), due to a diminished NO dilatory influence as shown by a smaller decrease in EDD with the NO inhibitor L-NAME (P < 0.05, Figure 3A and 3B). Curcumin restored NO-mediated EDD in old mice to levels not significantly different to young control values (Figure 3A and 3B). Curcumin had no effect on NO-mediated EDD in young treated mice. Acute ex vivo administration of the superoxide dismutase mimetic TEMPOL normalized ACh induced EDD in carotids from old animals, while not affecting responses in young control or young and old curcumin-treated animals (Figure 3C). Maximal endothelium-independent dilation to sodium nitroprusside was not different among the groups, although an effect of age was observed at the lower doses (Figure 3D). Protein expression of the NO-synthesizing enzyme eNOS was reduced in old compared with young control mice (P < 0.05, Table 3). Curcumin did not affect eNOS in either young or old mice.
Figure 3. Nitric oxide (NO)-mediated endothelium-dependent dilation (EDD).
(A) Acetylcholine (ACh)-induced carotid EDD in young and old control (YC and OC) and young and old curcumin supplemented (YCUR and OCUR) mice with or without the endothelial NO synthase (eNOS) inhibitor L-NAME. (B) NO-dependent dilation. (C) Maximal dilation to ACh with or without the superoxide dismutase mimetic, TEMPOL. (D) Endothelium-independent dilation to sodium nitroprusside (SNP) (n = 5–8/group). Values are mean ± S.E.M. * P < 0.05 vs. YC, YCUR, and OCCUR; ** Main effect of age.
Table III.
Aortic Protein Expressions
| YC | OC | YCUR | OCUR | |
|---|---|---|---|---|
| eNOS (AU) | 1.0 ± 0.2 | 0.6 ± 0.1* | 0.9 ± 0.1 | 0.5 ± 0.1* |
| ecSOD (AU) | 1.0 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| CuZnSOD (AU) | 1.0 ± 0.2 | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.5 ± 0.0 |
Values are mean ± SEM.
P < 0.05 vs. YC, YCUR.
YC, Young control; OC, Old control; YCUR, Young curcumin; OCUR, Old curcumin; AU, arbitrary units.
Arterial oxidative stress, superoxide production, NOX and SOD antioxidant enzymes
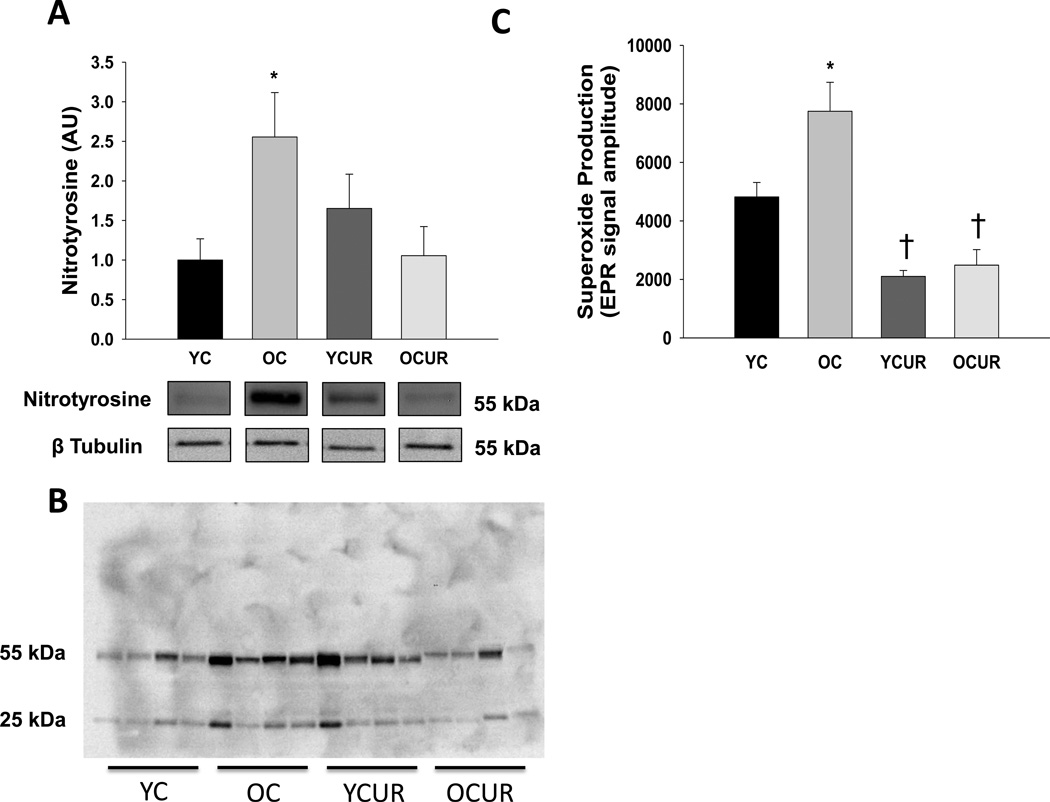
Arterial nitrotyrosine abundance at the 55kDa band and superoxide production were greater in old compared with young control mice (P < 0.05, Figure 4A, 4B and 4C). Curcumin ameliorated the excessive nitrotyrosine abundance of the 55kDA band in aorta of old mice (P < 0.05), without affecting nitrotyrosine in young treated animals. Curcumin reduced aortic superoxide production in both young and old treated animals to levels below young control values (P < 0.05). No group differences were observed in arterial nitrotyrosine staining at the 25kDa band (Figure 4B, quantification not shown).
Figure 4. Aortic nitrotyrosine abundance and superoxide production.
Aortic nitrotyrosine abundance quantified at the 55kDa band by western blot of whole artery lysates (A), representative western blot (B) and mean electron paramagnetic resonance signal (C) in young and old control (YC and OC) and young and old curcumin supplemented (YCUR and OCUR) mice (n = 3–8/group). Values are mean ± S.E.M. * P < 0.05 vs. YC, YCUR, and OCUR. † P < 0.05 vs. YC and OC.
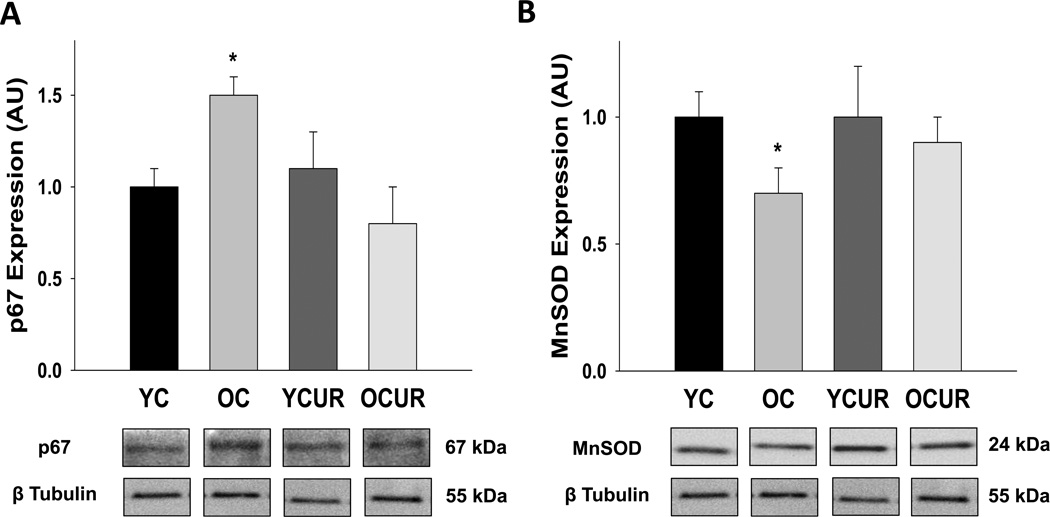
Expression of the p67 subunit of NOX was greater in aorta from old compared with young control mice (P < 0.05, Figure 5A). Curcumin treatment normalized p67 expression in old mice (P < 0.05), but had no effect in young treated animals. Antioxidant expression of MnSOD was reduced in old compared to young control mice (P < 0.05, Figure 5B), and curcumin supplementation increased MnSOD expression in old mice to levels not significantly different from young control animals. Neither ecSOD nor CuZnSOD were affected by age or curcumin treatment (Table 3).
Figure 5. Aortic NOX p67 and MnSOD protein expressions.
Aortic NOX p67 (A) and MnSOD (B) protein expressions assessed by western blot of whole artery lysates in young and old control (YC and OC) and young and old curcumin supplemented (YCUR and OCUR) mice (n = 3–4/group). Values are mean ± S.E.M. * P < 0.05 vs. YC, YCUR, and OCUR.
Discussion
The present study demonstrates for the first time that dietary curcumin supplementation ameliorates age-related large elastic artery stiffening and vascular endothelial dysfunction. Advancing age is the major risk factor for CVD and arterial dysfunction explains much of this increased risk (Lakatta and Levy, 2003). Therefore, these preclinical findings provide important evidence for curcumin as a novel, accessible and cost-effective intervention to improve arterial dysfunction and possibly reduce CVD risk with aging in humans. Our results also provide insight into the mechanisms by which curcumin may improve age-related arterial dysfunction. These include amelioration of oxidative stress, normalization of collagen I deposition and AGES, and restoration of NO bioavailability.
Large elastic artery stiffness
The present findings are consistent with previous work from our laboratory (Fleenor and others, 2012; Sindler and others, 2011) and others (Reddy and others, 2003; Soucy and others, 2006) showing that aging results in increased aPWV, the most important clinical measurement of large elastic artery stiffness in humans (Vlachopoulos and others, 2010). Our current observations extend these previous findings by demonstrating that 4 weeks of dietary curcumin treatment in old mice reduces aPWV to levels not significantly different from young control mice. Curcumin supplementation did not affect aPWV in young treated animals, suggesting that improvements with treatment were specific in old animals. These findings are of clinical significance for older humans because increased aPWV is a strong independent predictor of adverse cardiovascular events and all cause mortality (Mitchell and others, 2010; Vlachopoulos and others, 2010). Thus, our preclinical findings here provide the first support for the idea that curcumin may have efficacy for improving large elastic artery stiffness in middle-aged and older adults.
Age-associated large elastic artery stiffness is mediated in part by structural changes that include increased collagen I deposition, reductions in elastin and modifications of these proteins by AGEs (Kass and others, 2001; Lakatta and Levy, 2003). In the present study, collagen I, the major arterial collagen isoform, was increased overall and in the adventitial layer of the aorta as we have reported recently (Fleenor and others, 2010; Fleenor and others, 2012), whereas elastin is reduced overall and within the media (Csiszar and others, 2007; Fleenor and others, 2010; Fleenor and others, 2012; Wang and Lakatta, 2002). AGEs were increased overall and in both the medial and adventitial layers of aorta in old control mice as observed previously by our lab (Fleenor and others, 2012) and others (Qiu and others, 2007). Curcumin supplementation reversed these age-associated increases in collagen I and AGEs in a manner consistent with the decreases in aPWV observed. These results are in general agreement with prior reports that curcumin reverses collagen expression in vascular and lung injury models (Smith and others, 2010; Yang and others, 2006) and prevents AGEs accumulation in diabetic rats (Sajithlal and others, 1998). Although reported to prevent elastin degradation in a preclinical model of aneurysm (Parodi and others, 2006), curcumin did not influence elastin in the aortas of our old mice.
Vascular endothelial dysfunction
The current findings are in agreement with previous reports from our laboratory (Durrant and others, 2009; Fleenor and others, 2012; Rippe and others, 2010; Sindler and others, 2011) demonstrating EDD is impaired in large elastic arteries of old mice due to a smaller NO-mediated dilatory component that is associated with reduced eNOS protein expression. In the present study we show that curcumin supplementation ameliorated the impairment in EDD with aging by restoring NO-dependent dilation to levels not different than young mice. This observation is consistent with previous reports in animal models of clinical disorders including diabetes (Chai and others, 2005; Majithiya and Balaraman, 2005; Ramaswami and others, 2004; Rungseesantivanon and others, 2010). In contrast to results from disease models (Chai and others, 2005; Nakmareong and others, 2011a; Nakmareong and others, 2011b; Ramaswami and others, 2004), the present findings show that curcumin restored EDD in old mice without increasing expression of eNOS. The SOD mimetic, TEMPOL, restored the impaired EDD observed in old mice as reported previously by our laboratory (Durrant and others, 2009; Lesniewski and others, 2009; Lesniewski and others, 2011; Sindler and others, 2011) without having an effect in young or old curcumin treated mice. These data support the notion that the superoxide lowering effect of curcumin in old mice is responsible for the improvement in endothelial function.
Oxidative Stress
Our finding of increased arterial nitrotyrosine abundance, a cellular marker of oxidative stress, in old mice is consistent with our previous observations (Durrant and others, 2009; Fleenor and others, 2012; Sindler and others, 2011). Nitrotyrosine modifications are a result of a posttranslational nitration of tyrosine residues primarily mediated by peroxynitrite, which is a by-product of the reaction between superoxide and NO (Radi, 2004). The oxidative stress seen in old mice is associated with greater aortic superoxide production and p67 subunit expression of NOX, a pro-oxidant enzyme, and reductions in the mitochondrial anti-oxidant enzyme MnSOD, as we recently reported (Fleenor and others, 2012; Sindler and others, 2011).
The present results extend this prior work by demonstrating that curcumin supplementation normalizes aortic nitrotyrosine staining in old mice, indicating amelioration of age-associated vascular oxidative stress. This is consistent with previous work reporting reduced oxidative stress after treatment with curcumin in models of hemorrhage and cardiovascular disease, as well as in animals treated with lipopolysaccharide or L-NAME (Nakmareong and others, 2011a; Nakmareong and others, 2011b; Quiles and others, 2002; Sompamit and others, 2009; Wakade and others, 2009). Here we show that the antioxidant effect of curcumin in old mice is, at least in part, attributable to reductions in aortic superoxide production, directly measured by EPR spectroscopy. Results of previous reports assessing superoxide with fluorescent dyes in models of clinical disorders are in agreement with the present findings (Chai and others, 2005; Sompamit and others, 2009; Wakade and others, 2009). Curcumin restored expression of the p67 subunit of oxidant enzyme NOX and the mitochondrial antioxidant enzyme MnSOD in old mice to young control levels, suggesting that both production and dismutation of superoxide is enhanced with curcumin (Awad, 2011; Lakshmanan and others, 2011). In addition to altering pro- and anti-oxidant enzyme expressions, our data suggest that curcumin may also directly scavenge free radicals, as indicated previously (Chai and others, 2005; Ramaswami and others, 2004).
Curcumin
Asian cultures that widely use turmeric in cooking consume an estimated 1.5 – 2.5 g/d of this compound, which is ~60–100 mg/d of curcumin for a 60 kg person (Eigner and Scholz, 1999; Miriyala and others, 2007; Shah and others, 1999). In the current study, we used a dose of curcumin that has been reported to increase longevity in rodents (Kitani and others, 2004; Kitani and others, 2007), and the mice received the equivalent of 19 (young group) and 14 (old group) g of curcumin when compared with a 60 kg person. Because of curcumin’s poor absorption and rapid metabolism (Wahlstrom and Blennow, 1978), clinical trials in humans also have used high doses of curcumin (~8–12 g) similar to the amount our old mice consumed, while observing only infrequent, minor side effects (Cheng and others, 2001).
Although we did not assess plasma curcumin in the present study, the same dose of curcumin used here previously has been shown to produce measurable concentrations of this compound in plasma of mice (Perkins and others, 2002). Curcumin consumed orally is metabolized primarily in the intestine, resulting in the production of biologically active metabolites. An increase in these metabolites may be responsible for improvements in health and arterial function observed in the current study (Ahn and others, 2009; Nakmareong and others, 2011b). Moreover, it is important to note that curcumin is metabolized much more extensively in human compared with rodent intestinal tissues (Ireson and others, 2002; Sharma and others, 2005). Thus, it is possible that curcumin may have greater bioavailability and effects in rodents. To improve delivery and efficacy, manufacturers recently have developed curcumin formulations that enhance bioavailability and slow metabolism, thus allowing low-dose (e.g., 0.5 g) capsules to be used and further increasing the translational potential of the compound (Gota and others, 2010).
Finally, food intake and body mass were ~20% greater in curcumin-supplemented compared with chow-fed young mice in the present study. This effect of curcumin in young animals has been reported previously (Weisberg and others, 2008) and was not observed in the old mice in our study. Most importantly, the higher food intake and body mass in the young curcumin-treated animals was not associated with differences in arterial function or the other primary outcomes of the study.
Conclusions
Our results provide the first evidence that dietary curcumin supplementation ameliorates two clinically important markers of arterial dysfunction with aging: large elastic artery stiffening and endothelial dysfunction. Moreover, the present results provide insight into the mechanisms of action, including normalization of vascular superoxide production and oxidative stress, reductions in collagen I and AGES in the arterial wall, and restoration of NO bioavailability. Given its accessibility and safety, these pre-clinical findings provide the experimental basis for future translational studies assessing the potential for curcumin to treat arterial dysfunction with aging and reduce CVD risk in humans.
Highlights.
In old animals, 4 week curcumin supplementation:
Reverses large elastic artery stiffness
Ameliorates endothelial dysfunction
Attenuates arterial superoxide production and oxidative stress
Curcumin is a promising antioxidant therapy to treat age-related arterial dysfunction.
Acknowledgements
We thank Sanjana Ahsan, Jackson Kloor and Mandy Marziaz for their technical assistance. This work was supported by NIH AG13038, AG000279, HL007822, and the Japan Society for the Promotion of Science 238304
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn CM, Park B-G, Woo HB, Ham J, Shin W-S, Lee S. Synthesis of sulfonyl curcumin mimics exerting a vasodilatation effect on the basilar artery of rabbits. Bioorganic & Medicinal Chemistry Letters. 2009;19:1481–1483. doi: 10.1016/j.bmcl.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Awad AS. Effect of Combined Treatment With Curcumin and Candesartan on Ischemic Brain Damage in Mice. Journal of Stroke and Cerebrovascular Diseases. 2011;20:541–548. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovascular Research. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. Journal of the American College of Cardiology. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Chai H, Yan S, Lin P, Lumsden AB, Yao Q, Chen C. Curcumin Blocks HIV Protease Inhibitor Ritonavir-Induced Vascular Dysfunction in Porcine Coronary Arteries. Journal of the American College of Surgeons. 2005;200:820–830. doi: 10.1016/j.jamcollsurg.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Cheng A, Hsu C, Lin J, Hsu M, Ho Y, Shen T, Ko J, Lin J, Lin B, Ming-Shiang W, Yu H, Jee S, Chen G, Chen T, Chen C, Lai M, Pu Y, Pan M, Wang T, Tsai C, Hsieh C. Phase I clincial trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Research. 2001;21:2895–2900. [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective Effects of Anti-Tumor Necrosis Factor-{alpha} Treatment in Aging. Am J Pathol. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Uscio LV, Moreau P, Shaw S, Takase H, Barton M, Luscher TF. Effects of Chronic ETA-Receptor Blockade in Angiotensin II-Induced Hypertension. Hypertension. 1997;29:435–441. doi: 10.1161/01.hyp.29.1.435. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct Evidence of Endothelial Oxidative Stress With Aging in Humans: Relation to Impaired Endothelium-Dependent Dilation and Upregulation of Nuclear Factor-Κappa B. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. The Journal of Physiology. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigner D, Scholz D. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. Journal of Ethnopharmacology. 1999;67:1–6. doi: 10.1016/s0378-8741(98)00234-7. [DOI] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. The Journal of Physiology. 2010;588:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell. 2012 doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K, Currer JM, Harrison DE. The Mouse in Biomedical Research Second Edition. 2007. [Google Scholar]
- Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG. Safety and Pharmacokinetics of a Solid Lipid Curcumin Particle Formulation in Osteosarcoma Patients and Healthy Volunteers. Journal of Agricultural and Food Chemistry. 2010;58:2095–2099. doi: 10.1021/jf9024807. [DOI] [PubMed] [Google Scholar]
- Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11:105–111. [PubMed] [Google Scholar]
- Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved Arterial Compliance by a Novel Advanced Glycation End-Product Crosslink Breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, Santhanam L, Webb A, Camara A, Sikka G, Nyhan D, Shoukas AA, Ilies M, Christianson DW, Champion HC, Berkowitz DE. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol. 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani K, Yokozawa T, Osawa T. Interventions in Aging and Age-Associated Pathologies by Means of Nutritional Approaches. Annals of the New York Academy of Sciences. 2004;1019:424–426. doi: 10.1196/annals.1297.075. [DOI] [PubMed] [Google Scholar]
- Kitani K, Osawa T, Yokozawa T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology. 2007;8:567–573. doi: 10.1007/s10522-007-9100-z. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part I: Aging Arteries: A "Set Up" for Vascular Disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lakshmanan AP, Watanabe K, Thandavarayan RA, Sari FR, Meilei H, Soetikno V, Arumugam S, Giridharan VV, Suzuki K, Kodama M. Curcumin attenuates hyperglycaemia-mediated AMPK activation and oxidative stress in cerebrum of streptozotocin-induced diabetic rat. Free Radical Research. 2011;45:788–795. doi: 10.3109/10715762.2011.579121. [DOI] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H on behalf of the European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 Mice Are a Suitable Model of Oxidative Stress-Mediated Impaired Endothelium-Dependent Dilation With Aging. J Gerontol A Biol Sci Med Sci. 2009;64A:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. American Journal of Physiology - Heart and Circulatory Physiology. 2011;301:H1025–H1032. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majithiya JB, Balaraman R. Time-Dependent Changes in Antioxidant Enzymes and Vascular Reactivity of Aorta in Streptozotocin-Induced Diabetic Rats Treated With Curcumin. Journal of Cardiovascular Pharmacology. 2005;46:697–705. doi: 10.1097/01.fjc.0000183720.85014.24. [DOI] [PubMed] [Google Scholar]
- Miriyala S, Panchatcharam M, Rengarajulu P. Cardioprotective effects of curcumin. Advances in Experimental Medicine and Biology. 2007;595:359–377. doi: 10.1007/978-0-387-46401-5_16. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial Stiffness and Cardiovascular Events: The Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Delp JM. Aging-Induced Adaptations of Microvascular Reactivity. Microcirculation. 2006;13:301–314. doi: 10.1080/10739680600619023. [DOI] [PubMed] [Google Scholar]
- Nakmareong S, Kukongviriyapan U, Pakdeechote P, Donpunha W, Kukongviriyapan V, Kongyingyoes B, Sompamit K, Phisalaphong C. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn-Schmiedeberg's Archives of Pharmacology. 2011a;383:519–529. doi: 10.1007/s00210-011-0624-z. [DOI] [PubMed] [Google Scholar]
- Nakmareong S, Kukongviriyapan U, Pakdeechote P, Kukongviriyapan V, Kongyingyoes B, Donpunha W, Prachaney P, Phisalaphong C. Tetrahydrocurcumin alleviates hypertension, aortic stiffening and oxidative stress in rats with nitric oxide deficiency. Hypertension Research. 2011b doi: 10.1038/hr.2011.180. [DOI] [PubMed] [Google Scholar]
- Parodi F, Mao D, Ennis TL, Pagano MB, Thompson RW. Oral adminstration of diferuloylmethane (Curcumin) suppresses proinflammatory cytokines and destructive connective tissue remodeling in experimental abdominal aortic aneurysms. Annals of Vascular Surgery. 2006;20:360–368. doi: 10.1007/s10016-006-9054-7. [DOI] [PubMed] [Google Scholar]
- Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, Williams ML, Steward WP, Gescher AJ. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002;11:535–540. [PubMed] [Google Scholar]
- Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10:1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Depre C, Ghosh K, Resuello RG, Natividad FF, Rossi F, Peppas A, Shen Y-T, Vatner DE, Vatner SF. Mechanism of Gender-Specific Differences in Aortic Stiffness With Aging in Nonhuman Primates. Circulation. 2007;116:669–676. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]
- Quiles JL, Mesa MD, Ramirez-Tortosa CL, Aguilera CM, Battino M, Gil A, Ramirez-Tortosa MC. Curcuma longa Extract Supplementation Reduces Oxidative Stress and Attenuates Aortic Fatty Streak Development in Rabbits. Arterioscler Thromb Vasc Biol. 2002;22:1225–1231. doi: 10.1161/01.atv.0000020676.11586.f2. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proceedings of the National Academy of Sciences. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami G, Chai H, Yao Q, Lin PH, Lumsden AB, Chen C. Curcumin blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. Journal of Vascular Surgery. 2004;40:1216–1222. doi: 10.1016/j.jvs.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Reddy AK, Li Y-H, Pham TT, Ochoa LN, Trevino MT, Hartley CJ, Michael LH, Entman ML, Taffet GE. Measurement of aortic input impedance in mice: effects of age on aortic stiffness. Am J Physiol Heart Circ Physiol. 2003;285:H1464–H1470. doi: 10.1152/ajpheart.00004.2003. [DOI] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics -- 2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungseesantivanon S, Thenchaisri N, Ruangvejvorachai P, Patumraj S. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complementary and Alternative Medicine. 2010;10:57. doi: 10.1186/1472-6882-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajithlal GB, Chithra P, Chandrakasan G. Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochemical Pharmacology. 1998;56:1607–1614. doi: 10.1016/s0006-2952(98)00237-8. [DOI] [PubMed] [Google Scholar]
- Schiffman SC, Li Y, Martin RCG. The Association of Manganese Superoxide Dismutase Expression in Barrett's Esophageal Progression With MnTBAP and Curcumin Oil Therapy. Journal of Surgical Research. 2012 doi: 10.1016/j.jss.2011.11.1013. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clinical Science. 2012;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BH, Nawaz Z, Pertani SA, Roomi A, Mahmood H, Saeed SA, Gilani AH. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochemical Pharmacology. 1999;58:1167–1172. doi: 10.1016/s0006-2952(99)00206-3. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Gescher AJ, Steward WP. Curcumin: The story so far. European Journal of Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Gangireddy SR, Narala VR, Hogaboam CM, Standiford TJ, Christensen PJ, Kondapi AK, Reddy RC. Curcumin inhibits fibrosis-related effects in IPF fibroblasts and in mice following bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;298:L616–L625. doi: 10.1152/ajplung.00002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompamit K, Kukongviriyapan U, Nakmareong S, Pannangpetch P, Kukongviriyapan V. Curcumin improves vascular function and alleviates oxidative stress in non-lethal lipopolysaccharide-induced endotoxaemia in mice. European Journal of Pharmacology. 2009;616:192–199. doi: 10.1016/j.ejphar.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol. 2006;101:1751–1759. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and Endothelial Function in Normotensive Subjects and Patients With Essential Hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness: A Systematic Review and Meta-Analysis. Journal of the American College of Cardiology. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacologica et Toxicologica. 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- Wakade C, King MD, Laird MD, Alleyne CH, Jr, Dhandapani KM. Curcumin attenuates vascular inflammation and cerebral vasospasm after subarachnoid hemorrhage in mice. Antioxidants and Redox Signaling. 2009;11:35–45. doi: 10.1089/ars.2008.2056. [DOI] [PubMed] [Google Scholar]
- Wang M, Lakatta EG. Altered Regulation of Matrix Metalloproteinase-2 in Aortic Remodeling During Aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, Leibel R, Tortoriello DV. Dietary Curcumin Significantly Improves Obesity-Associated Inflammation and Diabetes in Mouse Models of Diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Thomas DP, Zhang X, Culver BW, Alexander BM, Murdoch WJ, Rao MNA, Tulis DA, Ren J, Sreejayan N. Curcumin Inhibits Platelet-Derived Growth Factor-Stimulated Vascular Smooth Muscle Cell Function and Injury-Induced Neointima Formation. Arterioscler Thromb Vasc Biol. 2006;26:85–90. doi: 10.1161/01.ATV.0000191635.00744.b6. [DOI] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, Pathophysiology, and Therapy of Arterial Stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]