Abstract
The corneal epithelial tissue is a layer of rapidly growing cells that are highly glycolytic and express GLUT1 as the major glucose transporter. It has been shown that GLUT1 in L929 fibroblast cells and other cell lines can be acutely activated by a variety agents.However, the acute regulation of glucose uptake in corneal cells has not been systematically investigated. Therefore, we examined glucose uptake in an immortalized human corneal–limbal epithelial (HCLE) cell line and compared it to glucose uptake in L929 fibroblast cells, a cell line where glucose uptake has been well characterized. We report that the expression of GLUT1 in HCLE cells is 6.6-fold higher than in L929 fibroblast cells, but the HCLE cells have a 25-fold higher basal rate of glucose uptake. Treatment with agents that interfere with mitochondrial metabolism, such as sodium azide and berberine, activate glucose uptake in L929 cells over 3-fold, but have no effect on glucose uptake HCLE cells. Also, agents known to react with thiols, such cinnamaldehyde, phenyarsine oxide and nitroxyl stimulate glucose uptake in L929 cells 3 to 4-fold, but actually inhibit glucose uptake in HCLE cells. These data suggest that in the fast growing HCLE cells, GLUT1 is expressed at a higher concentration and is already highly activated at basal conditions. These data support a model for the acute activation of GLUT1 that suggests that the activity of GLUT1 is enhanced by the formation of an internal disulfide bond within GLUT1 itself.
Keywords: Glucose uptake, HCLE cells, L929 fibroblast cells, GLUT1, acute regulation
1. Introduction
Corneal epithelial cells are rapidly growing cells that have a life cycle of 7-10 days. They originate from stem cells in the limbal basal region at the edge of the cornea and migrate across basement membrane of the anterior cornea forming a basal corneal epithelial layer. Cell division occurs in the basal layer and the daughter cells migrate anteriorly, differentiating to wing cells and squamous superficial cells that are eventually shed from the ocular surface, thereby maintaining an epithelium that is 5-7 cell layers thick [1]. Corneal epithelial cells are reported to have few mitochondria and are known to be heavily dependent on glycolysis. The predominant or only glucose transporter responsible for glucose uptake by corneal epithelial cells is GLUT1 [2-5]. GLUT1 expression and glucose uptake are enhanced during the corneal epithelial wound healing process, but little else is known about the regulation of GLUT1 activity [4, 6]. While GLUT1 is responsible for a basal level of glucose uptake in a wide variety of cells, data from cells that exclusively or predominately express GLUT1 reveal that this transporter can be acutely activated, that is, activated within 15 minutes, independent of new GLUT1 biosynthesis. Conditions such as glucose deprivation [7, 8], hyperposmolarity[9, 10] or exposure to azide[11, 12], methylene blue [13], C-peptide [14], or berberine[15], and thiol active agents such as cinnamaldehye[16], phenylarsine oxide [17], and nitroxyl[18] all activate glucose uptake via GLUT1.
An immortalized human corneal–limbal epithelial (HCLE) cell line has been developed [19-21], that forms stratified layers resembling the in vivo corneal epithelium and expresses the mucins known to be expressed by superficial corneal epithelial cells. We have used these cells to investigate the protective effects of potassium ions against UVB damage [22, 23]. The HCLE cell line is relatively new and glucose uptake has not been measured, nor has its response to acute stress been determined. Regulation of glucose uptake in corneal cells is relevant to diabetic patients where the disease is associated with an increased fragility of the corneal epithelium and a slowing of wound healing [24-26]. Therefore, the purpose of this study was to measure glucose uptake in HCLE cells, to confirm the expression of GLUT1,and to determine if glucose uptake is acutely regulated in a similar fashion to the regulation of GLUT1 in L929 fibroblast cells [11, 13, 27].The GLUT1 protein is recognized by the same antibody, which indicates that the transporter is very similar in the two species. Therefore, this suggests any differences in the regulation of GLUT1 are more likely a function of different cell types than of different species.
2. Materials and Methods
2.1 Chemicals
Angeli’s salt (AS) was a generous gift of Dr. John P. Toscano (Johns Hopkins University) and was stored at −4 °C under nitrogen. Phenylarsineoxide (PAO), cinnamaldehyde (CA), berberine, cytochalasin B, quercetin, 2-deoxy-D-glucose-[1,2-3H] (2DG) and D-mannitol-1-14C were purchased from the Sigma-Aldrich Chemical Company (St. Louis, MO, USA).
2.2 Cell culture
The immortalized human corneal–limbal epithelial (HCLE) cell line was obtained from Dr. Ilene Gipson (Department of Opthalmology, Harvard Medical School) and maintained asmonolayer cultures in Keratinocyte-Serum Free medium (K-SFM)(Invitrogen, Carlsbad, CA), as previously described[19]. The L929 mouse fibroblast cells were obtained from the American Type Culture Collection. To initiate each experiment, a 24-well plate was seeded either 2 or 3 days (HCLE cells) or 1 day (L929 cells) prior to experimentation. Experiments were done with cells near confluency, which is about 1.0 × 105cells per well for HCLE cells and 3.2 × 105for L929 fibroblast cells. The cells were grown at 37 °C in an incubator supplied with humidified room air with 5% CO2.
2.3 Western Blotting
HCLE or L929 cells from two wells of a 24-well plate were isolated and lysed in 50 μL of 0.5% SDS. The concentration of proteins in the whole cell extract was determined using a protein assay from Pierce. Proteins (20 μg) were separated on a 12% SDS-PAGE gel. GLUT1 was detected by Western blotting using a primary anti-GLUT1 rabbit polyclonal antibody (Millipore, Temecula, CA) and a secondary goat anti-mouse (IRDye™ 800 CW, Odyssey). The membrane was imaged with an OdesseyLiCor scanner (Lincoln, NE) and GLUT1 was quantified using the Odyssey Infrared ImaginingSystem software (version 3.0.25).
2.4 General experimental design
To initiate an experiment, the medium from cells in 24-well plates was removed and the cells were incubated in 0.8 mL of fresh treatment DMEM media plus glucose and the chemical of interest at the concentrations indicated. Cells were maintained at 37 °C for 30 minutes. In experiments using AS or PAO there was no treatment phase, but reagents were added directly to the glucose uptake buffer (see below). In the experiments using AS, the solid compound was quickly dissolved in uptake medium at room temperature and immediately applied to the cells in the 24-well plate (process took about 30 seconds). The cells were then returned to the incubator and the medium was allowed to warm to 37 °C. All other reagents were added to the media from 100-200x stock aqueous (sodium azide), or ethanol (cinnamaldehyde, cytochalasin B) or DMSO (berberine, phenylarsine oxide, quercetin) solutions. Ethanol and DMSO have no effect on glucose uptake at the concentrations added[16, 17].
2.5 Glucose uptake assay
Glucose uptake was measured using the radiolabeled glucose analog 2-deoxyglucose (2DG) as previously described [28]. Briefly, the medium was replaced with 0.4 mL of glucose-free HEPES buffer (140 mMNaCl, 5 mMKCl, 20 mM HEPES/Na pH=7.4, 2.5 mM MgSO4, 1 mM CaCl2, 2 mM pyruvate, 1 mMmannitol) supplemented with 1.0 mM (0.3 μCi/mL) 2-DG (1,2-3H) and 1.0 mM (0.02 μCi/mL) mannitol (1-14C) at 37 °C. Uptake medium was supplemented with additional compounds, such as AS or PAO, as indicated in the figure legends. After a 10-minute incubation, cells were washed twice with cold glucose-free HEPES. The cells were lysed in 0.5 mL lysis buffer (10 mMTris pH=7.4, 150 mMNaCl, 5 mM EDTA, 1.0% triton X-100, 0.4% SDS) and the 3H-2 DG uptake with 14C-mannitol was measured using scintillation spectrometry. Mannitol is not taken up by these cells, so the uptake of 14C-mannitol only occurs if there is non-specific cell surface binding or the cell membrane is compromised by the treatment conditions.
2.6 Data reporting and statistical analysis
Experimental conditions were repeated in triplicate or quadruplicate and glucose uptake was measured as nmol/10 min/well ±standard error. Uptakes reported in Figure 1 were expressed as uptakes per 105 cells. In the remaining figures, data from several experiments were each normalized to basal conditions, combined and reported as relative 2DG uptake. Data reported represent the mean from 12-24 samples. Statistical significance was determined by either ANOVA followed by a post-hoc Dunnett test or a two-tailed t-test. Experiments were repeated 3-5 times and results from a representative experimentor normalized data from multiple experiments are reported.
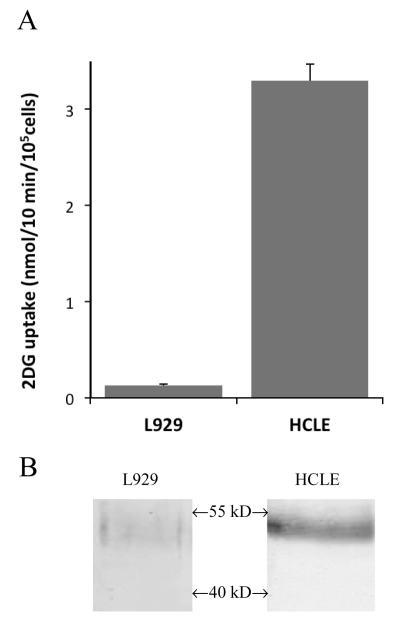
Figure 1.
Comparison of basal 2DG uptake rates and GLUT1 expression of HCLE and L929 cells. A. 2DG uptake in L929 and HCLE cells expressed as nmol/10min/105 cells. B. Proteins (20 μg) from total cell extracts from L929 and HCLE cells were separated by SDS gel electrophoresis and probed for GLUT1 by Western blotting. This analysis was done three times and a representative result is shown.
3. Results
3.1 HCLE cells have higher basal glucose uptake rates and expression of GLUT1
The major glucose transporter in corneal epithelial cells is GLUT1. We initially measured glucose uptake in HCLE cells under basal conditions and the results are shown in Figure 1A. The 2DG uptake expressed per 105 cells is 25 times greater in HCLE cells than in L929 fibroblast cells. The higher glucose uptake rate in HCLE cells could simply be attributed to agreater expression of GLUT1, so we quantified the GLUT1 protein in both cell lines. TheWestern blot analysis of whole cell extracts from L929 and HCLE cells revealed a single band for GLUT 1 just below 55 kD, which is shown in Figure 1B. The quantitative analysis of the GLUT1 band indicates that the expression of GLUT1 is 6.6-fold higher in HCLE cells than in L929 fibroblast cells. The 6.6-fold increased expression in HCLE cells is not sufficient to fully account for the 25-fold increase in the basal glucose uptake. Either GLUT1 is in a higher active state in HCLE cells or other glucose transporters, not yet documented, are responsible for the additional uptake rate. To ascertain the involvement of GLUT1, we measured the glucose uptake in both cells lines in the presence of maximally effective concentrations of cytochalasin B, an inhibitor of the GLUT family of proteins, orquercetin, a known competitive inhibitor of GLUT1 [29]. The results from each cell line were normalized to control and are shown on Table 1. The inhibition of glucose uptake was virtually identical in both cell lines with cytochalasin B reduceduptakes to8% and 9% in HCLE and L929 cells respectively and quercetin reduced uptakes to 29% and 27%. This result suggests that the uptake activity we are measuring can be attributed to GLUT1 and it suggests that some of the 25-fold higher glucose uptake rate is due to increased activity of GLUT 1. Therefore, we wanted to look more closely at the acute activation of GLUT1 in HCLE cells.
Table 1.
Effects of cytochalasin B and quercetin on glucose uptake
| Cell | Control | Cytochalasin B | Quercetin |
|---|---|---|---|
| HCLE | 1.00 ± 0.05 | 0.08 ± 0.01* | 0.29 ± 0.02* |
| L929 | 1.00 ± 0.10 | 0.09 ± 0.01* | 0.27 ± 0.07* |
2DG uptakes in both HCLE cells and L929 fibroblasts cells were measured in the absence (control) or presence of either 40μM cytochalasin B or 100μM quercetin. Uptakes from quadruplicate samples were normalize to control and reported as fractions ± S.E.
Significantly different from control at P<0.01.
3.2 Glucose uptake in HCLE cells is activated by glucose deprivation
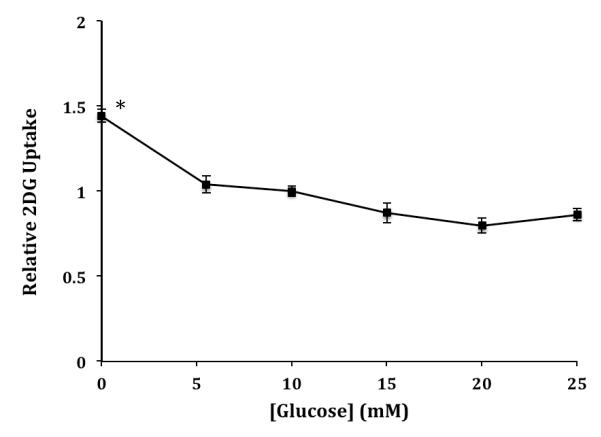
We have previously shown that glucose uptake in L929 cells is activated up to 20-fold by a brief, prior exposure to media with reduced glucose concentrations [7]. We were interested to determine if glucose uptake by HCLE cells is also sensitive to a brief glucose deprivation period. HCLE cells in a 24-well plate were exposed to media containing various concentrations of glucose for 20 minutes prior to measurement of glucose uptake. The results shown in Figure 2 indicate that there is not a strong dependence of glucose uptake on prior exposure to glucose. Complete deprivation does significantly activate glucose uptake by 1.47 times, but there was no difference if the 20-minute treatment phase contained 5.5, 10, 15, 20 or 25 mM glucose.
Figure 2.
Effects of glucose concentration on 2DG uptake by HCLE cells. Cells were incubated in DMEM medium containing 0, 5.5, 10, 15, 20, or 25 mM glucose for 30 minutes. 2DG uptake data are means ± S.E normalized to uptake at 10 mM glucose for 8-16 samples. *Significantly different from all other data at P < 0.01.
3.3 Cell stressors do not activate glucose uptake in HCLE cells
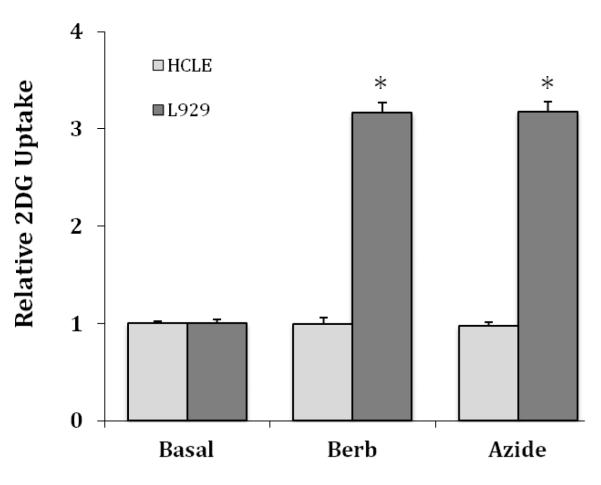
Stressors that have been reported to inhibit mitochondrial function, such as sodium azide and berberine, have been shown to increase the activity of GLUT1 in L929 cells [15, 18]. We measured the effects of 5.0 mMazide and 50 μM berberine on glucose uptake in HCLE cells, which are maximally effective concentrations in L929 cells. The results are shown in Figure 3 with data from L929 cells also shown for comparison. Data sets from L929 and HCLE cells are each normalized to their respective basal uptakes. In sharp contrast to L929 cells, where both azide and berberine stimulated glucose uptake 3.2-fold, these agents had no effect on glucose uptake in HCLE cells. Doubling the concentrations of azide and berberine also had no effect (data not shown).
Figure 3.
Comparative effects of berberine and sodium azide on 2DG uptake in HCLE and L929 cells. HCLE and L929 cells were exposed to either 50 μM berberine or 5.0 mM sodium azide for 30 minutes. Data are means ± S.E for 8-12 samples with each cell line normalize to its respective basal uptakes. *Significantly different from respective basal uptake at P < 0.01.
3.4Thiol active compounds inhibit glucose uptake in HCLE cells
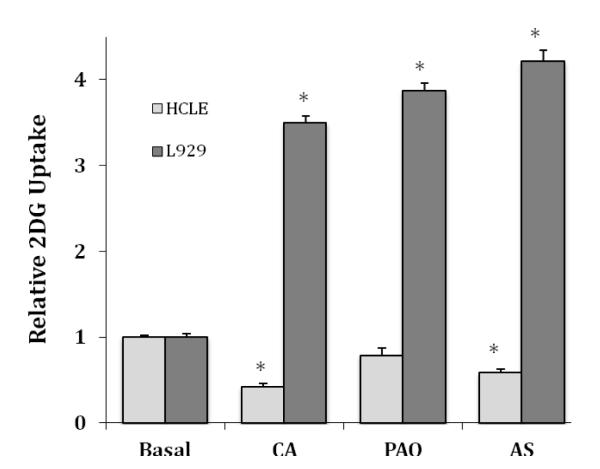
We have shown that a brief treatment with thiol active compounds, such as cinnamaldehyde, phenylarsine oxide, and nitroxyl, via treatment with Angeli’s salt, all activate glucose uptake in L929 fibroblast cells [16-18]. The data from these studies and others suggest that GLUT1 can be activated by the formation of a disulfide bond within GLUT1. We tested the effects of 2 mM CA, 10 μM PAO and 5 mM AS, all maximally effective concentrations in L929 cells, on glucose uptake in HCLE cells. The results are shown in Figure 4 along with data from L929 cells each normalized to its respective basal uptake. In L929 fibroblast cells, glucose uptake was significantly increased 3.5-fold by CA, 3.9-fold by PAO, and 4.2-fold by AS, whereas in HCLE cells uptake was significantly decreased upon exposure to CA and AS, 0.41 and 0.58 of control respectively (P < 0.01), and slightly reduced by PAO to 0.79 of control, but the difference was not statistically significant (P < 0.07).
Figure 4.
Comparative effects of CA, PAO, and AS on 2DG uptake in HCLE and L929 cells. HCLE and L929 cells were exposed to either 2.0 mMcinnamaldehyde (CA) for 30 minutes prior to the measurement of 2DG uptake or exposed to either or 10 μM PAO or 5 mM AS during uptake. 2DG uptakes rates are means ± S.E for 8-12 samples with each cell line normalized to its respective uptake under basal or untreated conditions. *Significantly different from respective basal uptake at P < 0.01.
4. Discussion
Corneal epithelial cells rely heavily on glucose uptake via GLUT1 and glucose metabolism to support their rapid growth [2-6]. In spite of the importance of glucose to corneal epithelial cell maintenance, there are no systematic studies investigating the regulation of glucose uptake in these cells. GLUT1 is widely expressed and it is generally thought that this transporter only responsible for basal glucose uptake. However, recent data from studies in a number of cells lines where GLUT1 is either the only or the predominant glucose transporter have demonstrated that this transporter can be acutely activated by number of reagents or environmental conditions [7-13, 15-18, 30].
In this study, we have investigated the glucose transport activity in a transformed human corneal–limbal epithelial cell line. HCLE cells have a 6.6 higher concentration of GLUT1 than L929 cells but have over a 25-fold higher basal rate of glucose uptake (see Figure 1). This discrepancy suggests that either there is either another glucose transporter at play in HCLE cells, or that GLUT1 in HCLE cells is in a more active state. However, it does not seem likely that another glucose transporter is involved. While glucose transporters expression in HCLE cells has not been systematically measured, a survey of the literature reveals that only GLUT1 expression has been documented in corneal cells. In addition, the observation that cytochalasin B, an inhibitor of the GLUT family of transporters, and quercetin, a competitive inhibitor of GLUT1, identically inhibit uptake in HCLE cells and L929 cells, a cell line where expression of only GLUT1 has been documented [27], strongly suggests that we are also measuring GLUT1 activity in HCLE cells (see Table 1).Therefore, we suggest that the 25-fold higher rate of glucose uptake in HCLE cells is a result of higher expression and higher activity of GLUT1. This is supported by the relative inability to activate glucose uptake in HCLE cells. Glucose deprivation only slightly activates glucose uptake in HCLE cells (1.5-fold, see Figure 2) compared to a robust 10-20-fold activation observed in L929 cells [7]. Berberine and sodium azide are compounds that activate glucose uptake via interference with mitochondrial function and possible activation of AMP kinase [11, 12, 15, 18, 30-32]. These agents activate glucose uptake over 3-foldin L929 cells, but they have no effect on glucose uptake in HCLE cells (see Figure 3). Given the high glycolytic dependence and the reported low number of mitochondria in corneal epithelial cells, it may not be surprising that agents that stress mitochondrial function do not activate glucose uptake in this cell line. However, thiol reactive agents, which are not known to affect mitochondrial function, acutely activate glucose uptake in L929 cells, but inhibituptake in HCLE cells. In L929 cells, CA and AS stimulate glucose uptake 3.5-fold and 4.2-fold respectively, but reduce uptake by 58%and 42% respectively in HCLE cells. PAO stimulates uptake 3.9-fold in L929 cells but has no effect in HCLE cells (see Figure 4). It should benoted that the 3- to 4-fold activation by the wide variety of activators in L929 cells coupled to the 6.6-fold higher expression of GLUT1 in HCLE cells nicely accounts for the 25-fold difference observed in the two cell lines.
The high concentration and high intrinsic activity of GLUT1 in HCLE cells suggest a plausible explanation for the observations that the corneal epithelium from diabetic patients are more fragile and have slower wound healing [24-26]. The high glucose uptake rates via GLUT1 coupled with high blood glucose concentrations in diabetic patients would produce higher intracellular concentrations of glucose. This would be expected to increase the production of intracellular advanced glycationend-products and thereby compromise cell function [33].
The inhibition of glucose uptake by CA and AS is consistent with a proposed mechanism for the acute activation of GLUT1 based on data from erythrocytes and L929 cells [34-36]. This model, shown in Figure 5, suggests that GLUT1 can exist in multiple states ranging from low activity monomers, containing reduced cysteine residues, to high activity oligomers, likely tetramers. The more active tetramer is a noncovalent complex that is stabilized by the conformation change that occurs when an internal disulfide bond forms within GLUT1.If the bulk of the GLUT1 transporters in HCLE cells are in a highly activated state, that is, tetramers with oxidized cysteine residues, the model predicts, as we observe, that reagents that react with thiols would stabilize a less active form of GLUT1 and shift the overall equilibrium away from the highly active state. Unfortunately, the denaturing conditions of SDS electrophoresis prevent the detection of a noncovalenthomotetramer of GLUT1 by Western blotting. Therefore, additional studies, beyond the scope of this study,are needed to confirm the formation of a tetramer.
Figure 5.
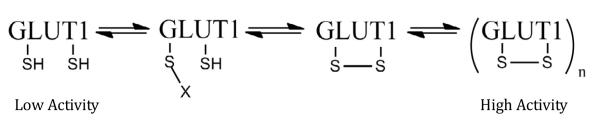
Mechanism for activation of GLUT1. This model for activation suggests that GLUT1 exists in a number of activation states. A fully reduced, monomer form would have the lowest transport activity, while the oligomer (likely a tetramer), would have the highest activity. The modified form of GLUT1 (with X attached) may be more active than the lowest state, but the modification would prevent conversion to the highest active state if the modification is not readily reversible.
It is interesting to note that the characteristics of fast growth rate, high expression of GLUT1, and a heavy dependence on glycolysis in corneal epithelial cells are also characteristics of cancer cells. There is accumulating evidence that many types of cancer overexpress GLUT1 and have high glucose uptake and glycolytic activity [37-43]. GLUT1 has been identified as a potential anticancer target [37, 44]. The observation that thiol reactive compounds inhibit glucose uptake in HCLE cells suggests that it may be important to determine thiol reactive compounds have efficacy as anticancer agents.
5. Conclusions
This study demonstrates that corneal epithelial cells have high glucose uptake rates due to both high expression and high intrinsic activity of GLUT1. In contrast to L929 cells, glucose uptake is only slightly activated by glucose deprivation, not activated by sodium azide, berberine, or phenylarsine oxide and inhibited by compounds that react with thiols such as cinnamaldehyde, and nitroxyl. The data reveal that GLUT1 is acutely regulated in different ways in different tissues. These data are also consistent with a proposed mechanism for GLUT1 activation that involves the formation of a disulfide bond within GLUT1.
Research Highlights.
Human corneal epithelial cells (HCLE) have very high basal rates of glucose uptake.
HCLE cells have 6.6-fold higher expression of GLUT1 than L929 cells.
GLUT1 in HCLE cells has high intrinsic activity and cannot be further activated.
Thiol reactive compounds inhibit rather than activate glucose uptake in HCLE cells.
Acknowledgements
This research was supported by a NIH R15 grant (DK08193-1A1) to Louters and a NIH R01 (EY018100) to Ubels. Special thanks to John P. Toscano for the gift of the Angeli’s salt.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–1443. [PubMed] [Google Scholar]
- [2].Bildin VN, Iserovich P, Fischbarg J, Reinach PS. Differential expression of Na:K:2Cl cotransporter, glucose transporter 1, and aquaporin 1 in freshly isolated and cultured bovine corneal tissues. Exp Biol Med (Maywood) 2001;226:919–926. doi: 10.1177/153537020122601007. [DOI] [PubMed] [Google Scholar]
- [3].Kumagai AK, Glasgow BJ, Pardridge WM. GLUT1 glucose transporter expression in the diabetic and nondiabetic human eye. Invest Ophthalmol Vis Sci. 1994;35:2887–2894. [PubMed] [Google Scholar]
- [4].Takahashi H, Kaminski AE, Zieske JD. Glucose transporter 1 expression is enhanced during corneal epithelial wound repair. Exp Eye Res. 1996;63:649–659. doi: 10.1006/exer.1996.0159. [DOI] [PubMed] [Google Scholar]
- [5].Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H. Ultracytochemical localization of the erythrocyte/HepG2-type glucose transporter (GLUT1) in the ciliary body and iris of the rat eye. Invest Ophthalmol Vis Sci. 1991;32:1659–1666. [PubMed] [Google Scholar]
- [6].Takahashi H, Ohara K, Ohmura T, Takahashi R, Zieske JD. Glucose transporter 1 expression in corneal wound repair under high serum glucose level. Jpn J Ophthalmol. 2000;44:470–474. doi: 10.1016/s0021-5155(00)00222-7. [DOI] [PubMed] [Google Scholar]
- [7].Roelofs B, Tidball A, Lindborg AE, TenHarmsel A, Vander Kooy TO, Louters LL. Acute activation of glucose uptake by glucose deprivation in L929 fibroblast cells. Biochimie. 2006;88:1941–1946. doi: 10.1016/j.biochi.2006.08.004. [DOI] [PubMed] [Google Scholar]
- [8].Kumar A, Xiao YP, Laipis PJ, Fletcher BS, Frost SC. Glucose deprivation enhances targeting of GLUT1 to lipid rafts in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2004;286:E568–576. doi: 10.1152/ajpendo.00372.2003. [DOI] [PubMed] [Google Scholar]
- [9].Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK) J Cell Sci. 2002;115:2433–2442. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- [10].Barros LF, Barnes K, Ingram JC, Castro J, Porras OH, Baldwin SA. Hyperosmotic shock induces both activation and translocation of glucose transporters in mammalian cells. Pflugers Arch. 2001;442:614–621. doi: 10.1007/s004240100577. [DOI] [PubMed] [Google Scholar]
- [11].Shetty M, Loeb JN, Vikstrom K, Ismail-Beigi F. Rapid activation of GLUT-1 glucose transporter following inhibition of oxidative phosphorylation in clone 9 cells. J Biol Chem. 1993;268:17225–17232. [PubMed] [Google Scholar]
- [12].Rubin D, Ismail-Beigi F. Distribution of Glut1 in detergent-resistant membranes (DRMs) and non-DRM domains: effect of treatment with azide. Am J Physiol Cell Physiol. 2003;285:C377–383. doi: 10.1152/ajpcell.00060.2003. [DOI] [PubMed] [Google Scholar]
- [13].Louters LL, Dyste SG, Frieswyk D, Tenharmsel A, Vander Kooy TO, Walters L, Whalen T. Methylene blue stimulates 2-deoxyglucose uptake in L929 fibroblast cells. Life Sci. 2006;78:586–591. doi: 10.1016/j.lfs.2005.05.082. [DOI] [PubMed] [Google Scholar]
- [14].Meyer JA, Froelich JM, Reid GE, Karunarathne WK, Spence DM. Metal-activated C-peptide facilitates glucose clearance and the release of a nitric oxide stimulus via the GLUT1 transporter. Diabetologia. 2008;51:175–182. doi: 10.1007/s00125-007-0853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cok A, Plaisier C, Salie MJ, Oram DS, Chenge J, Louters LL. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie. 2011;93:1187–1192. doi: 10.1016/j.biochi.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Plaisier C, Cok A, Scott J, Opejin A, Bushhouse KT, Salie MJ, Louters LL. Effects of cinnamaldehyde on the glucose transport activity of GLUT1. Biochimie. 2011;93:339–344. doi: 10.1016/j.biochi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Scott J, Opejin A, Tidball A, Stehouwer N, Rekman J, Louters LL. Dual action of phenylarsine oxide on the glucose transport activity of GLUT1. Chemico-biological interactions. 2009;182:199–203. doi: 10.1016/j.cbi.2009.08.008. [DOI] [PubMed] [Google Scholar]
- [18].Salie MJ, Oram DS, Kuipers DP, Scripture JP, Chenge J, MacDonald GJ, Louters LL. Nitroxyl (HNO) acutely activates the glucose uptake activity of GLUT1. Biochimie. 2012;94:864–869. doi: 10.1016/j.biochi.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- [20].Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, De Luca M, Catricala C, O’Toole KM. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol. 2002;22:5157–5172. doi: 10.1128/MCB.22.14.5157-5172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 2009;284:23037–23045. doi: 10.1074/jbc.M109.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schotanus MP, Koetje LR, Van Dyken RE, Ubels JL. Stratified corneal limbal epithelial cells are protected from UVB-induced apoptosis by elevated extracellular K(+) Exp Eye Res. 2011;93:735–740. doi: 10.1016/j.exer.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Singleton KR, Will DS, Schotanus MP, Haarsma LD, Koetje LR, Bardolph SL, Ubels JL. Elevated extracellular K+ inhibits apoptosis of corneal epithelial cells exposed to UV-B radiation. Exp Eye Res. 2009;89:140–151. doi: 10.1016/j.exer.2009.02.023. [DOI] [PubMed] [Google Scholar]
- [24].Chikama T, Wakuta M, Liu Y, Nishida T. Deviated mechanism of wound healing in diabetic corneas. Cornea. 2007;26:S75–81. doi: 10.1097/ICO.0b013e31812f6d8e. [DOI] [PubMed] [Google Scholar]
- [25].McLaughlin PJ, Sassani JW, Klocek MS, Zagon IS. Diabetic keratopathy and treatment by modulation of the opioid growth factor (OGF)-OGF receptor (OGFr) axis with naltrexone: a review. Brain Res Bull. 2010;81:236–247. doi: 10.1016/j.brainresbull.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Alves MC, Carvalheira JB, Modulo CM, Rocha EM. Tear film and ocular surface changes in diabetes mellitus. Arq Bras Oftalmol. 2008;71:96–103. doi: 10.1590/s0004-27492008000700018. [DOI] [PubMed] [Google Scholar]
- [27].Liong E, Kong SK, Au KK, Li JY, Xu GY, Lee YL, Kwok TT, Choy YM, Lee CY, Fung KP. Inhibition of glucose uptake and suppression of glucose transporter 1 mRNA expression in L929 cells by tumour necrosis factor-alpha. Life Sci. 1999;65:PL215–220. doi: 10.1016/s0024-3205(99)00408-7. [DOI] [PubMed] [Google Scholar]
- [28].Van Dyke DA, Walters L, Frieswyk D, Kokmeyer D, Louters LL. Acute effects of troglitazone and nitric oxide on glucose uptake in L929 fibroblast cells. Life Sci. 2003;72:2321–2327. doi: 10.1016/s0024-3205(03)00119-x. [DOI] [PubMed] [Google Scholar]
- [29].Vera JC, Reyes AM, Velasquez FV, Rivas CI, Zhang RH, Strobel P, Slebe JC, Nunez-Alarcon J, Golde DW. Direct inhibition of the hexose transporter GLUT1 by tyrosine kinase inhibitors. Biochemistry. 2001;40:777–790. doi: 10.1021/bi001660j. [DOI] [PubMed] [Google Scholar]
- [30].Kim SH, Shin EJ, Kim ED, Bayaraa T, Frost SC, Hyun CK. Berberine activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes. Biol Pharm Bull. 2007;30:2120–2125. doi: 10.1248/bpb.30.2120. [DOI] [PubMed] [Google Scholar]
- [31].Cheng Z, Pang T, Gu M, Gao AH, Xie CM, Li JY, Nan FJ, Li J. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim Biophys Acta. 2006;1760:1682–1689. doi: 10.1016/j.bbagen.2006.09.007. [DOI] [PubMed] [Google Scholar]
- [32].Zhou L, Yang Y, Wang X, Liu S, Shang W, Yuan G, Li F, Tang J, Chen M, Chen J. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism. 2007;56:405–412. doi: 10.1016/j.metabol.2006.10.025. [DOI] [PubMed] [Google Scholar]
- [33].Chibber R, Molinatti PA, Kohner EM. Intracellular protein glycation in cultured retinal capillary pericytes and endothelial cells exposed to high-glucose concentration. Cell Mol Biol (Noisy-le-grand) 1999;45:47–57. [PubMed] [Google Scholar]
- [34].Graybill C, van Hoek AN, Desai D, Carruthers AM, Carruthers A. Ultrastructure of human erythrocyte GLUT1. Biochemistry. 2006;45:8096–8107. doi: 10.1021/bi060398x. [DOI] [PubMed] [Google Scholar]
- [35].Carruthers A, DeZutter J, Ganguly A, U S. Devaskar, Will the original glucose transporter isoform please stand up! Am J Physiol Endocrinol Metab. 2009;297:E836–848. doi: 10.1152/ajpendo.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zottola RJ, Cloherty EK, Coderre PE, Hansen A, Hebert DN, Carruthers A. Glucose transporter function is controlled by transporter oligomeric structure. A single, intramolecular disulfide promotes GLUT1 tetramerization. Biochemistry. 1995;34:9734–9747. doi: 10.1021/bi00030a011. [DOI] [PubMed] [Google Scholar]
- [37].Furuta E, Okuda H, Kobayashi A, Watabe K. Metabolic genes in cancer: their roles in tumor progression and clinical implications. Biochim Biophys Acta. 2010;1805:141–152. doi: 10.1016/j.bbcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Amann T, Hellerbrand C. GLUT1 as a therapeutic target in hepatocellular carcinoma. Expert Opin Ther Targets. 2009;13:1411–1427. doi: 10.1517/14728220903307509. [DOI] [PubMed] [Google Scholar]
- [39].Marin-Hernandez A, Gallardo-Perez JC, Ralph SJ, Rodriguez-Enriquez S, Moreno-Sanchez R. HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev Med Chem. 2009;9:1084–1101. doi: 10.2174/138955709788922610. [DOI] [PubMed] [Google Scholar]
- [40].Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- [41].Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84:949–957. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Young CD, Lewis AS, Rudolph MC, Ruehle MD, Jackman MR, Yun UJ, Ilkun O, Pereira R, Abel ED, Anderson SM. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PLoS One. 2011;6:e23205. doi: 10.1371/journal.pone.0023205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- [44].Ojeda PG, Perez AA, Ojeda L, Vargas-Uribe M, Rivas CI, Salas M, Vera JC, Reyes AM. Non-Competitive Blocking of Human Glut1 Hexose Transporter by Methylxanthines Reveals an Exofacial Regulatory Binding Site. Am J Physiol Cell Physiol. 2012 doi: 10.1152/ajpcell.00145.2012. [DOI] [PubMed] [Google Scholar]