Abstract
Recent advances in a number of systems suggest many genes involved in orchestrating regeneration are redeployed from similar processes in development, with others being novel to the regeneration process in particular lineages. Of particular importance will be understanding the architecture of regenerative genetic regulatory networks and whether they are conserved across broad phylogenetic distances. Here, we describe the role of the conserved TALE class protein PBX/Extradenticle in planarians, a representative member of the Lophotrocozoa. PBX/Extradenticle proteins play central roles in both embryonic and post-embryonic developmental patterning in both vertebrates and insects, and we demonstrate a broad requirement during planarian regeneration. We observe that Smed-pbx has pleiotropic functions during regeneration, with a primary role in patterning the anterior-posterior (AP) axis and AP polarity. Smed-pbx is required for expression of polarity determinants notum and wnt1 and for correct patterning of the structures polarized along the AP axis, such as the brain, pharynx and gut. Overall, our data suggest that Smed-pbx functions as a central integrator of positional information to drive patterning of regeneration along the body axis.
Keywords: TALE class, Planarian, Polarity, Regeneration, Stem cells
INTRODUCTION
The ability of some extant taxa to regenerate adult tissues and organs after injury remains poorly understood at the molecular level. Among the Bilateria, planarian flatworms arguably have the most prodigious capacity for regeneration and are potentially immortal as a result (Aboobaker, 2011; Tan et al., 2012). Adult animals consist of many organized differentiated tissues and cell types that are basal to the Bilaterian lineage. These undergo constant replacement and renewal from a pool of totipotent adult stem cells (Wagner et al., 2011) called neoblasts. These life history traits make them suited to investigating the control of stem cell self-renewal and maintenance (Guo et al., 2006; Solana et al., 2009; Wagner et al., 2012), the global control of stem cell differentiation into particular cell types (Scimone et al., 2010), tissues and organs (Lapan and Reddien, 2011; Rink et al., 2011; Scimone et al., 2011), and the signals that underpin the polarity and position of structures along the body axes (Gurley et al., 2008; Iglesias et al., 2008; Petersen and Reddien, 2008; Petersen and Reddien, 2009; Rink et al., 2009; Yazawa et al., 2009; Felix and Aboobaker, 2010; Gaviño and Reddien, 2011; Iglesias et al., 2011; Molina et al., 2011).
Here, we focus on the last of these processes and describe a new component of AP axis regeneration. Previous work has uncovered the central roles of Wnt signaling and Hh signaling in setting posterior polarity. Posterior polarity and regeneration require active Wnt signaling and this, in turn, requires active Hh signaling to be correctly established at posterior wound sites (Gurley et al., 2008; Iglesias et al., 2008; Petersen and Reddien, 2008; Rink et al., 2009; Yazawa et al., 2009). Both ectopic Hh and Wnt signals are able to reprogram anterior wounds such that they produce tails instead of heads (Gurley et al., 2008; Iglesias et al., 2008; Petersen and Reddien, 2008; Rink et al., 2009; Yazawa et al., 2009). Brain regeneration is independent of polarity at early stages of regeneration, as brain tissues differentiate even in the presence of ectopic posteriorizing Wnt and Hh signals (Evans et al., 2011; Iglesias et al., 2011). Abrogation of Wnt signaling by β-catenin-1(RNAi) leads to anterior fate being adopted at all wound sites, suggesting that this is a default fate for regeneration in planarians (Iglesias et al., 2008). It has not been convincingly demonstrated that Hh signaling leads to ectopic anterior fates (Rink et al., 2009; Yazawa et al., 2009; Evans et al., 2011), suggesting its primary role with respect to polarity is to correctly establish a second phase of Wnt signaling after wounding (Petersen and Reddien, 2009). During normal regeneration, notum normally acts at anterior wounds to inhibit the establishment of Wnt signals and thus promote anterior regeneration (Petersen and Reddien, 2011). In the absence of notum, anterior wound sites form tails. Finally, the TALE (three amino acid loop extension) class homeodomain transcription factor prep is required for promoting anterior fates, with knockdown leading to a lack of anterior structures in the anterior blastema but without conversion to posterior fates (Felix and Aboobaker, 2010).
Here, we describe the role of another TALE class homeodomain protein, PBX/Extradenticle, in S. mediterannea. This transcription factor has been shown to have broad roles in embryonic and post-embryonic patterning events in vertebrates (Karlsson et al., 2010; Capellini et al., 2011; Vitobello et al., 2011) and members of the Ecdysozoa protostome clade (Peifer and Wieschaus, 1990; González-Crespo et al., 1998; Van Auken et al., 2002; Merabet et al., 2005; Yang et al., 2005; Tanaka and Truman, 2007; Prpic and Telford, 2008). Given the growing evidence for roles of other TALE class proteins in regeneration, this gene represented a good candidate for a central role in planarian regeneration (Mercader et al., 2005; Felix and Aboobaker, 2010; Shaikh et al., 2011). We find that Smed-pbx is broadly expressed in stem cells and their progeny, and that it is required for AP patterning along the body axis. Our data place PBX as a central regulator that interprets signals along the AP axis, but is not involved in stem cell maintenance, renewal or pluripotency/differentiation. Overall, we describe a key function for this important conserved transcription factor during regeneration in a metazoan and in a representative member of the Lophotrocozoan clade of animals.
MATERIALS AND METHODS
Planarian culture and irradiation
A clonal asexual line of Schmidtea mediterranea established from a worm provided by Emili Salo's laboratory in Barcelona was maintained as previously described (Felix and Aboobaker, 2010). The animals were starved for 7 days prior to experiments and not fed for the duration of the experiments. Animals were irradiated as previously described (González-Estévez et al., 2012).
Cloning Smed-pbx/extradenticle
Smed-pbx (pbx for short) was identified previously in a screen for orthologs of TALE class proteins (Felix and Aboobaker, 2010). The complete ORF was assembled and cloned by combining this genomic data with transcriptomic data (Blythe et al., 2010) and confirmed by sequence data generated by RACE (Ambion RLM RACE Kit). Two non-overlapping fragments were amplified by the primer pairs Fwd1-AATAATCATCGATTGAAGCCTGCG and Rev1-CCTTATGCGCTT - ATTGCCAAACCA, and Fwd2-GCACAGGAAGAAGCTAAT and Rev2-GCTATCAAGGATCAAACAC, and cloned as templates for the synthesis of dsRNA and in situ hybridization probes. Both fragments produced identical RNAi phenotypes and staining patterns by in situ hybridization (Fig. 1; Fig. 2; supplementary material Fig. S1). The complete pbx sequence has been submitted to GenBank with Accession Number KC353351.
Fig. 1.
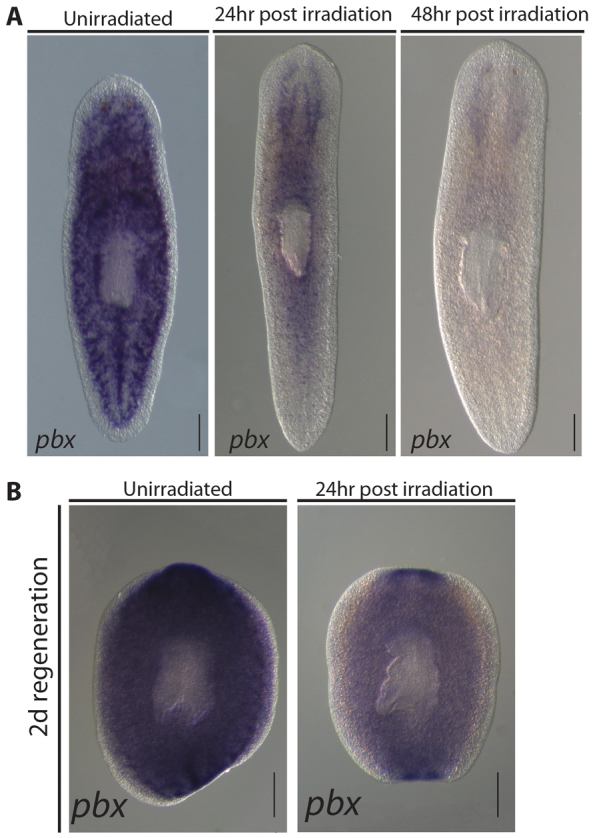
pbx is expressed in irradiation-sensitive cells, the cephalic ganglia and regenerating blastemas. (A) pbx was expressed throughout the parenchyma of whole worms and resembles the pattern observed for neoblast-expressed genes. Twenty-four hours after a 100 Gy dose of γ-irradiation, most pbx-expressing cells are lost, revealing clear expression in the CG. By 48 hours post-irradiation, the distinctive parenchymal expression pattern of pbx was completely lost. (B) pbx expression was broadly observed in both anterior and posterior blastemas of regenerating pieces. Regenerating fragments were γ-irradiated with a dose of 100 Gy at 1 dpa to aid visualization of pbx in cells other than neoblasts. Expression was bilateral in both anterior and posterior blastemas, suggesting expression in the regenerating CNS.
Fig. 2.
pbx(RNAi) disrupts regeneration. (A) Posterior blastemas of control head and trunk pieces regenerated tails, whereas those of pbx(RNAi) head and trunk pieces failed to do so (75/75, across three experiments). Anterior blastemas of control trunk and tail pieces regenerated a head and photoreceptors, whereas those of pbx(RNAi) trunk and tail pieces failed to do so (75/75, across three experiments). In addition, all pbx(RNAi) head and tail pieces failed to regenerate the pharynx correctly. (B) Staining of the nervous system shows that the VNCs of regenerated tails meet at an acute angle in control head (35/35 across three experiments) and trunk pieces (35/35), whereas those of pbx(RNAi) pieces regenerated aberrantly (35/35 in heads, 35/35 in tails, across three experiments, red arrowheads). Staining of the nervous system also showed that the CG of control trunk and tail pieces regenerated normally when compared with the intact CG of head pieces (35/35, across three experiments). CG regeneration was greatly reduced in pbx(RNAi) trunk pieces (35/35, across three experiments, shown by red arrows) and failed completely in tail pieces (33/35, across three experiments, red arrowheads). (C) The expression of pharynx-specific smed-laminin observed in control regenerated tail pieces (15/5) was absent in pbx(RNAi) regenerates (15/15). (D) Control regenerated trunk pieces expressed sFRP-1 (10/10) at the anterior margin and FZ4 at the posterior (10/10), whereas regenerated pbx(RNAi) trunk pieces failed to express either marker (10/10 for both markers). Scale bars: 200 μm.
RNAi
Template DNA was generated by PCR from plasmid templates and this was used as a template for synthesis of dsRNA by reverse transcription using T7 and Sp6 polymerase (New England Biolabs). DsRNA was diluted to the appropriate concentration and microinjected (Sánchez Alvarado and Newmark, 1999). In single and double RNAi experiments, a concentration of 2 μg/μl dsRNA was maintained for each gene injected (supplementary material Fig. S2).
In situ hybridization
Whole mount in situ hybridization and in situ hybridization on paraffin sections were performed as described previously (Umesono et al., 1997; Cardona et al., 2005; González-Estévez et al., 2009). The following probes were used: Smed-laminin-, Smed-sFRP, Smed-FZ4, Smed-GPAS, Smed-NB.21.11.e, Smed-AGAT1, Smed-septin, Smed-porcn1, Smed-Wnt1, Smed-Wnt11-2, Smed-slit, Smed-H2B and Smed-notum (Cebrià and Newmark, 2005; Cebrià et al., 2007; Cebrià and Newmark, 2007; Eisenhoffer et al., 2008; Gurley et al., 2008; Iglesias et al., 2011; Petersen and Reddien, 2011; Solana et al., 2012). RNA in situ probes were generated as described previously (González-Estévez et al., 2009). Bright-field images were taken on a Zeiss Discovery V8 using an Axiocam MRC (Carl Zeiss). Bright-field images of adjacent sections were false colored and overlaid to demonstrate colocalization of pbx and Smed-H2B in neoblasts. For quantification of Smed-NB.21.11.e- and Smed-AGAT1-expressing cells by fluorescent in situ hybridization, maximum projections were created from ∼20 1 μm optical sections from the anterior-dorsal domain. ImageJ was used to quantify the number of cells. Confocal laser scanning microscopy was performed using a Leica SP2 confocal microscope (CLSM, Leica Lasertechnik).
Immunohistochemistry
Intact and regenerating planarians were fixed and processed as previously described (Cebrià and Newmark, 2005). Primary antibodies used were: 3C11 (anti-SYNORF1) (Developmental Studies Hybridoma Bank, dilution 1:50), anti-phospho-serine 10 Histone H3 (H3P) (Upstate, dilution 1:500) and anti-acetylated tubulin (Sigma, dilution 1:200). Secondary antibodies used were: Alexa 488-conjugated goat anti-mouse (Molecular Probes, used at 1:400) and Alexa 568-conjugated goat anti-rabbit (Molecular Probes, used at 1:1000). Fluorescent images were acquired using a Leica MZ16F fluorescent stereomicroscope and DFC 300Fx camera (Leica Lasertechnik).
Pharynx amputation
Removal of the pharynx was performed using sharpened tungsten dissection needles. Animals were placed ventral upwards on glass slides on a bed of ice. A needle with a hooked end was used to pull the pharynx out of the body. The pharynx was then amputated using a second needle.
Analysis of proliferation
Mitotic figures were visualized by anti-H3P immunostaining and counted. Adobe Photoshop CS4 was used to determine the area of each sample analyzed and the number of mitoses/mm2 was calculated.
RESULTS
pbx is expressed in neoblasts, the regenerating blastema and the central nervous system
A single ortholog of the extradenticle/pbx family was identified and named Smed-pbx (pbx for short). pbx expression in multiple RNAseq experiments was reduced by irradiation or loss of neoblasts induced by genetic means, and enriched in stem cells and stem cell progeny in FACS sorted populations (Blythe et al., 2010; Labbé et al., 2012; Onal et al., 2012; Solana et al., 2012). In situ hybridization demonstrated that pbx is expressed broadly in intact planarians in a pattern that resembled that exhibited by neoblasts (Fig. 1A; supplementary material Fig. S1A). We found pbx expression was depleted from the parenchyma 24 hours after γ-irradiation to remove neoblasts, revealing the remaining expression of pbx in the cephalic ganglia (CG) (Fig. 1A). pbx was expressed throughout the parenchyma of regenerating pieces amputated anterior and posterior to the pharynx (Fig. 1B), and within and adjacent to the regenerating blastemas (Fig. 1B). As regeneration proceeded, pbx expression was broadly observed throughout anterior and posterior blastemas, indicative of expression in neoblast progeny forming this structure (supplementary material Fig. S1B).
To reveal expression in post-mitotic differentiated cells, regenerating pieces were irradiated 24 hours prior to fixing. In these animals at 2 days of regeneration (dR), bilateral expression was observed within both anterior and posterior blastemas, indicative of expression in the regenerating central nervous system (CNS) (Fig. 1B). We also observed expression in the regenerating pharynx region in head and tail fragments from 3 dR (supplementary material Fig. S1B). This pattern of expression continued to be evident through to 5 dR (supplementary material Fig. S1B).
Taken together, these data suggest that pbx has a complex pattern of expression that includes neoblasts, neoblast progeny and the CG. In addition, during regeneration pbx is also expressed in the regenerating CNS in both anterior and posterior blastemas, and in the region of the regenerating pharynx.
pbx is required for regeneration along the AP axis
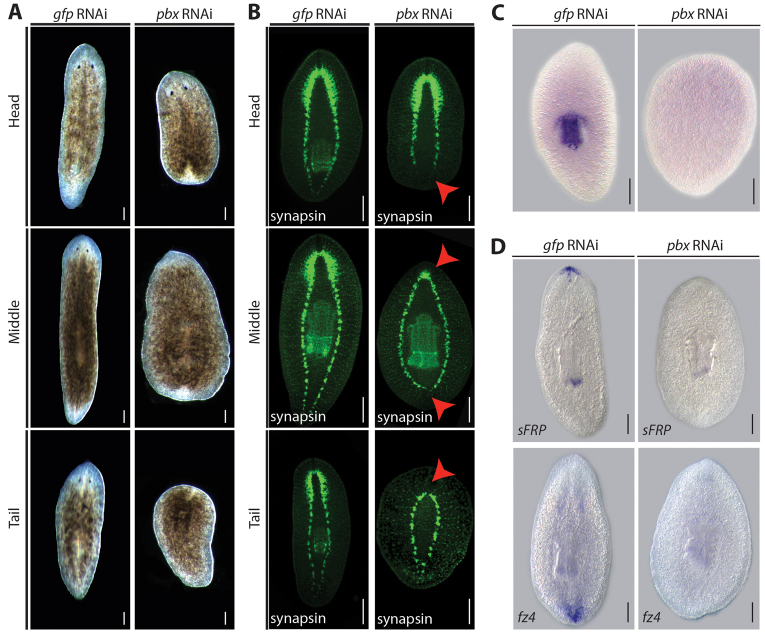
RNA interference (RNAi) was used to knock down pbx expression (see supplementary material Fig. S2A). We observed clear defects in the ability of pbx(RNAi) animals to regenerate correctly. Animals formed regeneration blastemas but head pieces failed to regenerate a tail or a pharynx, tail pieces failed to regenerate a head or a pharynx, and trunk pieces failed to regenerate a head or a tail (Fig. 2A). Using a pan-neural marker against synapsin, we observed that anterior blastemas had highly reduced CG labeling, and head and trunk pieces failed to correctly regenerate ventral nerve cords (VNCs) posteriorly after 14 days post amputation (dpa) (Fig. 2B). VNCs failed to extend into the posterior blastema in head pieces and were prematurely rounded and fused behind the pre-existing pharynx in trunk pieces (Fig. 2B). To confirm failure in pharynx regeneration, we used the pharynx-specific marker Smed-laminin (Cebrià and Newmark, 2007) and observed that characteristic expression in the pharynx is absent in pbx(RNAi)-regenerating animals (Fig. 2C). Regeneration in all control gfp(RNAi) animals was normal.
Taken together, these data indicate that pbx(RNAi) leads to regenerative defects along the planarian body axis. Supporting this hypothesis, pbx(RNAi) led to loss of sFRP-1 and Fz4 (Gurley et al., 2008), which are markers of anterior and posterior fate, respectively (Fig. 2D).
We assessed the maintenance and proliferative capacity of neoblasts by monitoring cell proliferation and the characteristic peaks of neoblast proliferation associated with amputation (supplementary material Fig. S3A) (Saló and Baguñà, 1984; Wenemoser and Reddien, 2010). Proliferation was also unaffected after the completion of regeneration. We also observed no significant difference in the generation of nb.21.11.e- or agat-1-expressing neoblast progeny (Eisenhoffer et al., 2008) between gfp(RNAi) and pbx(RNAi) animals (supplementary material Fig. S3B,C).
Together, these data suggest that defects in stem cell maintenance, proliferation and differentiation do not contribute to the pbx phenotype. Instead our data suggest that the observed phenotype results from a failure to regenerate the head/brain and tail/VNCs correctly.
Smed-pbx is required to pattern the anterior compartment and regenerating CG
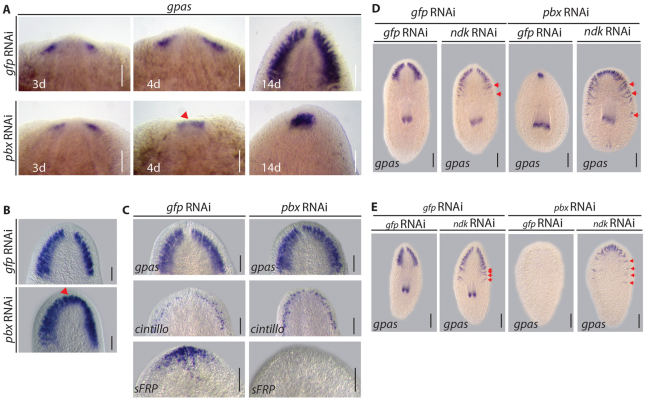
The pbx(RNAi) phenotype highlights a role in CG regeneration and anterior regeneration. Previously, it has been demonstrated that CG regeneration consists of early and late phases, with the early phase being independent of Wnt signaling-mediated polarity cues (Evans et al., 2011; Iglesias et al., 2011). Early CG regeneration was investigated in pbx(RNAi) trunk pieces (Fig. 3A), which exhibit a less comprehensive failure of CG regeneration than tail pieces (see Fig. 2B). At 3 days post-amputation, discrete bilateral brain primordia were observed in anterior blastemas (Fig. 3A). Whereas these structures remained discrete in gfp(RNAi) regenerates, by 4 days they had started to coalesce in pbx(RNAi) regenerates (Fig. 3A). Compared with gfp(RNAi) regenerates the fused CG of pbx(RNAi) regenerates did not enlarge significantly by 14 dpa (Fig. 3A).
Fig. 3.
pbx is required for CG patterning. (A) By 3 dpa, CG primordia expressed the neural marker GPAS in the anterior blastema of regenerating trunk pieces in control (10/10) and pbx(RNAi) (10/10). At 4 dpa, these structures remained separate in control regenerates (8/8), whereas they started to coalesce in pbx(RNAi) (8/8) (red arrowhead). By 14 dpa, the CG was completely regenerated in controls (10/10), whereas in pbx(RNAi) regenerates the fused CG did not enlarge significantly (10/10). (B) Following longitudinal amputation the structure of the CG was correctly regenerated by 14 dpa in control lateral regenerates (12/12). Following pbx(RNAi), CG tissue was regenerated to the same extent as in controls; however, the structure was fused at the midline (17/17 fused, red arrowhead). (C) The CG structure was comparable in intact control (12/12) and pbx(RNAi) (14/14) planarians, as was the presence of cintillo-expressing sensory cells (8/8 and 8/8, respectively). In control planarians, sFRP was expressed at the anterior margin (13/13), whereas it was lost following pbx(RNAi) within 3 weeks of homeostasis (9/13). (D,E) In control gfp(RNAi) background, ndk(RNAi) resulted in expansion of the regenerated CG in 14 dpa tail and trunk pieces (17/17 control animals normal, 18/18 tails and 20/20 trunks in ndk(RNAi) expanded, red arrows). CG regeneration was not observed following pbx/gfp(RNAi) in tails (22/22) and was greatly reduced in trunks (20/20). Tail and trunk pieces regenerated mis-patterned CG at the anterior following combined pbx/ndk(RNAi) (20/20 animals). Scale bars: 100 μm.
A similar encroachment of CG tissue into midline territory is observed following Smed-slit RNAi (Cebrià et al., 2007), prompting us to assess the regeneration of the midline following pbx RNAi. Consistent with a secondary role for pbx in the re-establishment of the midline, the characteristic stripe of Smed-slit expressing cells did not extend into either the anterior or posterior blastemas following pbx(RNAi) (supplementary material Fig. S4A).
These data suggest that subsequent to the early initiation of CG differentiation, pbx is required for later expansion and patterning of the CG, as well as having an effect on midline establishment.
The defect in midline patterning was also observed when assessing the regeneration of the dorsal cilia. On the dorsal surface of pbx(RNAi) animals, the stripe of prominent cilia along the midline is absent (supplementary material Fig. S4B). Cilia are regenerated normally on the ventral surface of pbx(RNAi) animals and on the dorsal surface away from the midline (supplementary material Fig. S4B), suggesting that defects observed in the regeneration of cilia on the dorsal surface result from a failure to re-establish the pattern of the dorsal midline stripe rather than from a general defect in ciliogenesis.
When amputated longitudinally, lateral regeneration re-established the bilateral organization of the CG following gfp(RNAi) (Fig. 3B). By contrast, whereas the CG of pbx(RNAi) animals regenerated to a comparable extent, the ganglia were fused at the midline (Fig. 3B). Smed-slit expression was observed along the midline in pbx (RNAi) lateral regenerates; however, expression did not extend as far anteriorly as in gfp (RNAi) controls (supplementary material Fig. S4C). These results suggest that pbx is required for the complete anterior regeneration of CG and for correct patterning, but not required for lateral regeneration. The fusion of CG may reflect a requirement for pbx to re-establish the midline as reflected by changes in Smed-slit expression.
During homeostasis, the CG are maintained following pbx(RNAi) (Fig. 3C), as are mechanosensory cintillo-positive cells (Oviedo et al., 2003) that are associated with the CG (Fig. 3C), despite loss of the anterior marker Smed-sFRP-1 (Fig. 3C) (Gurley et al., 2008) and of the dorsal stripe of cilia (supplementary material Fig. S4D). These data suggest that pbx activity is required for appropriate regeneration and patterning of the CG during anterior regeneration, rather than being required for CG maintenance or pattern during homeostasis.
To test whether pbx was absolutely required for the differentiation of stem cells to CG tissue following decapitation, we performed double RNAi experiments with noudarake (ndk). The FGF-like receptor (ndk) normally restricts CG differentiation to the anterior compartment (Cebrià et al., 2002; Felix and Aboobaker, 2010). We reasoned that if pbx were required for stem cell to CG differentiation, pbx/ndk(RNAi) would not lead to ectopic CG regeneration. However, we observed that pbx/ndk(RNAi) leads to the regeneration of extensive, albeit mis-patterned, CG fused at the anterior midline both in trunks and even in tails, which normally fail to regenerate any detectable CG after pbx(RNAi) (Fig. 3D,E).
We next wished to assess the role of pbx for other characteristic features of the anterior compartment apart from the CG. To do this, we investigated the expression of markers with characteristic distribution with respect to the anterior. The septin gene is expressed by gland cells exclusively on the dorsal surface of the animal (Molina et al., 2011), but is also absent from the anterior compartment (Fig. 4A). However, in pbx(RNAi) regenerates, septin expression extended into the regenerated anterior region of trunk pieces (Fig. 4A), despite the regeneration of limited CG (Fig. 3). This suggests that patterning of the anterior compartment is disrupted. We found no evidence for ectopic ventral expression of septin in pbx(RNAi) animals (Fig. 4A). This is consistent with the anterior ectopic expression of septin resulting from a failure to define the anterior compartment.
Fig. 4.
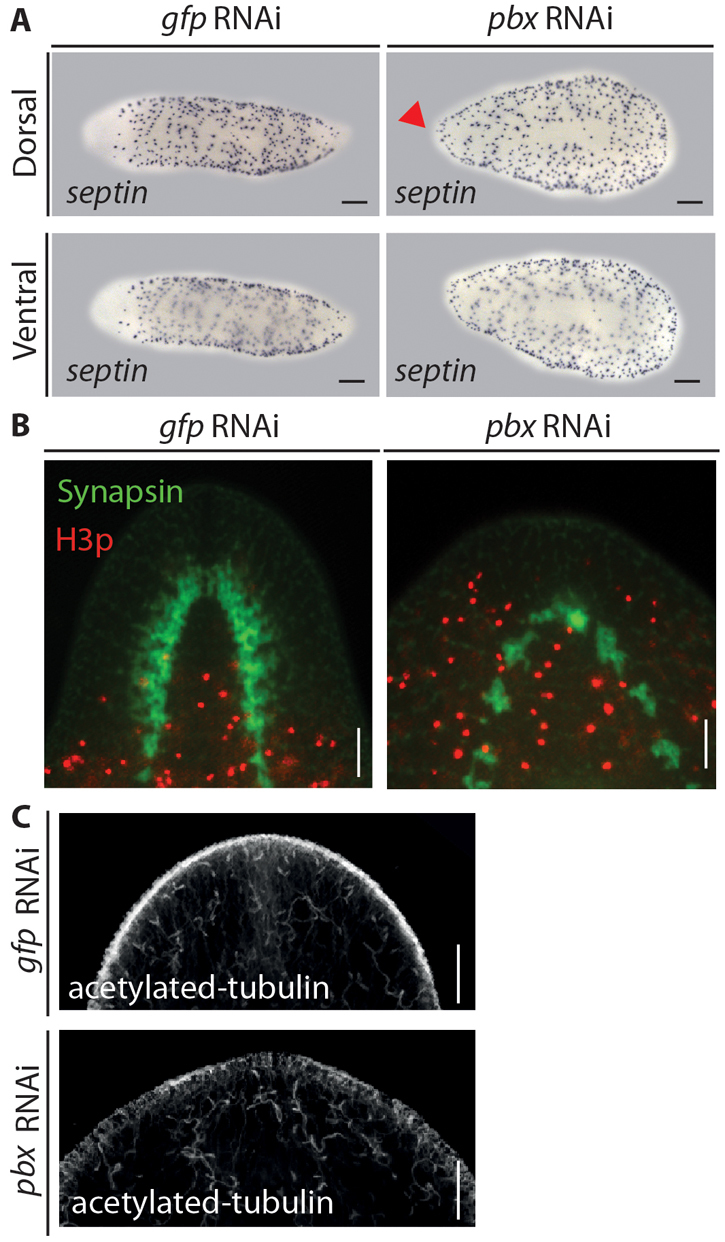
The anterior compartment is improperly patterned following pbx(RNAi). (A) The dorsal marker septin was characteristically absent form the anterior compartment of controls (6/6), whereas it was expressed ectopically at the anterior margin of pbx(RNAi) regenerates (7/7) (red arrowhead). No ventral expansion of septin expression is observed. (B) H3p-positive mitotic neoblasts (red) were characteristically absent from the anterior compartment of control regenerates at 14 dpa (15/15). The regenerated CG is shown by 3C11 staining (green). H3p-positive mitotic neoblasts failed to be excluded from the anterior compartment of pbx(RNAi) regenerates in which the mis-patterned CG differentiates (15/15). (C) Protonephridia labeled by anti-acetylated tubulin immunostaining were observed at the anterior margin of control and pbx(RNAi) regenerates (7/7 and 15/15). Scale bars: 100 μm in B,C; 200 μm in A.
In order to further investigate the defects in the patterning of the regenerating anterior compartment following pbx(RNAi), we investigated the distribution of mitotic cells in the anterior compartment of 14 dpa trunk pieces. H3p+ neoblasts are characteristically absent from the most anterior regions of gfp(RNAi) blastemas, where the regenerated bi-lobed CG is visualized by 3C11 staining (Fig. 4B). By contrast, H3p+ neoblasts are located in the anterior regions of pbx(RNAi) animals, surrounding the reduced and mis-patterned CG (Fig. 4B).
These data support two major conclusions regarding the pbx phenotype. First, as previously shown, mitotic neoblasts are maintained. Second, as suggested by the ectopic encroachment of normally excluded cell types (septin+) and the loss of structures and markers specific to the anterior compartment, the anterior compartment fails to regenerate appropriate identity so as to exclude mitotic neoblasts following pbx(RNAi).
Protonephridia are also distributed along the AP axis but without any obvious AP differences (Rink et al., 2011; Scimone et al., 2011). We observed normal protonephridia in the regenerative blastemas of pbx(RNAi) regenerates (Fig. 4C). Taken together, these data show that despite the failure to regenerate anterior and posterior structures as well as the pharynx, other cells, tissues and organs are regenerated correctly in pbx(RNAi) animals.
Smed-pbx is required to pattern the regenerating gut and pharynx
Another planarian tissue that displays polarity along the AP axis is the gut, which in Triclad species has one major anterior branch and two major posterior branches. During regeneration, the gut is coordinately remodeled in existing tissue and regenerated in new tissue to reconstitute this characteristic pattern (Forsthoefel et al., 2011). We investigated the role of pbx in this process, as well as that of prep, another member of the TALE-class family previously shown to be required specifically for anterior patterning (Felix and Aboobaker, 2010). Using Smed-porcupine as a gut marker (Gurley et al., 2008), we observed regeneration of this structure in tail fragments. Both prep(RNAi) and pbx(RNAi) animals had defects in gut regeneration. The gut completely fails to remodel in pbx(RNAi) animals (Fig. 5A). prep(RNAi) regenerating tail pieces join and remodel gut branches correctly around the regenerating pharynx; however, a clearly defined anterior branch and an anterior compartment absent of gut tissue do not form (Fig. 5A). These data demonstrate that pbx is required for regeneration and remodeling of the gut along the AP axis, while prep is required for this process specifically in the anterior.
Fig. 5.
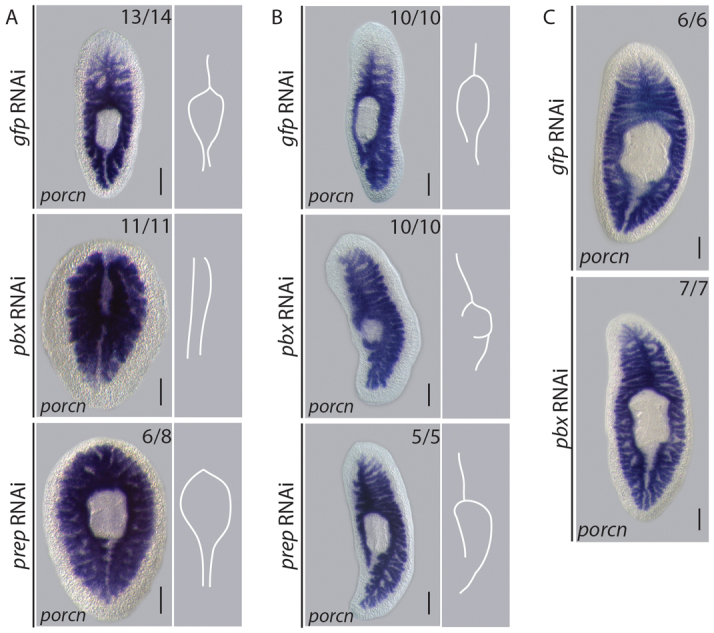
pbx coordinates AP patterning of the regenerating gut.
(A) The anterior gut branch was regenerated and the existing posterior gut branches re-patterned by 14 dpa in control tail pieces (14/14), revealed by gut-specific Smed-porcupine expression. The anterior gut branch failed to regenerate and the existing posterior gut did not re-model following pbx(RNAi) (11/11). The posterior gut remodeled in prep(RNAi) tail pieces; however, the anterior gut branch did not (8/8). Schematic representations of gut morphology summarize the phenotypes observed. (B) By 14 dpa, the posterior gut branch was restored in control lateral regenerates (10/10). The gut extended into the lateral blastema following pbx(RNAi); however, patterning of the posterior branch was not regenerated (10/10). Regeneration of the posterior gut was comparable with controls following prep(RNAi) (10/10). Schematic representations of gut morphology summarize the phenotypes observed. (C) Gut morphology is maintained during 3 weeks of homeostasis following pbx(RNAi) (17/17), resembling controls (16/16). Scale bars: 200 μm.
Longitudinal amputation requires that regeneration restore tissue identities along the whole AP axis. Following pbx(RNAi), the gut regenerated into the lateral blastema; however, appropriate AP patterning was not restored as the missing posterior gut branch did not form (Fig. 5B). By contrast, the posterior gut branch was restored in gfp(RNAi) and prep(RNAi) regenerates (Fig. 5B). After 3 weeks of homeostasis, no abnormalities were observed in the organization of the gut in pbx(RNAi) animals when compared with gfp(RNAi) controls (Fig. 5C). We conclude from these experiments that pbx is required to direct the appropriate patterning of the gut structures across the AP axis during regeneration, whereas prep is required only to pattern the anterior gut. Smed-porcupine-positive gut tissue was regenerated in blastemas of laterally regenerating animals, even though it was incorrectly patterned, suggesting that the capacity for stem cells to form gut cells per se does not require pbx.
We next tested this premise for pharynx regeneration. Pharynx regeneration de novo in regenerating heads and tails pieces fails entirely (Fig. 2). However, when we removed the pharynx from otherwise intact pbx(RNAi) animals we observed the formation of a pharynx anlage (supplementary material Fig. S5A,B). Whereas control animals fully regenerated a normal pharynx, pbx(RNAi) animals could generate only a cluster of Smed-laminin-expressing cells. This suggests that PBX is likely to be required for both establishment of pharynx regeneration and late patterning and morphogenesis of this key organ.
pbx activity promotes brain regeneration independently of anterior polarity specification
As the Wnt/β-catenin 1 pathway has been clearly demonstrated by a number of studies to play an integral role in AP patterning and CG regeneration, we investigated whether our observations could be explained in the context of this signaling pathway. Wnt signaling inhibits both anterior polarity specification and CG differentiation. pbx has the opposite activities: being required to promote anterior polarity and CG differentiation. We performed double pbx/β-catenin-1(RNAi) to investigate their relationship in each of these contexts. β-catenin-1/pbx(RNAi) did not restore pbx-dependent expression of the anterior marker sFRP-1 and we observed the regeneration of mis-patterned CG tissue, in contrast to pbx/gfp(RNAi) regenerates, which failed to differentiate detectable neural tissue (Fig. 6). The anteriors of control gfp/gfp(RNAi) and gfp/β-catenin-1(RNAi) tails pieces were normal with respect to sFRP expression and CG regeneration.
Fig. 6.
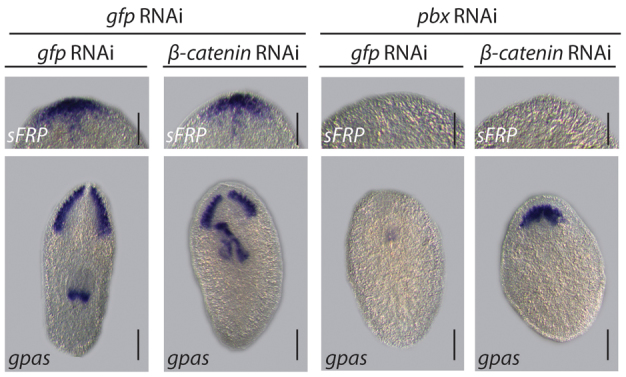
pbx functions independently during CG regeneration and anterior polarity specification. CG regenerated in gfp/gfp(RNAi) and gfp/β-catenin-1(RNAi) (33/33 and 20/20) 14 dpa tail pieces. These animals also expressed sFRP-1 correctly in the anterior (20/22 and 9/9). CG regeneration was not observed following pbx/gfp(RNAi) (38/39). Fused and mis-patterned CG were regenerated following pbx/β-catenin-1(RNAi) (18/18). sFRP-1 was not expressed at the anterior margin of 14 dpa pbx/gfp(RNAi) (27/27) or pbx/β-catenin(RNAi) (21/21) tail pieces. Scale bars: 200 μm (except for those concerning sFRP-1 expression, 100 μm).
Taken together, these data suggest that the role of pbx in establishing anterior polarity could be separated from its role in promoting CG tissue differentiation. Furthermore, we interpret these data to suggest that pbx activity normally promotes CG differentiation in the anterior by opposing the inhibitory activities of β-catenin-1 and ndk (Fig. 3D,E).
We used the combined knockdown of pbx and β-catenin-1 to further investigate the role of pbx during eye regeneration. By observing eye regeneration in different scenarios using combinations of β-catenin-1(RNAi), prep(RNAi) and pbx(RNAi) we found that that pbx, but not prep, has a role in the regeneration and maintenance of the eye that is independent of its role in the regeneration of CG tissue (see supplementary material Fig. S6).
Smed-pbx is required for the expression of polarity determinants
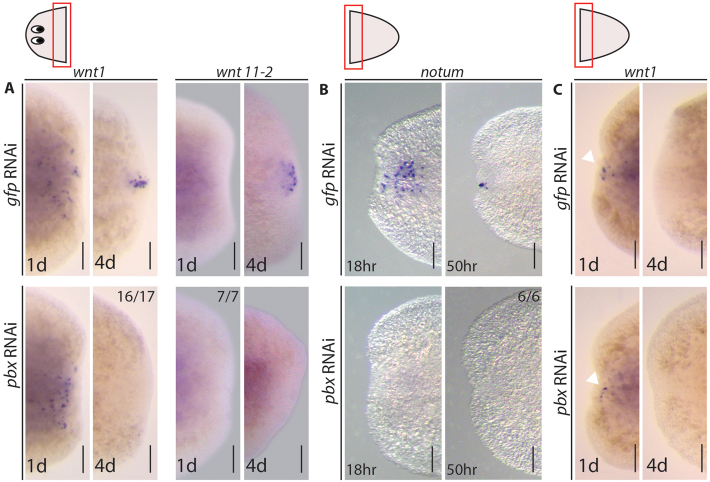
The range of defects observed following pbx(RNAi) are indicative of a broad axis wide role for pbx in AP patterning, and possibly subsequent establishment of the midline. We wished to determine whether these defects could be explained in terms of known AP axis polarity determination events that occur early in regeneration. wnt1 is expressed by differentiated cells at early wound sites, independent of orientation (Petersen and Reddien, 2009; Gurley et al., 2010), and later, through the activity of notum (Petersen and Reddien, 2011) and Hh signaling (Rink et al., 2009; Yazawa et al., 2009), becomes confined to posterior-facing wounds. Wnt1 activity in the posterior blastema drives β-catenin-mediated differentiation of neoblasts into further wnt1-expressing cells and cells expressing the posterior determinant wnt11-2 (Petersen and Reddien, 2009). Following pbx(RNAi), early posterior wnt1 expression was detected in the blastema 24 hours post-amputation, demonstrating that the early stem cell independent phase of expression of Wnt1 is not affected (Fig. 7A). The later phase of wnt1 expression observed 96 hours post-amputation was not detected in pbx(RNAi) regenerates (Fig. 7A). Correspondingly, the induction of wnt11-2 was also not detected following pbx(RNAi) (Fig. 7A). These data suggest that the failure in tail regeneration observed in pbx(RNAi) worms is due to a failure to establish posterior polarity correctly through the previously described Wnt-dependent program (Petersen and Reddien, 2009) and that pbx is required to establish the correct posterior program of Wnt expression during regeneration. Indeed, the posterior tailless phenotype of pbx(RNAi) mimics that described for wnt1(RNAi) (Petersen and Reddien, 2009).
Fig. 7.
pbx is required for expression of AP polarity determinants. (A) wnt1 was expressed in cells adjacent to the posterior wound in control regenerating head pieces (12/12) and following pbx(RNAi) (11/11) at 1 dpa. The sustained posterior wnt1 expression observed at 4 dpa in control animals (16/18) was absent following pbx(RNAi) (16/17). wnt11-2 expression was not observed at 1 dpa in control or pbx(RNAi) animals (8/8 and 7/7), became expressed within the posterior blastema of controls by 4 dpa (17/17) and remained absent from pbx(RNAi) animals (17/17). (B) notum was expressed in cells adjacent to the anterior wound within 18 hours of regeneration in control tail pieces (6/6) and became localized to the anterior tip of the blastema by 50 hours (6/6), whereas its expression was not observed at either time point following pbx RNAi (16/16 and 16/16). (C) wnt1 expression was observed adjacent to the anterior wound in control (6/7) and pbx(RNAi) (6/7) regenerating tail pieces at 1 dpa. Anterior wnt1 expression did not persist to 4 dpa in control (6/6) or pbx RNAi (8/8) regenerating tail pieces. Scale bars: 100 μm.
During anterior regeneration, the normal expression of notum observed 18 hours and 50 hours post-amputation was not detected following pbx(RNAi) (Fig. 7B). This suggests pbx activity is required for notum expression. notum(RNAi) leads to posteriorization of anterior wounds owing to sustained anterior expression of wnt1 (Petersen and Reddien, 2011). However, as already demonstrated, pbx(RNAi) leads to loss of anterior structures, but not posteriorisation. We reasoned that this could be explained if loss of notum expression in the anterior was not accompanied by ectopic wnt1 expression in pbx(RNAi) animals. Similar to controls, the normal anterior wnt1 expression observed at 24 hours post-amputation was not sustained at 96 hours post-amputation in pbx(RNAi) blastemas (Fig. 7C). From these data, we conclude that pbx is required for a stem-cell dependent phase of wnt1 expression, irrespective of position in the anterior or posterior, and independently of its requirement for notum expression. Loss of expression of these two polarity determinants explains much of the pbx(RNAi) phenotype, particularly the failure to regenerate both anterior and posterior structures.
DISCUSSION
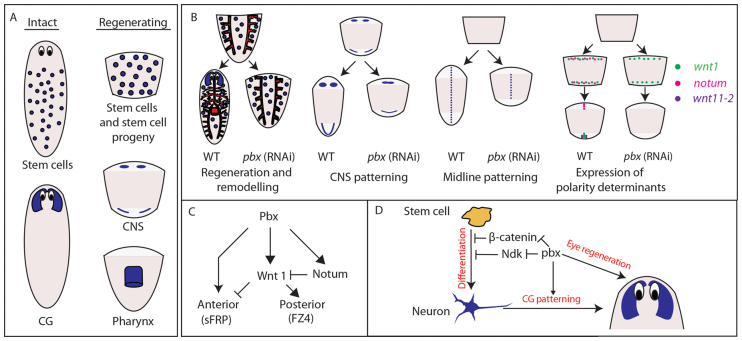
In this work, we present a key role in specifying positional identity for a conserved transcription factor expressed in stem cells, stem cell progeny and the regenerating CNS (Fig. 1; Fig. 8A for summary). In order for stem cells to replace missing tissues correctly during regeneration, they must be able to self-renew, produce progeny capable of differentiation into all the missing cell types and correctly interpret their position with regard to missing tissue. We have established that the TALE class homeobox gene pbx/extradenticle is essential for the last of these processes, demonstrating at the same time that the last of these processes can be separated out from the other two at a regulatory level in planarian stem cells and their progeny. Animals with reduced pbx do not show defects in stem cell maintenance or their general capacity to differentiate into missing cell types, rather they fail to restore positional identity across the AP axis. This includes failure to regenerate the anterior, posterior, the pharynx or the gut appropriately. In the case of the gut and pharynx, we demonstrate that cell types associated with these tissues can regenerate per se (Fig. 5; supplementary material Fig. S5), but they are not patterned correctly. In addition, we show that wnt1 and notum, which are early determinants of anterior and posterior polarity, require pbx for their expression to be established correctly (Fig. 7). Coupled to this, we also observe that the midline fails to be correctly specified during regeneration, leading to fusion of any remaining CG, loss of the dorsal midline cilia, ectopic fusion of VNCs and failure to re-establish expression of the midline determinant slit. Finally, our data also highlight a distinct role for pbx in brain/CG differentiation that is independent of its role in specifying AP polarity (all summarized in Fig. 8). This suggests that pbx may be a central regulator that is required for both interpreting pre-existing polarity signals and subsequently driving regeneration of cell types expressing polarity determinants (see Fig. 8C for summary). Overall, we propose a model in which PBX is required for the establishment of AP polarity and that failures to establish this at the poles leads to subsequent defects in midline establishment and remodeling along the body axis.
Fig. 8.
Summary of pbx expression and function. (A) pbx is expressed in stem cells and the CG of intact worms; and in stem cells, stem cell progeny, the CNS and pharynx of regenerating worms. (B) pbx is required for correct regeneration and remodeling of the pharynx, gut and anterior compartment; correct patterning of the regenerating CNS, including the CG/brain; correct re-establishment of expression of the midline determinant slit; and for the correct expression of previously described anterior and posterior polarity determinants. (C) A model of pbx function with respect to known regulatory logic of AP polarity specification. pbx is required for expression of both wnt1 and notum, and has an instructive role in promoting anterior polarity that is independent of the previously described wnt1/notum circuit. (D) pbx promotes CG/brain regeneration by inhibiting the activities of β-catenin-1 and ndk independently of its role in patterning the growing CG. pbx is also required for eye regeneration independently of its roles during CG regeneration and patterning.
A requirement of pbx for polarity determinant expression explains defects in anterior and posterior regeneration
pbx is required for the correct establishment of expression of notum and sFRP-1 in the anterior and wnt1, wnt11-2 and Fz4 in the posterior. This lack of expression clearly correlates with failures in anterior and posterior regeneration. These data imply a key role for pbx in establishing and generating regenerative polarity. Loss of both wnt1 and wnt11-2 expression explains the lack of posterior regeneration caused by pbx(RNAi). We also observe that loss of expression of the anterior determinant notum correlates with a loss of the anterior compartment caused by pbx(RNAi). notum has been previously described as being dependent on early wnt1 expression and itself is required for inhibition of wnt1 expression at anterior blastemas to prevent posteriorisation (Petersen and Reddien, 2011). However, despite loss of notum in pbx(RNAi), anterior blastemas do not regenerate with anterior or posterior fate. We show that this is because wnt1 expression is not established in the anterior in the pbx(RNAi) background, even though the suppressive effect of notum is absent. Thus, the requirement of pbx/extradenticle for wnt1 expression is independent of wound orientation with respect to the AP axis. This suggests a model where pbx is normally required for expression of both notum and wnt1, with anterior notum subsequently inhibiting the establishment of wnt1 in the anterior but not the posterior (Fig. 8C). Simultaneous wnt1/notum knockdown has been shown to lead to correct regeneration of the anterior but not the posterior (Petersen and Reddien, 2011). Both wnt1 and notum expression are also lacking following pbx(RNAi), but the anterior fails to regenerate; thus, we uncover a specific requirement for pbx in establishing anterior polarity, apart from its role in promoting notum expression (Fig. 8C).
β-catenin-1 and pbx control brain regeneration independently of Wnt expression and AP polarity
Knockdown of wnt1 expression can sometimes also lead to the ectopic differentiation of neural tissue within posterior blastemas as opposed to a tailless phenotype (Adell et al., 2009; Petersen and Reddien, 2009). However, following pbx knockdown, this was never observed despite the loss of wnt1 expression, suggesting that pbx may be required for ectopic neural structures in wnt1(RNAi). β-catenin-1 is the downstream effector of wnt1 and is itself required for establishment of the stem cell-dependent expression of wnt1 (Petersen and Reddien, 2009). Thus, the loss of wnt1 after pbx(RNAi) might also reflect a loss of β-catenin-1 activity. β-catenin-1 may also be required for the reduction or absence of brain tissue caused by pbx(RNAi), because, when combined, pbx/β-catenin-1(RNAi) enhances brain regeneration (Fig. 6). This indicates that a balance between pbx and β-catenin-1 activity may control this process.
pbx activity is required for appropriate CG patterning either when regenerating CG tissue de novo following decapitation or when integrating with existing tissue following longitudinal amputation. In contrast to the reduced CG regeneration observed following decapitation, laterally regenerating CG is restored to the same extent as controls. This indicates in the context of lateral regeneration signals promoting neural regeneration are present that are absent following decapitation. However, similar to anterior regeneration, the CG is fused at the midline. Combined ndk/pbx(RNAi) restores CG tissue, but this is also mis-patterned and shows fusion at the midline (Fig. 3). Following combined pbx/β-catenin-1(RNAi), CG regeneration is also enhanced; however, it is fused at the midline similar to lateral regenerates (Fig. 6). Together, these experiments demonstrate that the extent of CG regeneration after decapitation depends on the ability of pbx activity to overcome inhibitory effects mediated by ndk and β-catenin-1, and is independent of the process governing patterning of CG (Fig. 8D). The observation of fused CG correlates with loss of slit expression in both anterior and posterior regenerates, and a failure of slit expression to extend to the anterior in lateral regenerates (supplementary material Fig. S4A). Thus, either pbx has a further role in establishing the midline or the establishment of anterior and posterior polarity is required as a prerequisite to re-establishment of the midline in regenerating animals. In addition, pbx/β-catenin-1 RNAi does not restore anterior sFRP-1 expression, further evidence that the process of anterior polarity specification and the extent of CG regeneration are functionally separable.
Conserved roles for TALEs in axial regeneration?
We report for the first time the crucial role of an ortholog of PBX/Extradenticle during animal regeneration and present evidence that it is essential for appropriate tissue patterning and restoration of organismal integrity. Future work with improved experimental tools, particularly transgenesis, will help uncover which components of pbx expression are related to which of the functions we have described. We have added to a limited body of work detailing the activities of other members of the TALE class family during regeneration (Mercader et al., 2005; Felix and Aboobaker, 2010; Shaikh et al., 2011), and further highlight the role these proteins play in the orchestration of pattern formation. The interaction of PBX with other members of the TALE class family, Homothorax/Meis and Prep, regulates their nuclear localization and ability to regulate target gene expression by acting as transcription factors (Berthelsen et al., 1999). Dimers of TALE class proteins also interact with various other homeodomain-containing proteins, particularly Hox proteins, diversifying their regulatory potential (Karlsson et al., 2010; Noro et al., 2011). The planarian ortholog of Prep has been shown to be a crucial determinant of anterior fate during regeneration, being required both for anterior polarity specification and CG regeneration (Felix and Aboobaker, 2010). The salamander Homothorax/Meis ortholog has been show to mediate the specification of proximodistal (PD) identity of blastema cells during limb regeneration, as is the case during limb development (Mercader et al., 1999; Mercader et al., 2005). However, both in the case of limb regeneration and limb development a functional interaction between Homothorax/Meis and Extradenticle/PBX remains to be clarified. Salamander Homothorax/Meis orthologs also directly regulate the expression of the PD determinant Prod1 during regeneration through binding to cis-regulatory elements in the prod1 promoter, one of which is closely linked to a potential PBX-binding site (Shaikh et al., 2011). These data suggest that regulatory interactions between TALE homeodomains may be key in regenerative patterning. The similarities of anterior regeneration phenotypes following pbx and prep RNAi make it tempting to envisage that the two proteins interact functionally to coordinate anterior patterning, and this possibility is now open to investigation.
Supplementary Material
Footnotes
Funding
This work was funded by project grants from the Medical Research Council (MRC) [G0601133 to A.A.A.] and the Biotechnology and Biological Sciences Research Council (BBSRC) [BBE01030X1 to A.A.A.]. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.082982/-/DC1
References
- Aboobaker A. A. (2011). Planarian stem cells: a simple paradigm for regeneration. Trends Cell Biol. 21, 304-311 [DOI] [PubMed] [Google Scholar]
- Adell T., Salò E., Boutros M., Bartscherer K. (2009). Smed-Evi/Wntless is required for beta-catenin-dependent and -independent processes during planarian regeneration. Development 136, 905-910 [DOI] [PubMed] [Google Scholar]
- Berthelsen J., Kilstrup-Nielsen C., Blasi F., Mavilio F., Zappavigna V. (1999). The subcellular localization of PBX1 and EXD proteins depends on nuclear import and export signals and is modulated by association with PREP1 and HTH. Genes Dev. 13, 946-953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blythe M. J., Kao D., Malla S., Rowsell J., Wilson R., Evans D., Jowett J., Hall A., Lemay V., Lam S., et al. (2010). A dual platform approach to transcript discovery for the planarian Schmidtea mediterranea to establish RNAseq for stem cell and regeneration biology. PLoS ONE 5, e15617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capellini T. D., Zappavigna V., Selleri L. (2011). Pbx homeodomain proteins: TALEnted regulators of limb patterning and outgrowth. Dev. Dyn. 240, 1063-1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona A., Fernández J., Solana J., Romero R. (2005). An in situ hybridization protocol for planarian embryos: monitoring myosin heavy chain gene expression. Dev. Genes Evol. 215, 482-488 [DOI] [PubMed] [Google Scholar]
- Cebrià F., Newmark P. A. (2005). Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development 132, 3691-3703 [DOI] [PubMed] [Google Scholar]
- Cebrià F., Newmark P. A. (2007). Morphogenesis defects are associated with abnormal nervous system regeneration following roboA RNAi in planarians. Development 134, 833-837 [DOI] [PubMed] [Google Scholar]
- Cebrià F., Kobayashi C., Umesono Y., Nakazawa M., Mineta K., Ikeo K., Gojobori T., Itoh M., Taira M., Sánchez Alvarado A., et al. (2002). FGFR-related gene nou-darake restricts brain tissues to the head region of planarians. Nature 419, 620-624 [DOI] [PubMed] [Google Scholar]
- Cebrià F., Guo T., Jopek J., Newmark P. A. (2007). Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Dev. Biol. 307, 394-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhoffer G. T., Kang H., Sánchez Alvarado A. (2008). Molecular analysis of stem cells and their descendants during cell turnover and regeneration in the planarian Schmidtea mediterranea. Cell Stem Cell 3, 327-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Owlarn S., Tejada Romero B., Chen C., Aboobaker A. A. (2011). Combining classical and molecular approaches elaborates on the complexity of mechanisms underpinning anterior regeneration. PLoS ONE 6, e27927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix D. A., Aboobaker A. A. (2010). The TALE class homeobox gene Smed-prep defines the anterior compartment for head regeneration. PLoS Genet. 6, e1000915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsthoefel D. J., Park A. E., Newmark P. A. (2011). Stem cell-based growth, regeneration, and remodeling of the planarian intestine. Dev. Biol. 356, 445-459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaviño M. A., Reddien P. W. (2011). A Bmp/Admp regulatory circuit controls maintenance and regeneration of dorsal-ventral polarity in planarians. Curr. Biol. 21, 294-299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Crespo S., Abu-Shaar M., Torres M., Martínez-A C., Mann R. S., Morata G. (1998). Antagonism between extradenticle function and Hedgehog signalling in the developing limb. Nature 394, 196-200 [DOI] [PubMed] [Google Scholar]
- González-Estévez C., Arseni V., Thambyrajah R. S., Felix D. A., Aboobaker A. A. (2009). Diverse miRNA spatial expression patterns suggest important roles in homeostasis and regeneration in planarians. Int. J. Dev. Biol. 53, 493-505 [DOI] [PubMed] [Google Scholar]
- González-Estévez C., Felix D. A., Smith M. D., Paps J., Morley S. J., James V., Sharp T. V., Aboobaker A. A. (2012). SMG-1 and mTORC1 act antagonistically to regulate response to injury and growth in planarians. PLoS Genet. 8, e1002619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Peters A. H., Newmark P. A. (2006). A Bruno-like gene is required for stem cell maintenance in planarians. Dev. Cell 11, 159-169 [DOI] [PubMed] [Google Scholar]
- Gurley K. A., Rink J. C., Sánchez Alvarado A. (2008). Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science 319, 323-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley K. A., Elliott S. A., Simakov O., Schmidt H. A., Holstein T. W., Sánchez Alvarado A. (2010). Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Dev. Biol. 347, 24-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias M., Gomez-Skarmeta J. L., Saló E., Adell T. (2008). Silencing of Smed-betacatenin1 generates radial-like hypercephalized planarians. Development 135, 1215-1221 [DOI] [PubMed] [Google Scholar]
- Iglesias M., Almuedo-Castillo M., Aboobaker A. A., Saló E. (2011). Early planarian brain regeneration is independent of blastema polarity mediated by the Wnt/β-catenin pathway. Dev. Biol. 358, 68-78 [DOI] [PubMed] [Google Scholar]
- Karlsson D., Baumgardt M., Thor S. (2010). Segment-specific neuronal subtype specification by the integration of anteroposterior and temporal cues. PLoS Biol. 8, e1000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé R. M., Irimia M., Currie K. W., Lin A., Zhu S. J., Brown D. D., Ross E. J., Voisin V., Bader G. D., Blencowe B. J., et al. (2012). A comparative transcriptomic analysis reveals conserved features of stem cell pluripotency in planarians and mammals. Stem Cells 30, 1734-1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapan S. W., Reddien P. W. (2011). dlx and sp6-9 Control optic cup regeneration in a prototypic eye. PLoS Genet. 7, e1002226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet S., Ebner A., Affolter M. (2005). The Drosophila Extradenticle and Homothorax selector proteins control branchless/FGF expression in mesodermal bridge-cells. EMBO Rep. 6, 762-768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercader N., Leonardo E., Azpiazu N., Serrano A., Morata G., Martínez C., Torres M. (1999). Conserved regulation of proximodistal limb axis development by Meis1/Hth. Nature 402, 425-429 [DOI] [PubMed] [Google Scholar]
- Mercader N., Tanaka E. M., Torres M. (2005). Proximodistal identity during vertebrate limb regeneration is regulated by Meis homeodomain proteins. Development 132, 4131-4142 [DOI] [PubMed] [Google Scholar]
- Molina M. D., Saló E., Cebrià F. (2011). Organizing the DV axis during planarian regeneration. Commun. Integr. Biol. 4, 498-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noro B., Lelli K., Sun L., Mann R. S. (2011). Competition for cofactor-dependent DNA binding underlies Hox phenotypic suppression. Genes Dev. 25, 2327-2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onal P., Grün D., Adamidi C., Rybak A., Solana J., Mastrobuoni G., Wang Y., Rahn H. P., Chen W., Kempa S., et al. (2012). Gene expression of pluripotency determinants is conserved between mammalian and planarian stem cells. EMBO J. 31, 2755-2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M., Wieschaus E. (1990). Mutations in the Drosophila gene extradenticle affect the way specific homeo domain proteins regulate segmental identity. Genes Dev. 4, 1209-1223 [DOI] [PubMed] [Google Scholar]
- Petersen C. P., Reddien P. W. (2008). Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 319, 327-330 [DOI] [PubMed] [Google Scholar]
- Petersen C. P., Reddien P. W. (2009). A wound-induced Wnt expression program controls planarian regeneration polarity. Proc. Natl. Acad. Sci. USA 106, 17061-17066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. P., Reddien P. W. (2011). Polarized notum activation at wounds inhibits Wnt function to promote planarian head regeneration. Science 332, 852-855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prpic N. M., Telford M. J. (2008). Expression of homothorax and extradenticle mRNA in the legs of the crustacean Parhyale hawaiensis: evidence for a reversal of gene expression regulation in the pancrustacean lineage. Dev. Genes Evol. 218, 333-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J. C., Gurley K. A., Elliott S. A., Sánchez Alvarado A. (2009). Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science 326, 1406-1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J. C., Vu H. T., Sánchez Alvarado A. (2011). The maintenance and regeneration of the planarian excretory system are regulated by EGFR signaling. Development 138, 3769-3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saló E., Baguñà J. (1984). Regeneration and pattern formation in planarians. I. The pattern of mitosis in anterior and posterior regeneration in Dugesia (G) tigrina, and a new proposal for blastema formation. J. Embryol. Exp. Morphol. 83, 63-80 [PubMed] [Google Scholar]
- Sánchez Alvarado A., Newmark P. A. (1999). Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proc. Natl. Acad. Sci. USA 96, 5049-5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimone M. L., Meisel J., Reddien P. W. (2010). The Mi-2-like Smed-CHD4 gene is required for stem cell differentiation in the planarian Schmidtea mediterranea. Development 137, 1231-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimone M. L., Srivastava M., Bell G. W., Reddien P. W. (2011). A regulatory program for excretory system regeneration in planarians. Development 138, 4387-4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh N., Gates P. B., Brockes J. P. (2011). The Meis homeoprotein regulates the axolotl Prod 1 promoter during limb regeneration. Gene 484, 69-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana J., Lasko P., Romero R. (2009). Spoltud-1 is a chromatoid body component required for planarian long-term stem cell self-renewal. Dev. Biol. 328, 410-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana J., Kao D., Mihaylova Y., Jaber-Hijazi F., Malla S., Wilson R., Aboobaker A. (2012). Defining the molecular profile of planarian pluripotent stem cells using a combinatorial RNAseq, RNA interference and irradiation approach. Genome Biol. 13, R19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T. C., Rahman R., Jaber-Hijazi F., Felix D. A., Chen C., Louis E. J., Aboobaker A. (2012). Telomere maintenance and telomerase activity are differentially regulated in asexual and sexual worms. Proc. Natl. Acad. Sci. USA 109, 4209-4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Truman J. W. (2007). Molecular patterning mechanism underlying metamorphosis of the thoracic leg in Manduca sexta. Dev. Biol. 305, 539-550 [DOI] [PubMed] [Google Scholar]
- Umesono Y., Watanabe K., Agata K. (1997). A planarian orthopedia homolog is specifically expressed in the branch region of both the mature and regenerating brain. Dev. Growth Differ. 39, 723-727 [DOI] [PubMed] [Google Scholar]
- Van Auken K., Weaver D., Robertson B., Sundaram M., Saldi T., Edgar L., Elling U., Lee M., Boese Q., Wood W. B. (2002). Roles of the Homothorax/Meis/Prep homolog UNC-62 and the Exd/Pbx homologs CEH-20 and CEH-40 in C. elegans embryogenesis. Development 129, 5255-5268 [DOI] [PubMed] [Google Scholar]
- Vitobello A., Ferretti E., Lampe X., Vilain N., Ducret S., Ori M., Spetz J. F., Selleri L., Rijli F. M. (2011). Hox and Pbx factors control retinoic acid synthesis during hindbrain segmentation. Dev. Cell 20, 469-482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. E., Wang I. E., Reddien P. W. (2011). Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 332, 811-816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. E., Ho J. J., Reddien P. W. (2012). Genetic regulators of a pluripotent adult stem cell system in planarians identified by RNAi and clonal analysis. Cell Stem Cell 10, 299-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenemoser D., Reddien P. W. (2010). Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Dev. Biol. 344, 979-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Sym M., Kenyon C. (2005). The roles of two C. elegans HOX co-factor orthologs in cell migration and vulva development. Development 132, 1413-1428 [DOI] [PubMed] [Google Scholar]
- Yazawa S., Umesono Y., Hayashi T., Tarui H., Agata K. (2009). Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. Proc. Natl. Acad. Sci. USA 106, 22329-22334 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.