Abstract
Background/Aims
Vitamin D deficiency is highly prevalent in obese children. Obese children tend to respond poorly to vitamin D supplementation. The objective of the study was to compare the response to vitamin D3 supplementation (2000 IU once daily for 12 weeks) between obese and non- obese Caucasian adolescents.
Methods
The study design was open label non-randomized. It was carried out at a single center. Eighteen obese adolescents (age 12-18 years) and the same number of age, gender and season matched non-obese adolescents received Vitamin D3 (2000 IU/day) orally for 12 weeks. Total serum 25(OH) D, PTH, calcium and phosphorus were measured at baseline and at the end of the 12 week period.
Results
The mean baseline 25 (OH)D level was higher in the non-obese subjects compared to the obese subjects (mean, 28.9 vs. 25.2 ng/mL, p=0.029). The increment in 25(OH) D levels following vitamin D supplementation was significantly lower in the obese adolescents (mean change, 5.8 vs. 9.8 ng/mL, p=0.019).
Conclusions
Higher doses of vitamin D are required to treat vitamin D deficiency in obese adolescents than in their non-obese peers.
Introduction
Vitamin D deficiency is common in children 1-4 and adults 5-8 and it is significantly more prevalent in obese children9-13. A plausible explanation for the association of Vitamin D deficiency and obesity is the sequestration of this lipid-soluble vitamin or degradation in the adipose tissue causing a lower bioavailability9.
The recommended dietary intake of vitamin D for children was recently increased from 200 to 600 IU by several expert groups including the American Academy of Pediatrics and Institute of Medicine14-17. While the guidelines by the Endocrine Society suggest higher dose of vitamin D in obese adults 16, the pediatric guidelines do not recommend adjusting doses of vitamin D for prevention or treatment of vitamin D deficiency in obese children14, 15, 17. Limited studies in obese children and adults suggest that obese individuals tend to have a poor response to vitamin D supplementation9, 10, 18. High dose weekly vitamin D supplementation regimen (50,000 IU once a week for 6-8 weeks) in vitamin D deficient primarily Hispanic and African American adolescents was found to normalize 25(OH)D levels in only a minority of subjects10,23 A study in preadolescent African American children also concluded that vitamin D3 supplementation with 400 IU/day for 1 month was not effective in increasing the 25(OH)D levels in majority of the subjects 18. The objective of the study was to compare the response to vitamin D3 between obese and non-obese Caucasian adolescents.
METHODS
Subjects
Healthy adolescents, obese and non-obese, age 12-18 years were recruited through local advertisement. Subjects were considered obese if BMI was at or greater than 95th percentile for age and gender and non-obese if BMI was between the 5th and 85th percentile for age and gender and considered. The group of obese adolescents were part of a pilot double-blind placebo controlled randomized clinical trial examining the effect of vitamin D3 supplementation on insulin resistance and lipids. The non-obese adolescents were age, gender and season matched to the obese adolescents. The study was carried out between September 2008 and Jan 2011. Patients were evenly distributed throughout the four seasons. Exclusion criteria were: serum 25 (OH)D levels >80 ng/mL, serum calcium >10.8 mg/dL, serum phosphorus > 5.5 mg/dl, pregnancy or nursing, current cancer, ongoing multivitamin supplementation, dietary calcium intake exceeding 1500 mg/day, hepatic or renal disorders, Type 1 or Type 2 diabetes mellitus, ongoing use of insulin, metformin, or oral hypoglycemic medications, and malabsorption disorders (celiac disease, cystic fibrosis, and inflammatory bowel disease). Research protocol was approved by Institutional Review Board of Mayo Clinic. Informed consent and assents were obtained from participants and parents.
Study Design
Study design was an open label non randomized pre-post comparison of the effect of vitamin D supplementation in obese vs non-obese adolescents. All study participants received vitamin D3 (cholecalciferol) 2000 IU (1 capsule of 2000 IU) daily for a period of 12 weeks. Compliance was assessed at the 12 week visit by counting the number of pills remaining in the pill bottle. All study participants received the same vitamin D3 capsules compounded at Mayo clinic pharmacy. Baseline and post treatment 25(OH)D , calcium, phosphorus and parathyroid hormone (PTH) levels were compared between non-obese and obese subjects.
Biochemical Measurements
Blood samples were drawn after at least 12 hours of fasting. Blood tests were analyzed in the same laboratory at Mayo Clinic, Rochester. Measurement of 25(OH) D was performed using liquid chromatography-tandem mass spectrometry (LC-MS/MS). An internally developed and validated method in a single laboratory (Mayo Medical Laboratories, MML) was used in order to assure uniform measurements. The total 25-OHD concentration of each sample was calculated by summing the measured values of 25(OH) D2 and 25(OH) D3. This method has been shown to be accurate and precise with excellent correlation between 25(OH) D3 and 25(OH) D2 measured by this method and high pressure liquid chromatography with ultraviolet quantitation 19. Vitamin D deficiency was defined as a 25(OH)D level of less than 20 ng/mL and vitamin D insufficiency as a 25(OH)D level of 20–29 ng/mL 20. A 25(OH)D level of 30 ng/mL or higher was considered as sufficient vitamin D status 20.
Calcium was measured by Photometric, O-Cresolphthalein assay (Roche Diagnostics, Indianapolis, IN). Phosphorus was measured by Photometric, Ammonium Molybdate assay (Roche Diagnostics, Indianapolis, IN).
PTH was measured by a two-site chemiluminescent immunometric assay on the Immulite automated immunoassay system (Diagnostic Products Corp. Los Angeles, Ca 90045).
Statistical analysis
Age- and sex-specific BMI percentiles were determined using the 2000 Center for Disease Control growth charts.21 Baseline data (age, weight, BMI, and laboratory values) and changes following supplementation (post – pre) were summarized using mean ± standard deviation (SD) and median and range, separately for the two groups (non-obese vs obese). Relationship between 25(OH)D levels and other variables were assessed graphically using scatter plots and a smoothed line was generated using a cubic spline routine. Paired comparisons between the matched patients in the two groups were evaluated using the paired t-test or Wilcoxon signed rank test. All calculated p-values were two-sided and p-values less than 0.05 were considered statistically significant. Analyses were performed using the version 9.2 SAS software package (SAS Institute, Cary, NC).
RESULTS
Clinical characteristics
Twenty- seven obese adolescents (15 female, 12 male) who were recruited and randomized to receive Vitamin D3 in a separate study had their baseline laboratory studies drawn between September 18, 2008 and July 9, 2010; 20 obese adolescents completed the study. For each obese adolescent, 1 non-obese adolescent of the same gender, similar age (within 1.5 years), and enrolled in the same season was recruited. Twenty-two non-obese patients (9 female, 13 male) were recruited. Their baseline laboratory tests were drawn between November 13, 2009 and October 6, 2010. The study was completed by 18/22 non-obese adolescents. A total of 18 matched pairs for age, sex and season completed the study and were analyzed (Table 1). All patients except 1 in the non-obese category were Caucasian. The ethnic distribution of the subjects in this study reflects that of the population seen in the community (Rochester, MN). The mean (±SD) age was 15.2 ± 2 and 15.0 ±2.3 years in the non-obese and obese group respectively. Each group comprised 10 males and 8 females. As expected, the BMI (20.3±2.0 kg/m2, 35.4±7.5 kg/m2) and BMI percentile for age and sex (52.7±19.7, 98.5±1.0) were significantly different between the non-obese and obese groups.
TABLE 1.
Baseline Characteristics of Study Subjects.
| Non obese (N=18) | Obese (N=18) | |
|---|---|---|
| Gender, n (%) | ||
| Female | 8 (44.4%) | 8 (44.4%) |
| Male | 10 (55.6%) | 10 (55.6%) |
| Age at first visit (years) | ||
| Mean (SD) | 15.2 (2.0) | 15.0 (2.3) |
| Median | 14.8 | 15.0 |
| Range | (12.4-18.6) | (11.8-18.6) |
| Baseline Weight (kg) | ||
| Mean (SD) | 57.7 (8.8) | 103.4 (25.3) |
| Median | 56.4 | 100.7 |
| Range | (45.5-81.8) | (64.6-148.8) |
| Baseline BMI | ||
| Mean (SD) | 20.3 (2.0) | 35.4 (7.5) |
| Median | 20.1 | 32.2 |
| Range | (16.5-25.1) | (25.6-53.3) |
| Baseline BMI age & sex specific %ile | ||
| Mean (SD) | 52.7 (19.7) | 98.5 (1.0) |
| Median | 49.1 | 98.5 |
| Range | (4.8-83.6) | (96.7-99.8) |
Prevalence of vitamin D deficiency/insufficiency
The prevalence of vitamin D deficiency or insufficiency (< 30 ng/mL) was 78 % in obese subjects and 61% in non-obese subjects. Three (17%) of the obese subjects were vitamin D deficient (< 20 ng/mL) and 1 (6 %) of the non-obese subjects were vitamin D deficient. The mean baseline 25 (OH)D level was higher in the non-obese subjects compared to the obese subjects (mean, 28.9 vs. 25.2, p=0.029; Table 2). There were no significant differences in serum calcium, phosphorus or PTH between the obese and non- obese groups.
Table 2.
Baseline Laboratory Data for Study Subjects
| Non-obese (N=18) | Obese (N=18) | P-value† | |
|---|---|---|---|
| Baseline Vitamin D(ng/mL) | 0.086 | ||
| Mean (SD) | 28.9 (8.2) | 25.2 (4.9) | |
| Median | 28.5 | 25.0 | |
| Range | (7.9-44.0) | (15.0-32.0) | |
| Baseline Calcium(mg/dL) | 0.25 | ||
| Mean (SD) | 9.8 (0.3) | 9.9 (0.3) | |
| Median | 9.8 | 9.8 | |
| Range | (9.2-10.3) | (9.5-10.5) | |
| Baseline Phosphorus (mg/dL) | 0.71 | ||
| Mean (SD) | 4.3 (0.7) | 4.2 (0.6) | |
| Median | 4.2 | 4.2 | |
| Range | (3.1-5.5) | (3.2-5.3) | |
| Baseline PTH(pg/mL) | 0.96 | ||
| Mean (SD) | 32.3 (13.6) | 35.6 (25.9) | |
| Median | 29.5 | 28.0 | |
| Range | (11.0-61.0) | (16.0-132.0) |
P-values are based on the paired t-test for all comparisons, except the Wilcoxon signed rank test was utilized to evaluate baseline PTH.
Blood samples for 25(OH)D were obtained during fall (September-November) in 5 pairs (28 %), during winter (December-February) in 2 pairs (11 %), during spring (March-May) in 4 pairs (22%) and during summer (June-August) in 7 pairs (39 %).
As to be expected, nearly half of the subjects (7/18 per group) were enrolled during the summer months when the subjects were off from school. Among the obese subjects, the mean 25OH(D) values were 24.0, 25.5, 25.7, and 25.5 ng/mL in winter, spring, summer, and fall. Whereas among the non-obese subjects, the mean 25OH(D) values were 24.5, 31.8, 33.4, and 20.3 ng/mL in winter, spring, summer, and fall. So based on this limited sample, it appears the seasonable variation is slightly more dramatic in the non-obese subjects. However, the seasonal variation needs to be interpreted with caution as there was limited number of subjects per season.
Response to supplementation with vitamin D3
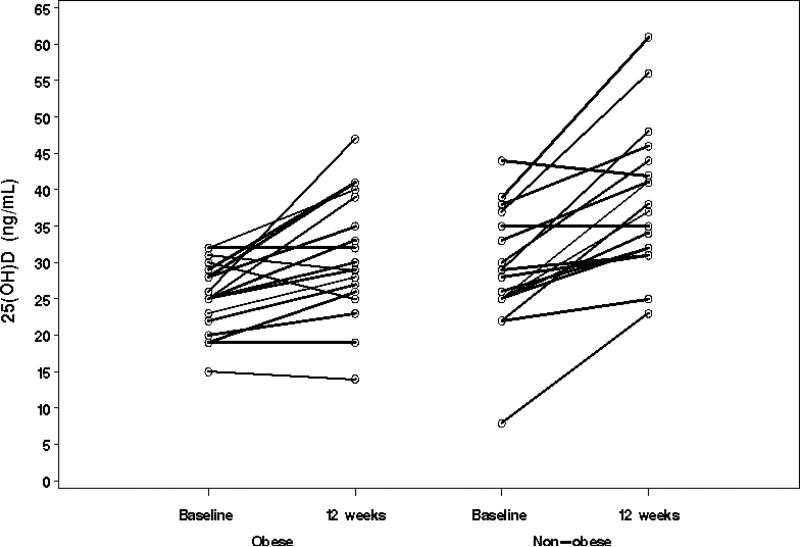
The median duration between the two study visits was 94 and 95.5 days in the non-obese and obese groups, respectively. Over this time period, the mean change in weight was 1.0 and 2.1 kg in the non-obese and obese groups, respectively. Tables 1 and 2 delineate mean 25(OH)D levels at baseline and following supplementation with 2000 IU vitamin D3 daily for 12 weeks. The increment in 25(OH) D levels was significantly greater in the non-obese adolescents (mean change, 9.8 vs. 5.8, p=0.019) (Table 3 and Figure 1). 25(OH)D levels normalized ( > 30 ng/mL) in 16/18 (89%) of the non obese subjects but only 9/18 (50%) of the obese subjects.
Table 3.
Change in Laboratory Data Following Supplementation.
| Change (12 weeks – baseline) | Non-obese (N=18) | Obese (N=18) | P-value† |
|---|---|---|---|
| 25(OH) vitamin D (ng/mL) | 0.019 | ||
| Mean (SD) | 9.8 (7.13) | 5.8 (6.44) | |
| Median | 8.5 | 5.0 | |
| Range | (-2.0-22.0) | (-5.0-21.0) | |
| Calcium (mg/dL) | 0.004 | ||
| Mean (SD) | 0.2 (0.27) | 0.0 (0.19) | |
| Median | 0.2 | 0.0 | |
| Range | (-0.4-0.7) | (-0.4-0.3) | |
| Phosphorus (mg/dL) | 0.26 | ||
| Mean (SD) | 0.1 (0.61) | -0.1 (0.36) | |
| Median | 0.2 | -0.1 | |
| Range | (-1.0-1.3) | (-0.6-0.7) | |
| PTH (pg/mL) | 0.10 | ||
| Mean (SD) | 0.1 (17.36) | -11.2 (23.62) | |
| Median | 2.0 | -5.5 | |
| Range | (-44.0-22.0) | (-96.0-14.0) |
P-values are based on the paired t-test for all comparisons, except the Wilcoxon signed rank test was utilized to evaluate baseline PTH.
Figure 1.
Increment in 25(OH)D levels following vitamin D3 supplementation in study subjects (n=18 for both groups).
There was no clear relationship between the baseline level of 25(OH)D and the changes in 25(OH)D levels following vitamin D3 supplementation for the obese and non-obese subjects. Additionally, the change in 25(OH)D did not correlate with body weight in either of the groups.
There were no significant differences between the change in levels of PTH and phosphorus after 12 weeks(Table 3). The change in serum calcium levels, though statistically significant did not appear to be of clinical significance (Table 3).
Relationship between Compliance and Response to Vitamin D Supplementation
In the non-obese group, we were not able to determine compliance in 2 patients in the non-obese group and in 5 subjects in the obese group, as they did not bring the pillbox to the return visit end of the study.
Of the 16 non obese subjects who did bring the pill box at the return visit, 10 had no pills left in the pill box, 2 had 3 pills left, 1 had 5 pills left, 1 had 7 pills left, 1 had 8 pills left and 1 had 17 pills remaining at the end of the study out of 12 weeks worth of pills ( 84 total) Of the 13 obese subjects who did bring the pill box at the return visit, 5 had no pills left in the pill box, 4 had 3 pills remaining, 1 had 5 pills remaining, 1 had 7 pills remaining , 1 had 12 pills remaining and 1 had 20 pills remaining at the end of the study. Among the patients who brought their pill box at the return visit, there was no statistically significant difference in the number of pills left between the obese and non-obese (Wilcoxon rank sum test, p=0.31). Based on the Spearman rank correlation coefficient, the correlation between the number of pills left and the change in 25(OH) D level was - 0.30, suggesting that patients with more pills remaining at the end of the study tended to have less of a change in the 25(OH) D level.
No adverse effects were reported during the course of the study by any of the study subjects. There was no correlation between the compliance as measured by the number of pills remaining at the end of the study and the change in 25(OH)D level.
DISCUSSION
In this study, we compared response to 3 month long vitamin D3 supplementation with 2000 IU daily in obese and non-obese Caucasian adolescents. We found that the response to vitamin D supplementation with this dose of vitamin D was modest in both obese and non-obese children. More importantly, the response to vitamin D supplementation was approximately 1.7 fold lower in obese adolescents compared to non-obese adolescents. Despite using a dose of vitamin D that is 3 and half times the recommended dietary intake, we found that supplementation with 2000 IU daily for 3 months only resulted in an increment in the 25(OH)D level by 5.8 ng/mL in obese adolescents. The increment in the 25(OH)D levels in the non-obese group was higher at 9.8 ng/mL and is similar to another study utilizing the same dose of vitamin D over a period of 8 weeks in children between the ages of 10 and 17 years22. The results of this study suggest that obese children and adolescents should be supplemented with doses of vitamin D that are approximately 2 fold higher than those used for non-obese children. To our knowledge, this is the first study that has examined the impact of obesity itself without any other confounding variables such as ethnicity, age, gender or season on response to vitamin D3 supplementation in Caucasian adolescents.
Our results of almost 2 fold lower response to vitamin D3 supplementation in Caucasian obese adolescents compared to non- obese adolescents are similar to those in a previous study of Caucasian obese and non obese adults involving a single oral dose of 50,000 IU of vitamin D29. We noted that oral administration of vitamin D3 at a dose of 2000 IU /day was able to normalize 25(OH)D levels in only half of the obese adolescents. Other investigators have also found poor response to vitamin D2 or D3 supplementation in African American 10, 23 and Hispanic obese adolescents 10. In a retrospective medical record review, weekly vitamin D supplementation regimen (50,000 IU once a week for 6-8 weeks) in vitamin D deficient primarily Hispanic and African American adolescents also normalized 25(OH)D in only a minority of subjects 10. Ashraf et al in a prospective study of obese African American adolescents receiving ergocalciferol (vitamin D2) 50,000 IU for 8 weeks also noted that the serum 25(OH)D concentration increased to >20 ng/mL in only two thirds of subjects23. The poor response to vitamin D supplementation in obese children and adolescents is likely secondary to a greater degree of sequestration of lipid-soluble vitamin D in the adipose tissue of obese adolescents as well as potential differences in vitamin D metabolism in the obese state24.
We did not find a correlation between baseline 25(OH)D level and increment in 25(OH)D level in obese adolescents. In contrast, a study in 6-10 year old African American children receiving 400 IU of vitamin D3 daily for 1 month suggested a different threshold level of 25(OH)D level for an increase in 25(OH)D by > 5ng/mL in obese relative to non-obese children 18. These differences may be secondary to differences in age and ethnicity of the subjects as well as to the dose and duration of vitamin D supplementation.
The strengths of our study include the prospective, pre-post comparison design of the study aimed at eliminating several confounding variables such as age, gender and season. The 25(OH)D levels were measured using the highly accurate liquid chromatography mass spectrophotometry in an nationally recognized laboratory. We used a dose of vitamin D that is recommended by several expert groups for treatment of vitamin D deficiency14, 16 and is more than three times the recommended dietary intake. Another strength was the inclusion of only one ethnic group given the impact ethnic differences may have on vitamin D levels and metabolism 25. Our study is limited by the small sample size and lack of assessment of vitamin D intake for the study subjects. We also did not have information on sun exposure or sunscreen use by the study subjects. However, that we matched for season likely diminished the effect of sun exposure on vitamin D levels. While we did count remaining pills to measure compliance, several patients in both groups did not return their pill bottles, making it difficult to determine their degree of compliance. We did not measure bone turnover markers and therefore no conclusions can be drawn regarding impact of vitamin D supplementation on skeletal health in obese and non-obese children and adolescents.
We conclude that obese adolescents with vitamin D deficiency and insufficiency require doses that are at least 2 fold higher than those recommended for non- obese adolescents as has been recommended in the adult population. Longitudinal studies involving a larger number of children and adolescents from different ethnic groups are warranted. In these studies, treatment of patients with higher doses of vitamin D would clarify whether these doses not only increase serum vitamin D levels, but also improve skeletal health.
Acknowledgments
This project was supported by the Pediatric and Adolescent Medicine Research Fund, Mayo Clinic College of Medicine and by NIH/NCRR CTSA Grant Number UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
No Conflict of interest or financial disclosures
References
- 1.Rovner AJ, O'Brien KO. Hypovitaminosis D among healthy children in the United States: a review of the current evidence. Arch Pediatr Adolesc Med. 2008;162(6):513–519. doi: 10.1001/archpedi.162.6.513. [DOI] [PubMed] [Google Scholar]
- 2.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105(6):971–974. doi: 10.1016/j.jada.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009;124(3):e362–370. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginde AA, Liu MC, Camargo CA., Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 8.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7(5):439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 9.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 10.Harel Z, Flanagan P, Forcier M, Harel D. Low Vitamin D Status Among Obese Adolescents: Prevalence and Response to Treatment. J Adolesc Health. 2011;48(5):448–452. doi: 10.1016/j.jadohealth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Smotkin-Tangorra M, Purushothaman R, Gupta A, Nejati G, Anhalt H, Ten S. Prevalence of vitamin D insufficiency in obese children and adolescents. J Pediatr Endocrinol Metab. 2007;20(7):817–823. doi: 10.1515/jpem.2007.20.7.817. [DOI] [PubMed] [Google Scholar]
- 12.Rajakumar K, de las Heras J, Chen TC, Lee S, Holick MF, Arslanian SA. Vitamin D status, adiposity, and lipids in black American and Caucasian children. J Clin Endocrinol Metab. 2011;96(5):1560–1567. doi: 10.1210/jc.2010-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57(2):183–191. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 15.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 17.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 18.Rajakumar K, Fernstrom JD, Holick MF, Janosky JE, Greenspan SL. Vitamin D status and response to Vitamin D(3) in obese vs. non-obese African American children. Obesity (Silver Spring) 2008;16(1):90–95. doi: 10.1038/oby.2007.23. [DOI] [PubMed] [Google Scholar]
- 19.Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK. HPLC method for 25-hydroxyvitamin D measurement: comparison with contemporary assays. Clin Chem. 2006;52(6):1120–1126. doi: 10.1373/clinchem.2005.064956. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 22.Maalouf J, Nabulsi M, Vieth R, Kimball S, El-Rassi R, Mahfoud Z, et al. Short- and long-term safety of weekly high-dose vitamin D3 supplementation in school children. J Clin Endocrinol Metab. 2008;93(7):2693–2701. doi: 10.1210/jc.2007-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashraf AP, Alvarez JA, Gower BA, Saenz KH, McCormick KL. Associations of serum 25-hydroxyvitamin D and components of the metabolic syndrome in obese adolescent females. Obesity (Silver Spring) 2011;19(11):2214–2221. doi: 10.1038/oby.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]