Abstract
The ascertainment of serum free light chain levels (sFLC) has been shown to be valuable in screening for the presence of plasma cell dyscrasia as well as for baseline prognosis in newly diagnosed patients. For patients with amyloidosis and those with oligo- or non-secretory multiple myeloma (MM), serial measurement of sFLC has also been shown to be valuable in monitoring disease status. However, in patients with a measureable, intact monoclonal protein by immunofixation (M protein), the serial measurement of sFLC remains undefined and is currently not recommended in professional guidelines. Herein, we provide data comparing sFLC to M protein as biomarkers of response in newly-diagnosed patients with MM undergoing induction therapy with the novel agents thalidomide, lenalidomide and/or bortezomib. We show that while M protein appears to outperform sFLC comparatively over the course of induction therapy, the addition of FLC to M-protein further informs the characterization of residual disease status post-induction. Moreover, sFLC at the time of stem cell mobilization appears to hold prognostic power for survival endpoints following HDC/SCT. These findings suggest potentially novel roles for sFLC in patients with MM with an intact M-protein receiving novel agent-based induction strategies followed by HDC/SCT.
Keywords: Multiple myeloma, biomarker, serum free light chains
Introduction
Multiple myeloma (MM) remains relatively unique among human malignancies in that the monoclonal immunoglobulin measured by immunofixation (“M protein”), produced by most MM tumors, has been an established biomarker of the disease for over 30 years [1]. The quantitative relationship between MM tumor cell mass and production of M-protein was originally demonstrated as early as 1958, and subsequent reports of serial measurement of M protein over time provided validation of this biomarker [2–4].
In the last 10 years, the availability of an assay to measure serum immunoglobulin-free light chains (sFLC) has developed and gained wide-spread use in the clinical setting [5,6]. Three major applications of FLC monitoring have been recommended: as a screening tool in combination with M protein obviating the need for 24-hour urine studies (except in screening for AL amyloidosis), as a prognostic variable at initial, baseline diagnosis, and as a biomarker for oligo- or non-secretory MM patients [7]. sFLC monitoring has become routinely incorporated into clinical practice in the care of patients with MM and associated plasma cell dyscrasias, and current work is extending its use into other B-cell lymphoproliferative disorders [8]. Any potential role of sFLC in assessing response in patients with an intact M protein, however, has remained largely undefined, to date [7]. The largest study examining sFLC in this context, assessing response 2 months into induction in newly-diagnosed patients receiving alkylator-based therapy, failed to support a routine role for the test in such a setting and patient population [9].
In the present work, we sought to compare sFLC to M protein as a potential response variable in newly-diagnosed patients with MM receiving induction regimens incorporating novel anti-MM therapies, including the proteosome inhibitor, bortezomib, and the immunomodulatory agents (IMIDS), thalidomide and lenalidomide. These novel therapies have virtually replaced chemotherapy-based induction regimens prior to high-dose chemotherapy (HDC) and autologous stem cell transplant (SCT) in eligible patients with MM, and may improve outcomes post-HDC/SCT [10]. We show that while M protein appears superior to sFLC as a biomarker of disease status over the course of induction therapy, the addition of sFLC to M protein adds further accuracy to the characterization of residual disease post-induction treatment. Moreover, assessment of sFLC post-induction may serve as a unique, practical, and a priori prognostic variable for survival outcomes following consolidative HDC/SCT. These findings potentially define a novel clinical application for sFLC in the response assessment of MM following induction therapy with novel agents in patients with MM going onto HDC/SCT consolidation.
Methods
Patient population
Under an Institutional Review Board (IRB)-approved protocol and using an IRB-approved database, data were collected on patients with MM who received novel-agent based induction therapies prior to stem cell mobilization, HDC and autologous SCT. Data regarding patient demographics and disease characteristics at diagnosis (Table 1) and following induction were collected. Specifically, patients’ M protein, sFLC, and kappa / lambda free light chain ratio were collected, as well as bone marrow plasmacytosis (as assessed by aspirate and core biopsy) at diagnosis and post-induction therapy. Progression free survival (PFS) and overall survival (OS) following SCT were determined. Disease characteristics as well as the nature of induction therapy received were also recorded. 24-hour excretion of monoclonal protein and free light chains in urine was not assessed in the present work due to lack of paired diagnosis and post-induction samples in a number of subjects.
Table 1.
Patient demographics and disease characteristics
| Patient characteristics | |
|---|---|
| Gender (%, female) | 42 |
| Age (mean, SD) | 55 (+/− 9) |
| KPS (median) | 90 |
| Disease characteristics | |
| Durie-Salmon (%) | |
| I | 12 |
| II | 18 |
| III | 70 |
| M-protein isotype (%) | |
| - IgG | 58 |
| - IgA | 22 |
| - Light chain (%) | 20 |
| M-protein at diagnosis (mean +/− SD, g/dL) | |
| - IgG | 3.0 (+/− 1.6) |
| - IgA | 1.5 (+/− 0.9) |
| Cytogenetic FISH (high risk, %) | 60 |
| Serum creatinine (>2mg/dL, %) | 22 |
| Hemoglobin (mean, range, g/dL) | 11.2 (6.9 – 15.8) |
| Beta-2 microglobulin (mean, range, g/mL) | 4.4 (1.3 – 26) |
| C-reactive protein (mean, range, mg/L) | 21 (0.24 – 120) |
Statistical considerations
Linear regression models were run with either one or two primary predictor variables as described using residual disease burden (as defined by bone marrow aspirate plasmacytosis or core biopsy plasmacytosis) post-induction as the outcome variable. Outcomes for each model were natural log transformed in order to meet the assumptions of normality and equal variance across groups. Coefficients shown in tables are based on log-transformed outcomes and are for a 100-unit change in the delta, diagnosis or post-induction values. The coefficient for percent change is relevant to a 10-unit change. Reported p-values test if the coefficient is statistically different from zero. Covariates were included in the models if shown to be potential confounding variables defined as altering the primary predictor coefficient by +/− 10%, independent of statistical significance. Correlation analyses and Kaplan-Meier analyses of PFS and OS were performed with MedCalc ® Software, version 9.5.2.0, © Frank Schoojans, 1993–2008.
As in similar studies characterizing the role of sFLC quantification as a potential response assessment tool, we limited analyses to involved FLC (as the serial assessment of the difference between involved and uninvolved FLC and kappa/lambda ratio may be confounded by direct, immunosuppressive effects of induction therapy on production of the uninvolved FLC, per se) [7,9].
Results
Patient population
Data were analyzed regarding n=73 patients. (N=14 patients in the study population exhibited only FLC as a biomarker and these patients were not included in the direct comparisons shown below regarding M-protein versus FLC.). Patients were stratified by the type of induction therapy received: n=25 received bortezomib-based induction, n=18 received immunomodulatory agent (IMID, i.e., thalidomide or lenalidomide)-based induction, and n=17 were treated with bortezomib/IMID combination (e.g., bortezomib, thalidomide, dexamethasone or bortezomib, lenalidomide, dexamethasone). (The remaining patients (n=13) received bortezomib and IMID therapies sequentially but not in combination.) As a function of induction regimen received (summarized in Table 2), patients did not differ in age, performance status, Durie-Salmon stage, or the presence or absence of high-risk cytogenetics. No differences were observed across induction groups relating to initial monoclonal protein, involved free light chain, or bone marrow plasmacytosis. Patients received comparable numbers of cycles of induction therapy and had similar time from diagnosis to transplant across induction groups. Most patients had achieved partial response to induction based on formal criteria [11].
Table 2.
Patient and disease characteristics by induction strategy utilized
| Variable | bortezomib-based | IMID-based | combination |
|---|---|---|---|
| Patient characteristics: | |||
| Age (mean, SD) | 52 (+/−9) | 56 (+/−7) | 57 (+/−9) |
| Gender (% female) | 28 | 50 | 58 |
| KPS (median) | 90 | 90 | 90 |
| Induction length: | |||
| - cycles (median, range) | 4 (2–7) | 4 (3–6) | 3 (2–4) |
| - months (median, range) | 5 (4–11) | 5 (3–12) | 5 (3–10) |
| Response to induction (%): | |||
| - CR | 8 | 0 | 0 |
| - PR | 84 | 88 | 94 |
| - less than PR | 8 | 12 | 6 |
| Disease characteristics: | |||
| Durie-Salmon stage (median) | 3 | 3 | 3 |
| High-risk cytogenetics present (%) | 40 | 44 | 70 |
| M-protein (mean, range, g/dl) | 2.9 (0.4–4.2) | 2.7 (0.2–5.3) | 2 (0.2–5.2) |
| Involved FLC (mean, range, mg/l) | 748 (9–3850) | 224 (12–1150) | 590 (23–4910) |
| Bone marrow plasmacytosis | |||
| - aspirate (mean, SD, %): | 33 (19) | 30 (17) | 28 (13) |
| - core biopsy (mean, SD, %): | 49 (24) | 52 (27) | 46 (27) |
Correlation between M protein, involved FLC and MM tumor at diagnosis and post-induction
We first assessed the correlation between the biomarkers M protein and involved FLC with bone marrow MM disease burden (by marrow aspirate and core biopsy as gold standards) at diagnosis and post-induction therapy. M protein correlated with MM disease burden at diagnosis (by aspirate, p = 0.02, and core biopsy, p = 0.001). Following induction therapy, M protein correlated with MM disease burden (by aspirate, p < 0.001 and core biopsy, p < 0.0001). Involved FLC correlated with disease burden at diagnosis (by aspirate, p = 0.02, but not core biopsy, p = 0.07). Following induction, involved FLC correlated with disease burden (by core biopsy, p = 0.01, but not by aspirate, p = 0.33).
Kinetics of biomarkers from diagnosis to mobilization
The average change in M protein from diagnosis to post-induction was 1883 mg/dL (+/− SD 1147) for patients receiving bortezomib-based induction, 1801 mg/dL (+/− 1068) for patients receiving IMID-based induction, and 1382 mg/dL (+/− 1539) for patients receiving combination-based induction. The average change in involved FLC from diagnosis to post-induction was 586 mg/L (+/− 1100) for patients receiving bortezomib, 23 mg/L (+/− 436) for patients receiving IMIDs, and 637 mg/L (+/− 1248) for patients receiving combination therapy. In neither case were changes statistically different between induction groups. No differences were observed across induction strategies in baseline or post-induction marrow plasmacytosis.
M protein more reliably predicts disease status than involved FLC
Utilizing bone marrow plasmacytosis (by both aspirate and core biopsy) as a gold standard, we compared the reliability of M protein and involved serum FLC as biomarkers for MM. Linear regression models in which either M protein or involved FLC served as the primary predictor of residual marrow plasmacytosis by aspirate or core biopsy as the outcome measure. In these models, described in depth below, M protein performed better as a biomarker of MM than involved FLC.
16 individual, multivariable-regression models were created to determine residual MM disease presence in marrow (by aspirate or core biopsy) with M protein or involved FLC serving as the primary predictor (as characterized by either value at diagnosis, value at mobilization, absolute change, or percent (%) change over the course of induction therapy). Statistical significance was observed in 6 of the 8 models regarding M protein as the primary predictor, whereas statistical significance was observed in only 2 of the 8 models regarding involved FLC as the primary predictor. These data are not shown, as for most results, the adjusted R2 and associated p-value were perhaps not clinically relevant. In models utilizing M protein, consistent covariates included degree of marrow plasmacytosis at diagnosis, type of induction received, and patient age. Of any variable examined, the absolute M protein and involved FLC at diagnosis were found to be the poorest individual predictor of disease status post-induction. The best predictor of residual disease status post-induction was the absolute M protein following therapy with adjusted R2 = 0.377 (p < 0.001) using residual marrow plasma cell burden by aspirate and adjusted R2 = 0.28 (p < 0.001) using residual marrow plasma cell burden by core biopsy.
We further studied the effects of various induction regimens (bortezomib-based, IMID-based, and combination) on reduction of M protein and involved FLC. By Kruskal-Wallis (non-parametric ANOVA), no differences were observed in M protein reduction as a function of induction regimen; however, patients receiving bortezomib-based induction regimens did show a greater reduction in involved FLC as compared to patients receiving IMID-based induction (p = 0.09 for absolute change and p = 0.041 for percent change in involved FLC). No differences in residual marrow plasmacytosis was observed across induction groups by aspirate or core biopsy assessment. In summary, these data suggest that M protein appears to be a better biomarker for MM as compared to involved FLC in assessing response to induction therapy with novel agents.
Involved FLC adds to the value of M protein for characterizing residual disease following induction
Given that M protein appears to be superior to involved FLC as individual biomarkers of response to induction therapy, we next examined whether or not the addition of involved FLC to M protein as a biomarker following induction therapy could further improve the evaluation of residual MM following induction treatment. 8 additional linear regression models were created (4 to predict bone marrow plasmacytosis by aspirate and 4 to predict plasmacytosis by core biopsy) where M protein and involved FLC served as co-primary predictors (by absolute change, % change, and absolute values at diagnosis and post-induction). Models were adjusted for covariates with confounding attributes (specifically, type of induction received and degree of marrow disease at diagnosis). These findings are summarized in Table 3. Here the p-value (from the partial F-test) indicates if the one predictor significantly adds to the model given the other primary predictor is already in the model. Interestingly, in these models, the % change from diagnosis to post-induction of both M protein and involved FLC performed best at predicting residual disease, measured by either marrow aspirate and core biopsy, in both cases accounting for about a third of the variability observed in the primary outcome measures analyzed.
Table 3.
Involved FLC adds to M-protein in predicting residual MM post-induction
| Outcome | Primary Predictors | Coefficient | p-value | Adjusted* R2 |
|---|---|---|---|---|
| Mobilization | delta M-protein | 0.97 | 0.003 | 0.200 |
| % aspirates | delta involved FLC | 0.99 | 0.346 | |
| Mobilization | % change M-protein | 0.87 | <0.001 | 0.337 |
| % aspirates | % change involved FLC | 0.97 | 0.004 | |
| Mobilization | Diagnosis M-protein | 1.01 | 0.386 | 0.054 |
| % aspirates | Diagnosis involved FLC | 0.99 | 0.555 | |
| Mobilization | Mobilization M-protein | 1.04 | <0.001 | 0.353 |
| % aspirates | Mobilization involved FLC | 1.10 | 0.248 | |
| Mobilization | Delta M-protein | 0.97 | 0.006 | 0.221 |
| % core | Delta involved FLC | 0.98 | 0.224 | |
| Mobilization | % change M-protein | 0.86 | 0.003 | 0.270 |
| % core | % change involved FLC | 0.96 | 0.012 | |
| Mobilization | Diagnosis M-protein | 0.99 | 0.667 | 0.070 |
| % core | Diagnosis involved FLC | 0.98 | 0.289 | |
| Mobilization | Mobilization M-protein | 1.06 | 0.001 | 0.292 |
| % core | Mobilization involved FLC | 1.19 | 0.118 | |
Adjusted for induction (bortezomib, IMID, Combination) and diagnosis % aspirates (or core) depending on whether or not the outcome is mobilization % aspirates or mobilization % core
In summary, these data suggest that the % change in involved FLC over the course of induction in addition to the % change in M protein increases the effectiveness of M protein in characterizing the nature of residual disease following induction therapy prior to HDC/SCT.
Change in involved FLC during induction therapy predicts overall survival following HDC/SCT
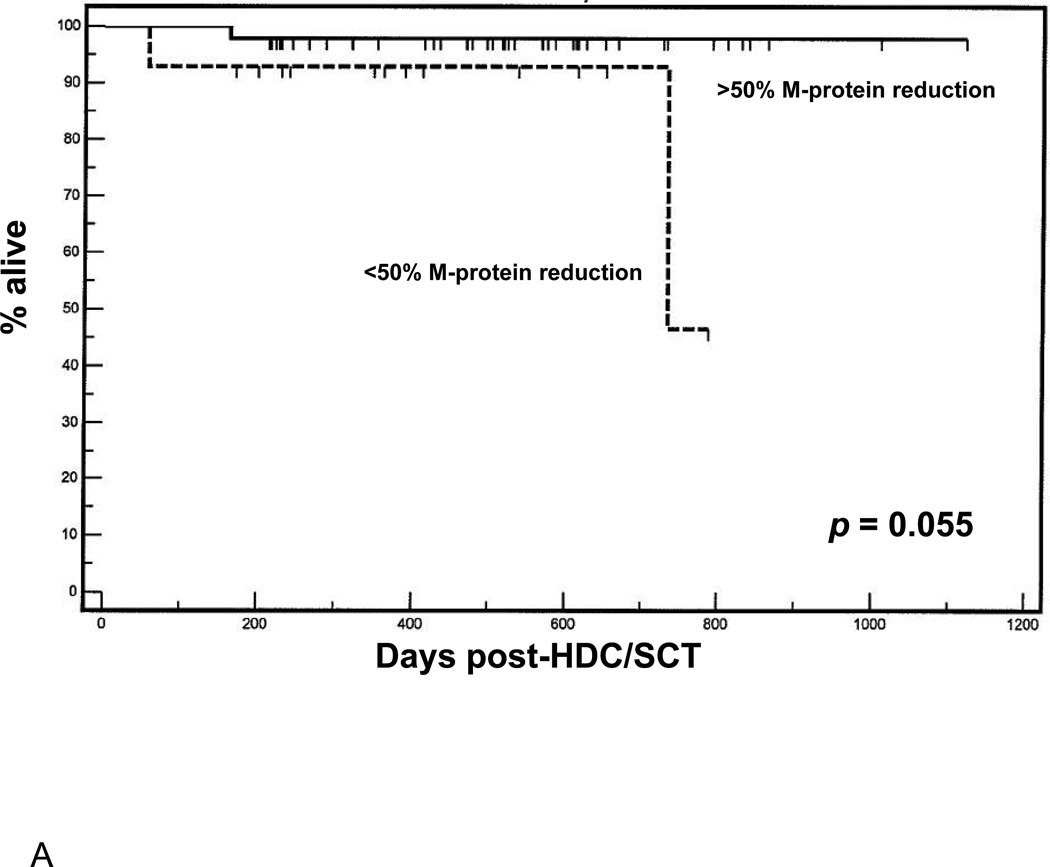
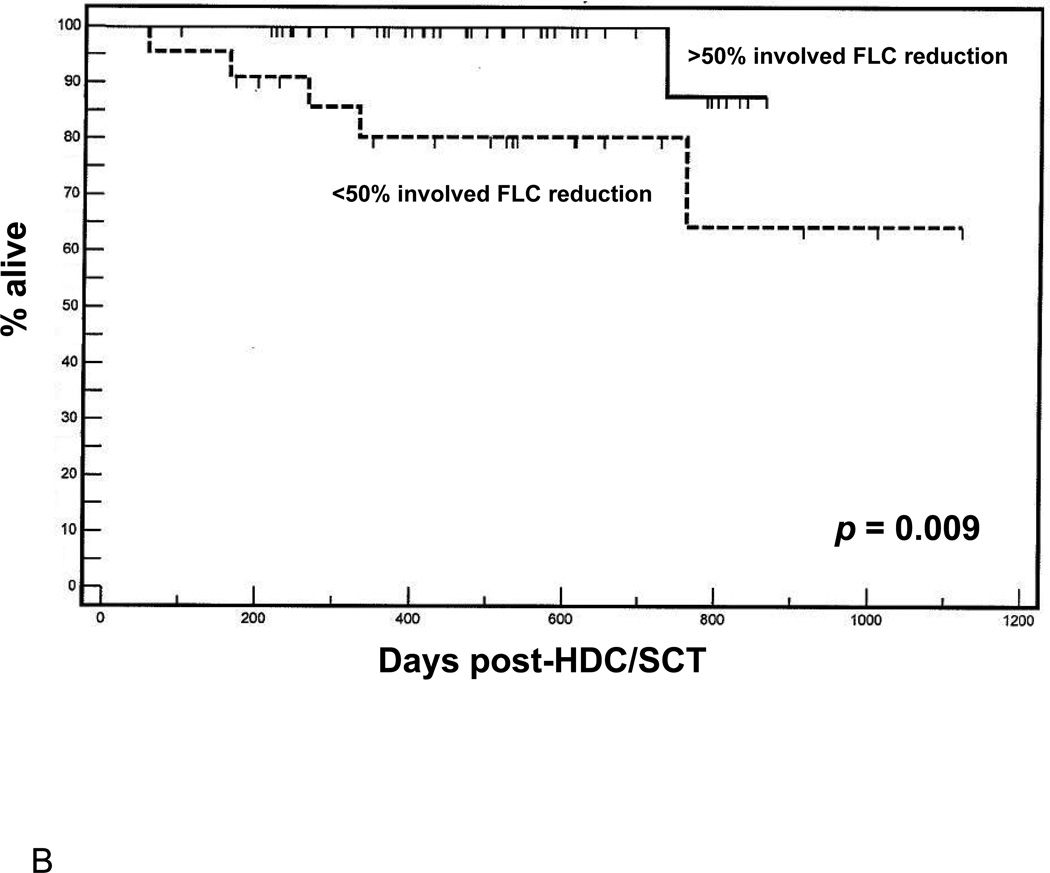
The improvement in quality of response to induction therapy related to the use of novel agents pre-transplant has been posited as a predictor of improved outcomes following subsequent HDC/SCT [10]. We hypothesized, thus, that changes observed in M protein and involved FLC, per se, may be useful a priori predictors of outcome following HDC/SCT. Indeed, objective response rates pre-mobilization correlated with observed % change in involved FLC (two-tailed T-test = 2.54, p = 0.01). Patients were first dichotomized by greater or less than 50% reduction in M protein or involved FLC (based on prior work) [9] over the course of pre-transplant induction therapy, and groups were compared in terms of survival endpoints following consolidative HDC/SCT. Regarding depth of response measured by M protein (Figure 1A), a trend towards favorable OS was observed in patients in whom M protein by immunofixation fell by over 50% as compared to patients with less than 50% reduction in M protein (p = 0.055). However, a clear difference was observed in OS (Figure 1B) in patients in whom involved FLC fell by more than 50% during induction as compared with patients in whom involved FLC decreased by less than 50% (p = 0.009).
Figure 1.
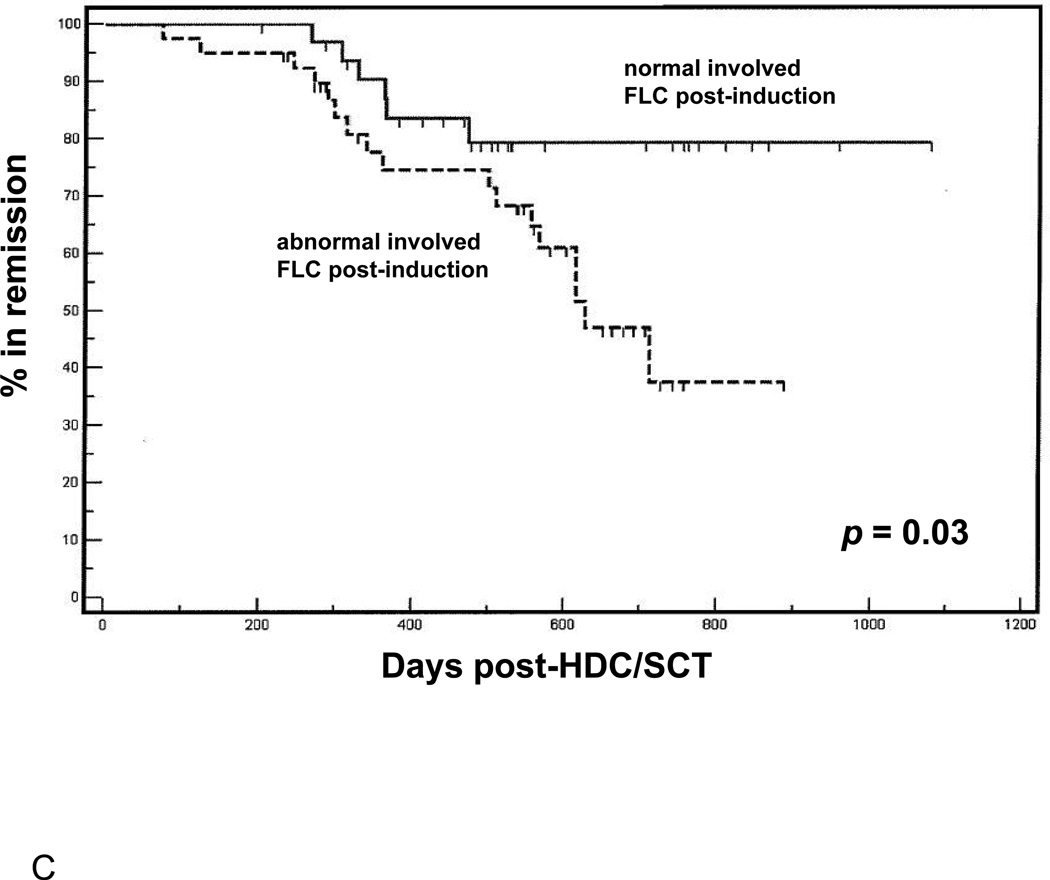
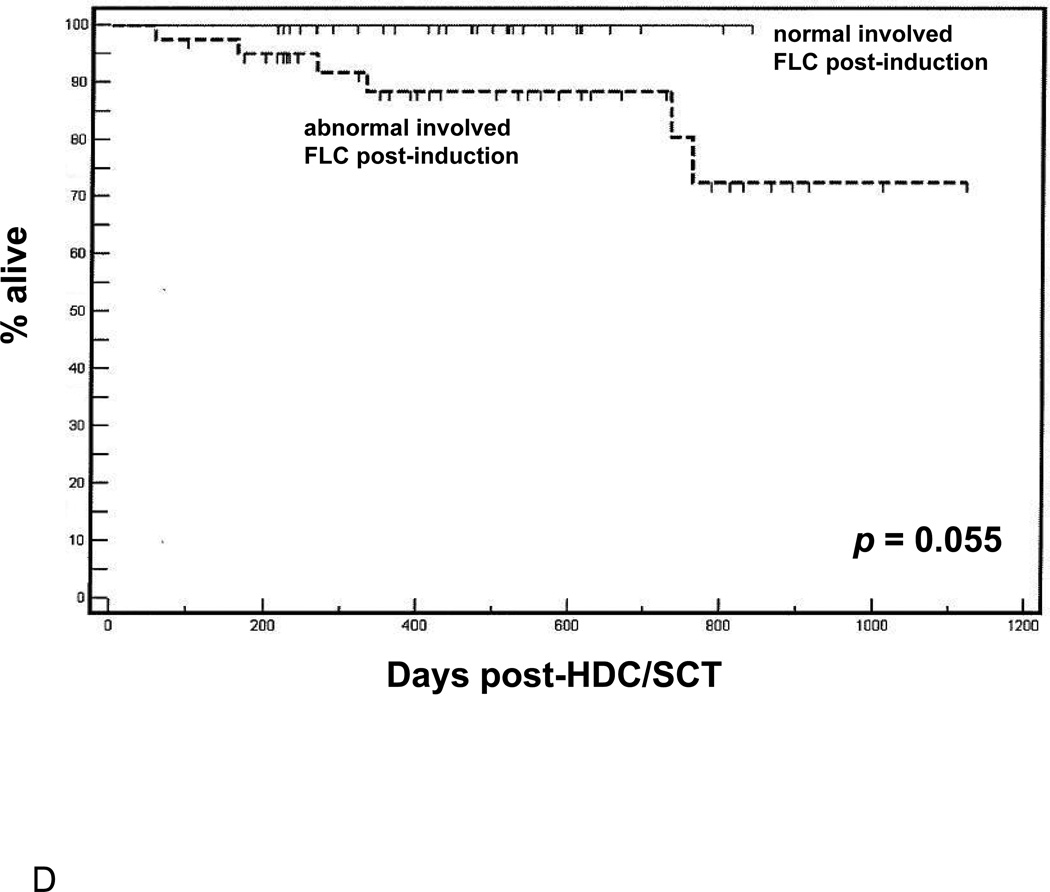
(A) Overall survival following HDC/SCT stratified by M-protein response during induction. (B) Overall survival following HDC/SCT stratified by involved FLC response during induction. (C) Progression free survival following HDC/SCT comparing patients with normalization of involved FLC during induction and patients with abnormal involved FLC after induction. (D) Overall survival following HDC/SCT comparing patients with normalization of involved FLC during induction and patients with abnormal involved FLC after induction.
We also studied whether or not normalization of involved FLC pre-HDC/SCT could be a useful predictor of survival outcomes post-transplant. As shown in Figure 1C, patients who achieved normalization of involved FLC pre-transplant had significantly better PFS following HDC/SCT than those who did not (p = 0.009). Also, with an average follow up of 500 days (range 100–1101), patients who showed normalization of involved FLC post-induction have demonstrated 100% OS following HDC/SCT (p = 0.055 compared to patients without normalization of involved FLC, Figure 1D). Interestingly, neither the presence of negative immunofixation nor reduction of marrow plasmacytosis to < 5% (by either aspirate or core biopsy) following induction therapy held prognostic value for either PFS or OS following HDC/SCT (respective p-values are not significant at the 0.05 level, data not shown).
These findings suggest that assessment of post-induction response regarding either normalization of involved FLC, or at least 50% decrease from diagnosis, may be a unique, a priorivariable to consider in predicting survival outcomes post-HDC/SCT.
Discussion
MM remains incurable and is increasing in prevalence [12]. Despite the advent of effective, novel therapies, MM remains the top indication for HDC/SCT worldwide [13]. Ongoing controversy exists regarding whether or not novel agents will replace HDC/SCT, but in the short term, effective incorporation of new agents into a treatment paradigm including HDC/SCT may lead to the greatest improvements in outcome for transplant-eligible patients with MM [10].
In addition to novel therapies, evolving clinical practice regarding care of patients with MM has also included the wide-spread availability of technology to measure FLC in serum [5,6]. Assessment of sFLC has been validated in a number of settings in the care of patients with plasma cell dyscrasias; however, to date, controversy exists in regards to the optimal role of this test as a potential additional, or complementary, biomarker of MM in the majority of cases where an intact M-protein by immunofixation may be followed [7,9,14–19]. Specifically, while sFLC has been validated in a number of established clinical settings, the utility of this biomarker in characterizing response to therapy remains unclear. To date, to our knowledge, only one study has assessed the utility of serum FLC as a response marker, in patients treated from 1988 – 1992 with an alkylator-based combination chemotherapy (vincristine, carmustine, melphalan, cyclophosphamide, and prednisone).[9] In that setting, sFLC did not add to the role of M protein as a biomarker of response following two months of induction therapy [9]. The present work thus adds to the current literature by describing the potential role of sFLC in the contemporary setting of newly-diagnosed MM patients treated entirely with novel agents in preparation for HDC/SCT consolidation.
Our results contribute several important points to the ongoing characterization of sFLC as a biomarker of response assessment in MM. First, by limiting analysis to a patient population receiving contemporary, novel agent-based induction, we contribute data to the examination of sFLC as a response biomarker relevant to the current management of SCT-eligible patients with MM. In this context, we show that, while both M protein and involved FLC both correlate with disease burden, involved FLC is individually inferior to M protein at predicting the status of residual disease post-induction therapy. However, the addition of involved FLC to M protein adds to the latter’s accuracy as an established biomarker of MM in characterizing response and residual disease pre-HDC/SCT. Interestingly, in patients receiving induction treatment with a bortezomib-based regimen, measurement of involved FLC in addition to M protein may be particularly useful in characterizing residual disease state post-induction therapy in the pre-mobilization setting. The reason for this finding is unclear but may be related to the different mechanisms of action of bortezomib and IMIDs.
We and others have shown that quality of response to induction therapy may be an important determinant of survival following subsequent HDC/SCT [10,20]. Induction with novel agents may lead to better disease control prior to HDC/SCT which, in turn, may prolong survival following consolidative HDC/SCT [10,21,22]. In this context, we provide evidence for a potentially novel role for involved FLC as a practical and convenient a priori prognostic marker for survival outcomes following HDC/SCT in patients receiving induction with a novel agent-based therapy. Specifically, greater than 50% reduction in, or normalization of, involved FLC to induction therapy appears to predict for enhanced PFS and potentially OS following subsequent HDC/SCT. This finding is particularly interesting in light of recent data from another group suggesting that sFLC are not prognostic survival factors when measured 3 months after HDC/SCT [23]. The reasons for this discrepancy are unclear but may relate to the substantial differences in regards to therapies received by patients between the respective manuscripts. In our study, all patients received induction with novel agents whereas patients examined by Giarin, et al.,[23] all received vincristine, adriamycin and dexamethasone (VAD) induction which is no longer a standard of care. In addition, all patients in the present work received a standardized preparative regimen (melphalan 200mg/m2 (or 140mg/m2 in the setting of renal insufficiency) intravenously on day-2 followed by autologous SCT on day 0) and single autograft whereas only 7% of patients in Giarin, et. al.,[23] were treated in this manner. 71% of patients in Giarin, et. al.,[23] received tandem autografts, 6% received 3 autografts, and 15% underwent tandem autologous/allogeneic SCT. None-the-less, our finding that change in involved FLC over the course of induction therapy may provide clinicians with a novel, practical biomarker with which to assist in judgment of response to induction therapy pre-mobilization and perhaps develop further plans for additional therapy pre-HDC/SCT to maximize post-transplant outcomes.
Several limitations warrant caution in the interpretation of the present findings. Being retrospective in nature and including a relatively small sample size of patients (n=73), the work should rightly be considered hypothesis-generating in nature and observations need confirmation in prospective studies. Also, in n=16 patients with intact M-protein, the post-induction value was less than 0.2 g/dL (range 0 – 186mg/dL), which may have made accurate measurement difficult. Still, the results contribute novel information to the potential, specific role of involved FLC as a response biomarker, an application of this biomarker which has not, to date, been recommended for routine clinical utilization [7]. In addition, these results extend on prior work investigating serum FLC as a response variable, by contributing data in patients receiving novel agent-based inductions followed by HDC/SCT.
Acknowledgments
Supported by: Multiple Myeloma Opportunities for Research and Education (MMORE) Grant (DMB Jr, CCH, YE)
Footnotes
Portions of this work were presented at the 6th international symposium on Clinical Applications of Serum Free Light Chain Analysis, Bath, UK, September 23–24, 2010.
Conflict of interest statement: No authors have conflicts of interest to declare.
References
- 1.Durie BGM, Salmon SE. A clinical staging system for multiple myeloma: Correlation of measured myeloma cell mass with presenting clinical features, response to treatment and survival. Cancer. 1975;36(3):842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Nathans D, Fahey JL, Potter M. The formation of myeloma protein by mouse plasma cell tumor. J Exp Med. 1958;108(1):121–130. doi: 10.1084/jem.108.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salmon SE, Smith B. Immunoglobulin synthesis and total body tumor cell number in multiple IgG myeloma. J Clin Invest. 1970;49:1114–1121. doi: 10.1172/JCI106327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan PW, Salmon SE. Kinetics of tumor growth and regression in IgG multiple myeloma. J Clin Invest. 1970;49(6):1114–1121. doi: 10.1172/JCI106971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47(4):673–680. [PubMed] [Google Scholar]
- 6.Bradwell AR. Serum free light chain measurements move to center stage. Clin Chem. 2005;51(5):805–807. doi: 10.1373/clinchem.2005.048017. [DOI] [PubMed] [Google Scholar]
- 7.Dispenzieri A, Kyle R, Merlini G, Miguel JS, Ludwig H, Hajek R, et al. International myeloma working group guidelines for serum free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215–224. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- 8.Martin W, Abraham R, Shanafelt T, Clark RJ, Bone N, Geyer SM, et al. Serum-free light chain- a new biomarker for patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Trans Res. 2007;149(4):231–235. doi: 10.1016/j.trsl.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Dispenzieri A, Zhang L, Katzmann JA, Snyder M, Blood E, Degoey R, et al. Appraisal of immunoglobulin free light chain as a marker of response. Blood. 2008;111(10):4908–4915. doi: 10.1182/blood-2008-02-138602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson DM, Panzner K, Hamadani M, Hofmeister CC, Bakan CE, Smith MK, et al. Effects of induction with novel agents versus conventional chemotherapy on mobilization and autologous stem cell transplant outcomes in multiple myeloma. Leuk Lymphoma. 2010;51(2):243–251. doi: 10.3109/10428190903480728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haematopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. Brit J Haematol. 1998;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 13.Catley L, Anderson K. Strategies to improve the outcome of stem cell transplantation in multiple myeloma. Hematol J. 2004;5(1):9–23. doi: 10.1038/sj.thj.6200322. [DOI] [PubMed] [Google Scholar]
- 14.Abraham RS, Clark RJ, Bryant SC, Lymp JF, Larson T, Kyle RA, et al. Correlation of sreum immunoglobulin free light chain quantification with urinary Bence Jones protein in light chain myeloma. Clin Chem. 2002;48(9):655–657. [PubMed] [Google Scholar]
- 15.Smith A, Wisloff F, Samson D. Guidelines on the diagnosis and management of multiple myeloma 2005. Brit J Haematol. 2006;132(4):410–451. doi: 10.1111/j.1365-2141.2005.05867.x. [DOI] [PubMed] [Google Scholar]
- 16.Fulton RB, Fernando SL. Serum free light chain assay reduces the need for serum and urine immunofixation electrophoresis in the evaluation of monoclonal gammopathy. Ann Clin Biochem. 2009;46(Pt 5):407–412. doi: 10.1258/acb.2009.009038. [DOI] [PubMed] [Google Scholar]
- 17.Giarin MM, Giaccone L, Sorasio R, Sfiligoi C, Amoroso B, Cavallo F, et al. Serum free light chain ratio, total kappa/lambda ratio, and immunofixation results are not prognostic factors after stem cell transplantation for newly diagnosed multiple myeloma. Clin Chem. 2009;55(8):1510–1516. doi: 10.1373/clinchem.2009.124370. [DOI] [PubMed] [Google Scholar]
- 18.Siegel DS, McBride L, Bilotti E, Lendvai N, Gonsky J, Berges T, et al. Inaccuracies in 24-hour urine testing for monoclonal gammopathies: serum free light chain analysis provides a more accurate measure of light chain burden than urine protein electrophoreses. Lab Med. 2009;40(6):341–344. [Google Scholar]
- 19.Singhal S, Stein R, Vickrey E, Mehta J. The serum-free light chain assay cannot replace 24-hour urine protein estimation in patients with plasma cell dyscrasias. Blood. 2007;109(8):3611–3612. doi: 10.1182/blood-2006-11-060368. [DOI] [PubMed] [Google Scholar]
- 20.Palumbo A, Rajkumar V. Multiple myeloma: chemotherapy or transplantation in the era of new drugs. Eur J Haematol. 2010;84(5):379–390. doi: 10.1111/j.1600-0609.2010.01431.x. [DOI] [PubMed] [Google Scholar]
- 21.Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, et al. Bortezomib plus dexamethasone is supper ior to vincrinstine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28(20):4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 22.Lokhorst HM, Schmidt-Wolf I, Sonneveld P, van der Holt B, Martin H, Barge R, et al. Thalidomide in induction treatment increases the very good partial response rate before and after high-dose therapy in previously untreated multiple myeloma. Haematologica. 2008;93(1):124–127. doi: 10.3324/haematol.11644. [DOI] [PubMed] [Google Scholar]
- 23.Giarin MM, Giaccone L, Sorasio R, Sfiligoi C, Amoroso B, Cavallo F, et al. Serum free light chain ratio, total kappal/lambda ratio and immunofixation results are not prognostic factors after stem cell transplantation for newly diagnosed multiple myeloma. Clin Chem. 2009;55(8):1510–1516. doi: 10.1373/clinchem.2009.124370. [DOI] [PubMed] [Google Scholar]