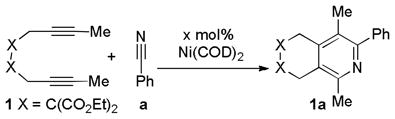
Abstract
Xanted Nickel!
The catalytic combination of Ni(COD)2 and Xantphos was used to access pyridines. The reaction proceeds under ambient conditions to provide excellent yields of products. Comparison of this catalyst with the other state-of-the-art catalysts is also provided.
Keywords: cycloaddition, nickel/phosphine, catalyst, diynes, nitriles, pyridines
A variety of transition metal complexes (i.e., Co, Ru, Rh, Ni, and Fe) catalyze the cycloaddition of alkynes and nitriles to form pyridines.[1–7] Most of the catalysts generate pyridines through a mechanism that involves initial oxidative coupling of the alkynes and then subsequent insertion of the nitrile (i.e., formation of A, Figure 1).[8] In contrast, nickel-mediated cycloadditions are thought to undergo hetero-oxidative coupling between a single alkyne and the heteroatom-containing substrate, such as a nitrile, before insertion of the second alkyne (i.e., formation of B, Figure 1).[9] Thus, a Ni/Ln-based system (especially where Ln=phosphine) that provided pyridines remained absent due to the difficulty associated with forming the required azametallacyclopentadiene intermediate until two notable solutions were developed. Takahashi accessed the azametallacyclopentadiene intermediate stoichiometrically through transmetallation between Ni(PPh3)2Cl2 and an azazirconacyclopentadiene complex.[9] Alternatively, catalytic pyridine formation was achieved at 100 °C between activated alkynes such as benzyne with Ni/DPPE as the catalyst.[10]
Figure 1.
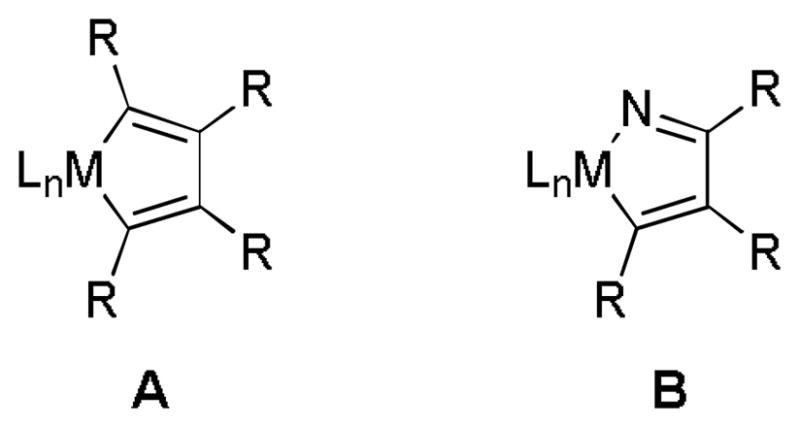
Transition metal intermediates in the cycloaddition of alkynes and nitriles
We successfully developed an efficient Ni-cycloaddition catalyst by replacing the phosphines with N-heterocylic carbene (NHC) ligands.[6] Thus, enhancing the nucleophilicity of the nickel center appeared to be the key for promoting oxidative coupling of the alkyne and nitrile. Recently, we reported a Ni-catalyzed cycloaddition of diynes and ketenes.[11] In our efforts to expand this chemistry to ketenimines, we serendipitously discovered the combination of Ni(0) and Xantphos serves as a general catalyst system for the cycloaddition of diynes and nitriles. Furthermore, these cycloadditions proceed, in many cases, more effectively than current state-of-the-art catalytic methods.
 |
(1) |
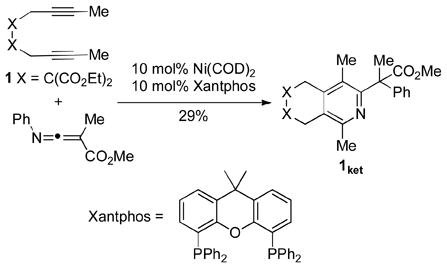
Upon investigating conditions to promote the cycloaddition between diyne 1 and methyl 2-methyl-3-(phenylimino)acrylate, we found that pyridine 1ket (rather than the expected carbocycle) was obtained in moderate yield when Xantphos was used as the ligand (eq 1).[11–13] Surprised by this result, particularly in light of previous difficulties associated with pyridine formation using Ni/PR3 catalysts (vide supra), we evaluated a variety of monodentate and bidentate phosphines as potential ligands in the Ni-catalyzed cycloaddition of diyne 1 and the simple, unactivated nitrile a at 100 °C (eq 2, Table 1). As expected, in most cases, little to no pyridine product was observed despite good conversion of the diyne.[14] From the pool of monodentate phosphines screened (entries 1–5), only moderate catalytic activity was observed in reactions run with PPh3 (Θ=145°) and PCy3 (Θ=170°). Even worse catalytic activity was observed when the slightly larger monodentate phosphine, P(o-tol)3 (Θ=194°), was used.[15]
Table 1.
| ||||||
|---|---|---|---|---|---|---|
| Entry | Ligand (Ln) | Catalyst loading | Temp. | 1a % Yieldc | ||
| 1 | PCy3 | 10 mol% | 100 °C | 27 | ||
| 2 | PBu3 | 10 mol% | 100 °C | - | ||
| 3 | PPh3 | 10 mol% | 100 °C | 23 | ||
| 4 | P(o-tol)3 | 10 mol% | 100 °C | 11 | ||
| 5 | P(O-iPr)3 | 10 mol% | 100 °C | - | ||
| 6 | DPPE | 10 mol% | 100 °C | - | ||
| 7 | DPPF | 10 mol% | 100 °C | 21 | ||
| 8 | rac-BINAP | 10 mol% | 100 °C | 7 | ||
| 9 | Xantphos | 10 mol% | 100 °C | >99 (86)d | ||
| 10 | Xantphos | 5 mol% | 100 °C | >99 | ||
| 11 | Xantphos | 3 mol% | 100 °C | >99 | ||
| 12 | Xantphos | 3 mol% | RT | >99 (98%)d,e | ||
For Entries 1–5, 20 mol% Ln was used. For entries 6–9, 10 mol% Ln was used. For entry 10, 5 mol% Ln was used. For entries 11–12, 3 mol% Ln was used.
The reactions from entries 1–11 were analysed after 24h and >99% diyne conversion was observed for all the entries.
Analysed by GC using naphthalene as an internal standard.
Isolated yields in parentheses.
The reaction was completed in 3 h.
In general, bidentate phosphines did not fare any better. DPPE (β= 85°) was ineffective towards pyridine formation (entry 6). Although pyridine was observed when bidentate ligands with larger bite angles such as BINAP (β = 92°) or DPPF (β= 96°) were employed (entries 7–8), yields were still marginal. Surprisingly, a dramatic increase in pyridine formation occurred when Xantphos (β= 111°), a bidentate ligand with a large bite angle, was evaluated (entry 9).[15–16] Further optimization (entries 10–12) led to a system that provided pyridine 1a in excellent yields under mild reaction conditions (3 mol% Ni-catalyst @ RT for 3h). Thus, in contrast to our previous hypothesis,[6a] sterics may play an equally or more important role in promoting both the challenging oxidative coupling and reductive elimination of C-N bond.[15]
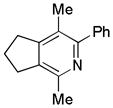
The reaction has a broad substrate scope and tolerates a variety of functional groups (Table 2). Yields obtained using the Ni/NHC catalyst system are provided in parantheses for direct comparison.[6a] Diyne 1 reacted with benzonitrile a to afford the pyridine in excellent yields (entry 1). Electron withdrawing groups (4-CF3, 3,5-F2, entries 4–5) on the arylnitrile afforded the cycloadduct in higher yields than arylnitriles bearing electron donating groups (2-Me, 4-OMe, entries 2–3). 2-Napthylnitrile f also afforded the pyridine in excellent yields (entry 6). Interestingly, diyne 2 reacted with a variety of nitriles to provide 5,6-membered fused-ring systems in higher yields than those obtained with the Ni/SIPr catalyst system (entries 7–11). In addition, similar trends in pyridine yields from those obtained from diyne 1 were observed when the electronics on the arylnitrile were modified (i.e., electron-withdrawing substituents gave higher yields than substrates bearing electron-donating substituents). However, these effects were not as pronounced as they were with the original Ni/SIPr system (entry 9 vs. entry 10). Nitrile g bearing an activated olefin successfully reacted to afford 2g (entry 12) in excellent yield, although elevated temperature was required. Notably, no product arising from incorporation of olefin was observed.[17] The challenging alkyl nitriles (h and i) also afforded the desired pyridines in excellent yields (entries 13–14). Similary, sulfonamide diyne and diyne-ether reacted with nitrile a to yield products in good yields (entries 15–16). The Thorpe-Ingold effect was not a necessity of this reaction as diyne 5 reacted to afford the cycloadduct in excellent yields (entry 17). It is important to note that all nitriles evaluated were not purified prior to use; instead, they were used as received from commercial suppliers.
Table 2.
| Entry | Diyne | Nitrile | Product | Yieldc |
|---|---|---|---|---|
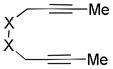 X = C(CO2Et)2 |
|
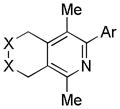
|
||
| 1 | 1 | a (Ar = Ph) | 1a | 98% |
| 2 | 1 | b (Ar = 2-Me-Ph) | 1b | 87% |
| 3 | 1 | c (Ar = 4-OMe-Ph) | 1c | 90% |
| 4 | 1 | d (Ar = 4-CF3-Ph) | 1d | 99% |
| 5 | 1 | e (Ar = 3,5-F2-Ph) | 1e | >99% |
| 6 | 1 | f (Ar = 2-Naphthyl) | 1f | 96% |
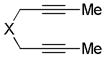 X = C(CO2Me)2 |
|
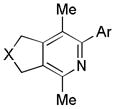
|
||
| 7 | 2 | a (Ar = Ph) | 2a | 92% (86%)d |
| 8 | 2 | b (Ar = 2-Me-Ph) | 2b | 85% (81%)d |
| 9 | 2 | c (Ar = 4-OMe-Ph) | 2c | 89% (64%)d |
| 10 | 2 | d (Ar = 4-CF3-Ph) | 2d | 98% (94%)d |
| 11 | 2 | e (Ar = 3,5-F2-Ph) | 2e | >99% |
|
|

|
|||
| 12 | 2 | g | 2g | >99%e |
|
|
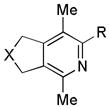
|
|||
| 13 | 2 | h (R = Me) | 2h | 94% (69%)d |
| 14 | 2 | i (R = i-Pr) | 2i | 90% (72%)d |
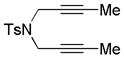
|
||||
| 15 | 3 | a | 3a | 73% (78%)d |
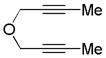
|
||||
| 16 | 4 | a | 4a | 80% (93%)d |
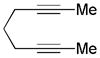
|
|
|||
| 17 | 5 | a | 5a | 93% |
Reaction Conditions: 3 mol % Ni(COD)2, 3 mol % Xantphos, diyne (1 equiv., 0.1 M), nitrile (1.5 equiv.), toluene, RT, 3 h.
Nitriles were neither distilled nor degassed. Nitriles were used as received.
Isolated yields.
Yields from reference 6a.
The reaction was run at 100 °C.
The efficacy of the Ni/Xantphos cycloaddition catalyst system was compared to other, state-of-the-art transition metal catalysts based on Co, Ru, Rh, and Ni (Table 3, entry 6). Specifically, diyne 1 and benzonitrile a were subjected to a variety of catalytic conditions and monitored for pyridine formation. Even after 24 h in presence of a large excess nitrile (20 equiv), full conversion of the diyne was not reached with 5 mol% [Co] catalyst (Table 3, entry 1). Also, the desired cycloadduct was formed in only 70% yield.[18]
Table 3.
Comparison of Metal-Catalysts for Cycloaddition of Diynes and Nitrile.
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | [M] Catalyst x mol% | equiv. of Nitrile a | Temp | Time | Conva 1 | Yielda,b 1a | ||
| 1 | 5 mol% [Co]c | 20 | RT | 24h | 86% | 70% | ||
| 2 | 3 mol% [Rh]d | 1.5 | 60 °C | 3h | >99% | >99% | ||
| 3 | 3 mol% [Rh]e | 1.5 | RT | 3h | 32% | 15% | ||
| 4 | 10 mol% [Ru]f | 1.5 | 60 °C | 24h | 6%g | - | ||
| 5 | 3 mol% [Ni/SIPr]h | 1.5 | RT | 3h | >99% | 80% | ||
| 6 | 3 mol% [Ni/Xant]i | 1.5 | RT | 3h | >99% | >99% (98%) | ||
Analysed by GC using naphthalene as an internal standard.
Isolated yield in parenthesis.
5 mol% CoCl2, 6 mol% DPPE, xs Zn, diyne (1 equiv., 1 M), nitrile (20 equiv), NMP, RT.
3 mol% [Rh(COD)]BF4, 3 mol% Segphos, diyne (1equiv., 0.1 M), nitrile (1.5 equiv.), DCE, 60 °C.
Same conditions as in [d], but the reaction was run @ RT.
10 mol% Cp*Ru(COD)Cl, diyne (1 equiv., 0.1 M), nitrile (1.5 equiv.), DCE, 60 °C, 24 h.
Analysed by NMR.
3 mol% Ni(COD)2, 3 mol% SIPr, diyne (1 equiv., 0.1 M), nitrile (1.5 equiv.), THF, RT.
3 mol% Ni(COD)2, 3 mol% Xantphos, diyne (1 equiv., 0.1 M), nitrile (1.5 equiv.), toluene, RT.
A Rh/BINAP catalyst[5a] developed by Tanaka et al is known for exhibiting excellent catalytic activity for activated nitriles (an approach complementary to Ru-catalyzed cycloaddition developed independently by Yamamoto and Saa).[4] However, replacement of BINAP to Segphos was required when simple nitriles, such as acetonitrile or benzonitrile, were employed. Although excellent yield and conversion were observed at 60 °C, the reaction afforded the pyridine in lower yield at RT with the Rh-Segphos catalyst (Table 3, entries 2–3 vs. entry 6). The [Ru] catalyst[4a] was found to be completely ineffective towards pyridine product formation (entry 4). Although excellent conversion of diyne 3 was observed with Ni/SIPr catalyst, 1a was produced in lower yields than Ni-Xantphos system (entry 5 vs 6). Thus, lower catalyst loadings, milder reaction conditions, and equimolar substrate stoichiometry make Ni-Xantphos a more efficient catalyst for coupling of diynes and unactivated nitriles.
We recently reported Ni/NHC catalyzes the cycloaddition of diynes and cyanamides to access 2-aminopyridines.[19] These cycloadditions possessed an expanded substrate scope as terminal diynes were successfully converted to pyridine products. However, SIPr was required as a ligand rather than the standard IMes ligand employed for the coupling of internal diynes and cyanamides. The Ni/Xantphos system was evaluated and proved effective for the coupling of particularly challenging substrates (Figure 2). Specifically, 2,7-diynes (i.e., 1, 6) were successfully converted to 6,6-fused bicyclic products (1j, 6k, 1l). In addition, the same Ni/Xantphos system could be used for both internal and terminal diynes. Importantly, diallyl cyanamide, a cyanamide that is unreactive with the Ni/IMes catalyst, gave 1l in good yield.
Figure 2.
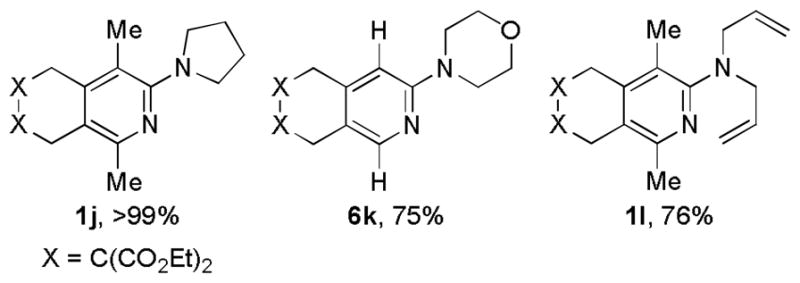
Ni-Catalyzed Cycloaddition of Diynes and Cyanamides
Treatment of an unsymmetrical diyne (7) with nitrile a led to the regioselective formation of the cycloadduct 7a (Figure 3). Despite the higher reactivity of terminal diynes, the regioselectivity is governed by the insertion of a less-sterically hindered alkyne (bearing -H on the terminal) in the azametallacycle formed by initial oxidative coupling of the internal alkyne (bearing -Me on the terminal) and nitrile a. Analogous to the reaction of diyne 7 and nitrile a, N,N-dimethylcyanamide was also regioselectively coupled to afford 2-aminopyridine, 7m (Figure 3).
Figure 3.
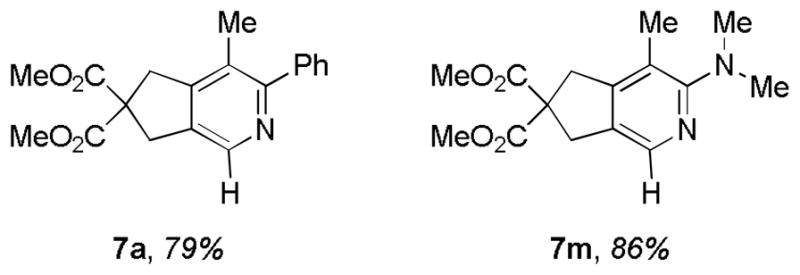
Regioselectivity in Ni-Catalyzed Cycloaddition of Diynes and Nitriles
The combination of high yields, mild reaction conditions, and, importantly, efficient stoichiometry led us to investigate the use of this system to rapidly click together a diyne with a complex nitrile. Specifically, equimolar amounts of both diyne 2 and δ-tocopherol-derived nitrile n were subjected to the Ni/Xantphos catalyst at RT. After only 3 h, pyridine 2n was obtained in 94% yield (eq 4). Thus, excess nitrile is not required to obtain high yields of potential pyridine bio-conjugates.
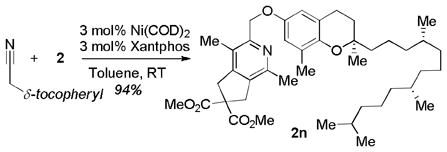 |
(4) |
In summary, we discovered a Ni/phosphine system that promotes both oxidative coupling between an alkyne and a nitrile as well as C-N bond-forming reductive elimination. The combination of catalytic amounts of Xantphos and Ni(0) produced a variety of pyridines from unactivated nitriles and diynes under mild and efficient reaction conditions. Studies focused on understanding the mechanism of this reaction and the application of this methodology toward the synthesis of bio-conjugates is ongoing.
Experimental Section
Representative Procedure
In nitrogen filled glove-box, diyne (1 equiv., 0.1M) and nitrile (1.5 equiv.) were added to an oven-dried screw-cap vial equipped with a magnetic stir bar. In a separate vial Ni(COD)2 and Xantphos were weighed (in 1:1 molar ratio) and dissolved in toluene. 3 mol% Catalyst solution was added to the reaction mixture. The vial was sealed and brought out of the glove-box. The reaction was stirred @ RT for 3 h. The resulting reaction mixture was concentrated and purified by flash column chromatography using first 15%, 30%, and finally 50% EtOAc/hexanes.
Further experimental details are available in the Supporting Information.
Supplementary Material
Footnotes
The NIGMS (5RO1GM076125) and the NSF (0911017) are acknowledged for financial support. S.P. thanks Technische Universitat braunschweig and Deutscher Akademischer Austausch Dienst (DAAD) for fellowship.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.For general reviews on metal catalyzed [2+2+2] cycloaddition reactions, see: Leboeuf D, Gandon V, Malacria M. In: Handbook of Cyclization Reactions. Ma S, editor. Vol. 1. Wiley-VCH; Weinheim, Germany: 2009. pp. 367–406.Lautens M, Klute W, Tam W. Chem Rev. 1996;96:49–92. doi: 10.1021/cr950016l.Chopade P, Louie J. Adv Synth Catal. 2006;348:2307–2327.Louie J. Curr Org Chem. 2005;9:605–623.Nakamura I, Yamamoto Y. Chem Rev. 2004;104:2127–2198. doi: 10.1021/cr020095i.Domínguez G, Perez-Castells J. Chem Soc Rev. 2011;40:3430–3444. doi: 10.1039/c1cs15029d.
- 2.For excellent reviews on synthesis of pyridine by metal-catalyzed [2+2+2] cycloaddition reaction, see: Volhardt KPC. Angew Chem, Int Ed Engl. 1984;23:539–556.Bönnenmann H. Angew Chem, Int Ed Engl. 1985;24:264–279.Varela JA, Saà C. Chem Rev. 2003;103:3787–3802. doi: 10.1021/cr030677f.Heller B, Hapke M. Chem Soc Rev. 2007;36:1085–1094. doi: 10.1039/b607877j.Weding N, Hapke M. Chem Soc Rev. 2011;40:4525–4538. doi: 10.1039/c0cs00189a.
- 3.For [Co]-catalyzed approaches to synthesize pyridines via cycloaddition strategy, see: Wakatsuki Y, Yamazaki H. J Chem Soc, Chem Commun. 1973:280a–280a.Wakatsuki Y, Yamazaki H. Tetrahedron Lett. 1973;14:3383–3384.Wakatsuki Y, Yamazaki H. J Chem Soc, Dalton Trans. 1978:1278–1282.Vollhardt KPC, Bergman RG. J Am Chem Soc. 1974;96:4996–4998.Bönnemann H, Brinkmann R, Schenkluhn H. Synthesis. 1974:575–577.Bönnemann H, Brinkmann R. Synthesis. 1975:600–602.Moretto AF, Zhang H-C, Maryanoff BF. J Am Chem Soc. 2001;123:3157–3158.Young DD, Dieters A. Angew Chem, Int Ed Engl. 2007;119:5187–5190. doi: 10.1002/anie.200700802.Geny, Agenet N, Iannazzo L, Malacria M, Aubert C, Gandon V. Angew Chem Int Ed. 2009;48:1810–1813. doi: 10.1002/anie.200806001.Hapke M, Weding N, Spannenberg A. Organometallics. 2010;29:4298–4304.Yuan C, Chang CT, Axelrod A, Siegel D. J Am Chem Soc. 2010;132:5924–5925. doi: 10.1021/ja101956x.
- 4.For [Ru]-catalyzed approaches to synthesize pyridines via cycloaddition strategy, see: Yamamoto Y, Ogawa R, Itoh K. J Am Chem Soc. 2001;123:6189–6190. doi: 10.1021/ja003890r.Yamamoto Y, Okuda S, Itoh K. Chem Commun. 2001:1102–1103.Varela JA, Carlos L, Saà C. J Org Chem. 2003;68:8595–8598. doi: 10.1021/jo035050b.Yamamoto Y, Kinpara K, Saigoku T, Takagishi H, Okuda S, Nishiyama H, Itoh K, Tanaka K, Suzuki N, Nishida G. J Am Chem Soc. 2005;127:605–613. doi: 10.1021/ja045694g.Yamamoto Y, Kinpara K, Ogawa R, Nishiyama H, Itoh K. Chem Eur J. 2006;12:5618–5631. doi: 10.1002/chem.200600176.
- 5.For [Rh]-catalyzed approaches to synthesize pyridines via cycloaddition strategy, see: Tanaka K, Suzuki N, Nishida G. Eur J Org Chem. 2006:3917–3922.Tanaka K, Hara H, Nishda G, Hirano M. Org Lett. 2007;9:1907–1910. doi: 10.1021/ol070456m.Wada A, Noguchi K, Hirano M, Tanaka K. Org Lett. 2007;9:1295–1298. doi: 10.1021/ol070129e.Shibata T, Uchiyama T, Endo K. Org Lett. 2009;11:3906–3908. doi: 10.1021/ol9014893.Komine Y, Kamisawa A, Tanaka K. Org Lett. 2009;11:2361–2364. doi: 10.1021/ol900802d.Komine Y, Tanaka K. Org Lett. 2010;12:1312–1315. doi: 10.1021/ol100182u.
- 6.For [Ni]-catalyzed approaches to synthesize pyridines via cycloaddition strategy, see: McCormick MM, Duong HA, Zuo G, Louie J. J Am Chem Soc. 2005;127:5030–5031. doi: 10.1021/ja0508931.Tekavec TN, Zuo G, Simon K, Louie J. J Org Chem. 2006;71:5834–5836. doi: 10.1021/jo0608669.
- 7.For [Fe]-catalyzed approaches to synthesize pyridines via cycloaddition strategy, see: D’Souza BR, Lane TK, Louie J. Org Lett. 2011;13:2936–2939. doi: 10.1021/ol2009939.Wang C, Li X, Wu F, Wan B. Angew Chem, Int Ed. 2011;50:7162–7166. doi: 10.1002/anie.201102001.
- 8.For Mechanistic discussions of Co-, Rh-, Ru- catalyzed cycloaddition reactions, see: Dazinger G, Torres-Rodrigues M, Kirchner K, Calhorda MJ, Costa PJ. J Organomet Chem. 2006;691:4434–4445.For studies involving [Ru], see: Yamamoto Y, Arakawa T, Ogawa R, Itoh K. J Am Chem Soc. 2003;125:12143–12160. doi: 10.1021/ja0358697.Yamamoto Y, Kinpara K, Saigoku T, Takagishi H, Okuda S, Nishiyama H, Itoh K. J Am Chem Soc. 2005;127:605–613. doi: 10.1021/ja045694g.For studies involving [Co], see: Diercks R, Eaton BE, Gurtzgen S, Jalisatgi S, Matzger AJ, Radde RH, Vollhardt KPC. J Am Chem Soc. 1998;120:8247–8248.
- 9.For Mechanistic discussion of Ni-catalyzed cycloaddition reactions, see: Takahashi T, Tsai F-Y, Kotora M. J Am Chem Soc. 2000;122:4994–4995.Takahashi T, Tsai FY, Li Y, Wang H, Kondo Y, Yamanaka M, Nakajima K, Kotora M. J Am Chem Soc. 2002;124:5059–5067. doi: 10.1021/ja017507+.Eisch JJ, Ma X, Han KI, Gitua JN, Kruger C. Eur J Inorg Chem. 2001:77–88.
- 10.Hsieh J-C, Cheng C-H. Chem Commun. 2008:2992–2994. doi: 10.1039/b801870g. [DOI] [PubMed] [Google Scholar]
- 11.Kumar P, Troast DM, Cella R, Louie J. J Am Chem Soc. 2011;133:7719–7721. doi: 10.1021/ja2007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For ligand screening, see supporting information.
- 13.For ketenimine-nitrile rearrangement, see: Lee K-W, Horowitz N, Ware J, Singer LA. J Am Chem Soc. 1977;99:2622–2627.Kim SS, Liu B, Park CH, Lee KH. J Org Chem. 1998;63:1571–1573.DeKorver KA, Hsung RP, Lohse AG, Zhang Y. Org Lett. 2010;12:1840–1843. doi: 10.1021/ol100446p.Prober M. J Am Chem Soc. 1956;78:2274–2277.
- 14.In most of the cases, dimerization of diyne was observed.
- 15.For cone angle and bite angle values, see: Tolman CA. Chem Rev. 1977;77:313–348.Kamer PCJ, Van Leeuwen PWNM, Reek JNH. Acc Chem Res. 2001;34:895–904. doi: 10.1021/ar000060+.Van Leeuwen PWNM, Kamer PCJ, Reek JNH, Dierkes P. Chem Rev. 2000;100:2741–2769. doi: 10.1021/cr9902704.
- 16.At the suggestion of one of the reviewers, a similar bidentate ligand, DPEphos was evaluated (3 mol% [Ni], 3 mol% DPEphos, toluene, RT). However, only 67% conversion of the diyne and a 9% yield of pyridine was detected by GC after 3 h.
- 17.a) Grigg R, Scott R, Stevenson P. J Chem Soc, Perkin Trans. 1988;1:1365–1369. [Google Scholar]; b) Wakatsuki Y, Yamazaki H. Synthesis. 1976:26–28. [Google Scholar]
- 18.Kase K, Goswami A, Ohtaki K, Tanabe E, Saino N, Okamoto S. Org Lett. 2007;9:931–934. doi: 10.1021/ol070037p. [DOI] [PubMed] [Google Scholar]
- 19.a) Heller B, Sundermann B, Buschmann H, Drexler H-J, You J, Holzgrade U, Heller E, Oehme G. J Org Chem. 2002;67:4414–4422. doi: 10.1021/jo011032n. [DOI] [PubMed] [Google Scholar]; b) Bonaga LVR, Zhang HC, Maryanoff BE. Chem Commun. 2004:2394–2395. doi: 10.1039/b410012c. [DOI] [PubMed] [Google Scholar]; c) Stolley RM, Maczka MT, Louie J. Eur J Org Chem. 2011;20–21:3815–3824. doi: 10.1002/ejoc.201100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.