Abstract
MM is the top indication for high-dose chemotherapy (HDC) with autologous stem cell transplantation (SCT), a strategy which improves progression-free survival and potentially overall survival. Novel induction regimens incorporating the immunomodulatory (IMID) agents thalidomide and lenalidomide, and the proteosome inhibitor bortezomib improve response rates and survival for newly diagnosed patients. Recent data temper enthusiasm for these treatments by illustrating difficulty in some circumstances with mobilizing CD34(+) hematopoietic stem cells for subsequent HDC/SCT. We compare conventional induction regimens with novel-agent based induction strategies and the associated effects on stem cell mobilization and HDC/SCT outcome in 397 patients. Although patients exposed to novel agent inductions collected generally fewer CD34(+) cells than patients induced with chemotherapy, these differences did not translate into adverse consequences with subsequent HDC/SCT. We show that an improvement in overall survival following HDC/SCT may be related to induction therapy with novel agents as opposed to chemotherapy. Our data extrapolate on prior work and expand on ongoing controversies about optimal induction regimens for MM patients planned for subsequent HDC/SCT and optimal sequencing of therapies.
Keywords: Multiple myeloma, stem cell transplant, stem cell mobilization
Introduction
Multiple myeloma (MM) is an incurable plasma cell malignancy with about 20,000 new cases in the United States in 2008 [1]. The recent development of novel agents including thalidomide, lenalidomide, and bortezomib, have revolutionized treatment options and improved response rates for both newly diagnosed and relapse/refractory myeloma patients [2–11].
Despite these advances, MM remains the number one indication world-wide for high-dose chemotherapy (HDC) with autologous stem cell transplantation (SCT) [12]. HDC/SCT confers superior progression-free survival and may also provide overall survival benefit [13–15]. In the pre-IMID (immuno-modulatory drugs), pre-bortezomib era, recognition of impaired hematopoietic (CD34+) stem mobilization following alkylator-based (e.g. melphalan) chemotherapy, promoted wide-spread use of induction regimens with moderate or no negative impact on the ability to collect hematopoietic stem cells [16–21]. Recent reports suggesting that some novel induction regimens may confer deleterious effects on the ability to mobilize CD34+ stem cells for subsequent HDC/SCT have reignited this controversy [22–25]. For instance, Mazumder et al. concluded that patients receiving a lenalidomide-based induction should go on to high-dose cyclophosphamide based mobilization strategy rather than granulocyte-colony stimulating factor (G-CSF) alone [24].
Herein, we summarize our institutional experience in 397 consecutive patients with MM who received either traditional chemotherapy-based induction or induction regimens containing novel agents prior to HDC/SCT. We describe our experience in stem cell mobilization and outcomes following HDC/SCT as a function of patient and disease characteristics, induction and mobilization regimens utilized, and the role of prior radiation therapy. We provide the first data, to our knowledge, supporting the role of pre-mobilization bone marrow plasmacytosis, as a practical and accurate, a priori predictive variable for mobilization success, particularly in patients receiving induction regimens containing novel agents. Additionally, our data extend on prior work by including assessments of novel induction regimens beyond lenalidomide-containing therapies, as well.
METHODS
Patient-specific Variables
We reviewed our institutional experience of patients with MM undergoing HDC/SCT from 1992 to 2009. All investigation was approved by The Ohio State University Cancer Institutional Review Board and Clinical Scientific Review Committee prior to initiation. Patients’ baseline characteristics, details of MM-specific variables, induction therapies, stem cell mobilization and transplantation outcomes were collected from a prospectively maintained electronic database of all patients undergoing SCT at our institution.
Disease-specific Variables
Information was extracted regarding the patients’ disease characteristics including: Durie-Salmon stage, immunoglobulin isotype, serum creatinine, serum beta-2 microglobulin, and serum C-reactive protein at diagnosis. Cytogenetic data by conventional karyotype and/or FISH were also collected. FISH data were stratified by “standard” risk and “high” risk as defined previously [26].
Treatment-specific Variables
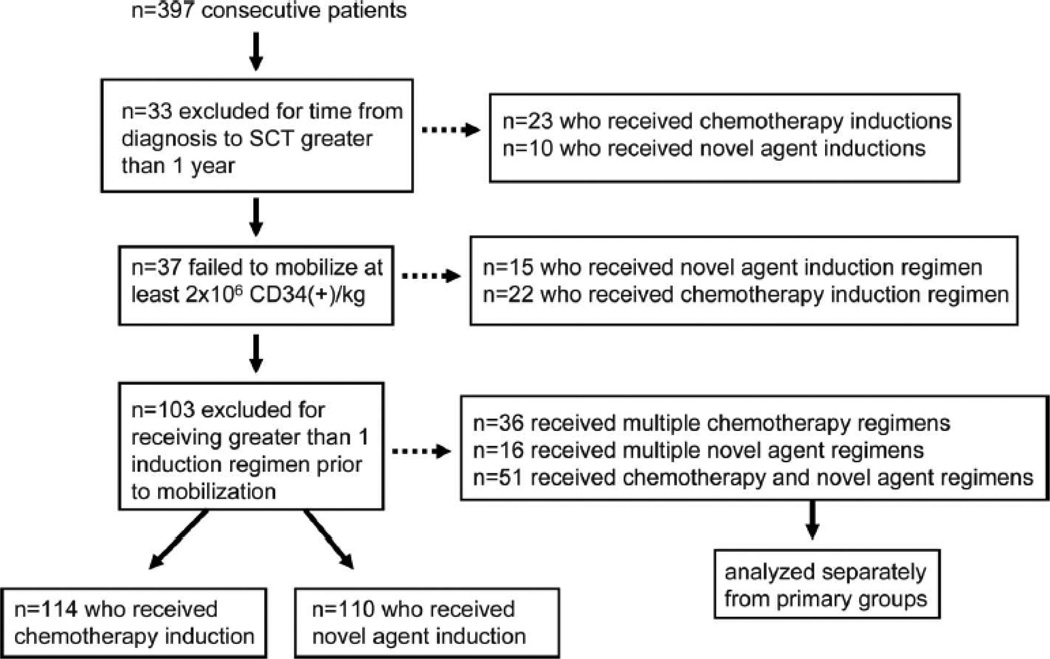
In order to create more homogeneous groups for comparision, only patients who received one induction regimen were considered, and only patients who underwent SCT within a year of diagnosis were included.
Data regarding the induction therapy a patient received were collected and stratified by type: “chemotherapy” (mainly vincristine, doxorubicin, and dexamethasone (VAD) or liposomal doxorubicin, vincristine and dexamethasone (DVD) or “novel therapy” regimen (containing lenalidomide, thalidomide, or bortezomib). The number of cycles of induction treatment were recorded as was the total time from diagnosis to SCT for each patient. Note was made if a patient also received radiation therapy prior to HDC/SCT and, if so, how much bone marrow was in the field.
Regarding mobilization, it is institutional policy to routinely collect sufficient CD34+ cells (i.e., ≥9x106 CD34+ cell/kg patient body weight) in order to administer two rounds of HDC/SCT. (However, tandem autografts were not routinely performed if patients achieved greater than partial response to initial autograft.) Data were extracted regarding mobilization strategy utilized: G-CSF alone, cyclophosphamide-based, or mobilization that included plerixafor (AMD-3100). In order to account for differences in number of collection days across patients, data were collected regarding peak peripheral blood CD34+ count and CD34+ stem cell collection on day 1 only. “Successful” stem cell collection was defined as a total of ≥2x106 CD34+ cell/kg patient body weight in the final product. In regards to HDC/SCT, the preparative regimen used and CD34+ cell dose utilized were also collected.
Outcomes
Patients’ disease status at the time of mobilization was evaluated by standard criteria [27]. Bone marrow aspiration and core biopsy specimen obtained prior to mobilization were reviewed to assess total cellularity and degree of plasmacytosis. Neutrophil engraftment was defined as first of 3 successive days with ANC ≥ 0.5×109/L and ANC > 1.0×109/L after post-transplantation nadir. Platelet engraftment was considered to have occurred on the first of three consecutive days with platelet count 20×109/L or higher, in the absence of platelet transfusion. Progression free survival (PFS) and overall survival (OS) following transplantation as well as cause of death, if applicable, were also recorded.
Statistical considerations
Student’s t-test and ANOVA were used to compare differences in dependent variables of interest as described in the results with statistical significance assigned when the probability of a result was p < 0.05. Chi-square was used to compare remission status at time of mobilization between groups. PFS and OS were analyzed by the Kaplan-Meier method. For data collected on integer or non-ratio scales, appropriate non-parametric statistical analyses were utilized where indicated.
RESULTS
Patient Population
Data on 397 consecutive MM patients undergoing attempted stem cell mobilization at our institution were collected for this study. In limiting our analyses to patients receiving one induction therapy (chemotherapy or a novel regimen) and undergoing HDC/SCT within a year of diagnosis, 224 patients in total were available for inclusion in analysis. Only patients who successfully collected at least 2x106 CD34(+) cells / kg body mass were included in the analyses. Summary demographic and disease-related data on 114 patients who received chemotherapy induction and 110 who received novel therapy induction are presented in Table 1. Patients were well matched in terms of age, gender, Durie-Salmon stage, ISS stage, isotype, and cytogenetics. However, the time from diagnosis to HDC/SCT was significantly different between the groups, p < 0.001. The average time from diagnosis to transplant for patients receiving chemotherapy was 232 (+/− SD 53) days and for patients receiving novel induction regimens was 110 (+/− 82) days.
Table 1.
Patient demographics and disease characteristics
| Induction strategy | ||
|---|---|---|
| Variable | Chemotherapy (n=114) | Novel agent (n=110) |
| Patients: | ||
| Age, years, mean (SD) | 56 (9) | 55 (9) |
| Female, % | 42 | 41 |
| KPS, median: | 90 | 90 |
| Diagnosis to SCT, d, mean (SD) | 232 (53) | 110 (82) |
| Disease: | ||
| ISS stage, med: | 2 | 2 |
| Durie-Salmon stage, %: | ||
| 1: | 9 | 10 |
| 2: | 16 | 13 |
| 3: | 77 | 77 |
| Isotype, %: | ||
| IgG: | 58 | 51 |
| IgA: | 24 | 23 |
| Light chain only: | 13 | 25 |
| IgD: | 5 | 1 |
| Cytogenetics,%: | ||
| Standard risk: | 74 | 74 |
| High risk: | 26 | 26 |
| Radiation, %: | 22 | 18 |
Induction Treatments
Patients included in the analysis received one line of induction therapy, classified as either “chemotherapy-based” or “novel-agent based”. Most patients in the chemotherapy group received either vincristine, adriamycin, and dexamethasone (VAD, n=63) or liposomal doxorubicin, vincristine, and dexamethasone (DVD, n=42). Patients in the novel agent group primarily received bortezomib (V) and dexamethasone (n=33), thalidomide (T) and dexamethasone (n=31), or lenalidomide (R) and dexamethasone (n=21). Some patients in the novel agent group received VTD (n=10) or VRD (n=15). As expected, patients that received chemotherapy-based inductions were primarily treated earlier in the overall sequential cohort (1992 – 2007) whereas patients receiving novel agent inductions received therapy later in the cohort (2004 – 2009). Although the median number of cycles of induction therapy were similar between groups (median = 4 cycles for both groups), a statistically significant difference was observed in time from diagnosis to SCT between groups (232 +/− 53 days for chemotherapy group and 110 +/− 82 for the novel agent group, p < 0.001). 22% of the patients in the chemotherapy group received involved field radiation as part of their induction whereas 18% of the patients receiving novel agent inductions received involved field radiation prior to transplant. Radiation was primarily directed to plasmacytomas and spine in both groups with no patients in either group receiving pelvic or sternal radiation.
At the time of mobilization, a statistically significant difference was observed between the induction groups in terms of disease status (p = 0.004). In the chemotherapy group, 8% of patients were in CR, 70% were in PR and 22% had stable or progressive disease. In the novel agent induction group, 9% were in CR, 85% had achieved PR, and 6% had stable or progressive disease.
All patients in both groups underwent bone marrow aspiration and biopsy prior to stem cell mobilization. We studied total marrow cellularity and marrow plasmacytosis as a function of type of induction therapy received. Patients who received chemotherapy induction had an average total marrow cellularity of 51% (+/−24) and patients who received novel induction regimens had an average total marrow cellularity of 44% (+/−19), p = 0.03. Plasmacytosis was evaluated by core biopsy and marrow aspiration. Patients in the chemotherapy group had an average core plasmacytosis of 20% (+/−25) and patients in the novel agent group had an average core plasmacytosis of 17% (+/−12), p = 0.01. The difference in marrow aspirate plasmacytosis (chemotherapy = 14% +/−19 and novel agent = 10 +/− 8) approached statistical significance, p = 0.07.
Given recent data by other groups suggesting a potentially deleterious effect of novel induction regimens on stem cell mobilization, we compared the pre-mobilization bone marrow findings in successfully mobilized patients exposed to novel agent inductions to those were unable to mobilize to G-CSF (neupogen, filgrastim) alone. We identified n=15 patients exposed to a novel agent induction who failed to mobilize at least 2x106 CD34(+) cells / kg body mass. In this group, the median ISS stage was 2 and median Durie-Salmon stage was 3 and 5 patients had high risk cytogenetics. 4 patients received involved field radiation prior to mobilization attempt. 5 patients received R, 3 received T, 3 received V, and 4 received either VRD or VTD. 9 patients were in PR, 6 had SD. The median number of cycles received prior to mobilization attempt was 4. The average total marrow cellularity was 39% (+/− 24, p = n/s as compared to successfully mobilized novel induction patients), and average core plasmacytosis was 27% (+/−26, p = 0.01 compared to successfully mobilized patients). The average aspirate plasmacytosis was 17% (+/− 17, p = 0.01 compared to successfully mobilized patients).
CD34+ Stem Cell Mobilization Results
To control for differences in the number of collection days experienced by each patient, we limited our analyses to peripheral blood CD34(+) cell count on day 1 of collection and total CD34(+) cells collected on day 1.
Patients who received chemotherapy inductions had an average peripheral blood CD34(+) cell count of 94 (+/− 108) and patients who received novel agent inductions had an average of 54 (+/−50, p = 0.003). Overall, the patients receiving chemotherapy induction regimens collected an average of 10.8 (+/− 12) CD34(+) cells on day 1 whereas the patients receiving novel induction regimens collected an average of 4.7 (+/− 4.9, p = 0.0001). These differences were likely largely accounted for by the difference between the groups in terms of stem cell mobilization strategy. Whereas 82% of patients who received chemotherapy inductions were mobilized with high-dose intravenous cyclophosphamide, 78% of patients who received novel agent inductions were mobilized with G-CSF alone, p < 0.01.
In the chemotherapy group, no correlations were observed between marrow plasmacytosis and peripheral blood CD34(+) cell count or marrow plasmacytosis and CD34(+) collection on day 1. In the novel agent group, however, overall statistically significant inverse correlations were observed between core marrow plasmacytosis and peripheral blood CD34(+) cell count on day 1 (p = 0.02) and day 1 collection (p = 0.02).
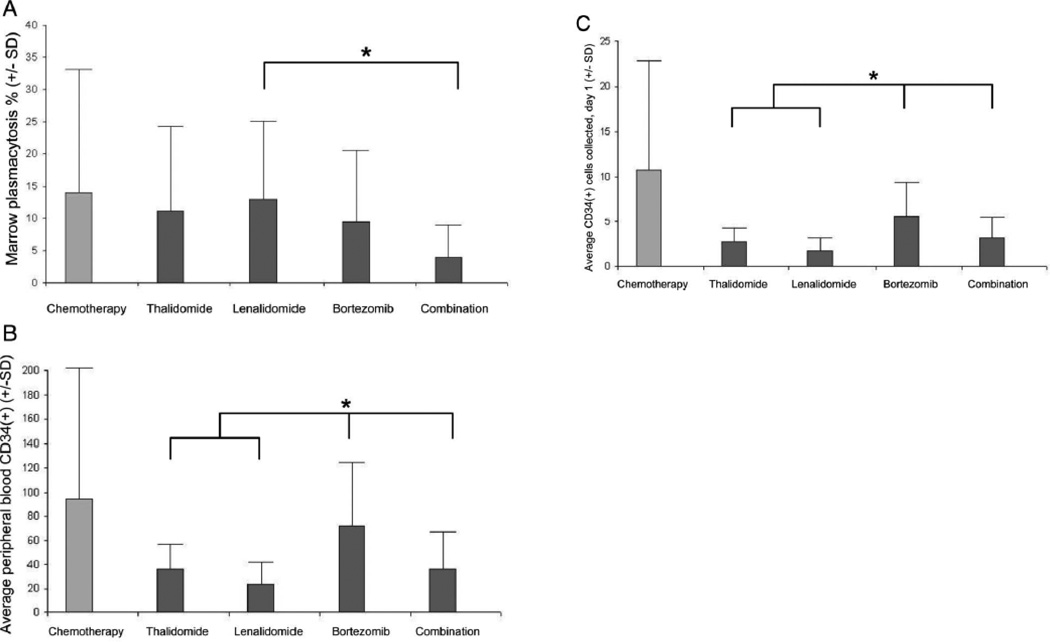
We subdivided patients in the novel agent induction group by type of induction received: thalidomide-based (T), lenalidomide-based (R), bortezomib-based (V), or bortezomib-based combination (VRD/VTD) in order to assess the relationship between pre-mobilization marrow plasmacytosis and stem cell mobilization as a function of specific novel induction strategy utilized. Total marrow cellularity was similar between groups; however, statistically significant differences were observed in pre-mobilization marrow plasmacytosis between groups: T = 11.2 (+/−13), R = 13 (+/−12), V = 9.5 (+/−11), and VTD/VRD = 4 (+/−5, p = 0.048). Planned comparisons showed VTD/VRD was less than R. These differences were associated with differences between peripheral day1 CD34(+) cell count between groups: T = 36 (+/−21), R = 23 (+/−19), V = 72 (+/−53), and VTD/VRD = 36 (+/−31, p = 0.0001). Planned comparisons showed V was greater than T, R, and VTD/VRD. Also, groups differed in mean day 1 CD34(+) cell collection: T = 2.8 (+/−1.5), R = 1.7 (+/− 1.4), V = 5.7 (+/− 3.8), and VTD/VRD = 3.1 (+/−2.5, p < 0.0001). These findings are summarized in Figure 1.
Figure 1.
A. Differences in pre-mobilization marrow plasmacytosis as a function of novel induction regimen utilized. Patients were stratified by the type of novel induction agent received, either thalidomide-, lenalidomide-, bortezomib-, or combination (i.e., VTD or VRD)- based. A significant difference was observed between groups. Planned comparisons revealed that the combination induction regimens led to significantly less residual marrow plasmacytosis than lenalidomide-based induction (*).
Figure 1B. Differences in day 1 peripheral blood CD34(+) cell count as a function of novel induction regimen utilized. Patients were stratified as in Fig 1A. A significant difference was observed between groups and planned comparisons revealed that patients receiving bortezomib-based induction had higher peripheral blood CD34(+) cell counts than others (*).
Figure 1C. Differences in day 1 CD34(+) cell collection as a function of novel induction regimen utilized. Patients were stratified as in Fig 1A. A significant difference in day 1 CD34(+) cell collection was observed between groups and planned comparisons revealed that patients receiving bortezomib-based induction had higher day 1 CD34(+) cell collections than others (*).
Multivariate models to predict peripheral blood CD34(+) cell count as well as day 1 CD34(+) cell collection were constructed utilizing the following predictor variables: induction therapy received (chemotherapy or novel agent), patient age, exposure to radiation prior to mobilization, pre-mobilization core biopsy plasmacytosis, pre-mobilization marrow aspirate plasmacytosis, and type of mobilization utilized (cyclophosphamide-based or G-CSF alone). In both models, only type of mobilization was found to be a statistically significant predictor of collection variables (p < 0.001 as a predictor of peripheral blood CD34(+) cell count and day 1 CD34(+) cell collection).
Similar multivariate analyses were conducted in patients receiving novel agent inductions stratified by specific type of induction received (T, R, or V). As in the models above, patient age, exposure to prior radiation, pre-mobilization plasmacytosis, and mobilization strategy (cyclophosphamide-based or G-CSF alone) were analyzed in addition to type of induction received (T, R, or V). Moblization strategy remained a statistically significant predictor of peripheral blood CD34(+) cell count as well as day 1 collection (p < 0.001 for both peripheral blood CD34(+) cell count and day 1 CD34(+) cell collection). However, in addition, the specific type of novel induction therapy received also was found to be statistically significant.
Transplant Associated Outcomes
No differences were observed in engraftment following SCT between the groups receiving chemotherapy and novel agent induction regimens. There were no instances of graft failure reported in either group and length of stay was similar.
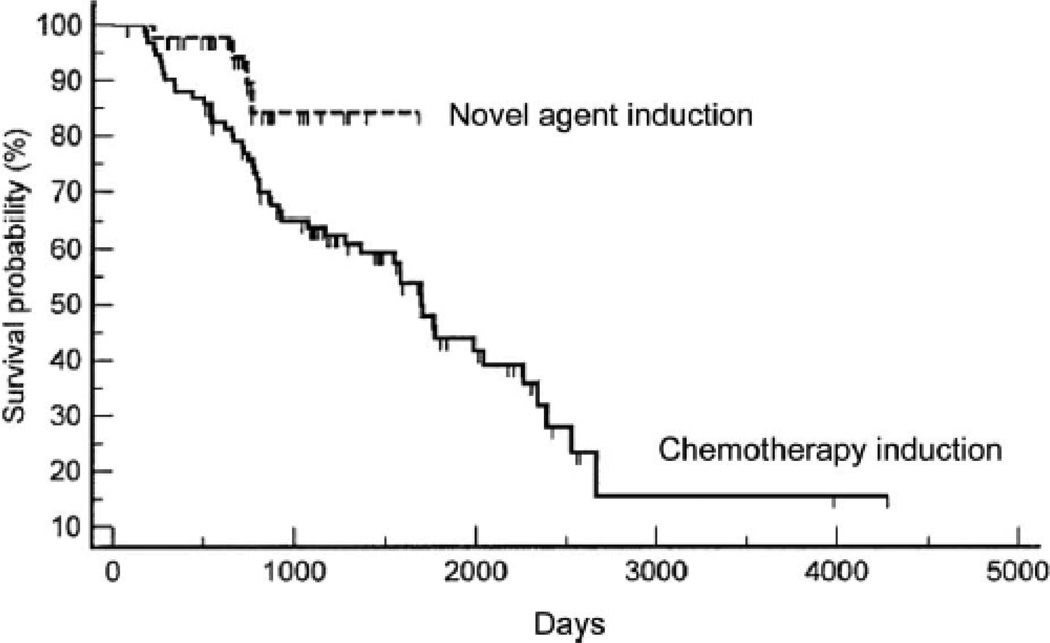
Kaplan Meier curves were plotted for both progression free and overall survival as a function of whether patients received chemotherapy based induction or novel agent induction. No difference was observed in progression-free survival between the groups; however, as shown in Fig 2a statistically significant difference was observed in overall survival following transplant between the groups, with patients receiving novel induction agents (med = not reached) surviving longer than patients who received chemotherapy based inductions prior to HDC/SCT (med = 1672 days, p = 0.03).
Figure 2. Overall survival as a function of induction regimen utilized prior to HDC/SCT.
Patients were assessed for overall survival following HDC/SCT as a function of induction regimen received – chemotherapy-based or novel agent-based. A significant difference was observed between groups (p = 0.03) in favor of the novel agent induction group (med survival = not reached vs med survival = 1672 days for the chemotherapy induction group).
DISCUSSION
The introduction of novel agents including thalidomide, lenalidomide and bortezomib have revolutionized the treatment options for patients with MM [2–5, 9, 13, 28]; however, recent data have tempered this enthusiasm somewhat because of a potential deleterious effect on stem cell mobilization reported with some of these agents [23–25]. This notion has complicated the optimal integration of these agents into a treatment sequence including HDC/SCT and raised questions as to the preferred strategy to mobilize stem cells from patients previously exposed to these therapies.
Findings present herein extrapolate on recent studies in this area regarding lenalidomide by including patients treated with thalidomide and bortezomib based inductions. Using conventional chemotherapy regimens as a comparator to novel induction regimens, we summarize herein our institutional experience in a large sequential cohort of patients with MM undergoing HDC/SCT. Patients receiving chemotherapy-based inductions were well matched to those who received novel agent-based inductions in terms of demographic-related and disease-related variables. Two findings in comparing chemotherapy inductions to novel agent inductions warrant further discussion, however. First, novel agent induction regimens were associated with improved remission rates at time of transplant as compared to chemotherapy inductions. Whereas only 78% of patients who received chemotherapy based induction achieved at least partial remission prior to HDC/SCT, 94% of patients who received novel induction regimens achieved at least partial remission prior to HDC/SCT. Second, despite limiting our analysis to patients who had less than 1 year from diagnosis to HDC/SCT, a statistically significant difference in time to SCT was found between the groups, as well. Patients who received novel induction regimens went onto transplant an average of 4 months sooner than patients who received chemotherapy inductions, despite a comparable median number of cycles between groups. These novel findings serve to reinforce the importance of novel induction strategies compared to chemotherapy inductions in newly diagnosed transplant-eligible patients.
Although patients who received chemotherapy inductions had more robust stem cell mobilization as compared to patients who received novel induction regimens, this finding should be interpreted with caution as most chemotherapy-induced patients received cyclophosphamide mobilizations in our analysis whereas most patients who received novel agent inductions were mobilized with G-CSF alone. Despite this difference, the mean total CD34(+) cell / kg collection for patients receiving novel induction regimens was 6.1 (+/− 4.3) and 80% of patients who received novel induction regimens successfully collected enough stem cells to support two SCTs. 88% of patients who received novel induction regimens were successful in mobilizing stem cells with G-CSF alone in our series. Of the 12% who failed G-CSF mobilization alone on their first attempt, 80% were subsequently collected successfully with a second mobilization attempt (40% with AMD-3100, 33% with cyclophosphamide, and 6% with re-attempt with G-CSF alone). In comparing patients who received novel induction regimens who mobilized successfully to G-CSF alone in their first attempt or not, we found a statistically significant relationship between pre-mobilization bone marrow residual plasmacytosis. This simple a priori evaluation may be clinically useful in predicting which patients will mobilize to G-CSF alone and which may require cyclophosphamide or AMD-3100. Taken together, in our experience, these findings provide some degree of reassurance that most patients receiving novel agent inductions may in fact be mobilized successfully without the addition of toxic (cyclophosphamide) or expensive (AMD-3100) agents.
Perhaps the most provocative finding in our analysis is the statistically significant difference in overall survival observed between patients who received chemotherapy based inductions and novel agent inductions prior to HDC/SCT. The reason for this finding is not entirely clear, but may be related to the better quality of remission in which patients who received novel agent inductions were in prior to mobilization. Most patients in both groups did not routinely receive any maintenance therapy post-SCT.
Several limitations warrant caution in the interpretation and application of our data. First, although our study analyzed consecutive patients, the work was retrospective in nature and should be considered hypothesis-generating. Second, a rigorous interpretation of mobilization success following chemotherapy or novel agent based induction is limited in our dataset as most patients who received chemotherapy inductions also received cyclophosphamide-based mobilizations. This accounted for most of the differences observed between groups in peripheral blood CD34(+) cell count and day 1 CD34(+) collections. Third, the notion that pre-mobilization plasmacytosis as a predictor variable of mobilization success in novel agent induced patients did not hold significance in multivariate analysis and should be considered one potential factor of success among others.
The question of whether novel agents will replace HDC/SCT in the management of patients with MM seems misdirected at least at present. The integration of novel agents into a treatment strategy incorporating HDC/SCT seems most likely to provide the best chances of disease control, duration of remission and quality of life to patients with MM. In our study, that novel agent inductions were associated with better disease control, a shorter time to SCT, and improved overall survival following transplant are encouraging findings that may reconcile some of the concerns regarding the integration of novel agent induction strategies into the HDC/SCT platform of therapy for MM. In our series overall, 98% of patients who received novel agent induction regimens mobilized enough stem cells for at least one HDC/SCT. Opportunities to cryopreserve stem cells and defer HDC/SCT as well as the ongoing evolution of effective, non-toxic maintenance therapies following SCT will likely also provide new avenues to “personalize” care for patients with MM in the future, optimizing outcomes until a cure is discovered.
Acknowledgments
Supported by: American Cancer Society Grant # IRG-67-003-44 (DMB)
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer.J.Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N.Engl.J.Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 3.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N.Engl.J.Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 4.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N.Engl.J.Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 5.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Rosinol L, Hussein M, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J.Clin.Oncol. 2008;26:2171–2177. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Rosinol L, Hussein M, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J.Clin.Oncol. 2008;26:2171–2177. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J.Clin.Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 10.Oakervee H, Popat R, Cavenagh JD. Use of bortezomib as induction therapy prior to stem cell transplantation in frontline treatment of multiple myeloma: impact on stem cell harvesting and engraftment. Leuk.Lymphoma. 2007;48:1910–1921. doi: 10.1080/10428190701540991. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Richardson PG, Sonneveld P, et al. Bortezomib is associated with better health-related quality of life than high-dose dexamethasone in patients with relapsed multiple myeloma: results from the APEX study. Br.J.Haematol. 2008;143:511–519. doi: 10.1111/j.1365-2141.2008.07378.x. [DOI] [PubMed] [Google Scholar]
- 12.Catley L, Anderson K. Strategies to improve the outcome of stem cell transplantation in multiple myeloma. Hematol.J. 2004;5:9–23. doi: 10.1038/sj.thj.6200322. [DOI] [PubMed] [Google Scholar]
- 13.Barlogie B, Jagannath S, Vesole DH, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–793. [PubMed] [Google Scholar]
- 14.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N.Engl.J.Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 15.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N.Engl.J.Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 16.Prince HM, Imrie K, Sutherland DR, et al. Peripheral blood progenitor cell collections in multiple myeloma: predictors and management of inadequate collections. Br.J.Haematol. 1996;93:142–145. doi: 10.1046/j.1365-2141.1996.448987.x. [DOI] [PubMed] [Google Scholar]
- 17.Knudsen LM, Rasmussen T, Jensen L, Johnsen HE. Reduced bone marrow stem cell pool and progenitor mobilisation in multiple myeloma after melphalan treatment. Med.Oncol. 1999;16:245–254. doi: 10.1007/BF02785870. [DOI] [PubMed] [Google Scholar]
- 18.Jansen J, Thompson J, Dugan M, et al. Impaired PBPC collection in patients with myeloma after high-dose melphalan. Cytotherapy. 2004;6:498–504. doi: 10.1080/14653240410005023. [DOI] [PubMed] [Google Scholar]
- 19.de la Rubia J, Blade J, Lahuerta JJ, et al. Effect of chemotherapy with alkylating agents on the yield of CD34+ cells in patients with multiple myeloma. Results of the Spanish Myeloma Group (GEM) Study. Haematologica. 2006;91:621–627. [PubMed] [Google Scholar]
- 20.Boccadoro M, Palumbo A, Bringhen S, et al. Oral melphalan at diagnosis hampers adequate collection of peripheral blood progenitor cells in multiple myeloma. Haematologica. 2002;87:846–850. [PubMed] [Google Scholar]
- 21.Goldschmidt H, Hegenbart U, Wallmeier M, Hohaus S, Haas R. Factors influencing collection of peripheral blood progenitor cells following high-dose cyclophosphamide and granulocyte colony-stimulating factor in patients with multiple myeloma. Br. J.Haematol. 1997;98:736–744. doi: 10.1046/j.1365-2141.1997.2783095.x. [DOI] [PubMed] [Google Scholar]
- 22.Mark T, Stern J, Furst JR, et al. Stem cell mobilization with cyclophosphamide overcomes the suppressive effect of lenalidomide therapy on stem cell collection in multiple myeloma. Biol.Blood Marrow Transplant. 2008;14:795–798. doi: 10.1016/j.bbmt.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paripati H, Stewart AK, Cabou S, et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia. 2008;22:1282–1284. doi: 10.1038/sj.leu.2405100. [DOI] [PubMed] [Google Scholar]
- 24.Mazumder A, Kaufman J, Niesvizky R, Lonial S, Vesole D, Jagannath S. Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia. 2008;22:1280–1281. doi: 10.1038/sj.leu.2405035. author reply 1281-2. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 26.Dispenzieri A, Rajkumar SV, Gertz MA, et al. Treatment of newly diagnosed multiple myeloma based on Mayo Stratification of Myeloma and Risk-adapted Therapy (mSMART): consensus statement. Mayo Clin.Proc. 2007;82:323–341. doi: 10.4065/82.3.323. [DOI] [PubMed] [Google Scholar]
- 27.Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br.J.Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 28.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N.Engl.J.Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]