Abstract
The term “macromolecular crowding” denotes the combined effects of high volume fractions of nominally unrelated macromolecules upon the equilibrium and transport properties of all macrosolutes, dilute as well as concentrated, in the crowded medium. We present a formal partitioning of the total crowding effect into contributions from steric exclusion (excluded volume) and weak, nonspecific attractive interactions between a concentrated “crowding agent” and reactant and product species present at trace concentration. A numerical example of the combined effect of both steric and chemical interactions between crowder and tracer upon the reversible dimerization of tracer is presented, based upon reasonable estimates of the magnitude of both repulsive and attractive interactions between tracer and crowder species.
KEYWORDS/PHRASES: Excluded volume, steric repulsion, weak binding, equivalent hard particle model
INTRODUCTION
The stability, reactivity, and transport properties of dilute proteins and other macromolecules have been found to be significantly affected by the presence of high concentrations of nominally unrelated and presumably unreactive proteins and polymers that occupy a substantial fraction of total solution volume 1–3 . These effects have been referred to as consequences of “macromolecular crowding”. Many effects of crowding upon reaction equilibria involving isomerization or association of dilute “test” macromolecules may be accounted for qualitatively and even semi-quantitatively by simple statistical-thermodynamic models invoking entropic changes resulting from the size- and shape- dependent reduction in solution volume available to the test molecule by the concentrated “background” macromolecules due to mutual impenetrability 4. Although it has long been recognized that long-ranged chemical interactions between background and test molecules may in principle and in certain cases do contribute significantly to crowding effects 5, until recently attention has been focused upon the excluded volume consequences of crowding due to their ubiquity and generality, since excluded volume effects do not depend upon the chemical identity of test and background molecules, but only upon their relative sizes and shapes. However, in recent years additional examples of phenomena that cannot be satisfactorily accounted for on the basis of excluded volume alone have been reported 6–8. These findings emphasize a need to provide a quantitative treatment of chemical interactions between tracer and background molecules to supplement the existing treatment of excluded volume interactions, and hence provide a more comprehensive framework for understanding and interpreting the thermodynamic consequences of macromolecular crowding. In what follows, we shall develop this treatment as it applies to a simple assocation equilibrium. Generalizations to more complex association and isomerization equilibria will become evident as we proceed.
GENERAL STATISTICAL-THERMODYNAMIC CONSIDERATIONS
Consider the specific association of two protein monomers in solution to form a homodimer:
| [1] |
Under a given set of experimental conditions (temperature, pressure, solvent composition), the conversion of two moles of A1 to one mole of A2 in dilute (thermodynamically ideal) solution will result in a change in the Gibbs free energy of the solution amounting to , a quantity referred to as the standard state free energy change or the standard state free energy of the reaction in ideal solution. Under the same set of experimental conditions, the conversion of two moles of A to one mole of A2 in a “crowded” (thermodynamically nonideal) solution containing a concentration cB of a background species will result in a corresponding standard state free energy change . According to the thermodynamic cycle depicted in Figure 1,
Figure 1.
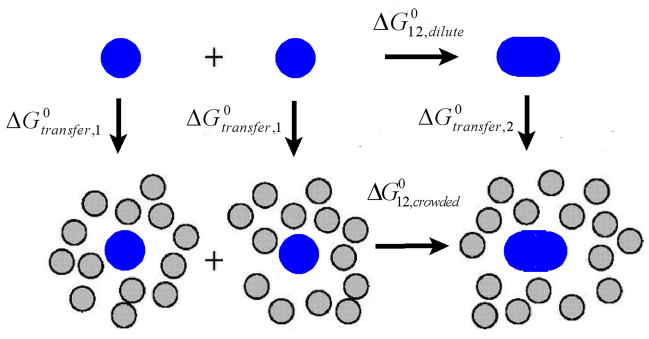
Thermodynamic cycle indicating free energy changes accompanying dimerization in dilute and crowded media, and transfer of monomer and dimer from a dilute to a crowded medium.
| [2] |
where denotes the free energy change accompanying the transfer of one mole of Ai from a fixed position in the ideal dilute solution to a fixed position in the nonideal solution containing concentration cB of the background species B, referred to as the standard state transfer free energy. This quantity is related to the thermodynamic activity coefficient of each species by
| [3] |
It follows from equations [2] and [3] that
| [4] |
where Ko and K denote the equilibrium association constant in the ideal and nonideal solutions respectively. It is stressed that while Ko is truly constant at constant T, P, and solvent conditions, K is subject to variation with cB as shown below. We refer to effect of changing cB upon the equilibrium association constant as the “crowding effect”, and define a quantitative measure of the crowding effect called the “crowding factor” 9:
| [5] |
One may conceptually decompose the process of transferring a molecule of solute species i from an ideal to a nonideal solution into the following steps:
Turn off all chemical interactions in the nonideal solution. This has the effect of turning background molecules into hard particles, and is associated with a free energy change of .
Create a cavity in the hard particle fluid into which a molecule of species i can fit without overlapping any part of any background molecule. This is associated with a purely negentropic free energy change of which is always > 0.
Take a molecule from a fixed location in the dilute solution and place it in the cavity formed in step 2. This step may be accomplished with no free energy change since in the absence of chemical interactions (step 1) there is no interaction between the molecule of species i in the cavity and any background molecule.
Turn on chemical interactions in the nonideal solution, which include interactions between the newly introduced molecule and background molecules and interactions between the background molecules themselves. This process is thus associated with a free energy change of .
Thus the total transfer free energy is
| [6a] |
where
| [6b] |
Thus the thermodynamic activity coefficient may be partitioned into contributions from excluded volume and from chemical interactions
| [7a] |
where
| [7b] |
and
| [7c] |
It is evident that when chemical interactions between the test species i and the background molecules are predominantly attractive ( ) then ln γi < ln γexvoli,i, i.e., attractive chemical interactions can compensate excluded volume effects to a greater or lesser degree.
Equation [7] may be expanded in terms of enthalpic and entropic contributions to the transfer free energy:
| [8] |
where . Equation [8] informs us that a significant dependence of ln γi upon temperature is a hallmark of significant chemical interactions between species i and the background molecules present in solution.
Combination of equations [5] and [7] yields
| [9] |
where
and
A NUMERICAL EXAMPLE BASED UPON SIMPLIFIED STRUCTURAL MODELS AND “REALISTIC” THERMODYNAMIC PARAMETERS CHARACTERIZING ATTRACTIVE INTERACTION BETWEEN TRACE SPECIES AND BACKGROUND MOLECULES
It has been shown that the nonideal behavior of several proteins in highly concentrated and/or crowded solutions may be well described by simple structural models in which globular proteins and other macromolecules are represented by equivalent convex hard particles (see for example 10–12). In what follows we shall present an extension of the equivalent hard particle model to treat the case of significant attractive nonspecific chemical interactions between test molecules and background molecules that can, depending upon their magnitude, attenuate or even override excluded volume effects upon reaction equilibria.
To facilitate numerical computation we shall make the following simplifying assumptions. In reversible dimerization scheme [1] we shall represent the monomer A1 by a sphere of radius r1, and the dimer A2 by a spherocylinder of cylindrical radius r2 = r1 and a cylinder length equal to L times the cylinder diameter. In order for the protein volume to be conserved upon dimerization, L = 2/3. A comparison between this equivalent hard particle model and a more detailed atomic model for the acid dimerization of α-chymotrypsin in shown in Figure 2. It may be seen that for the purpose of calculating volume excluded sterically to molecules of comparable size, the representation of molecular shape by simple convex particles is a reasonably accurate approximation. In addition, we represent the background species as another spherical particle of radius rB = r1. In order to calculate concentrations in molar units, we shall assume that monomer and crowder Bhave molar masses equal to that of α-chymotrypsin, 25,000. The specific excluded volume of all species is taken to be vexc = 1 cm3/g3. It follows that rB = r1 = 21.5 Å, and the surface areas of spherical monomer (s1) and spherocylindrical dimer (s2) are respectively equal to 5755 and 9591 Å2.
Figure 2.
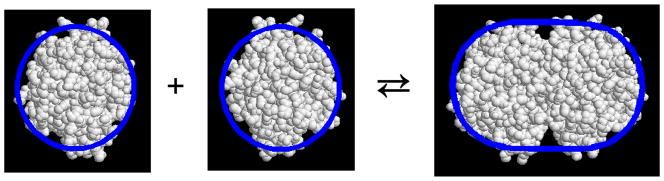
Equivalent convex particles described in text superimposed on molecular models of monomeric and dimeric alpha-chymotrypsin (PDB 4CHA).
Using this structural model, the excluded volume contribution to the free energy of transfer of monomer and dimer from ideal to crowded solution may be estimated using the scaled particle theory of hard particle mixtures 13–15. According to this theory, the negentropic work associated with the insertion of a single hard spherocylinder with cylindrical radius rC and cylindrical axial ratio L into a suspension of hard spheres of radius rB that occupy a fraction φ of total solution volume is given by
| [10a] |
where
| [10b] |
| [10c] |
| [10d] |
| [10e] |
and R = rC/rB. Note that equation [10] also applies to insertion of a sphere of radius rC in this fluid when L = 0.
The contribution of attractive interactions between crowder and test molecule to the free energy of transfer may be estimated by treating such interactions as formally equivalent to weak, unsaturable binding 16. Let i-mer contain nsite,i sites for the binding of background molecule B, each of which can independently “bind”, or attract, B according to the following scheme:
As an illustrative example of weak attractive intermolecular interactions describable as binding, we consider those between urea and an unfolded protein. At a fixed temperature, the dependence of the heat of binding of urea to unfolded ribonuclease A 17 may be well described by a simple independent binding site isotherm
| [11] |
where KA denotes the equilibrium association constant, and cU the molar concentration of urea. The results obtained by Makhatadze and Privalov 17 at multiple temperatures could be accurately described by equation [11] with a temperature-dependent equilibrium association constant given by
| [12] |
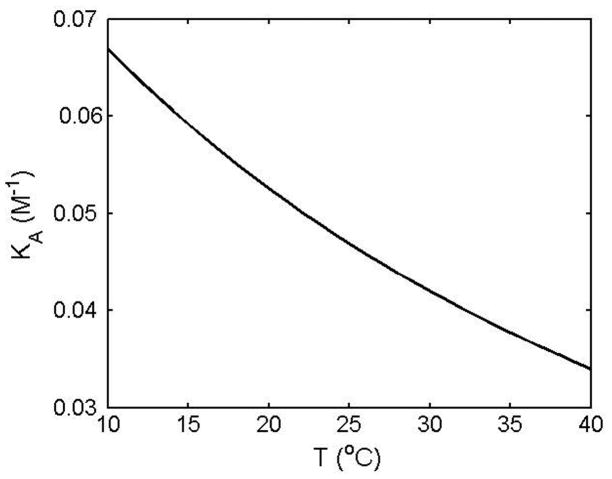
where R denotes the molar gas constant, T the absolute temperature, ΔHo = −2000R and ΔSo = −9.77R. The calculated value of KA is plotted as a function of temperature in Figure 3.
Figure 3.
Temperature dependence of the equilibrium association constant for binding of urea to unfolded ribonuclease A, calculated as described in text.
In the example presented here, we shall assume that weak nonspecific attractive interactions between background species B and i-mer may be approximated by that between urea and unfolded ribonuclease, with the same temperature-dependent site binding constant: KB,i(T)=KA(T). The free energy change associated with the binding of a nonideal ligand L to 1 mole of an ideal substrate S containing n identical and independent binding sites is given by 18
| [12] |
where γL and cL respectively denote the activity coefficient and molar concentration of ligand L. Equation [12] may be generalized to the case of a nonideal substrate 9:
| [13] |
The free energy of binding B to nonideal i-mer is then given approximately by
| [14] |
where . Since we have chosen B to have the same size and spherical shape as monomer, γexvol,B = γexvol,1 and γexvol,B:1 = γexvol,2. We shall in addition approximate the shape of the complex of dimer and B by a spherocylinder with r = r1 and a volume equal to three times that of monomer, such that L = 4/3. (Test calculations indicated that the final results are insensitive to the choice of shape). For the purpose of calculating the value of as a function of T and ϕ, the activity coefficients of monomer, dimer, monomer:B and dimer:B are estimated using equation [10]. Since the number of virtual binding sites for B on i-mer is in general unknown, we make the reasonable approximation that nsites,i is proportional to the surface area of i-mer, so equation [14] simplifies to
| [15] |
where α denotes a temperature-independent constant of proportionality that is equal for monomer and homo-dimer.
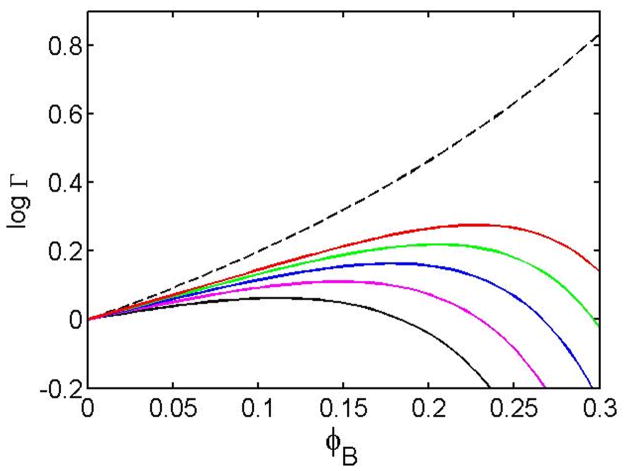
Given equations [10], [15], and the simplified structural and thermodynamic models described above, we may use equation [9] to estimate the values of Γexvol, Γchem, and Γ for dimer formation as a function of ϕB and temperature. In Figure 4, the calculated dependence of ln Γ upon ϕB is plotted for a series of temperatures. For purposes of illustration, the value of α, which scales the magnitude of ln γchem,i was selected so that the temperature dependence of the crowding effect qualitatively resembles that reported for the hetero-association of superoxide dismutase and catalase 6.
Figure 4.
Dependence of the crowding factor for dimerization of trace molecules upon volume fraction of background species B and temperature, calculated as described in text. Dashed curve: calculated for pure excluded volume – no chemical interaction. Solid curves: red, green, blue, magenta, and black curves are calculated for T = 40, 30, 20, 10, and 0°C respectively.
CONCLUSIONS
The example presented above demonstrates that the general statistical-thermodynamic partitioning of total crowding effects into independent contributions from excluded volume (steric repulsion) and weakly attractive interactions can account for reports of temperature-dependent crowding effects 6 and crowding effects at fixed temperature that are substantially smaller, or of opposite sign, than predicted on the basis of excluded volume alone 7,8,19. This is the result of competition between the tendency of nonspecific repulsive interactions to favor reactions that minimize surface exposure (e.g., protein folding and association) and the tendency of nonspecific attractive interactions to favor reactions that maximize surface exposure (e.g., protein unfolding and dissociation). Equation [15] suggests the intriguing but as yet experimentally unobserved possibility that in some systems (such as the numerical example presented here), the nonlinear dependence of γB upon ϕB may may lead to an increasing fractional contribution of chemical interactions to the total crowding effect as ϕB increases. Under these conditions, steric effects would dominate at lower values of ϕB and higher temperatures, so that crowding agents would enhance protein association. However, at higher values of ϕB and lower temperatures, attractive interactions between trace reactants and crowder would dominate, and the crowding agent would inhibit self-association. The combination of the two effects is illustrated in Figure 4.
It is evident that future progress toward accurate interpretation of the observed effects of crowding in a particular medium will require that the free energy of transfer of reactants and products from dilute to the crowded medium be characterized as a function of temperature as well as the composition of the medium. Methods for doing so have been developed 20,21. While preliminary studies aiming toward realization of this goal have been carried out 22,23, much work remains to be done.
Acknowledgments
The author thanks Dr. Peter McPhie (NIH) for helpful comments on a preliminary draft of this report. Research of the author is supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH.
Footnotes
DEDICATION
This work is dedicated to the memory of Professor Henryk Eisenberg, under whose guidance the author was first introduced to the field of physical biochemistry. The interest in obtaining a quantitative understanding of the behavior of biological macromolecules that he ignited in me endures to this day.
References
- 1.Hall D, Minton AP. Biochim Biophys Acta. 2003;1649:127–139. doi: 10.1016/s1570-9639(03)00167-5. [DOI] [PubMed] [Google Scholar]
- 2.Zhou HX, Rivas G, Minton AP. Ann Rev Biophys. 2008;37:375–397. doi: 10.1146/annurev.biophys.37.032807.125817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmerman SB, Minton AP. Ann Rev Biophys Biomol Struct. 1993;22:27–65. doi: 10.1146/annurev.bb.22.060193.000331. [DOI] [PubMed] [Google Scholar]
- 4.Minton AP. Methods in Enzymology. 1998;295:127–149. doi: 10.1016/s0076-6879(98)95038-8. [DOI] [PubMed] [Google Scholar]
- 5.Minton AP. Molecular and Cellular Biochemistry. 1983;55:119–140. doi: 10.1007/BF00673707. [DOI] [PubMed] [Google Scholar]
- 6.Jiao M, Li HT, Chen J, Minton AP, Liang Y. Biophys J. 2010;99:914–923. doi: 10.1016/j.bpj.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillip Y, Sherman E, Haran G, Schreiber G. Biophys J. 2009;97:875–885. doi: 10.1016/j.bpj.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miklos AC, Sarkar M, Wang Y, Pielak GJ. J Am Chem Soc. 2011;133:7116–7120. doi: 10.1021/ja200067p. [DOI] [PubMed] [Google Scholar]
- 9.Minton AP. Biopolymers. 1981;20:2093–2120. [Google Scholar]
- 10.Fernández C, Minton AP. Biophys J. 2008;96:1992–1998. doi: 10.1016/j.bpj.2008.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minton AP. J Pharm Sci. 2007;96:3466–3469. doi: 10.1002/jps.20964. [DOI] [PubMed] [Google Scholar]
- 12.Minton AP. Biophys J. 2008;93:L57–L59. doi: 10.1529/biophysj.107.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boublík T. Mol Phys. 1974;27:1415–1427. [Google Scholar]
- 14.Cotter MA. J Chem Phys. 1977;66:1098–1106. [Google Scholar]
- 15.Lebowitz JL, Helfand E, Praestgaard E. J Chem Phys. 1965;43:774–779. [Google Scholar]
- 16.Hill TL, Chen YD. Biopolymers. 1973;12:1285–1312. [Google Scholar]
- 17.Makhatadze GI, Privalov PL. J Mol Biol. 1992;226:491–505. doi: 10.1016/0022-2836(92)90963-k. [DOI] [PubMed] [Google Scholar]
- 18.Peller L. J Am Chem Soc. 1959;63:1199–1206. [Google Scholar]
- 19.Minton AP. Biophys Chem. 1995;57:65–70. doi: 10.1016/0301-4622(95)00046-z. [DOI] [PubMed] [Google Scholar]
- 20.Fernández C, Minton AP. J Phys Chem B. 2011;115:1289–1293. doi: 10.1021/jp110285b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivas G, Fernández JA, Minton AP. Biochemistry. 1999;38:9379–9388. doi: 10.1021/bi990355z. [DOI] [PubMed] [Google Scholar]
- 22.Fodeke AA, Minton AP. J Phys Chem B. 2010;114:10876–10880. doi: 10.1021/jp104342f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fodeke AA, Minton AP. J Phys Chem B. 2011;115:11261–11268. doi: 10.1021/jp2049266. [DOI] [PMC free article] [PubMed] [Google Scholar]