Abstract
Background
Heart rate and rhythm disturbances are common after cardiac surgery. This study tests the hypothesis that inflammation caused by cardiac surgery is an underlying mechanism for postoperative changes in heart rate (HR), rhythm, and heart rate variability (HRV).
Method and Results
Normal canines (n=6/group) were divided into four groups: 1. anesthesia, 2. sternotomy and pericardiotomy, 3. atriotomy, and 4. corticosteroids combined with an atriotomy. Continuous ECG recordings were done preoperatively and for three post-operative days. Electrophysiologic testing was done at the initial and terminal surgeries. C-reactive protein (CRP) was assessed at each study day and tissue myeloperoxidase activity (MPO) was assessed at the terminal study. Measurements of HRV were determined daily to detect changes in autonomic tone. Postoperatively HR increased in the pericardiotomy (p=0.0005) and atriotomy (p=0.001) groups and HRV decreased in both groups. No significant change occurred in HR or HRV in the anesthesia (p=0.52) and steroid groups (p=0.16). HRV (triangular index) on POD3 was correlated with the tissue MPO levels (r= −0.83, p=0.0004) Autonomic blockade with atropine and esmolol resulted in a HR and HRV which were not significantly different between groups. Atrial premature beats occurred postoperatively in the all the groups except the anesthesia group and were independent of the degree of inflammation.
Conclusion
Cardiac surgery increases postoperative heart rate by reducing heart rate variability, due in most part to a reduction in vagal tone. Furthermore, the magnitude of these changes is dependent upon the degree of inflammation and is normalized by corticosteroids.
Keywords: Heart Rate, Heart Rate Variability, Inflammation, Surgery, Atrial Fibrillation
Background
Cardiac surgery is a complex and invasive procedure. Among the many complications that can occur, the most common involve the electrophysiology of the heart. New onset atrial fibrillation occurs in 30% of patients.1 Heart rate control is significantly altered, including sinus bradycardia, sinus tachycardia, and decreased heart rate variability.2–4 These negative sequela has been shown to be associated with inflammation.5–7 A meta-analysis has shown that corticosteroid prophylaxis reduces the incidence of post operative atrial fibrillation.8 However, it is not clear if the observed effects are caused by inflammation or are simply associated with the inflammation that occurs as result of the surgery. Multiple intraoperative and postoperative surgical interventions could independently affect the electrophysiology and the autonomic nervous system. These include management of fluids and the drugs used to maintain pressure; respiratory management, including ventilation times; renal and metabolic support, including electrolyte and endocrine disturbances; postoperative complications including neurologic, gastrointestinal, and infections; and a patients underlying diseases and medications.9
To test the hypothesis that inflammation caused by cardiac surgery is an independent mechanism for the changes in heart rate and heart rate variability, this study was designed to examine the affect of inflammation on heart rate, rhythm, and variability of anesthesia alone, anesthesia with a thoracotomy and pericardiotomy, both of these combined with an atriotomy, and in an atriotomy treated with corticosteroids.
Methods
Surgical Preparation
All animals received humane care in compliance with the Principles of Laboratory Animal Care, formulated by the National Society for Medical Research, and the Guide for the Care and Use of Laboratory Animals, prepared by the National Academy of Science and published by the National Institutes of Health (NIH publication 86–23, revised 1985). The Animal Studies Committee of the Washington University School of Medicine approved this study protocol. Twenty-four adult mongrel canines weighing between 25 and 30 kg were divided randomly into 4 groups: (1) anesthesia alone (n=6); (2) median sternotomy and pericardiotomy (n=6); (3) 5-cm lateral right atriotomy(n=6); and (4) 5-cm lateral right atriotomy with administration of methylprednisolone (2 mg/kg per day) for 1 week before atriotomy until the terminal study. All animals were anesthetized with intravenous propofol (5 to 7 mg/kg), intubated with a cuffed endotracheal tube, and mechanically ventilated with a pressure-controlled ventilator. An adequate level of anesthesia was maintained by inhaled isoflurane (1% to 3%). A limb-lead ECG was monitored. A femoral artery catheter was inserted to monitor systemic arterial pressure continuously. Arterial blood samples were drawn every 30 minutes to determine arterial oxygen tension, acid-base balance, and electrolyte levels. The total anesthesia time for all animals was standardized at 4 hours.
A median sternotomy was performed in the pericardiotomy, atriotomy, and anti-inflammatory groups. To mimic cardiac surgery, the pericardium was opened, and the right atrium was exposed to air for 1 hour. In the atriotomy and anti-inflammatory groups, a lateral right atrial incision (5 cm) was made with the use of the closed heart technique without the use of cardiopulmonary bypass, as described previously.10 No major atrial arterial branches were divided by the atrial incision. The chest was closed. The animals were given cefazolin sodium 20 mg/kg, for 3 days postoperatively. Three days after the initial surgery, each animal was anesthetized again with intravenous propofol (5 to 7 mg/kg), intubated with a cuffed endotracheal tube, and mechanically ventilated. Anesthesia was maintained by inhaled isoflurane (1% to 3%). The ECG and systemic arterial pressure were monitored continuously. Arterial blood samples were drawn every 30 minutes to determine arterial oxygen tension, acid-base balance, and electrolyte levels and normalized. The terminal study was performed on POD3, since this represents the day on which the peak incidence of arrhythmias occur in patients.1
Electrophysiological Studies
All animals had continuous 24 hour ECG recordings (Midmark Diagnostics Group, Gardena, CA) for all four days of the study. All data were manually reviewed to confirm and correct the classification of beats. Heart rate variability measurements were made according to published standards.11 The number of spontaneous premature atrial depolarizations (PAD) were determined for each day of the study. On POD2 each animal was treated with Esmolol (0.5 mg/kg iv loading dose 0.2 mg/kg/min iv for 20 min), Atropine (0.04 mg/kg iv), and a combined dose of Esmolol+Atropine to completely block the sympathetic or parasympathetic nerves or both. Holter data were analyzed for heart rate variability for 20 minutes before drug administration and for 20 minutes after treatment. Between each drug the animal was allowed to recover for one hour. At the start of each study, a bipolar catheter was introduced into the RA via the femoral vein. The sinus node recovery time (SNRT) was determined by over drive pacing for 60 seconds at 10 beats per minute (bpm) above the sinus rate. The SNRT was the first interval after termination of pacing. The sino-atrial conduction time (SACT) was determined by pacing for eight beats at 5 bpm above the sinus rate. The return cycle was then measured. The SACT was calculated by subtracting the average pre-paced cycle length from the return cycle length. Except for the anesthesia group these data were taken in the open chest dog. A bolus dose of isoproterenol was given (3 μg/kg iv) and electrograms were recorded for two minutes to determine the maximum heart rate response. This was then repeated with an adenosine bolus (12 mg/kg iv). This protocol was repeated at the terminal study on POD 3.
Myeloperoxidase Activity
Myeloperoxidase activity in the right atrial myocardium medial to the incision, including sinus node, was excised and frozen at the end of the study to quantify the degree of inflammation. In the animals without an atriotomy the sample was taken from the same site in the RA. Quantitative myeloperoxidase activity of the atrial tissue was determined as previously described.12
C-reactive Protein
Blood was collected each study day for determination of the serum CRP levels. C-reactive protein was measured by immunoassay using the Tridelta Phase™ range CRP - Canine Assay (Tridelta Development Ltd., Maynooth, Ireland).
Statistical Analysis
All continuous values were expressed as mean±1 SE. All data were checked for normality (Schapiro-Wilks test) and equality of variance (Bartlett's test). If needed, a log transform was done, and the data were retested for normality and equality of variances. Multigroup data were compared using ANOVA. Post hoc multiple comparisons were made with Tukey's HSD technique. ERP data were compared with the repeated ANCOVA model with one factor. Post hoc multigroup comparisons were made by contrasts with a Dunn-Sidak correction. Only the AF duration data failed the normality and equality of variance tests after the log transformation and were compared with the Kruskal-Wallis test. A Sidak correction was used to control the type I error for multiple comparisons. Correlation coefficients were calculated by the Spearman rank method. A significance level of 0.05 was considered statistically significant. All calculations were made with the use of SYSTAT version 13 (SYSTAT Software Inc).
Results
Electrophysiologic Studies
Table 1 summarizes the effects of the four experimental groups on heart rate, SACT, SNRT, and the heart rate response with isoproterenol and adenosine prior to surgery and at the terminal study on POD 3. All data were taken with the animal under anesthesia. Isoproterenol caused spontaneous ventricular fibrillation in first two animals treated with steroids (not included in the study). Drug challenges or blockade were not done in the steroid group. Anesthesia alone shortened SACTPAD. The HR increased in the steroid group between the control and terminal study. There were no other significant differences in the preoperative and POD 3 spontaneous HR, SACT, or SNRT. There was no difference between the preoperative and POD3 HR response to any of the drug boluses. (p=0.45) There was also no difference in the HR response between the three groups. (p=0.097)
Table 1.
Summary of Electrophysiologic Studies
| Anesthesia | Pericardiotomy | Atriotomy | Steroid | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
|
| ||||||||
| HR (bpm) | 118±10 | 134±9 | 119±10 | 132±5 | 118±6 | 113±13 | 118±5 | 125±3 |
| SNRT (ms) | 624±49 | 523±32 | 626±77 | 538±26 | 603±36 | 563±23 | 568±32 | 562±32 |
| SACT(ms) | 43±6 | 35±6* | 43±5 | 43±5 | 44±7 | 55±56 | 47±8 | 53±10 |
|
| ||||||||
|
Heart Rate Response to Drug
| ||||||||
| Isoproterenol (bpm) | 222±10 | 223±8 | 210±10 | 196±8 | 211±10 | 207±24 | - | - |
| Adenosine (bpm) | 106±9 | 127±8 | 111±6 | 111±6 | 111±3 | 109±7 | - | - |
| Iso+Aden (bpm) | 207±10 | 214±12 | 204±10 | 182±11 | 206±5 | 186±21 | - | - |
HR, SNRT, SACT, and duration expressed as mean ± sem; Atrial premature Depolarization expressed as median and range. Post vs pre,
p<0.05,
p<0.001.
Spontaneous Premature Atrial Depolarizations
Figure 1 shows the average number of PADs that occurred in each group for each day. Preoperatively they were rare. The number of PADs did not increase from control in the anesthesia group (p=0.68). The number of PADs increased from preoperative levels in the pericardiotomy (p=0.0002), atriotomy (p=0.0096), and steroid groups (p=0.0085). There was no difference between the pericardiotomy, atriotomy, or steroid groups (p=0.47).
Figure 1.
The average number of PADs in a 24 hour period is shown for each day of the study for the four experimental groups. The p values for the time effect and group effect are p<0.001and p=0.028 respectively.
Spontaneous Heart Rate
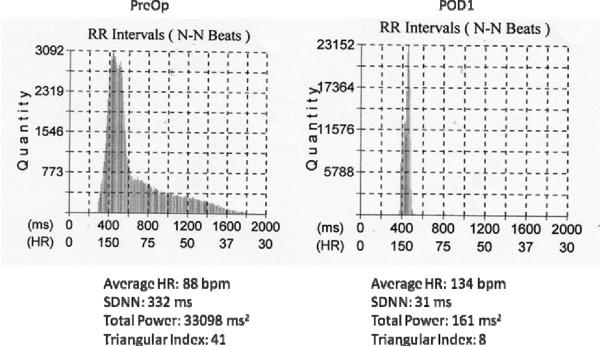
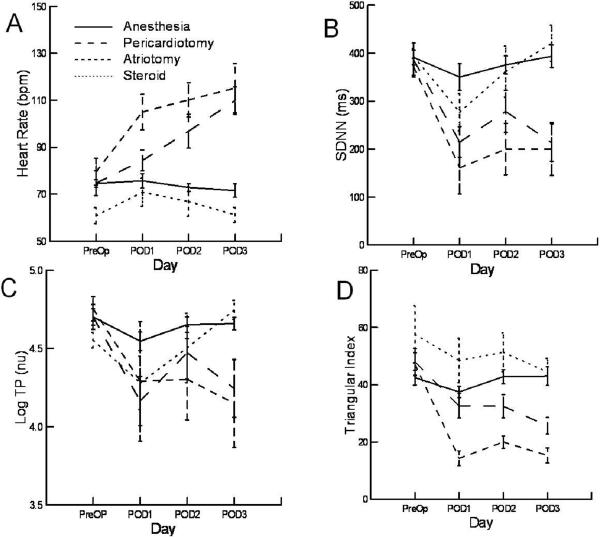
An example of the effect of an atriotomy on heart rate over a 24 hour period is illustrated in Figure 2. The tracing shows the 5 minute average heart rate over 24 hours. The upper panel shows the control preoperative recording and the lower panel the first post operative day. Figure 3 shows the histogram of RR intervals for the two recordings in Figure 2. There is an increase in the average rate and a loss in heart rate variability. Figure 4 quantify these by the experimental groups over time. Table 2 summarizes the significant changes. There were no differences in average heart rate preoperatively between the anesthesia, pericardiotomy, and atriotomy groups. (p=0.63) However, the group that had been treated with steroids for the week prior to surgery had a lower control rate (p=0.014). Compared to control, the average heart rate increased in the pericardiotomy (p=0.0005) and atriotomy (p=0.001) groups. There was no significant change in the heart rate in the anesthesia (p=0.52) and steroid groups (p=0.16). The maximum and minimum heart over 24 hours decreased and increased respectively compared to control in the pericardiotomy and atriotomy groups. There were no changes over time in the anesthesia or steroid groups. Time domain measures of variability showed no decrease in the anesthesia group compared to the preoperative values. The geometric and frequency measures show a small decrease in variability on POD1, which resolves by POD2. In the pericardiotomy group multiple domain measures of variability decreased from PreOp through POD2. Even more measures were decreased and to a greater degree and duration in the atriotomy group. With steroids there were almost no significant differences in any of the measures of variability in the post operative period compared to the control data.
Figure 2.
Examples of the heart rate trend for an animal from the atriotomy group is shown on the Preop day (upper panel) and on the POD1 (lower panel).
Figure 3.
Histograms are shown for the normal beat RR intervals derived from the heart rate trend graphs shown in figure 2. Below each histogram is the average HR, SDNN, Total Power, and Triangular Index.
Figure 4.
Panel A shows the average heart rate over a 24 hour period for each day of the study for the four experimental groups.. Panels B–D show the trends for three measures of heart rate variability, SDNN, logTP, and triangular index for each study day and each experimental group. The p values for the time effect and group effect for panels A–D are: A: p<0.001,p<0.001; B: p<0.001, p=0.001; C: p<0.001, p=0.026; D: p<0.001,p<0.001.
Table 2.
Summary of Heart Rate Variability
| Anesthesia | Pericardiotomy | Atriotomy | Steroids | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Time Domain | |||||||||
|
| |||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
|
| |||||||||
| HR | Preop | 75.00 | 1.51 | 75.00 | 4.78 | 80.00 | 5.14 | 61.00 | 3.02 |
| POD 1 | 76.00 | 2.74 | 84.00 | 9.90 | 105.00 | 6.86 * | 71.00 | 5.43 | |
| POD 2 | 73.00 | 1.43 | 97.00 | 16.00 * | 110.00 | 6.65 * | 67.00 | 5.67 | |
| POD 3 | 72.00 | 2.57 | 110.00 | 12.80 * | 115.00 | 9.43 * | 61.00 | 2.90 | |
|
| |||||||||
| HR min | Preop | 34.00 | 0.94 | 35.00 | 3.80 | 31.00 | 3.92 | 32.00 | 0.57 |
| POD 1 | 35.00 | 1.18 | 49.00 | 6.60 * | 63.00 | 8.12 * | 37.00 | 3.84 | |
| POD 2 | 30.00 | 2.98 | 42.00 | 8.10 * | 53.00 | 7.06 * | 35.00 | 2.98 | |
| POD 3 | 31.00 | 3.47 | 53.00 | 5.90 * | 68.00 | 8.57 * | 34.00 | 1.22 | |
|
| |||||||||
| HR max | Preop | 189.00 | 6.08 | 185.00 | 21.70 | 184.00 | 4.12 | 155.00 | 7.23 |
| POD 1 | 180.00 | 1.96 | 150.00 | 15.20 * | 149.00 | 5.63 * | 159.00 | 10.70 | |
| POD 2 | 196.00 | 3.67 | 191.00 | 7.80 | 172.00 | 8.45 | 148.00 | 7.72 | |
| POD 3 | 187.00 | 7.06 | 196.00 | 11.10 | 173.00 | 10.08 | 144.00 | 10.17 | |
|
| |||||||||
| SDNN | Preop | 391.00 | 13.88 | 386.00 | 78.00 | 372.00 | 15.92 | 393.00 | 14.70 |
| POD 1 | 350.00 | 25.31 | 215.00 | 70.00 * | 161.00 | 49.40 * | 277.00 | 34.70 * | |
| POD 2 | 376.00 | 17.15 | 278.00 | 97.00 | 200.00 | 48.17 * | 362.00 | 47.77 | |
| POD 3 | 394.00 | 20.82 | 213.00 | 86.00 * | 200.00 | 49.40 * | 434.00 | 30.21 | |
|
| |||||||||
| SDANN | Preop | 197.00 | 12.66 | 170.00 | 39.00 | 164.00 | 13.47 | 196.00 | 17.15 |
| POD 1 | 178.00 | 11.43 | 98.00 | 23.00 * | 78.00 | 15.11 * | 152.00 | 14.29 | |
| POD 2 | 199.00 | 15.92 | 133.00 | 36.00 | 111.00 | 26.13 | 148.00 | 13.88 | |
| POD 3 | 184.00 | 14.70 | 107.00 | 33.00 | 80.00 | 18.78 * | 149.00 | 16.33 | |
|
| |||||||||
| RMSSD | Preop | 404.00 | 23.27 | 415.00 | 89.00 | 402.00 | 20.00 | 465.00 | 27.76 |
| POD 1 | 381.00 | 35.11 | 266.00 | 106.00 | 205.00 | 71.04 * | 356.00 | 59.60 | |
| POD 2 | 398.00 | 19.60 | 332.00 | 140.00 | 216.00 | 57.15 * | 532.00 | 94.31 | |
| POD 3 | 408.00 | 31.03 | 243.00 | 130.00 | 252.00 | 66.54 | 655.00 | 65.73 | |
|
| |||||||||
| Geometric Domain | |||||||||
|
| |||||||||
| TI | Preop | 43.00 | 2.45 | 48.00 | 4.08 | 47.00 | 3.67 | 64.00 | 9.80 |
| POD 1 | 38.00 | 1.63 * | 33.00 | 3.67 | 14.00 | 2.45 * | 49.00 | 6.94 | |
| POD 2 | 43.00 | 2.04 | 33.00 | 3.67 * | 20.00 | 2.04 * | 51.00 | 6.12 | |
| POD 3 | 43.00 | 2.86 | 26.00 | 2.45 * | 15.00 | 2.45 * | 45.00 | 4.08 | |
|
| |||||||||
| Frequency Domain | |||||||||
|
| |||||||||
| log TP | Preop | 4.701 | 0.047 | 4.701 | 0.071 | 4.753 | 0.071 | 4.552 | 0.044 |
| POD 1 | 4.546 | 0.053 * | 4.164 | 0.142 * | 3.803 | 0.402 * | 4.280 | 0.154 | |
| POD 2 | 4.652 | 0.045 | 4.571 | 0.156 | 4.087 | 0.285 | 4.627 | 0.202 | |
| POD 3 | 4.659 | 0.036 | 4.244 | 0.166 | 4.003 | 0.390 | 4.746 | 0.055 | |
|
| |||||||||
| log HF | Preop | 4.513 | 0.045 | 4.519 | 0.072 | 4.573 | 0.070 | 4.368 | 0.045 |
| POD 1 | 4.369 | 0.054 * | 3.982 | 0.143 * | 3.604 | 0.408 * | 4.098 | 0.155 | |
| POD 2 | 4.472 | 0.047 | 4.382 | 0.157 | 3.907 | 0.287 | 4.445 | 0.201 | |
| POD 3 | 4.479 | 0.036 | 4.059 | 0.165 | 3.810 | 0.400 | 4.566 | 0.054 | |
|
| |||||||||
| log LF | Preop | 4.086 | 0.051 | 4.059 | 0.073 | 4.138 | 0.070 | 3.925 | 0.048 |
| POD 1 | 3.926 | 0.049 * | 3.542 | 0.143 * | 3.171 | 0.418 | 3.659 | 0.156 | |
| POD 2 | 4.021 | 0.040 | 3.946 | 0.152 | 3.440 | 0.284 | 4.010 | 0.208 | |
| POD 3 | 4.027 | 0.038 | 3.611 | 0.170 | 3.350 | 0.414 | 4.131 | 0.058 | |
|
| |||||||||
| log VLF | Preop | 3.740 | 0.058 | 3.761 | 0.071 | 3.744 | 0.078 | 3.587 | 0.036 |
| POD 1 | 3.527 | 0.056 * | 3.174 | 0.133 * | 2.888 | 0.371 | 3.287 | 0.149 | |
| POD 2 | 3.669 | 0.051 | 3.625 | 0.167 | 3.129 | 0.275 | 3.632 | 0.189 | |
| POD 3 | 3.683 | 0.040 | 3.299 | 0.162 | 3.089 | 0.329 | 3.739 | 0.054 | |
|
| |||||||||
| log ULF | Preop | 3.256 | 0.099 | 3.291 | 0.097 | 3.113 | 0.083 | 3.090 | 0.040 |
| POD 1 | 2.851 | 0.077 | 2.547 | 0.115 | 2.237 | 0.285 | 2.600 | 0.131 * | |
| POD 2 | 3.040 | 0.082 | 3.123 | 0.215 | 2.618 | 0.254 | 3.038 | 0.187 | |
| POD 3 | 3.154 | 0.070 | 2.778 | 0.165 | 2.581 | 0.257 | 3.123 | 0.156 | |
|
| |||||||||
| LF/HF | Preop | 0.375 | 0.009 | 0.348 | 0.005 | 0.367 | 0.002 | 0.361 | 0.002 |
| POD 1 | 0.361 | 0.008 | 0.363 | 0.004 | 0.370 | 0.012 | 0.364 | 0.002 | |
| POD 2 | 0.355 | 0.010 | 0.367 | 0.014 | 0.342 | 0.010 | 0.368 | 0.010 | |
| POD 3 | 0.353 | 0.005 | 0.356 | 0.004 | 0.348 | 0.013 | 0.368 | 0.007 | |
POD data are statistical different from Preop values (p<0.05). HR: Average heart rate over 24 hours; HRmin and HRmax: The minimum and maximum five minute average heart rate over a 24 hour period; SDNN: Standard deviation of all NN(normal-normal) intervals: SDANN: Standard deviation of the averages of NN intervals in all 5-minute segments over 24 hours; RMSSD: The square root of the mean of the sum of the squares of differences between adjacent NN intervals; TI: Triangular Index; TP: Total Power, <0.4 Hz; HF: Power in high frequency interval, 0.15–0.4 Hz; LP: Power in low frequency interval, 0.04–0.15 Hz; VLF: Power in very low frequency interval, 0.003–0.04 Hz; ULF: Power in ultra low frequency interval, <0.003 Hz.11
Sympathetic and Parasympathetic blockade
The change of heart rate following autonomic blockade by experimental group is shown in figure 5. There was a significant group effect in the response to atropine (p=0.003) and atropine plus esmolol (p=0.001). There was no group effect for esmolol alone (p=0.12). For the pericardiotomy (p=0.004) and atriotomy (p=0.002) groups atropine had a significantly smaller (~50%) effect than it did in the anesthesia group. There was no difference in the effect of atropine between the pericardiotomy and atriotomy groups (p=0.82). For atropine combined with esmolol there were similar effects between the groups (p=0.001). The pericardiotomy (p=0.01) and atriotomy (p=0.0003) groups had a significantly smaller effect than the anesthesia group. The difference between the pericardiotomy and atriotomy groups (p=0.09) trended towards a difference. In the atriotomy group, the combination of atropine and esmolol did not change heart rate (pre: 119±9 bpm, post: 120±12bpm, p=0.90). Esmolol, atropine, and the combination all significantly reduced HF and LF power (p<0.001). Esmolol reduced both the HF and LF by a factor of 10 whereas atropine and the combination of drugs reduced the HF and LF by a factor of 100. However there were no significant differences in HR, HF, and LF following complete blockade between the experimental groups (p=0.49, 0.47, and 0.45 respectively).
Figure 5.
The change in heart rate with autonomic blockade is shown for the Anesthesia, Pericardiotomy, and atriotomy group on POD3. Data were recorded in the conscious dog given Atropine, Esmolol, or the combination of both drugs. The p values for the drug and group effect are p<0.001 and p<0.001 respectively.
CRP and MPO levels
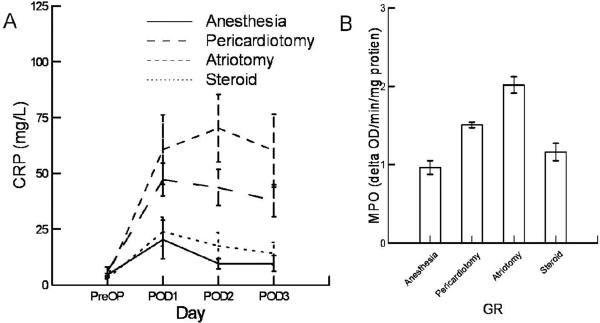
There was a significant difference in CRP levels (figure 6A) between groups (p<0.00001) and over time (p<0.0001).The CRP levels of the pericardiotomy (p=0.045) and atriotomy (p=0.002) were significantly higher than the anesthesia group. The anesthesia group was not different from the steroid group (p=0.83). The levels of right atrial MPO activity on postoperative day 3 (Figure 6b) were significantly different (p<0.0001). The pericardiotomy (p=0.0001) and atriotomy (p<0.0001) groups were greater than the anesthesia group. The MPO activity of the atriotomy group was greater than the pericardiotomy group (p=0.002). The MPO activity levels of the steroid group trended higher than the anesthesia group (p=0.09) and were significantly lower than the pericardiotomy (p=0.006) and atriotomy (p<0.0001) groups.
Figure 6.
Panel A shows the serum concentration of CRP for the four days of the study for the four experimental groups. The p values for the time effect and group effect are p<0.001and p<0.001 respectively. Panel B shows the MPO activity for each of the four groups on POD3. The levels were significantly different (p<0.001). The anesthesia group was different from the pericardiotomy (p=0.001) and atriotomy (p<0.001) groups. The anesthesia and steroid group were not significantly different (p=0.034). The steroid group was different from the atriotomy (p<0.001) and pericardiotomy group (p=0.032).
Relationship between MPO and CRP on Electrophysiology
Serum CRP levels correlated with tissue MPO levels on POD3 (r=0.81, p=0.0001). The heart rate variability (Figure 7) as measured by the Triangular Index (TI) on POD3 was correlated with the RA tissue MPO levels (r=−0.83, p=0.0004) Average heart rate on POD3 also correlated with tissue MPO levels (r=0.59, p=0.03). The Average daily heart rate and variability (TI) correlated with daily CRP levels (r=0.53, p<0.001 and r=−0.57, p<0.001 respectively).
Figure 7.
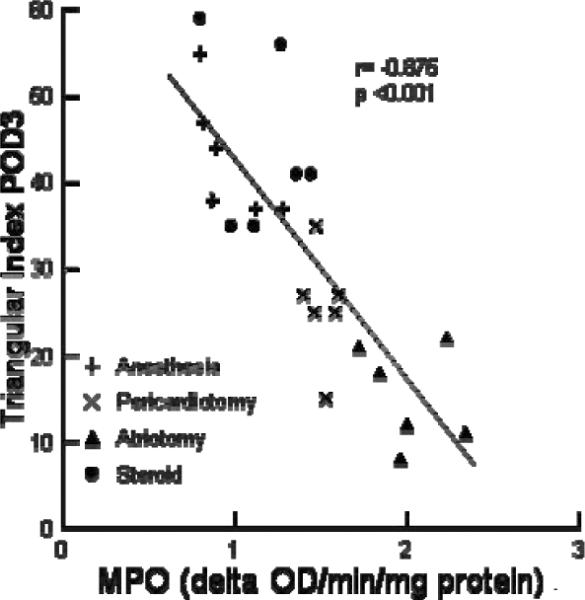
The relationship between MPO activity and the Triangle Index is shown. Different symbols represent the different experimental groups.
Discussion
Inflammation, in this canine model of cardiac surgery, reduces heart rate variability in a dose dependent fashion in conscious dogs. Steroids reduce the inflammation and normalize heart rate and heart rate variability. Both the atriotomy group and the steroid groups had the identical surgery interventions, except for treatment with steroids. This suggests that the changes are caused by inflammation. The reduction in heart rate variability caused a net increase in heart rate. This is mainly caused by an increase in the average minimum heart rate (HRmin, Table 2) reflecting a decrease in vagal tone. HRmin increased in the inflamed groups but is normal in the steroid group. The loss of variability and the increase in the HRmin is dramatically illustrated in Figures 2 and 3. The reduction in vagal tone caused by inflammation is further confirmed by the reduction in vagal measures of heart rate variability (Table 2) and in the response to atropine (Figure 5). Sympathetic tone is affected to a lesser degree than parasympathetic tone, increasing with increasing inflammation. Combined blockade with both atropine and esmolol shows that in the anesthesia group, the animals have high vagal tone. However, in the most inflamed group with an atriotomy, the sympathetic and parasympathetic effects are balanced such that, with blockade, there is no change in heart rate.
There was a significant correlation between the degree of inflammation and the reduction of heart rate variability. There was a correlation between the TI and tissue MPO (Figure 7) activity and with serum CRP levels. The correlation was highest for the tissue MPO activity suggesting that if the effects were primarily in the nerve tissue that it was most affected at the terminal end of the sympathetic and parasympathetic nerves or in the intrinsic nerves and ganglia on the heart. During anesthesia, there were no differences in any of the four experimental groups between preoperative day and postoperative day 3 heart rates or heart rate variability. Pre and post operative heart rate response to direct stimulation with isoproterenol and adenosine were also not significantly different. SACT and SNRT were also not different. Because isoflurane blocks most sympathetic and parasympathetic activity these measurement approximate the intrinsic function of the sinus node.13 Complete blockade with atropine and esmolol resulted in a HR and HRV which were not significantly different between groups. Taken together, these data also suggest that the surgical interventions which mediate changes in heart rate are mediated in the CNS, or within the intrinsic nervous system of the heart, and are not mediated by changes in the sinus node or autonomic receptors.
Anesthesia did not cause any increase in spontaneous PADs on any postoperative day. (Figure 1) Pericardiotomy and atriotomy caused an increase on POD1 and POD2. Steroids did not reduce significantly the premature depolarizations observed. This suggests that the spontaneous atrial premature beats seen in this study were not mediated by inflammation. It is unclear what the mechanism is for generation of spontaneous PADs in this model. Whatever the mechanism, it appears to be independent of inflammation. Interestingly steroids did sensitize the ventricles to isoproterenol causing ventricular fibrillation. It caused fibrillation prior to surgery and was also independent of inflammation. Rats pretreated with steroids also have been shown to go into ventricular fibrillation with beta adrenergic stimulation.14 This may suggest caution in the use of beta adrenergic stimulation in the presences of high dose steroids.
The data of this study also demonstrates that the dogs are very vagal. In the least inflamed group (anesthesia alone), atropine in the conscious dog increased heart rate by 100 bpm, whereas esmolol only decreased the heart rate by less than 10 bpm. This is similar to humans. When normal conscious individuals were given atropine heart rate increased by 50 bpm whereas propranolol decreased it by about 10 bpm.15 Although the magnitude is greater in dogs, the dominance of the vagal tone is apparent in both species. Humans also demonstrate a decrease in heart rate variability with cardiac surgery and inflammation.3 Impairment of heart rate variability is greatest in patients with the postoperative AF.2 In addition to the reduction in heart rate variability, human baroreflex sensitivity is also decreased suggesting that inflammation affects heart rate control by altering central nervous activity.16
A limitation of the present study is that all animals studied were young normal dogs. Still this study does show the electrophysiologic effects of surgery in normal animals are significant, altering heart rate and heart rate variability, and are consistent with human data.3, 4 Because a large number of statistical comparisons were made and corrections had to be made for multiple comparisons more subtle changes in heart rate and variability did not reach significance. With a larger sample size these may reach statistical significance. However, the trends in the data are consistent with the conclusions of the study.
Conclusion
In summary, cardiac surgery in this canine model significantly increases postoperative heart rate by reducing heart rate variability, which is caused by a reduction in vagal tone. Furthermore, the magnitude of these changes is dependent upon the degree of inflammation and is normalized by treatment with steroids. The study supports the concept that the effects of inflammation on heart rate are mediated within the nervous system and not by direct effects on the sinus node. However, the surgical intervention does directly change the underlying atrial myocardial substrates for AF.
Acknowledgments
This study was supported by NIH Grants R01 HL 032257, NIH R01 HL085113.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shen J, Lall S, Zheng V, Buckley P, Damiano RJ, Jr., Schuessler RB. The persistent problem of new-onset postoperative atrial fibrillation: a single-institution experience over two decades. J Thorac Cardiovasc Surg. 141(2):559–570. doi: 10.1016/j.jtcvs.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogue CW, Jr., Domitrovich PP, Stein PK, et al. RR interval dynamics before atrial fibrillation in patients after coronary artery bypass graft surgery. Circulation. 1998;98(5):429–434. doi: 10.1161/01.cir.98.5.429. see comment. [DOI] [PubMed] [Google Scholar]
- 3.Bauernschmitt R, Malberg H, Wessel N, Kopp B, Schirmbeck EU, Lange R. Impairment of cardiovascular autonomic control in patients early after cardiac surgery. Eur J Cardiothorac Surg. 2004;25(3):320–326. doi: 10.1016/j.ejcts.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Demirel S, Akkaya V, Oflaz H, Tukek T, Erk O. Heart rate variability after coronary artery bypass graft surgery: a prospective 3-year follow-up study. Ann Noninvasive Electrocardiol. 2002;7(3):247–250. doi: 10.1111/j.1542-474X.2002.tb00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madsen T, Christensen JH, Toft E, Schmidt EB. C-reactive protein is associated with heart rate variability. Ann Noninvasive Electrocardiol. 2007;12(3):216–222. doi: 10.1111/j.1542-474X.2007.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii Y, Schuessler RB, Gaynor SL, et al. Inflammation of Atrium After Cardiac Surgery is Associated with Inhomogeneity of Atrial Conduction and Atrial Fibrillation. Circulation. 2005;111:2881–2888. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Clemente JM, Vilardell C, Broch M, et al. Lower heart rate variability is associated with higher plasma concentrations of IL-6 in type 1 diabetes. Eur J Endocrinol. 2007;157(1):31–38. doi: 10.1530/EJE-07-0090. [DOI] [PubMed] [Google Scholar]
- 8.Ho KM, Tan JA. Benefits and risks of corticosteroid prophylaxis in adult cardiac surgery: a dose-response meta-analysis. Circulation. 2009;119(14):1853–1866. doi: 10.1161/CIRCULATIONAHA.108.848218. [DOI] [PubMed] [Google Scholar]
- 9.Kahalpey AI, Ganim RB, Rawn JD. Postoperative Care of Cardiac Surgery Patients. In: Cohn LA, editor. Cardiac Surgery in the Adult. 3rd ed McGraw Hill Medical; New York: 2008. pp. 465–487. [Google Scholar]
- 10.Lee R, Nitta T, Schuessler RB, Johnson DC, Boineau JP, Cox JL. The closed heart MAZE: a nonbypass surgical technique. Annals of Thoracic Surgery. 1999;67(6):1696–1702. doi: 10.1016/s0003-4975(99)00268-4. [DOI] [PubMed] [Google Scholar]
- 11.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 12.Ishii Y, Schuessler RB, Gaynor SL, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111(22):2881–2888. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 13.Skovsted P, Sapthavichaikul S. The effects of isoflurane on arterial pressure, pulse rate, autonomic nervous activity, and barostatic reflexes. Can Anaesth Soc J. 1977;24(3):304–314. doi: 10.1007/BF03005103. [DOI] [PubMed] [Google Scholar]
- 14.Green M, Guideri G, Lehr D. Role of alpha- and beta-adrenergic activation in ventricular fibrillation death of corticoid-pretreated rats. J Pharm Sci. 1980;69(4):441–444. doi: 10.1002/jps.2600690420. [DOI] [PubMed] [Google Scholar]
- 15.Katona PG, McLean M, Dighton DH, Guz A. Sympathetic and parasympathetic cardiac control in athletes and nonathletes at rest. J Appl Physiol. 1982;52(6):1652–1657. doi: 10.1152/jappl.1982.52.6.1652. [DOI] [PubMed] [Google Scholar]
- 16.Bauernschmitt R, Malberg H, Wessel N, et al. Autonomic control in patients experiencing atrial fibrillation after cardiac surgery. Pacing Clin Electrophysiol. 2007;30(1):77–84. doi: 10.1111/j.1540-8159.2007.00568.x. [DOI] [PubMed] [Google Scholar]