Abstract
The human infant face represents an essential source of communicative signals on the basis of which adults modulate their interactions with infants. Behavioral studies demonstrate that infants faces activate sensitive and attuned responses in adults through their gaze, face expression, voice, and gesture. In this study we aimed to identify brain responses that underlie adults’ general propensity to respond to infant faces. We recorded fMRI during adults’ (non-parents) processing of unfamiliar infant faces compared to carefully matched adult faces and infrahuman mammal infant and adult faces. Human infant faces activated several brain systems including the lateral premotor cortex, supplementary motor area, cingulate cortex, anterior insula and the thalamus. Activation of these brain circuits suggests adults’ preparation for communicative behavior with infants as well as attachment and caregiving. The same brain regions preferentially responded to human infant faces when compared to animal infant faces, indicating species-specific adult brain responses. Moreover, results of support vector machine based classification analysis indicated that these regions allowed above chance-level prediction of brain state during perception of human infant faces. The complex of brain responses to human infant faces appear to include biological mechanisms that underlie responsiveness and a caring inclination toward young children which appear to transcend adult’s biological relationship to the baby.
Keywords: Infant faces, fMRI, species-specific, premotor cortex, supplementary motor area
Introduction
Human infants are born with structural and functional characteristics that prompt adult proximity and care and that ensure child survival and wholesome development. Prominent infant features include a facial morphology and a suite of communicative signals that activate sensitive and attuned caregiving behaviors in adults (Bornstein 2002; Bornstein et al. 2008a; Bowlby 1969; Lorenz 1943, 1971). Lorenz (1943, 1971) hypothesized that an infant physiognomy generally - round and large head, big eyes, small nose and mouth, chubby cheeks - activates “innate releasing mechanisms” in human adults for care and affection of infants. In return, adult sensitive responsiveness fosters infants’ motivation to interact and has positive effects on infant development (Landry et al. 1997; Sander 2000; Sroufe 2000; Trevarthen & Aitken 2001; Tronick 2005; van IJzendoorn et al. 1995). Moreover, young infants display dramatic upsetness to failures at adult contingent responsiveness (Murray & Trevarthen 1986; Tronick et al. 1978). Adult-infant dyadic interactions are grounded in this mutual regulation as revealed, for example, in video microanalysis (Beebe et al. 2007; Feldstein et al. 1993; Stern 1985; Trevarthen 2003).
The long evolutionary history of adult-infant transactional relationships suggests that specific brain circuits might mediate adult responsiveness to infants. Behavioral studies demonstrate that infants trigger prompt and syntonic responses in adults through their gaze, facial expression, voice, and gesture (Bornstein 2002; Bornstein et al. 2008a). The human infant face thus represents a crucial source of emotional and communicative signals on the basis of which adults modulate their interactions with infants.
From an evolutionary perspective Seligman’s theory of ‘preparedness’ holds that stimuli which are critical for survival, such as threat stimuli, elicit automatic prepared responses (Seligman 1971, Ohman & Mineka 2001). Extension of this theory predicts that the human brain would show innate predispositions to react not only to threat stimuli but to all biologically salient stimuli independent of their valence (Sherer 2011). In this view, human infant faces represent highly biologically relevant stimuli that capture attention of and reward adults (Brosch et al. 2007, Kringelbach et al. 2008; Glocker et al. 2009) and to which humans might be prepared to respond. Guided by this line of thought we expected that infant faces would trigger specific brain mechanisms that subserve adaptive preparatory responses in adults.
To date, putative neural systems that mediate adults’ specific responses to human infant faces have escaped detection. Prior studies comparing adults’ reactions to their own infants versus other infants have revealed involvement of dopamine-associated reward-processing regions as well as areas related to social cognition and emotion (Lenzi et al. 2009; Ranote et al. 2004; Strathearn et al. 2008; Swain 2007). The extent to which these brain circuits, posited to underlie parenting behaviors and attachment, also subserve a general inclination to respond to infant cues (even in non parents looking at unfamiliar babies) is unclear. Moreover, comparing adults’ reactions to their own infants versus other infants cedes stimulus control and misses what may be specific in adults’ brain responses to human infants. Only one study, using magnetoencephalography, aimed to investigate specific brain responses in adults to unfamiliar infant faces compared to adult faces. In accord with Lorenz’s theory, the authors reported that unfamiliar infant faces with an emotional expression tend to be more rewarding and salient than adult faces (Kringelbach et al. 2008).
Here, in an attempt to identify the brain responses that underlie adults’ general propensity to respond to infant cues, we recorded fMRI from adults non-parents while they processed unfamiliar emotionally neutral infant faces and compared resultant patterns of brain activity to those of carefully matched adult faces and infrahuman mammal infant and adult faces.
We specifically hypothesized that generic human infant faces would activate adult brain regions critical for preparation for communicative and interactive responses such as the premotor cortex. Moreover, we expected to find activations of phylogenetically older reward circuits - the cingulate cortex and its projections to and from midbrain, basal ganglia, and thalamus. These regions have previously been associated with parental attachment (Glocker et al. 2009; Kringelbach et al. 2008; Ranote et al. 2004; Swain et al. 2007) and linked to “baby schema” features in artificially manipulated infant faces (Glocker et al. 2009). Additionally, by including animal faces in our comparisons, we aimed to clarify whether adults’ inclination to respond to infant forms is species specific.
To test the role of the hypothesized brain systems in mediating the responses to human infant faces further, we used a sensitivity method aimed at examining how well a statistical classifier can predict the brain state during human infant faces perception on the basis of activation from independent anatomical ROIs (Haxby et al. 2001; Poldrack et al. 2009). Evidence of human adults’ species-specific brain responses to human infants would constitute a biological signature of an adult instinct that is requisite to child survival and antecedes the development of attachment.
Materials and Methods
Participants
Sixteen healthy adults non-parents (M age = 28.06, SD = 5.66; 7 males) were recruited through the University of Trento webpage and local advertisement. Advertisement did not provide the details of the study. Exclusion criteria were: being a parent, specific phobias for cats and dogs, neurological or psychiatric disorders, including substance abuse/dependence, psychotropic medications, and pregnancy. Candidates were screened by a neurologist for compatibility with MRI scan. Occupation and level of education varied in the sample, but most participants had attended (44%) or completed university (38%). Thirty-eight percent of the sample owned (or had owned in the previous two years) cats and/or dogs. Participants were from the metropolitan area of Trento (Italy) and ethnically homogeneous of European heritage. All participants provided written informed consent for their participation. The experimental procedures were approved by the ethical committee for experiments involving humans at the University of Trento.
Visual Stimuli
A total of 56 color pictures of infant and adult human and animal faces were used (14 for each category). Pictures of infant and adult human and animal faces were adjusted for brightness and color-balance using Adobe Photoshop 8.0.1. Specifically, based on brightness histograms, pictures were modified so that the average brightness value of all pixels fell between 125 and 220 cd/m2. After editing, mean brightness of the four picture categories did not differ from one another (F(1, 3) = 2.00, p = .15). Pictures were also corrected using primary color curves by reducing eventual excess of the primary colors. All pictures of humans showed a frontally oriented, neutrally expressive face on a white background; head size was matched across stimuli. Human adult faces consisted of equal numbers of males and females; human infant faces had no cues to distinguish gender. Equal numbers of cat and dog faces were used and their faces were also frontally oriented. Face stimuli came from public domain databases (Nefian et al. 1997; Solina et al. 2003), Van Duuren and colleagues (2003), or were publicly available images edited by a private graphics company (Tommaso Sega). To exclude potential influence of attractiveness on brain activity (Yamamoto et al. 2009; Parsons et al. 2011), pictures were selected within a larger database (n = 96 with the same characteristics and sources) and rated by 42 adults (19 males, M age = 32.00, SD = 4.25) on a 4-point Likert scale assessing attractiveness. These participants did not report cat or dog phobias. They were recruited by public advertisement and participated in this behavioral experiment only. The stimuli were presented on a laptop (for 3 s each) in one of two possible random orders and were interleaved with a of a 4-point scale ranging from unattractive to attractive. Participants verbally responded to each picture, and their responses were recorded by an experimenter out of the participants’ view. We then selected 56 stimuli for the fMRI experiment that were the same in attractiveness [2-way Age X Species ANOVA on attractiveness scores, F(1, 41) = .30, ns].
fMRI protocol
During functional scanning, participants viewed pictures of infant faces and adult faces and they were instructed to attend to all stimuli. Pictures of faces were interspersed with pictures of various objects of common use (taken from the Amsterdam Library of Object Images; Geusebroek, Burghouts & Smeulders, 2005; e.g., cup, ball, shoe) to obviate habituation effects to faces. Stimuli were back-projected onto a screen by a liquid-crystal projector at a frame rate of 60 Hz and a screen resolution of 1280 × 1024 pixels (mean luminance: 109 cd/m2). Two fMRI time series were acquired for each participant in a single fMRI session. During each time series 70 faces of humans (14 infants (HI) and 14 adults (HA)) and domestic animals (14 infants (AI) and 14 adult cats and dogs (AA)) interspersed with 14 pictures of objects (Ob) appeared in pseudo-randomized sets of 5 (one image from each category). A time series consisted of a 10-s inter-stimulus interval (ISI) followed by a 4-s picture presentation. During each ISI, participants were presented with a black fixation.
fMRI data acquisition
Participants underwent MRI scanning at 4 Tesla in a MedSpec Biospin MR scanner (Bruker Ettlingen, Germany) and an 8-channel birdcage head coil. Mild external head restraint was used to minimize head movement during scanning. Before collecting functional images, a high-resolution T1 weighted image of the whole brain (MPRAGE: 176 slices, GRAPPA acquisition with an acceleration factor of 2, FOV=256×256 mm2, voxel size = 1×1×1 mm, TI = 1020 ms TE = 4.18 ms, TR = 2700 ms) was acquired for the purpose of spatial coregistration. Whole-brain functional data were acquired using echoplanar imaging, sensitive to BOLD contrast (34 slices, tilted 18° from intracommisural plane, FOV=192×192 mm, voxel size = 3×3×3 mm, slice gap = 15%, flip angle (FA), 73°, TE = 33 ms, TR = 2 s per volume). We performed an additional scan to measure the point-spread function (PSF) of the acquired sequence, which served for distortion correction that is expected with high-field imaging. The experimental session consisted of 998 whole brain images per participant; these included four dummy scans at the start of each time series to allow for T1 equilibration. The experiment lasted about 40 min.
Univariate fMRI data analysis
To correct for distortions in geometry and intensity in the EPI images, we applied distortion correction on the basis of the PSF data acquired before the EPI scans (Zeng & Constable 2002). The fMRI time series data were analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) on a Matlab platform (Mathworks Inc.). Echoplanar images were corrected for head movements, normalized to the Montreal Neurological Institute (MNI) stereotaxic standard space, and then spatially (9-mm full-width half-maximum Gaussian kernel) and temporally (cut-off period 256 s) smoothed. After motion correction two participants were excluded from further analysis because of large head movements. For each participant, an analytic design matrix was constructed, modeling onsets and duration of each trial as epochs convolved with a hemodynamic response function. The five trial types were modeled as separate regressors and interrogated to derive contrast images for second-level (group) mixed-effects analysis using a general linear model. For each participant, within-human/animal category contrast images of infants versus adults were created. Additionally, within-infant category cross-species contrast images were created. To remove potential differences in information processing of cross-species features, brain activity related to the contrast human-versus-animal within adults was subtracted from the within-infant category cross-species contrast: (human infant - animal infant) - (human adult – animal adult). These images were then entered into a second-level (random-effects) analysis to allow inferences across participants that generalize to the population. One sample t-tests on the contrast images imported from the first-level analysis were performed to assess group effects across all participants. The t-tests indicated whether observed differences between infants and adults, and human and animal infants, differed significantly from zero (Holmes & Friston 1998). The resulting SPM(t) maps were thresholded at p < 0.01 (cluster-wise family wise error (FWE) correction for multiple comparisons). The cluster-forming threshold was set to p < 0.001 uncorrected. To test for modulation of category-specific brain responses by human infant stimuli, we measured activity during each condition and subject with respect to the baseline in several regions of interest (ROIs). Parameter estimates in the lateral premotor cortex (PMC), supplementary motor area (SMA), middle cingulate gyrus (mCG), anterior insula (AI) and thalamus (Th) were extracted in spheres with 6-mm radius centered on peak activations derived from HI > HA contrast (center of mass coordinates (MNI): right SMA (6,−15,72), right PMC (36,−6,45), left mCG (−6,−15,48), left AI (−28,15,−9) and right Th (6,−27,−6)). ROI analysis was performed using the rfxplot toolbox (Gläscher, 2009). Moreover, to identify brain regions specifically activated by human infant faces independently of the condition used for SPM contrast, a conjunction analysis was performed (Minimum Statistic compared to the Conjunction Null (MS/CN), Nichols et al. 2005). This approach allowed us to test for the conjunction of HI > HA, HI > AI, and HI > Ob contrasts by testing the null hypothesis that one or more of the comparisons have not activated, the conjunction null (MS/CN). As the test for the conjunction null hypothesis of the effects is a quite conservative procedure, particularly in the contest of multiple comparisons, a threshold of p < 0.01 uncorrected was adopted (Nichols et al. 2005).
Multivariate fMRI data analysis
We applied a multivariate method based on a support vector machine classifier implemented by our group (SVM; Lee at al. 2010) to fMRI data to measure the degree to which activity in each region is predictive of the human infant face condition with respect to other conditions.
Anatomical regions were selected based on results of univariate conjunction analysis (Haxby et al 2001; Mitchel et al. 2004; Polyn et al. 2005; Walther et al. 2009). The use of anatomical masks led to ROI selection unbiased by an investigator. SVM classification was performed separately on independent anatomical regions (SMA, right PMC, mCG, left AI and Th) defined using the WFU PickAtlas toolbox as implemented in SPM8 (Maldjian et al. 2003). From each ROI, a mean BOLD value at each voxel of a ROI was acquired after averaging BOLD time-series at each voxel for a single trial (2TRs). The mean BOLD values in each ROI in each trial were collected into an input vector, whose design label was given based on its condition (1 for HI, 2 for HA, 3 for AA, 4 for AI, 5 for Ob). To account for the hemodynamic delay, the design label was shifted by two scans (4 s), and then the two sessions of data from all 14 participants were used to construct the input vectors of classifiers. To classify the data of pair-wise tests (HI vs HA, HI vs AA, HI vs AI, and HI vs Ob) across sessions, SVM (linear SVM with the regularization parameter C = 1, using SVMlight; Joachims 1999) were used in each separate session. Classification performance from data within a session was evaluated through 28-fold crossvalidation (Hastie et al 2001). In each fold, the data of 27 sessions were used to train the SVM classifier, and then the data of one remaining session were used to test the classifier. This process was repeated across all the folds without the testing sets overlapping across folds (leave-one-session-out cross-validation). Classification accuracies were calculated over all 28 repetitions, and the 28 values were used for a statistical test. One-tailed t-tests were used to assess whether decoding accuracy was significantly above chance level (50%).
Behavioral data
In a subsequent session, participants rated their feelings while viewing a sample of 24 faces derived from the fMRI experiment stimuli (6 stimuli for each category: infant and adult human faces, infant and adult animal faces). Feelings were rated according to four 7-point scales: positive emotions toward the organism, willingness to approach the organism, willingness to smile at the organism, willingness to communicate with the organism. All scales ranged from not at all to extremely and were selected on the basis of the literature on adult-infant interactions to assess the degree of adults’ typical and prominent responses to faces (Beebe et al. 2007; Feldstein et al. 1993; Stern 1985; Trevarthen 2003).
Results
Functional imaging data
Neuroimaging data were analyzed to compare brain responses to the four face categories. To test our main hypotheses, brain responses to human infant and adult faces were compared in intra-species contrasts. Moreover, to clarify whether adults’ brain responses to infant faces are species specific, cross-species contrasts were performed comparing responses to human and animal infant faces.
Intra-species comparisons
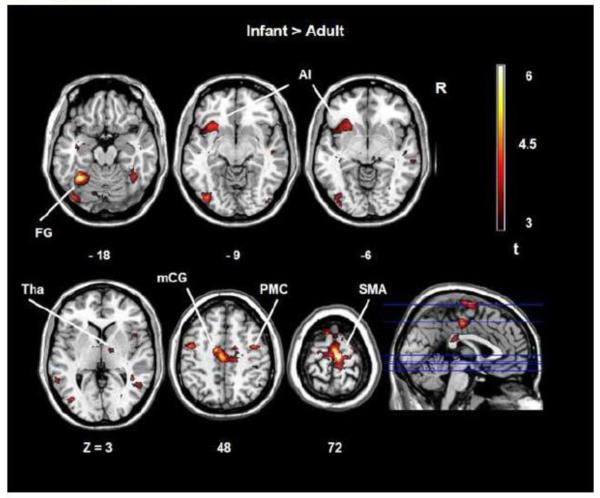
Human infant faces compared to human adult faces revealed a peak maximum in the supplementary motor area (SMA, BA 6) (Table 1, Figure 1). BOLD signal clusters included the fusiform gyrus (BA37, BA19), the precentral gyrus (BA 6), the middle cingulate cortex (BA 31, BA24), the left anterior insula (BA 48), and the thalamus. No significant effects in brain activity were observed when infant animal faces were compared to adult animal faces. Furthermore, no significant activation was observed when human adult faces were compared to human infant faces excluding possible residual variance effects between stimulus categories.
Table 1.
| Anatomical structure | Hemisphere | MNI coordinates (X Y Z) |
Brodmann area (BA) |
Cluster size | t value |
|---|---|---|---|---|---|
| Human: Infant > Adult | |||||
| Supplementary motor area | R | 6 −15 72 | BA 6 | 268 | 8.47 |
| Superior frontal gyrus | R | 15 −15 75 | BA 6 | 5.73 | |
| Pre-supplementary motor area |
L | −9 15 72 | BA 6 | 4.89 | |
| Fusiform gyrus | L | −33 −51 −18 | BA 37 | 143 | 6.62 |
| Cerebellum | L | −24 −57 −21 | BA 37 | 3.80 | |
| Precentral gyrus | R | 36 −6 45 | BA 6 | 78 | 6.55 |
| Precentral gyrus | R | 42 −3 51 | BA 6 | 5.14 | |
| Superior frontal gyrus | R | 36 −3 63 | BA 6 | 4.84 | |
| Fusiform gyrus | L | −39 −78 −12 | BA 19 | 133 | 6.38 |
| Inferior occipital gyrus | L | −42 −78 9 | BA 19 | 5.07 | |
| Inferior occipital gyrus | L | −39 −87 −6 | BA 19 | 3.87 | |
| Middle cingulate cortex | L | −6 −15 48 | BA 31 | 141 | 5.64 |
| Middle cingulum | L | −12 −9 48 | BA 24 | 5.45 | |
| Middle cingulum | R | 21 −15 45 | 4.78 | ||
| Cerebellum | R | 36 −57 −27 | 113 | 5.58 | |
| Cerebellum | R | 36 −45 −24 | 4.92 | ||
| Cerebellum | R | 36 −69 −27 | 3.50 | ||
| Anterior Insula | L | −28 15 −9 | BA 48 | 88 | 4.45 |
| Anterior Insula | L | −33 −18 −12 | BA 48 | 4.44 | |
| Anterior Insula | L | −27 27 −3 | BA 48 | 3.89 | |
| Thalamus | R | 6 −27 −6 | 69 | 3.31 | |
| Thalamus | R | 15 −9 3 | 3.24 | ||
| Midbrain | L | −3 −27 −3 | 3.21 |
Notes: Bold text indicates the peak of BOLD cluster.
Figure 1.
SPM t-maps of adults brain activations in response to infant faces compared to human adult faces. A = anterior. R = right. AI = anterior insula. Tha = thalamus. mCG = middle cingulate gyrus. PMC = premotor cortex. SMA = supplementary motor area.
Cross-species comparisons
Potential effects due to inter-species differences were appropriately controlled (see fMRI data analysis section). When compared to animal infant faces, human infant faces were associated with a peak maximum the middle frontal/precentral gyrus (BA 6) (Table 2). Clusters of BOLD activity were also observed in the fusiform gyrus, the supplementary motor area (BA 6), the superior temporal pole/anterior insula, and the middle cingulate cortex. No significant effects in brain activity were observed when faces of adult humans were compared to faces of adult animals and when animal infant faces were compared to human infant faces.
Table 2.
| Anatomical structure | Hemisphere | MNI coordinates (X Y Z) |
Brodmann area (BA) |
Cluster size | t - value |
|---|---|---|---|---|---|
| Human infant > Animal infant | |||||
| Middle frontal gyrus | R | 39 3 60 | BA 6 | 116 | 6.67 |
| Precentral gyrus | R | 24 −12 57 | BA 6 | 5.04 | |
| Precentral gyrus | R | 18 −3 51 | BA 6 | 4.58 | |
| Fusiform gyrus | R | 42 −48 −18 | BA 41 | 221 | 4.94 |
| Middle temporal gyrus | R | 36 −57 21 | BA 37 | 4.73 | |
| Middle temporal gyrus | R | 36 −57 −6 | BA 37 | 4.66 | |
| Supplementary motor area | R | 6 15 72 | BA 6 | 87 | 4.72 |
| Supplementary motor area | L | −15 15 72 | BA 6 | 4.24 | |
| Supplementary motor area | L | −6 15 72 | BA 6 | 4.23 | |
| Middle occipital gyrus | L | −51 −66 −3 | BA 37 | 212 | 4.48 |
| Inferior occipital gyrus | L | −36 −81 15 | BA 19 | 4.19 | |
| Middle occipital gyrus | L | −42 −78 9 | BA 19 | 4.16 | |
| Superior temporal pole | L | −51 15 −5 | BA 38 | 97 | 4.43 |
| Anterior Insula | L | −30 18 −9 | BA 48 | 3.98 | |
| Superior temporal pole | L | −54 12 −6 | BA 38 | 3.58 | |
| Middle cingulate cortex | L | −13 −9 51 | 75 | 4.02 | |
| Middle cingulum | 0 −21 45 | BA 23 | 3.93 | ||
| Middle cingulum | L | −6 15 33 | BA 24 | 3.87 |
Notes: Bold text indicates the peak of BOLD cluster. R = right hemisphere. L = left hemisphere. Montreal Neurological Institute (MNI) template was used as standard coordinate system for spatial normalization of individual subjects.
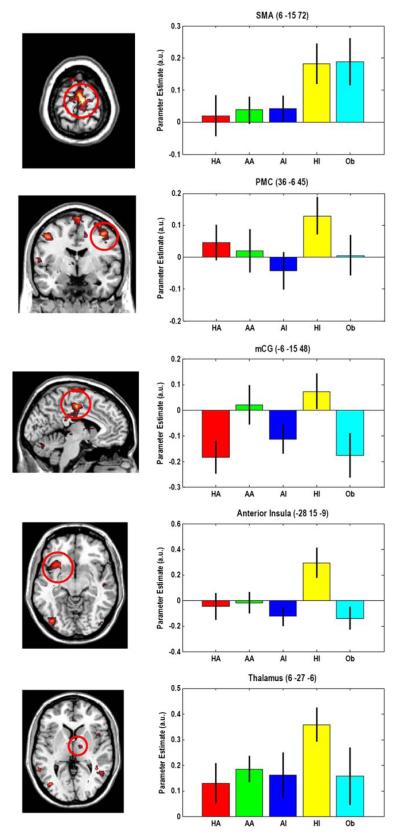
Parameters estimation on the selected regions (SMA, PMC, mCG, AI and Th) revealed that participants showed enhanced response to human infant with respect to human adult, animal adult and infant, and object. SPM beta values are plotted as effect size in Figure 2.
Figure 2.
The plot shows the mean effect in all five relevant conditions extracted form the right supplementary motor area, right premotor cortex, middle cingulate gyrus, left insula and thalamus (MNI coordinates are in brackets). See Results and Table 1 for additional significant areas during observation of human infant faces in comparison with human adult faces and animal infant faces. Bars represent group mean of parameters estimates and standard error of the mean (SEM) that were calculated using rfxplot toolbox for SPM (Gläscher 2009). a.u. = arbitrary units, HA = human adult; AA = animal adult; AI = animal infant; HI = human infant.
Conjunction analysis
The conjunction analysis of the three contrasts (HI > HA, HI > AI, and HI > Ob) revealed those areas that exhibit a group effect and that are significant (thresholded) in the three contributing SPM maps (Table 3). These areas were the left AI, left Th, middle temporal gyrus bilaterally, left mCG, left SMA, and right PMC.
Table 3.
| Anatomical structure | Hemisphere | MNI coordinates (X Y Z) |
Brodmann area (BA) |
Cluster size | t value |
|---|---|---|---|---|---|
| Anterior Insula | L | −28 15 −9 | BA 48 | 117 | 3.67 |
| Superior temporal pole | L | −39 18 −18 | BA 48 | 2.81 | |
| Anterior Insula | L | −42 12 −6 | BA 48 | 2.69 | |
| Thalamus | L | −4 −4 6 | 74 | 3.37 | |
| Thalamus | L | −9 −6 0 | 2.93 | ||
| Middle temporal gyrus | L | −39 −60 18 | BA 39 | 25 | 3.29 |
|
Middle temporal gyrus
(Occipitotemporal area) |
R | 39 −48 −21 | BA 37 | 17 | 3.13 |
| Middle temporal gyrus | R | 42 −51 12 | BA 21 | 42 | 3.09 |
| Middle cingulate cortex | L | −6 −15 33 | BA 23 | 13 | 2.97 |
| Supplementary motor area | L | −6 −12 75 | BA 6 | 10 | 2.94 |
| Middle temporal gyrus | L | −57 −54 9 | BA 21 | 22 | 2.92 |
| Middle frontal gyrus | R | 39 0 54 | BA 6 | 30 | 2.92 |
| Precentral gyrus | R | 51 6 12 | BA 6 | 60 | 2.88 |
| Inferior frontal gyrus | R | 57 9 21 | BA 6/45 | 2.70 |
Notes: Bold text indicates the peak of BOLD cluster. R = right hemisphere. L = left hemisphere. Montreal Neurological Institute (MNI) template was used as standard coordinate system for spatial normalization of individual subjects.
SVM Classification
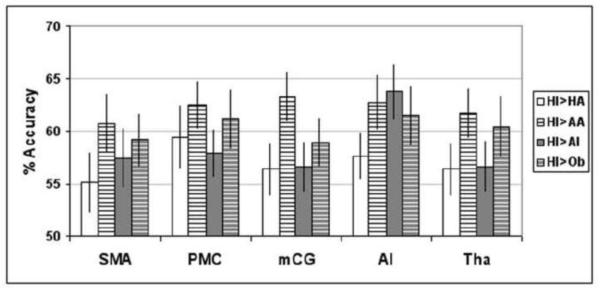
To test our hypotheses, we evaluated whether SVM could consistently classify the fMRI signals from SMA, PMC, mCG, AI, and Th between the HI condition and HA, AA, AI, and Ob conditions. Our analysis indicated that SVM could discriminate between HI and remaining conditions with a decoding accuracy significantly better than chance across all pair-wise comparisons and for all the selected ROIs (p < 0.0001). Average accuracy varied from 55.10% to 63.78% (Figure 3, Table 4).
Figure 3.
Classification accuracy of group data in the 28-fold cross validation (leave-one-subject-out approach; mean accuracy [%] ± standard error of the mean [%]) of pair-wise tests comparing HI vs HA, HI vs AA, HI vs AI and HI vs Ob for each separate ROIs.
Table 4.
| HI>HA | HI>AA | HI>AI | HI>Ob | |
|---|---|---|---|---|
| SMA | 55.10 | 60.71 | 57.40 | 59.18 |
| PMC | 59.44 | 62.50 | 57.91 | 61.22 |
| mCG | 56.38 | 63.27 | 56.63 | 58.93 |
| AI | 57.65 | 62.76 | 63.78 | 61.48 |
| Tha | 56.38 | 61.73 | 56.63 | 60.46 |
Behavioral data
Separate ANOVAs were carried out with the scale scores as dependent variables and with age (baby vs adult) and species (human vs animal) as within-subjects factors. Main effects of age for all scales indicated higher rates for infant compared to adult scores (positive emotions, F(1, 11) = 31.21, p < 0.01, η2p = .53; willingness to approach, F(1, 11) = 31.21, p < 0.001, η2p = .76; willingness to smile at, F(1, 11) = 20.05, p = 0.001, η2p = .67; willingness to communicate with, F(1, 11) = 8.17, p < 0.02, η2p = .39. No species main or interaction effects emerged.
Discussion
The present study aimed to assess whether generic human infant faces elicit activity in specific brain circuits underlying essential adult responsiveness to infants. We hypothesized that non-parents’ processing of unfamiliar infant faces compared to adult faces would activate brain circuits involved in preparation for communicative and interactive responses as well as reward circuits previously shown to mediate attachment and caregiving behaviors in parents towards their own children. We included animal faces in this study to assess whether hypothesized brain responses relate to a disposition to respond specifically to human children (a species-specific mechanism) or a more general inclination toward infants (a species-general mechanism). Using functional MRI, we identified brain regions that specifically respond to human infant faces. Brain regions showing enhanced BOLD response included the lateral premotor regions, the supplementary motor area, the thalamo-cingulate circuit, and the left anterior insula. Moreover, all these regions allowed significantly above chance-level prediction of brain state during human infant face perception. Results from our multivariate pattern analysis confirmed the results of univariate analysis and further support the assumption of a critical role of these brain systems in mediating responses to human infants.
In support of our first hypothesis, the observed large involvement of the supplementary motor area as well as the lateral premotor areas in response to unfamiliar and emotionally neutral infant faces suggests that human infants trigger adults’ preparatory behavior. The role of the supplementary motor area in the preparation of voluntary action has been demonstrated extensively (Nachev et al. 2008). The SMA is also implicitly activated in humans to facilitate behavioral responses to specific objects when they are simply observed without active movement being performed (Grezes & Decety 2005). Moreover, this brain region, along with lateral premotor areas, generates a negative potential known as the Bereitschaftspotential – “readiness potential” – that antecedes movement onset (Deecke & Kornhuber 1978; Goldberg, G. 1985; Jahanshahi et al. 1995). The Bereitschaftspotential is considered the neural correlate of intentional movement planning that can be measured even when people are unaware of their intention to move (Haggard & Eimer 1999). As participants in our study were not asked to execute any motor task, the observed premotor activity might reflect the implicit preparation to respond to infant faces. In conjunction with behavioral studies of early adult-infant transactions that highlight syntonic intuitive communicative behaviors, our neuroimaging results seem to confirm that infant faces activate a “readiness” to interact with babies. Supporting this interpretation, a behavioral study of implicit attention to infants (Brosch et al. 2007) reported faster response times to unfamiliar human infant pictures compared to adults pictures, in adults independent of gender; motor facilitation associated with enhanced activity in the premotor regions could help to explain the observed decreased time to respond to infants.
In a related way, the SMA along with the premotor cortex and the left anterior insula have been also implicated in preparation and intention to communicate (Alario et al. 2006; Brendel et al. 2010; Riecker et al. 2005). In particular the SMA underlies preparation for verbal utterance and initiating vocal tract movements during speech production (it is the so-called “starting mechanism of speech”; Ackermann & Riecker 2010; Ackermann & Ziegler 2010; Botez & Barbeau 1971; Brendel et al. 2010). In early dyadic interactions, adults readily speak to infants even though they know that babies cannot understand language and will not reply, and adults even speak to babies in a special speech register called “infant-directed speech” that includes multiple specific (prosodic, simplicity, redundancy, lexical, and content) modifications from adult-adult or even adult-child speech. Infant-directed speech is believed to be intuitive, nonconscious, and virtually universal; indeed, adults cannot help themselves from using it (Papoušek & Bornstein 1992).
The measured increased activity in the fusiform gyrus, a critical area for face perception, during the observation of unfamiliar infant faces compared to adult faces suggests enhanced visual inspection and attention devoted to infant stimuli. Brosch and colleagues (2007) reported that human infant faces, having high biological significance, selectively modulate adult attention.
Increased response in areas associated with face perception and attention, along with the insula and the thalamus, was previously reported when mothers (n=7) were observing unfamiliar (school-age) children compared to unfamiliar adults (Leibenluft et al. 2004). However, the authors did not observe activity in premotor areas perhaps because they also used pictures of participants’ own children that engendered an attention bias.
From an evolutionary perspective, adults’ responsiveness to human infant cues reflected in increased allocation of attention and readiness to respond, has a clear adaptive value as it favors offspring and species survival. Moreover, the adaptive value of adult responsiveness to infants not only pertains to their survival but might extend to fostering children’s mental development. During early dyadic interactions, adults and infants display mutually regulated intersubjectivity as evidenced by video microanalysis (Beebe et al. 2007; Feldstein et al. 1993; Stern 1985; Trevarthen 2003). These intuitive adult behaviors foster communicative motivation in children allowing the mutual regulation of one another’s interests and feelings into rhythmic patterns, based on the exchange of multimodal communicative signals and on imitations of vocal, facial, and gestural expressions (Bornstein et al. 2008a, 2008b, Manian & Bornstein 2009). These dyadic transactions are essential to infant psychological development, and they are regulated by adults’ attunement to baby communicative and emotional signals. Our results support the idea that baby faces represent salient stimuli that convey affective and communicative signals to which adults are prompt to respond and ready to modulate their interactive behaviors.
Consistent with our second hypothesis, human infant faces compared to human adult faces elicited activation of the thalamo-cingulate dopaminergic system and insula. These areas have previously been implicated in parents’ responses to their own children (Swain et al. 2007), but our results refine this conclusion and indicate that human infant faces in general are trigger features for adults. Neuroimaging studies in human adults focusing on maternal responses to their own versus other children reported stronger activity in striate and extrastriate visual areas and in reward-related areas to own versus other infant faces (Leibenluft et al. 2004; Ranote 2004; Strathearn et al. 2008). These studies contributed to contemporary brain models of human parenting: based on the literature on nonhuman mammal parenting and existing neuroimaging studies of human parents, Swain and colleagues (2007) hypothesized that human parenting behaviors might be mediated by a complex circuit of brain structures generally involved in social behavior but specifically responsive to stimuli that are relevant to parenting. In this model, the brain areas that underlie parental responses to infant stimuli include (a) the cingulate with feedback loops involving midbrain, basal ganglia, and thalamus, that are implicated in motivation and reward (Strathearn et al. 2008), (b) the frontal, insular, fusiform, and occipital areas, involved in complex planning and social emotional/empathic responsiveness (Bartels & Zeki 2004), and (c) detection centers for arousal and salience of emotional stimuli, including amygdala, hippocampus, and insula (LeDoux 2003). Here, we found that human infant faces, regardless of familiarity, activate essential components of this model of the parenting brain (Swain et al. 2007). Thus, our findings appear to support a general human adult inclination to respond sensitively to infants and may constitute a foundation of “intuitive parenting” (Papoušek & Papoušek 2002). This pattern of response also accords with the view that the human social brain evolved in a situation where alloparenting was common, as would be true in the environment of evolutionary adaptedness (Hrdy 1999; Lorenz 1971), that is where many adults shared responsibility for infant care.
The observed brain activity underlying adults’ responsiveness to infants stands as complementary to compelling evidence that young infants themselves anticipate that their adult interactants will respond. The “still-face” paradigm (Tronick et al. 1978) validates this expectation on the part of babies. An adult who is naturalistically interacting with an infant, and suddenly becomes non responsive, typically elicits demonstrative upsetness from the infant – more than does the adult physically departing the interaction altogether (Field et al. 2007; Tronick et al. 1978).
The observed brain activity in response to human infant stimuli accords with appraisal theories of emotion, positing that emotionally significant stimuli enhance visual processing and attention and elicit changes in the autonomic, motor, and motivational system to prepare the organism for adaptive responses (Scherer 2001). Such theories extend the concept of “preparedness” – that indicates an innate predisposition to selectively focus on threat stimuli (Seligman 1971, Ohman & Mineka 2001) – to all biologically salient stimuli independent of their valence.
With respect to cross-species comparisons, although we found no differences in brain responses to human and animal adult faces, we observed species-specific activation to human infant faces compared to animal infant faces. This pattern resembled the activation we found in the comparison of human infant with human adult faces. Specifically, the premotor regions, including SMA, the fusiform gyrus, the left anterior insula, and the middle cingulate cortex, were activated preferentially by human infant faces when compared to animal infant faces. These results suggest that adults’ preparation to respond and interact is specific to human infants. Lorenz (1943; 1971) hypothesized that features of infant physiognomy are similar across different species and elicit human adult responses of care and affection even towards infants of different species. Our participants’ subjective ratings of feelings of positive emotions and willingness to approach, to smile, and to communicate elicited by infant faces accord with Lorenz’s intuitions and with previous studies (Fullard & Reiling 1976; Sanefuji et al. 2007). Both human and animal infants scored higher compared to their respective adult forms in all dimensions, although all stimuli were balanced for attractiveness. Moreover, within infants no differences were found between species. Nonetheless, our fMRI findings showed adults’ brain responses only to human baby schema, in comparison with both human adult and animal infant. A possible explanation of the observed discrepancy between behavioral and neurobiological results may rely on the fact that the former is an explicit task whereas the latter is an implicit measurement. Behavioral studies using implicit tasks (e.g., “dot probe task”, Brosch et al 2007) have demonstrated that pictures of neutral human infant faces compared to animal faces (cats and dogs) increase the spatial deployment of attentional resources, indicating a perception bias to conspecifics.
In summary, our neuroimaging results suggest that adult brain responses to human infant faces are species-specific and point to a human predisposition for solicitude of very young children, rather than a general cross-species inclination to infant care and affection activated by infant forms generally. A limitation that arises in relation to this conclusion concerns the species considered in the contrast conditions in this study. To reduce the possible effects of differential familiarity, common domestic mammals (cats and dogs) were shown as non-human exemplars. In an extension of this line of research, we are investigating human brain responses to mammalian species (such as primates) closer to humans in the evolutionary line.
Conclusions
Prominent twentieth-century ethologists and developmental scientists (Ainsworth et al. 1974; Bowlby 1969; Lorenz, 1971) opined that infant morphology and communicative signals prompt adult proximity and care that in turn secure child survival and promote healthy development. In line with this overarching view, we identified brain circuits that subserve preparation to respond and reward in the presence of human infants. Visual processing of infant faces predisposes adults to interact with them, an attitude that is readily apparent in close observation of healthy adult-infant interactions. We observed these mechanisms in non-parent adults’ perceptions of unfamiliar infant faces, indicating that they transcend any adult relationship with the baby. The complex of species-specific brain responses we identified appears to include biological mechanisms that underlie responsiveness and a caring inclination toward young children.
Results from this fMRI inquiry broaden and deepen our understanding of the neural foundations of human caregiving and help to direct clinical work aimed to identify adults at risk for deviant parenting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest The authors declare no conflict of interest.
References
- Ackermann H, Riecker A. Functional brain imaging of speech motor control. In: Maassen B, van Lieshout PHHM, editors. Speech Motor Control: New Developments in Basic and Applied Research. Oxford University Press; Oxford, UK: 2010. pp. 85–111. [Google Scholar]
- Ackermann H, Ziegler W. Brain mechanisms underlying speech motor control. In: Hardcastle WJ, Laver J, Gibbons F, editors. The Handbook of Phonetic Sciences. 2nd ed. Blackwell Publishing Ltd; Oxford, UK: 2010. doi. 10.1002/9781444317251.ch6. [Google Scholar]
- Ainsworth MDS, Bell SM, Stayton D. Infant-mother attachment and social development. In: Richards MP, editor. The introduction of the child into a social world. Cambridge University Press; London: 1974. pp. 99–135. [Google Scholar]
- Alario FX, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Res. 2006;1076:129–43. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Beebe B, Jaffe J, Buck K, Chen H, Cohen P, Blatt S, Kaminer T, Feldstein S, Andrews H. Six-week postpartum maternal self-criticism and dependency and 4-month mother-infant self- and interactive contingencies. Dev Psychol. 2007;43:1360–1376. doi: 10.1037/0012-1649.43.6.1360. [DOI] [PubMed] [Google Scholar]
- Bornstein MH. Handbook of parenting. Vols 1-5. Lawrence Erlbaum New Jersey; Mahwah: 2002. [Google Scholar]
- Bornstein MH, Putnick DL, Heslington M, Gini M, Suwalsky JT, Venuti P, de Falco S, Giusti Z, Zingman de Galperín C. Mother-child emotional availability in ecological perspective: Three countries, two regions, two genders. Dev Psychol. 2008b;44:666–680. doi: 10.1037/0012-1649.44.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Tamis-Lemonda CS, Hahn CS, Haynes OM. Maternal responsiveness to young children at three ages: longitudinal analysis of a multidimensional, modular, and specific parenting construct. Dev Psychol. 2008;44:867–74. doi: 10.1037/0012-1649.44.3.867. [DOI] [PubMed] [Google Scholar]
- Botez MI, Barbeau A. Role of subcortical structures and particularly of the thalamus, in the mechanisms of speech and language. Int J Neurol. 1971;8:300–320. [PubMed] [Google Scholar]
- Bowlby J. Attachment and Loss. Vol 1 Attachment. Hogarth Press; London: 1969. [Google Scholar]
- Brendel B, Hertrich I, Erb M, Lindner A, Riecker A, Grodd W, Ackermann H. The contribution of mesiofrontal cortex (SMA) to the preparation and execution of repetitive syllable productions: An fMRI study. Neuroimage. 2010;50:1219–1230. doi: 10.1016/j.neuroimage.2010.01.039. [DOI] [PubMed] [Google Scholar]
- Brosch T, Sander D, Scherer KR. That Baby Caught My Eye … Attention Capture by Infant Faces. Emotion. 2007;7(3):685–689. doi: 10.1037/1528-3542.7.3.685. [DOI] [PubMed] [Google Scholar]
- Deecke L, Kornhuber HH. An electrical sign of participation of the mesial ‘supplementary’ motor cortex in human voluntary finger movement. Brain Res. 1978;159:473–476. doi: 10.1016/0006-8993(78)90561-9. [DOI] [PubMed] [Google Scholar]
- Feldstein S, Jaffe J, Beebe B, Crown CL, Jasnow M, Fox H, Gordon S. Coordinated interpersonal timing in adult-infant vocal interactions: A cross-site replication. Infant Behav Dev. 1993;16:455–470. [Google Scholar]
- Field T, Hernandez-Reif M, Diego M, Feijo L, Vera Y, Gil K, Sanders C. Still-face and separation effects on depressed mother-infant interactions. Infant Ment Health J. 2007;28:314–323. doi: 10.1002/imhj.20138. [DOI] [PubMed] [Google Scholar]
- Fullard W, Reiling AM. An investigation of Lorenz’s “babyness”. Child Dev. 1976;47:1191–1193. [Google Scholar]
- Gläscher J. Visualization of Group Inference Data in Functional Neuroimaging. Nueroinformatics. 2009;7:73–82. doi: 10.1007/s12021-008-9042-x. [DOI] [PubMed] [Google Scholar]
- Geusebroek JM, Burghouts GJ, Smeulders AWM. The Amsterdam library of object images. Int. J. Comput. Vision. 2005;61:103–112. [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdeza JN, Griffin MD, Sachser N, Gur RC. Baby schema modulates the brain reward system in nulliparous women. Proc Natl Acad Sci USA. 2009;106:9115–9119. doi: 10.1073/pnas.0811620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. Supplementary motor area structure and function - review and hypotheses. Behav. Brain Sci. 1985;8:567–588. [Google Scholar]
- Grezes J, Decety J. Does visual perception of object afford action? Evidence from a neuroimaging study. Neuropsychologia. 2002;40:212–222. doi: 10.1016/s0028-3932(01)00089-6. [DOI] [PubMed] [Google Scholar]
- Haggard P, Eimer M. On the relation between brain potentials and the awareness of voluntary movements. Exp Brain Res. 1999;126:128–133. doi: 10.1007/s002210050722. [DOI] [PubMed] [Google Scholar]
- Haxby JV, et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2429. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. Springer; New York, NY: 2001. [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage. 1998;7:S754–70. [Google Scholar]
- Hrdy S. Mother Nature: A history of mothers, infants and Natural Selection. Pantheon; New York: 1999. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Joachims T. Making large-scale SVM learning practical. In: Schölkopf B, Burges C, Smola A, editors. Advances in Kernel Methods: Support Vector Learning. MIT Press; Boston, MA: 1999. pp. 169–184. [Google Scholar]
- Kringelbach ML, Lehtonen A, Squire S, Harvey AG, Craske MG, Holliday IE, Green AL, Aziz TZ, Hansen PC, Cornelissen PL, Stein A. A Specific and Rapid Neural Signature for Parental Instinct. PLoS ONE. 2008;3:e1664. doi: 10.1371/journal.pone.0001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Miller-Loncar CL, Swank PR. Predicting cognitive-language and social growth curves from early maternal behaviors in children at varying degrees of biological risk. Dev Psychol. 1997;33:1040–1053. doi: 10.1037//0012-1649.33.6.1040. [DOI] [PubMed] [Google Scholar]
- Lee S, Halder S, Kübler A, Birbaumer N, Sitaram R. Effective Functional Mapping of fMRI Data with Support-Vector Machines. Human Brain Mapping. 2010;31(10):1502–11. doi: 10.1002/hbm.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft L, Gobbini MI, Harrison T, Haxby JV. Mothers’ Neural Activation in Response to Pictures of Their Children and Other Children. Biol Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, Ammaniti M. Neural Basis of Maternal Communication and Emotional Expression Processing during Infant Preverbal Stage. Cereb Cortex. 2009;9:1124–1133. doi: 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- Lorenz K. Die angeborenen Formen möglicher Erfahrung. (Innate form of potential experience) Zeitschrift für Tierpsychologie. 1943;5:235–309. [Google Scholar]
- Lorenz K. Studies in Animal and Human Behavior. vol. II. Methuen; London: 1971. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manian N, Bornstein MH. Dynamics of emotion regulation in infants of clinically depressed and nondepressed mothers. J Child Psychol Psychiatry. 2009;50:1410–1418. doi: 10.1111/j.1469-7610.2009.02166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TM, et al. Learning to decode cognitive states from brain images. Mach. Learn. 2004;5:145–175. [Google Scholar]
- Murray L, Trevarthen C. The infant’s role in mother-infant communication. J Child Lang. 1986;13:15–29. doi: 10.1017/s0305000900000271. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nefian AV, Khosravi M, Hayes MH., III Real-time human face detection from uncontrolled environments. SPIE Visual Communications on Image Processing. 1997 Available: http://www.anefian.com/research/face_reco.htm.
- Nichols T, Brett M, Andersson J, Wager T, Poline J. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The Functional Neuroanatomy of Maternal Love: Mother’s Response to Infant’s Attachment Behaviors. Biol Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness. Toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Parsons CE, Young KS, Kumari N, Stein A, Kringelbach ML. The Motivational Salience of Infant Faces Is Similar for Men and Women. PLoS ONE. 2011;6(5):e20632. doi: 10.1371/journal.pone.0020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoušek H, Bornstein MH. Didactic interactions. In: Papoušek H, Jurgens U, Papoušek M, editors. Nonverbal vocal communication: Comparative and developmental approaches. Cambridge University Press; Cambridge: 1992. pp. 209–220. [Google Scholar]
- Papoušek H, Papoušek M. Intuitive parenting. In: Bornstein MH, editor. Handbook of parenting. 2nd ed Vol. 2: Biology and ecology of parenting. Lawrence Erlbaum New Jersey; Mahwah: 2002. pp. 183–203. [Google Scholar]
- Polyn SM, et al. Category-specific cortical activity precedes recall during memory search. Science. 2005;310:1963–1966. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Halchenko Y, Hanson SJ. Decoding the large-scale structure of brain function by classifying mental states across individuals. Psychological Science. 2009;20:1364–1372. doi: 10.1111/j.1467-9280.2009.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JF, Appleby L. The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport. 2004;15:1825–1829. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, et al. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64:700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Sander L. Where are we going in the field of infant mental health? Infant Ment Health J. 2000;21:1–18. [Google Scholar]
- Sanefuji W, Ohgami H, Hashiya K. Development of preference for baby faces across species in humans (Homo sapiens) J Ethol. 2007;25:249–254. [Google Scholar]
- Scherer KR. Appraisal considered as a process of multilevel sequential checking. In: Scherer KR, Schorr A, Johnstone T, editors. Appraisal processes in emotion: Theory, methods, research. Oxford University Press; New York: 2001. pp. 92–120. [Google Scholar]
- Seligman MEP. Phobias and preparedness. Behavior Therapy. 1971;2:307–321. [Google Scholar]
- Solina F, Peer P, Batagelj B, Juvan S, Kovac J. Color-based face detection in the 15 seconds of fame art installation. Proceedings of Mirage INRIA Rocquencourt France. 2003:38–47. Available: http://web.mit.edu/emeyers/www/face_databases.html.
- Sroufe AL. Early relationships and the development of children. Infant Ment Health J. 2000;21:67–74. [Google Scholar]
- Stern D. The Interpersonal World of the Infant. Basic Books; New York: 1985. [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a Smile? Maternal Brain Responses to Infant Facial Cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarthen C. Conversations with a two month-old. In: Raphael-Leff J, editor. Parent-infant psychodynamics: Wild things, mirrors and ghosts. Whurr Publishers; Philadelphia: 2003. pp. 25–34. [Google Scholar]
- Trevarthen C, Aitken KJ. Infant intersubjectivity: Research, theory, and clinical applications. J Child Psychol Psychiatry. 2001;42:3–48. [PubMed] [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, Brazelton TB. J Am Acad Child and Adolesc Psychiatry. 1978;17:1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, Brazelton TB. The infant’s response to entrapment between contradictory messages in face-to-face interaction. J Am Acad Child and Adolesc Psychiatry. 1978;17:1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Tronick EZ. Why is connection with others so critical? The formation of dyadic states of consciousness and the expansion of individuals’ states of consciousness: coherence governed selection and the co-creation of meaning out of messy meaning making. In: Nadel J, Muir D, editors. Emotional development: Recent research advances. Oxford University Press; New York: 2005. pp. 293–315. [Google Scholar]
- Van Duuren M, Kendell-Scott L, Stark N. Early aesthetic choises: Infant preferences for attractive premature infant faces. Int J Behav Dev. 2003;27:212–219. [Google Scholar]
- van IJzendoorn MH, Dijkstra J, Bus AG. Attachment, intelligence, and language: A meta-analysis. Soc Dev. 1995;4:115–128. [Google Scholar]
- Yamamoto R, Ariely D, Chi W, Langleben DD, Elman I. Gender Differences in the Motivational Processing of Babies Are Determined by Their Facial Attractiveness. PLoS ONE. 2009;4(6):e6042. doi: 10.1371/journal.pone.0006042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DB, Caddigan E, Fei-Fei L, Beck DM. Natural scene categories revealed in distributed patterns of activity in the human brain. J Neurosci. 2009;29:10573–10581. doi: 10.1523/JNEUROSCI.0559-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Constable RT. Image distortion correction in EPI: Comparison of field mapping with point spread function mapping. Magn Reson Med. 2002;48:137–146. doi: 10.1002/mrm.10200. [DOI] [PubMed] [Google Scholar]