Abstract
Background
Infants of diabetic mothers (IDM) are at increased risk for metabolic complications. Type 1 and some type 2 diabetic patients have elevated levels of ketone bodies acetoacetate (AA) and β-hydroxybutyrate (BHB).
Objective
The aim of this study is to examine how hyperketonemia in diabetic mothers affects markers of inflammation and oxidative stress in their offspring.
Methods
Blood was obtained from 23 diabetic mothers and 13 healthy mothers, and their infants’ umbilical cords at the delivery. IL-8, MCP-1 and protein carbonyl (protein oxidation) levels were determined by ELISA. U937 human monocyte cell culture was used to examine the effect of AA and BHB on secretion of MCP-1.
Results
There was a significant increase in the levels of AA in cord blood of diabetic mothers compared with cord blood of healthy mothers. A significant increase in the levels of protein oxidation (p<0.05) and MCP-1 levels (p<0.05) were observed in the cord blood of IDMs. The level of MCP-1 significantly correlated (r=0.51, p=0.01) with the concentration of AA in the IDM. In further experiments with cultured monocytes treated with exogenous AA (0-4 mM), a significant increase in MCP-1 secretion was observed with AA but not in BHB-treated monocytes.
Conclusion
Blood levels of AA and MCP-1 are elevated in IDM, which may contribute to the development of the metabolic complications seen in IDM.
Keywords: Infant of diabetic mother, Oxidative stress, MCP-1, acetoacetate, Hyperketonemia
Introduction
Maternal hyperglycemia leads to elevated blood glucose levels in the fetus and a metabolically abnormal fetal milieu. This may result in birth defects, spontaneous abortions, macrosomia, asphyxia, respiratory distress syndrome and other metabolic complications [1-3]. Infants of diabetic mothers may be at an increased risk for developing diabetes and/or obesity later in life [1]. Controlling hyperglycemia during pregnancy reduces these complications in their offspring. In addition to hyperglycemia, hyperketonemia also occurs in type 1 and some type 2 diabetic patients [4]. Hyperketonemia can cause increased circulating levels of pro-inflammatory cytokines [5-7]. Vascular inflammation plays an important role in the development of many complications associated with diabetes mellitus during pregnancy and newborn period (8, 9). Even though ketonemia is associated with markers of inflammation in adult diabetic patients [5-7], the effect of ketonemia on the inflammatory markers in infants of diabetic mothers is unknown. Dahlgreen et al. reported that animals exposed prenatally to cytokines in utero can induce gender-specific programming of neuroendocrine regulation with subsequent sequele in adult life [10]. In another study, ketonemia during pregnancy was associated with a lower intelligence quotient in children [11]. Ketone bodies are increased in maternal and fetal plasma of streptozotocin-treated diabetic pregnant ewe [12]. However, no study has examined if cord blood of IDMs have elevated ketone levels. The objective of this study was to examine whether ketone levels are elevated in IDMs and possible effects of hyperketonemia on pro-inflammatory cytokines and oxidative stress markers. This study measured ketone blood levels (acetoacetate,β-hydroxybutyrate), the levels of inflammatory markers (IL-8, MCP-1) and protein oxidation in diabetic mothers and healthy mothers (controls) as well as in the blood from umbilical cords of their respective infants at the time of delivery.
Methods
The study was approved by the Louisiana State Health Sciences Center-Shreveport Institutional Review Board. Recruitment began in November 2008 and concluded in April 2010. Written informed consent was obtained from all study subjects. Subjects were divided into 2 groups: pregnant women with diabetes mellitus (study group, n=23) and healthy non-diabetic pregnant women (control group, n=13) and their infants’ umbilical cords at the delivery. The study group consisted of pregnant women with type I diabetes (n=12), type II diabetes (n=3), and gestational diabetes (n=8). All the study group patients showed ketonemia. Data forms were completed on all patients. They included: age, type of diabetes, complications during any previous pregnancy, medications, substance abuse, chorioamnionitis, premature rupture of membranes, meconium, mode of delivery, gestational age, Apgar score at 5, minutes and infant's complications at birth. Immediately prior to delivery, 10 mL of venous blood was drawn from all mothers and umbilical vein of placenta. The sample was divided into three aliquots: 2 mL for the HbA1c, 2 ml for the fructosamine measurements in mothers blood, the remaining 6 mL aliquot was centrifuged (2500 rpm for 20 minutes), plasma recovered and frozen at -80°C. Immediately after delivery of the placenta, 6 mL of cord blood was drawn from umbilical vein, centrifuged, and plasma was frozen at -80°C.
Human pro-monocytic cell line and treatment with acetoacetate and β-hydroxybutyrate
The U937 monocytic cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA). These cells were maintained at 37°C in RPMI 1640 medium containing 7 mM glucose, 10% (v/v) heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 12 mM sodium carbonate, 12 mM HEPES and 2 mM glutamine in a humidified atmosphere containing 5% (v/v) CO2 and air. Cells were washed once in plain RPMI 1640 before suspension in fresh medium containing serum and other supplements [5, 6]. The U937 cells (500,000 cells/ml) were treated with normal glucose (7 mM) without and with AA or BHB (0-4 mM). For cytokine secretion studies, cells were stimulated with lipopolysaccharide (LPS, 2 μg/ml) at 37°C for 24 h. Values obtained with cells incubated with LPS alone were considered as controls. All experiments were repeated four times.
HbA1c and fructosamine assays
HbA1c was determined using HPLC at the clinical laboratory of LSUHSC-Shreveport. This assay is standardized according to the National Glycohemoglobin Standardization Program (NGSP) used for analysis. Fructosamine level was determined using a commercially available automated colorimetric NitroBlue tetrazolium (NBT) method [6].
IL-8, MCP-1, ketone bodies and oxidative stress (protein oxidation) assays
IL-8 and MCP-1 levels were determined by the sandwich ELISA method using commercially available kits from Fisher Thermo Scientific Co. (Rockford, IL). All appropriate controls and standards as specified in the manufacturer's kit were used. During the cytokine assays, control samples were analyzed each time to check the variation from plate to plate on different days of analysis. The protein oxidation was assessed by determining the protein carbonyl levels in the plasma using an ELISA kit from ENZO Life Sciences International Inc. (Plymouth Meeting, PA). The concentrations of acetoacetate and β-hydroxybutyrate in plasma were determined as described previously [5]. The Viability of cells in cell culture experiments was determined using the Alamar Blue reduction bioassay (Alamar Biosciences, Sacramento, CA). This method is based upon Alamar Blue dye reduction by live cells. All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise mentioned. Data were analyzed statistically using one-way ANOVA among different groups using Sigma Stat and Sigma Plot software (Jandel Scientific, San Rafael, CA). A p value of less than 0.05 was considered significant.
Results
Table 1 gives the maternal age, fructosamine and HbA1c levels, and birth weight, gestational age and length of hospital stay of the newborns. Maternal age in the two groups was similar. Although IDM were larger than normal infants, the difference in their birth weights was not statistically significant. However, IDM had slightly shorter gestational ages compared to those of the normal infants. The HbA1c and fructosamine levels of diabetic mothers were higher compared with those of healthy mothers (p<0.01). Apgar scores were similar in the two groups, and none of the infants had meconium stained amniotic fluid. None of the mothers in the diabetes or the control (healthy) groups had cholrioamnionitis or history of substance abuse. Sepsis was neither suspected or diagnosed in the newborn infants. Amongst the infants born to diabetic mothers, six had hypoglycemia which resolved within a few hours, 5 had macrosomia and 3 had congenital malformations (1 cleft lip and palate, 1 hypospadius, 1 ear anomaly).
Table 1.
Age, HbA1C and fructosamine levels of mothers, and infant's birth weight, gestational age and length of stay. Data is given as Mean±SEM.
| Infants of diabetic mothers (n=23) | Infants of healthy mothers (n=13) | p | |
|---|---|---|---|
| Mother's age (yrs) | 26.8 ± 1.3 | 26.5 ± 1.7 | NS |
| Mother's HbA1C (%) | 7.0 ± 0.3 | 5.6 ± 0.1 | 0.01 |
| Mother's Fructosamine (mM) | 198±8 | 166±3 | 0.01 |
| Newborn birth weight (gm) | 3737±118 | 3221±81 | NS |
| Gestational age (wks) | 37.6 ± 0.2 | 39.3 ± 0.3 | 0.05 |
| Newborn Length of stay (days) | 3.9 ± 0.5 | 2.2 ± 0.1 | 0.02 |
Acetoacetate and β-hydroxybutyrate levels in infants of diabetic mothers and infants of healthy mothers are shown in Table II. There was a significant increase in plasma acetoacetate levels in IDM compared with those of infants born to healthy mothers. Total ketone levels were obtained by adding the concentrations of acetoacetate and β-hydroxybutyrate. There was a significant correlation in levels of total ketones (r=0.50, p=0.016) and β-hydroxybutyrate (0.49, p=0.018) but not acetoacetate (0.26, p=0.22) between mothers and their respective infants’ cord blood in the diabetic group. There was no relationship to any ketone levels between mothers and cord blood in the healthy group. Table II also gives MCP-1 and protein oxidation levels in infants of diabetic and infants of healthy mothers. There was a significant increase in protein oxidation and MCP-1 levels in IDM compared with infants born to healthy mothers (p<0.05). The level of MCP-1 significantly correlated with those of total ketone bodies (r=0.41, p=0.05) and acetoacetate (r=0.51, p=0.01). When data from cord blood of all diabetic patients was separated in to type 1 diabetics versus gestational and type 2 diabetes groups, there was an increase in MCP-1 levels in cord blood of type 1 mothers (713±211 pg/ml) versus cord blood of normal mothers (269±32 pg/ml) but it was not significant (p=0.097) in the subgroup of gestational and type 2 diabetes. The MCP-1 levels in cord-blood of mothers with gestational diabetes (493±162 pg/ml) was similar to healthy mothers. No relationship of gestational age was seen with MCP-1 (r= 0.15, p=0.50) in cord blood samples. Similarly, there was no relationship between blood MCP-1 levels and fetal birth weight in this study (r=0.14, p=0.51). Thus, differences in gestational age or fetal birth weight are unlikely to have any effect on changes in MCP-1.There was no relationship between MCP-1 levels with maternal fructosamine or HbA1c levels at delivery. The increase in plasma levels of IL-8 in IDM compared with those of infants of healthy mothers was not statistically significant (Table 2).
Table 2.
Plasma inflammatory markers and ketones levels in diabetic and healthy groups. Data is given as Mean±SEM.
| Diabetic Mothers (n=23) | Healthy Mothers (n=13) | p | IDM (n=23) | Infants of healthy mothers (n=13) | p | |
|---|---|---|---|---|---|---|
| IL-8 (pg/ml) | 7.95 ± 2.6 | 9.65 ± 2.2 | NS | 13.84 ± 3.04 | 9.27 ± 2.15 | NS |
| MCP-1(pg/ml) | 277 ± 51 | 328 ± 63 | NS | 608.4 ± 133.6 | 269.4 ± 32.1 | 0.04 |
| Acetoacetate (mM) | 0.38 ± 0.12 | 0.33 ± 0.12 | NS | 0.31 ± 0.06 | 0.15 ± 0.05 | 0.048 |
| â-hydroxybutyrate(mM) | 2.4 ± 0.47 | 1.38 ± 0.41 | NS | 1.68 ± 0.28 | 1.39 ± 0.46 | NS |
| Total Ketones (mM) | 2.8 ± 0.5 | 1.71 ± 0.52 | NS | 1.99 ± 0.33 | 1.54 ± 0.47 | NS |
| Protein Carbonyl (μg/ml) | 0.38 ± 0.07 | 0.31 ± 0.14 | NS | 0.30 ± 0.05 | 0.16 ± 0.04 | 0.047 |
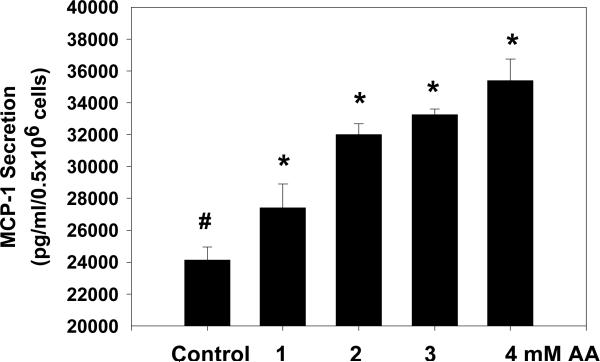
Figure 1 illustrates that treatment of monocytes with acetoacetate resulted in a concentration dependent increase in secretion of MCP-1, suggesting that acetoacetate can stimulate MCP-1 secretion. In contrast, BHB treatment (1-5 μM) did not show any effect on MCP-1 secretion (data not given here). AA or BHB treatment had no effect on cell viability (data not shown here). There was no change in pH of the culture medium after addition of acetoacetate. Air+CO2 was used during cell culture incubation studies. The present study observed AA levels in the range of 0.1-0.93 mM in cord blood of diabetic mothers and up to 1.96 mM in mother's blood. However, studies in literature have reported more than 4mM AA levels in the blood of diabetic patients (13). Thus, AA conc. of 1 mM is quite close to the physiological range. Our previous study has shown an increase in MCP-1 after AA treatment of monocyte-rich human peripheral blood mononuclear cells isolated from human blood without activation with LPS (7). Stimulation of monocyte with LPS has been used by many investigators for cell culture studies.
Figure 1.
The effect of acetoacetate (AA) on MCP-1 secretion in U937 monocytes. Values are Mean±SEM.
Discussion
Approximately 100,000 IDM are born in the United States annually, some of whom may be at increased risk for metabolic and other complications [1]. The biochemical mechanisms leading to these complications are not completely understood. Recent studies suggest that hyperketonemia, oxidative stress and vascular inflammation may each play a role in the development of fetal complications during diabetic pregnancies. Ketone bodies can cross the placenta and their levels have been found to be elevated in fetuses of diabetic mothers in pregnant Ewe [12]. At birth, an increase in inflammatory markers, such as CRP and ICAM-1 has been reported in the offspring of type I diabetic mothers [14, 15]. However, the effect of hyperketonemia on markers of vascular inflammation and oxidative stress in infants of diabetic mothers has not been investigated. For the first time, this study demonstrates increased levels of MCP-1 and protein oxidation levels in the cord blood of IDM (p<0.05) but not in cord blood of infants born to normal mothers. The level of MCP-1 significantly correlated (p=0.01) with the concentration of aceotoacetate in the cord blood of IDM. A significant correlation of MCP-1 with aceotoacetate levels in the blood and increased secretion of MCP-1 in acetoacetate treated monocytes suggests that hyperketonemia may contribute to elevated MCP-1 levels in IDM.
Monocyte chemotactic protein-1 (MCP-1) is a chemokine secreted by different cell types [16]. It attracts and monocytes into an area of inflammation and then activates them. Several studies have examined its role during normal and abnormal pregnancies and during the neonatal period [17, 18]. Denison et al. found that MCP-1 is important in the development of the placenta and thus, maintaining a normal pregnancy [18]. Briana et al estimated maternal and cord blood MCP-1 levels, and reported significantly lower levels in growth retarded infants compared to healthy controls [18]. MCP-1 levels are increased in women with type 1 and 2 diabetes [19, 20]. Two separate studies from Poland reported that women with gestational diabetes showed increased levels of the chemokine MCP-1, possibly leading to adverse pregnancy outcomes [21, 22]. This study did not find significant increase in the levels of IL-8 in the cord blood of IDM or the cord blood of infants born to normal mothers.
This study observed elevated levels of protein carbonyl, a marker of oxidative stress. Kinalski et al study in diabetic mothers suggest that their fetuses experience increased oxidative stress (23). The results show that acetoacetate, but not β-hydroxybutyrate, increased MCP-1 secretion in U937 monocytes exposed to high glucose. The reasons that one ketone body as opposed to the other affect inflammation differently remain unclear. We speculate that effects caused by AA may be due to the generation of oxygen radicals either directly or indirectly. It has been shown that AA but not BHB can generate oxygen radicals in a cell free system [24-26], which may ultimately lead to an oxidative environment. It is also possible that AA is indirectly producing ROS by being fed into the electron transport chain, creating an overload of electrons similar to that seen with hyperglycemia [24]. Since studies have also shown a decrease in the mitochondrial membrane potential in monocytes treated with AA, it seems likely that the mitochondria would be the second source of ROS in monocytes treated with AA. Another possibility is that AA is converted to BHB, which in turn can alter the redox state of the cell by affecting both NADH and GSH levels. Structurally the two compounds are very similar, but there is no ketone functional group present in BHB. AA contains two keto groups whereas BHB does not, suggesting that the difference in chemical structure of the two ketone bodies may play a role in mediating the effects caused by AA and not BHB. Acetoacetate is much less stable than β-hydroxybutyrate. Previous studies have also reported that the ketone body aceotoacetate can increase oxygen radical formation and oxidative stress [24-26]. However, we found no correlation between levels of acetoacetate and protein oxidation levels in IDM. The long term outcomes of IDM exposed to oxidative stress is unknown.
In conclusion, this study demonstrate that increase in circulating MCP-1 and protein oxidation levels in the cord blood of IDM compared to those of normal infants suggest increased inflammation and oxidative stress. The hyperglycemic environment reflected by higher HbA1c and fructosamine levels in diabetic mothers and the presence of the acetoacetate ketone may be a factor in the increased MCP-1 levels in the cord blood of IDM. The increase in oxidative stress may also result from elevated acetoacetate levels and/or glycosylation of antioxidative defense enzymes due to hyperglycemia. The role of elevated MCP-1 levels in the metabolic complications common to diabetic pregnancies needs further investigation in a larger patient population.
Acknowledgements
The authors thank Ms Georgia Morgan for excellent editing of this manuscript. The authors are supported by grants from NIDDK and the Office of Dietary Supplements of the National Institutes of Health RO1 DK072433, and the Malcolm Feist Endowed Chair in Diabetes.
Footnotes
None of the authors has any financial interest in the publication of this manuscript, nor have they received any money from any sources other than the NIH or LSUHSC.
References
- 1.Yogev Y, Visser GH. Obesity, gestational diabetes and pregnancy outcome. Semin Fetal Neonatal Med. 2009;14:77–84. doi: 10.1016/j.siny.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Agoudemos M, Reinking BE, Koppenhafer SL, Segar JL, Scholz TD. Programming fo adult cardiovascular disease following exposure to late-Gestation hyperglycemia. Neonatology. 2011;100:198–205. doi: 10.1159/000324863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koskinen A, Laiho A, Lukkarinen H, Kaapa P. Maternal hyperglycemia modifies extracellular matrix signaling pathways in neonatal rat lung. Neonatology. 2010;98:387–396. doi: 10.1159/000317010. [DOI] [PubMed] [Google Scholar]
- 4.Newton CA, Raskin P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: clinical and biochemical differences. Arch Intern Med. 2004;164:1925–31. doi: 10.1001/archinte.164.17.1925. [DOI] [PubMed] [Google Scholar]
- 5.Jain SK, Kannan K, Lim G, Matthews-Greer J, McVie R, Bocchini JA., Jr Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care. 2003;26:2139–43. doi: 10.2337/diacare.26.7.2139. [DOI] [PubMed] [Google Scholar]
- 6.Jain SK, Kannan K, Lim G, McVie R, Bocchini JA., Jr Hyperketonemia increases tumor necrosis factor-alpha secretion in cultured U937 monocytes and Type 1 diabetic patients and is apparently mediated by oxidative stress and cAMP deficiency. Diabetes. 2002;51:2287–93. doi: 10.2337/diabetes.51.7.2287. [DOI] [PubMed] [Google Scholar]
- 7.Rains JL, Jain SK. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM 1 expression in endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:E298–306. doi: 10.1152/ajpendo.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornton NL, Cody MJ, Yost CC. Toll-like receptor ½ stimulation induces elevated interleukin-8 secretion in polymorphonuclear leukocytes isolated from preterm and term newborn infants. Neonatology. 2012;101:140–146. doi: 10.1159/000330567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verbruggen SC, Landzaat LJ, Reiss IKM, van Goudoever JB, Joosten KFM. Efficacy and safety of a tight glucose control protocol in critically ill term neonates. Neonatology. 2012;101:232–238. doi: 10.1159/000330846. [DOI] [PubMed] [Google Scholar]
- 10.Dahlgren J, Nilsson C, Jennische E, Ho HP, Eriksson E, Niklasson A, Björntorp P, Albertsson Wikland K, Holmäng A. Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab. 2001;281:E326–34. doi: 10.1152/ajpendo.2001.281.2.E326. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo T, Metzger BE, Burns WJ, Burns K. Correlations between antepartum maternal metabolism and child intelligence. N Engl J Med. 1991;325:911–6. doi: 10.1056/NEJM199109263251303. 26. [DOI] [PubMed] [Google Scholar]
- 12.Clark KE, Miodovnik M, Skillman CA, Mimouni F. Review of fetal cardiovascular and metabolic responses to diabetic insults in the pregnant ewe. Am J Perinatol. 1998;5:312–8. doi: 10.1055/s-2007-999716. [DOI] [PubMed] [Google Scholar]
- 13.Candiloros HMS, Zeghari N, Donner M, Drouin P, Ziegler O. Decreased erythrocyte membrane fluidity in poorly controlled IDDM. Influence of ketone bodies. Diabetes Care. 1995;18:549–551. doi: 10.2337/diacare.18.4.549. [DOI] [PubMed] [Google Scholar]
- 14.Nelson SM, Sattar N, Freeman DJ, Walker JD, Lindsay R. Inflammation and endothelial activation is evident at birth in offspring of mothers with type 1 diabetes. Diabetes. 2007;56:2697–704. doi: 10.2337/db07-0662. [DOI] [PubMed] [Google Scholar]
- 15.Lindegaard ML, Svarrer EM, Damm P, Mathiesen ER, Nielsen LB. Increased LDL cholesterol and CRP in infants of mothers with type 1 diabetes. Diabetes Metab Res Rev. 2008;24:465–71. doi: 10.1002/dmrr.867. [DOI] [PubMed] [Google Scholar]
- 16.Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411:1570–9. doi: 10.1016/j.cca.2010.07.006. 11. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi N, Uehara R, Kobayashi M, Yada Y, Koike Y, Kawamata R, Odaka J, Honma Y, Momoi MY. Cytokine profiles of seventeen cytokines, growth factors and chemokines in cord blood and its relation to perinatal clinical findings. Cytokine. 2010;49:331–7. doi: 10.1016/j.cyto.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Denison FC, Kelly RW, Calder AA, Riley SC. Cytokine secretion by human fetal membranes, decidua and placenta at term. Hum Reprod. 1998;13:3560–5. doi: 10.1093/humrep/13.12.3560. [DOI] [PubMed] [Google Scholar]
- 19.Piemonti L, Calori G, Mercalli A, Lattuada G, Monti P, Garancini MP, Costantino F, Ruotolo G, Luzi L, Perseghin G. Fasting plasma leptin, tumor necrosis factor-alpha receptor 2, and monocyte chemoattracting protein 1 concentration in a population of glucose-tolerant and glucose-intolerant women: impact on cardiovascular mortality. Diabetes Care. 2003;26:2883–9. doi: 10.2337/diacare.26.10.2883. [DOI] [PubMed] [Google Scholar]
- 20.Nomura S, Shouzu A, Omoto S, Nishikawa M, Fukuhara S. Significance of chemokines and activated platelets in patients with diabetes. Clin Exp Immunol. 2000;121:437–443. doi: 10.1046/j.1365-2249.2000.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lappas M, Hiden U, Desoye G, Froehlich J, Hauguel-de Mouzon S, Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal. 2011;15:3061–100. doi: 10.1089/ars.2010.3765. [DOI] [PubMed] [Google Scholar]
- 22.Wender-Ozegowska E, Michalowska-Wender G, Zawiejska A, Pietryga M, Brazert J, Wender M. Concentration of chemokines in peripheral blood in first trimester of diabetic pregnancy. Acta Obstet Gynecol Scand. 2008;87:14–9. doi: 10.1080/00016340701698724. [DOI] [PubMed] [Google Scholar]
- 23.Kinalski M, Sledziewski A, Telejko B, Kowalska I, Kretowski A, Zarzycki W, Kinalska I. Lipid peroxidation, antioxidant defence and acid-base status in cord blood at birth: the influence of diabetes. Horm Metab Res. 2001;33:227–31. doi: 10.1055/s-2001-14953. [DOI] [PubMed] [Google Scholar]
- 24.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–75. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain SK, McVie R. Hyperketonemia can increase lipid peroxidation and lower glutathione levels in human erythrocytes in vitro and in type 1 diabetic patients. Diabetes. 1999;48:1850–5. doi: 10.2337/diabetes.48.9.1850. [DOI] [PubMed] [Google Scholar]
- 26.Jain SK, Kannan K, Lim G. Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic Biol Med. 1998;25:1083–8. doi: 10.1016/s0891-5849(98)00140-3. [DOI] [PubMed] [Google Scholar]