Abstract
How can adverse experiences in early life, such as maltreatment, exert such powerful negative effects on health decades later? The answer may lie in changes to DNA. New research suggests that exposure to stress can accelerate the erosion of DNA segments called telomeres. Shorter telomere length correlates with chronological age and also disease morbidity and mortality. Thus, telomere erosion is a potential mechanism linking childhood stress to health problems later in life. However, an array of mechanistic, methodological, and basic biological questions must be addressed in order to translate telomere discoveries into clinical applications for monitoring health and predicting disease risk. This paper covers the current state of the science and lays out new research directions.
Keywords: aging, childhood stress, mechanism, telomere erosion, telomere length
Introduction
Stress in early life is known to have a powerful direct negative effect on health in later life. This direct effect requires one or more underlying mechanisms that can maintain it throughout the course of life. Interest in the etiological pathways that mediate the effect of early-life stress on physical and mental health has focused on key biological systems, including the sympathetic nervous system, hypothalamus-pituitary-adrenal axis, immune system, and the epigenome, leading to important insights into the systemic effects of stress [1, 2]. Some of the adversities associated with early life trauma include neurological and respiratory problems, cardiovascular disease, and metabolic disorders, to name but a few [3, 4]. However, the questions of how and when childhood stress impacts at the cellular level, specifically in humans, remain to be answered.
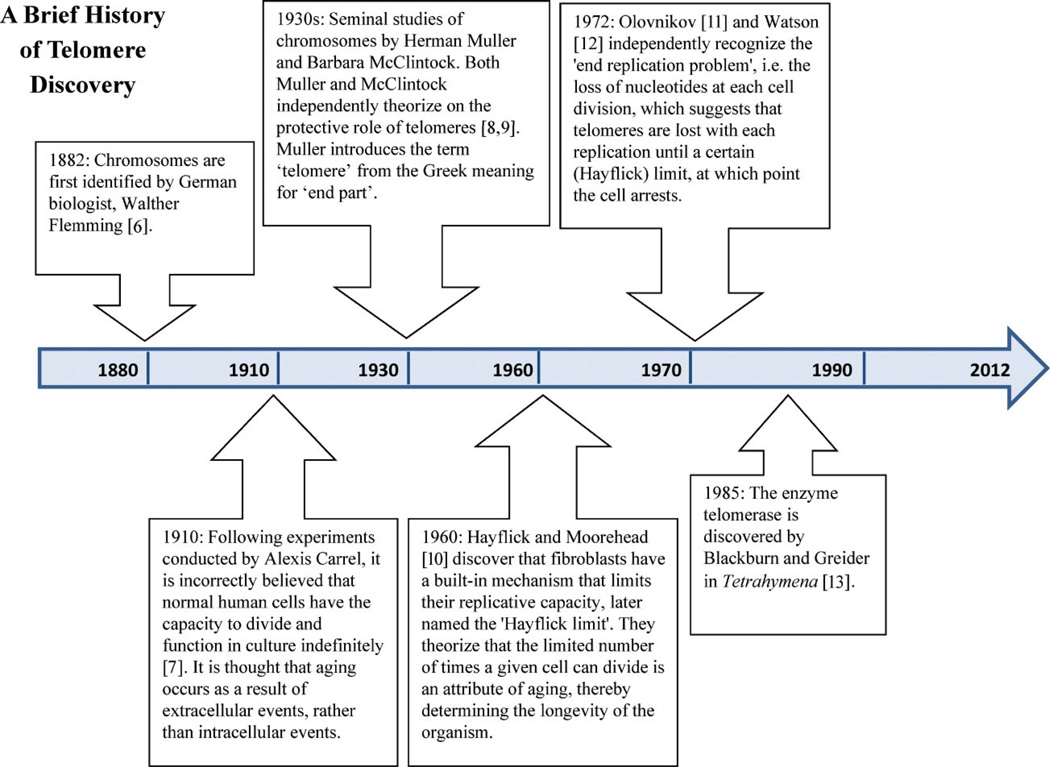
Previous studies have suggested telomere erosion as a potential mechanism linking stress to disease and mortality in humans. Telomeres are the repetitive TTAGGG sequences at the end of chromosomes that, together with the shelterin protein complex, function to cap and protect the ends of chromosomes from the DNA damage response. The repetitive non-coding sequence of telomeres is conserved in all vertebrates, and is thought to have arisen from a common ancestor over 400 million years ago [5]. In certain cell types, such as germ cells and stem cells, telomere length (TL) is maintained by the enzyme telomerase, which can add telomeric repeats to the ends of chromosomes.Most somatic cells, however, lack sufficient telomerase and, as a consequence, telomeres progressively shorten with each cell division. Upon reaching a critically short TL, cells enter a state of replicative arrest called senescence. The discovery of telomeres has a long and intriguing history dating back to the early 20th century [6–13] (see Fig. 1). Today, it is widely accepted that telomeres become shorter with each cell division. Indeed, they have been established as a molecular clock for cellular replicative aging [14, 15].
Figure 1.
Timeline – a brief history of telomere discovery.
The importance of telomere biology in mediating the effects of early life trauma stems from its ancient role in regulating cellular replication and from its vast interactions with other systems and factors that are thought to mediate these adverse effects (e.g. immune and endocrine systems, oxidative stress, and mitochondrial dysfunction). However, as more studies accumulate that link early life stress to shorter TL and a higher telomere erosion rate, important questions are emerging. How can short TL lead to health problems that only emerge after 30 or 40 years? Which types, or features, of stress matter the most? Given the plurality of different measurement methods to assess TL, can we recommend an optimal way to measure telomeres? Moreover, do we really understand the functional differences of TL in different types of tissue? Finally, since longitudinal studies suggest that, in certain individuals, telomeres can lengthen over time in telomerase-deficient cells, we need to critically assess the veracity of this observation. If true, what are the mechanisms and what are the implications? Given the potential of TL to predict disease risk, these and other questions are extremely important if we want to understand the underlying mechanisms and to push forward the application of TL in clinical settings.
By now telomeres, and the associated enzyme telomerase, have become one of the principal focuses of research into lifespan extension, for which they have gained a reputation as a potential “fountain of youth”. Indeed, the general consequences of unraveling the exact mechanisms by which TL influences lifespan and healthy aging could be immense. However, many obstacles still remain. In this paper, I try to clarify some of the topical questions in the field of early life stress and TL by integrating the most up-to-date literature. Due to its multi-disciplinary nature and the ever expanding literature on the study of telomeres, this paper is not an attempt for in-depth coverage of the field. Instead, I present potential mechanisms, raise important questions and caveats, and suggest several pathways for intervention in order to advance our understanding so that this information can be translated into clinical applications. For the interested reader, I provide relevant references for further reading on key aspects throughout this paper.
Telomere length is an important predictor of physical health and quality of life, but why?
Shorter TL and an increased telomere erosion rate are both associated with higher risk of morbidity and mortality. In vitro studies have established a causal link between telomere shortening and cellular senescence, leading to growth arrest [16]. Studies using animal models have provided support for the predictive utility of TL early in life on the actual lifespan of birds [17] and the potential to reverse tissue degeneration in aged, telomerase-deficient mice through telomerase activation [18]. Other studies have shown that shortened telomeres are also associated with a lower survival rate in humans [19]. Individuals aged 60 years or older with shorter TL in blood cells were at higher risk of mortality from heart disease and infectious disease [19]. In another study, centenarians and their offspring maintained longer leukocyte TL, on average, than unrelated controls of advancing age [20]. In addition, longer TL was related to a healthy profile including protection from age-related disease, better cognitive function and a healthy lipid profile [20]. However, another study failed to replicate the association between shorter TL and mortality; although longer TL in white blood cells was associated with better health status, as self-reported by participants [21].
The advent of high-throughput and cost-effective laboratory techniques that measure TL has paved the way toward more studies linking shorter TL in blood cells with a broad range of risk factors that predict disease morbidity, including smoking and obesity [22], psychosocial stress [23, 24], mood disorders [25], schizophrenia [26], and cancer [27]. Thus, it could be suggested that TL is more a marker for biological aging than a clock for chronological aging.
Some recent literature documents telomere erosion during childhood stress; other studies lack clarity as to cause and effect in later life
In the past two years, several studies of adult participants have provided support for an association between a childhood history of stress and shorter TL in blood cells [24, 28–30]. In contrast to these previous findings, another study failed to replicate the association between leukocyte TL and physical and sexual abuse in childhood in a large cohort of adult twins [31]. In the first study of children, greater exposure to institutional care was significantly associated with shorter TL in buccal cells in middle childhood [32].
Although these studies have advanced our understanding of the link between childhood stress and TL, they possess several limitations: (i) almost all studies of the violence-TL association have relied on retrospective self-reporting of childhood experiences; (ii) all studies have used a cross-sectional design measuring TL at one time-point and, thus, were unable to assess change in TL over time; and (iii) the elapsed time between putative exposure to violence and measurement of TL has been decades [24, 28–30]. For example, given that maltreated children often grow up to be in poor physical health as adults [3], telomere erosion could be a consequence of later health problems, as opposed to a proximal effect of the maltreatment itself.
The hypothesis that childhood exposure to violence would accelerate telomere erosion was recently tested in the first prospective longitudinal study in children [33]. Based on evidence that the effects of stress are cumulative [2], the hypothesis was that cumulative exposure to violence would be associated with accelerated telomere erosion. Indeed, only children who experienced two or more kinds of violence (exposure to maternal domestic violence, frequent bullying victimization, or physical maltreatment by an adult) showed significantly greater telomere erosion in buccal cells between age-5 (baseline) and age-10 (follow-up) measurements, even after adjusting for confounding factors [33]. This finding has provided the first evidence that stress-related accelerated telomere erosion in buccal cells can already be observed at a young age, while children are experiencing stress. However, questions remain about the mechanistic pathways that lead from telomere erosion during childhood to disease risk in later life.
What are the mechanistic pathways linking childhood stress to accelerated telomere erosion?
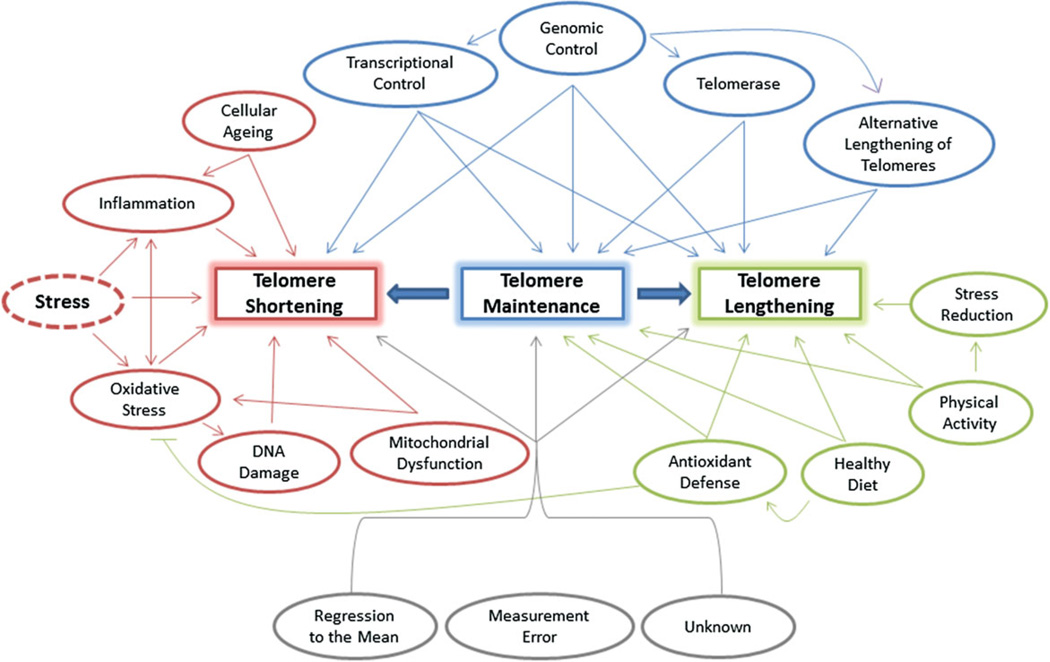
One of the most challenging questions concerns our understanding of the link between childhood stress, and stress in general, to telomere dynamics. Some factors that may influence the degree of telomere dynamics, and that link short TL to disease, involve genetic regulation [20, 34], epigenetic modification [35], or transcriptional control [36, 37] (Fig. 2). In the case of childhood stress, the effect of stress on TL during sensitive developmental periods and age-dependent maturation of the brain and immune system [2] may play a critical role in precipitating this long-term damage. Currently, most of the insights into mechanisms associated with telomere erosion originate from research on inflammation and oxidative stress, indicating that both have important influences on TL [38, 39].
Figure 2.
Schematic representation of TL regulation by different factors.
Inflammation and the inflammatory response are known to be triggered by stress. Chronic activation of the inflammatory response contributes to the pathophysiology of several chronic diseases and predicts elevated risks for cardiovascular disease, type-2 diabetes, major depression, and dementia [40, 41]. Several studies have shown that childhood stress causes elevated inflammation [42] and also that individuals with early life stress have a heightened inflammatory response to psychosocial stress [43]. Moreover, older adults that experienced childhood adversity have evidenced both higher amounts of inflammatory markers and shorter TL in blood cells [24]. Inflammation is also associated with increased proliferation of immune cells and, as a consequence, with greater telomere erosion [44]. These studies suggest a mediating role for inflammation in linking childhood stress to telomere erosion. However, another potential mechanism may suggest the opposite, whereby telomere erosion mediates the effects of early life trauma on inflammation. An important feature of telomere-induced senescent cells, apart from growth arrest, is the observation of increased secretion of chemokines and inflammatory factors (such as interleukins 6 and 8). This effect is known as the senescence-associated secretory phenotype (SASP) [45]. Thus, increased senescence rate, as a result of an increased rate of telomere erosion, will consequently increase the secretion of inflammatory markers associated with SASP. This cycle, if chronically activated, suggests a possible cause for the increased telomere erosion and inflammation levels observed in victims of violence.
Oxidative stress is another potential pathway that can lead to increased telomere erosion. Telomeres are sensitive to damage by oxidative stress, as demonstrated by experiments showing increased erosion under conditions of high levels of reactive oxygen species (ROS) in vitro [39]. In humans, oxidative stress has been associated with increased perceived stress and shorter TL in peripheral blood mononuclear cells [23]. Moreover, TL, oxidative stress and elevated ROS are tightly linked to mitochondrial function. The mitochondrial-dysfunction route to aging, stemming from free-radical damage to mitochondrial DNA (i.e. the free-radical theory of aging), is a well-recognized theory dating back to the 1950s [46]. Telomere dysfunction is associated with mitochondrial impairment, which induces more DNA damage by increased ROS production. Increased ROS production, in turn, induces more rapid erosion of telomeres, resulting in a domino effect of greater DNA damage and increased telomere erosion [47].
The endocrine system is another plausible route mediating the effects of early life stress. The connection between cortisol (the primary stress hormone), oxidative stress, and cell senescence is well established [48]. Cortisol has been associated with reduced telomerase activation of human T lymphocytes in culture [49]. Similarly, higher levels of cortisol in response to a laboratory stressor have been associated with shorter TL in buccal cells of 5-to-6-year old children [50]. Overall, stress-induced secretion of cortisol may down-regulate the activity of telomerase and increase oxidative stress which, in turn, can lead to more rapid erosion of telomeres. More research is needed to test whether the effects of stress on telomere erosion are mediated by changes in the immune and endocrine systems, oxidative stress, mitochondria dysfunction, or other factors in children.
Intriguingly, two recent studies have revealed another potential mechanism unrelated to telomere erosion [51, 52]. The authors showed that, in fibroblast cells, up to half of the DNA damage foci in stress-induced senescence, whether caused endogenously by oxidative stress or exogenously by DNA-damaging agents, were located at telomeres [51]. Moreover, the DNA damage which, in turn, leads to cell senescence, is irreparable at telomeres. In addition, a DNA damage response may arise independently of telomere erosion and can also be observed in long telomeres [52]. This mechanism seems to be conserved throughout evolution as it has been observed in yeast, rodent, and primate cells. Since longer telomeres are probably more exposed to DNA-damaging agents, it is tempting to suggest that very long TL may induce cell senescence at a similar rate as very short TL. This may imply a potential U-shaped response curve, with very short and very long TL predicting disease risk. More research is needed to test this conjecture.
The results of these studies imply that telomeres are important targets for stress both in vitro and in vivo, and raise more questions about the complex mechanism behind telomere-induced cell senescence. From an evolutionary standpoint, it appears to be an elegant mechanism to prevent cells that have accumulated genomic damage from dividing, thereby preventing de novo mutation and abnormal genomic function that may eventually lead to cancer and other types of diseases. It seems that telomeres act as cellular “sensors” for detecting DNA damage. By sensing this damage, telomeres send a warning signal to the cell that damage has potentially accumulated in other regions along chromosomes and other cell compartments. Thus, senescence-induced telomere damage can act as a tumor-suppressor to prevent disease and mortality.
Open questions in the study of childhood stress and telomere length
Although recent findings support the hypothesis of stress-related telomere erosion, even for the young, and more studies provide plausible mechanistic pathways, there are caveats and open questions that require further research. In the next sections, I present some of the questions that need to be answered in order to advance our understanding of how stress impacts telomeres.
Which types of stress matter the most? How does type, severity, and chronicity of stress exposure relate to telomere erosion?
Although studies have reported an association between different kinds of exposure to violence and telomere erosion, which types of stress matter the most is not entirely clear (e.g. physical abuse, domestic violence, bullying victimization, sexual abuse, emotional abuse, physical neglect, or emotional neglect). Some studies indicate that the effect might be seen most clearly when stress is cumulative, regardless of type [2, 29, 33]. It has been suggested that repeated exposure to stress, which results in chronic activation of the endocrine and immune systems, and the overall heightened chronic physiological overload of the body (allostatic load), may lead to telomere damage. However, other factors, such as severe single stressors, should not be excluded. For example, recent death of a first-degree relative or spouse has been associated with shorter leukocyte TL in women [53]. This raises an important caveat in stress research generally, i.e. that all exposures are not necessarily alike and that considerable care needs to be taken when attempting to synthesize and interpret research on the psychobiological sequelae of stress exposures. As more studies accumulate, it will be possible to determine the specific type and features of a stressor that matter most in relation to telomere erosion (e.g. duration, severity, physical harm, perceived threat). In addition, testing for the most sensitive periods for exposure and dose-response gradients (e.g. exposure in childhood vs. adolescence; single vs. recurrent events; single vs. multiple types of violence) can also be investigated.
What is the optimal method for measuring telomere length?
There are tradeoffs regarding the best way to measure TL [54]. The four main methods to assess TL are: (i) Southern blot analysis of the terminal restriction fragments (TRF) [55]; (ii) Quantitative RT-PCR to measure the ratio between single-copy gene and telomeric repeat regions (T/S ratio) [56]; (iii) Fluorescence in situ hybridization (FISH), Quantitative-FISH (Q-FISH) and flow-FISH methods [57, 58]; and (iv) single telomere length analysis (STELA) [59]. The “gold standard” southern blot technique uses restriction enzymes to measure TL in kilobases; not only of the telomeric region (i.e. the TTAGGG repeat), but also the sub-telomeric region. Both the T/S ratio and FISH methods measure the average TL of the telomeric region, whereas the STELA and FISH methods can also determine the specific TL in distinct cell populations or measure the specific length in individual cells or chromosomes. However, the successful application of FISH methods requires intact nuclei and metaphase cells, while STELA requires expertise in single molecule techniques, which limits their utility for high-throughput settings. Nevertheless, as telomere-induced senescence may rely on a few (or even single) critically short telomeres [60], future research will benefit by utilizing FISH and STELA for high resolution analyses of telomere dysfunction. The principal advantage of the T/S ratio over the other methods is that it can be performed at high-throughput and low cost. However, this method can suffer from greater measurement error compared to other methods.
In summary, although all methods are highly correlated [61, 62], each method has advantages and disadvantages and the optimal way to measure TL is still debated. The use of different methods makes it difficult to compare mean values between studies or to establish normative reference values for a particular age group. Table 1 provides an example of the heterogeneity between study designs, age groups, and tissue types for studies that have used the TRF method to measure TL. The first attempt to compare southern blot and T/S ratio methods, by two of the leading laboratories in the field, yielded a strong correlation between both methods [62]. However, the relationship of TL values between the methods deviated from linearity and the sample size was relatively small [62]. As has previously been suggested [63], an impartial evaluation of the different methods for measuring TL, using comparative studies, is needed to establish an appropriate framework for future studies.
Table 1.
Representative studies that have measured TL using southern blot analysis of TRF from different tissues
|
Reference |
N (male/female) |
Age |
Tissue |
Study outcome |
Mean telomere lengtha in Kbp |
|---|---|---|---|---|---|
| Glass et al. (2010) [31] |
520 Control (n/a) 20 Cases (n/a) |
Adults | Leukocyte | Physical and sexual abuse |
6.97 (SD = 0.67) 7.04 (SD = 0.58) |
| Aviv et al. (2009) [75] |
450 Caucasians 185 African-Americans |
31.4 (at baseline) 31.4 (at baseline) |
Leukocyte | Age-related longitudinal analysis |
7.29 (SD = 0.73) 7.85 (SD = 0.73) |
| Cherkas et al. (2008) [103] |
2,152 Female 249 Male |
48.6 (+/−12.8) 48.3 (+/−13.7) |
Leukocyte | Physical activity in leisure time |
7.0 (SD = 0.6) |
| Damjanovic et al. (2007) [104] |
41 Controls (11/30) 41 Cases (11/30) |
65 (+/−1) | PBMC T cells Monocyte |
Immune function of Alzheimer’s caregivers |
6.2 (SE = 0.10) case 6.4 (SE = 0.10) control 6.3 (SE = 0.20) case 6.5 (SE = 0.20) control 5.7 (SE = 0.10) case 5.9 (SE = 0.10) control |
| Simon et al. (2006) [25] |
44 Controls (25/19) 44 Cases (23/21) |
50.5 (+/−8.4) 51.1 (+/−7.7) |
Leukocyte | Mood disorder | 7.64 (SD = 1.10) 6.98 (SD = 0.84) |
| Andrew et al. (2006) [105] |
2,050 Female | 47.8 (+/−12.4) range 18 to 80 |
WBC | Linkage analysis | 7.1 (SD = 0.69) range 5.1–9.4 |
| Okuda et al. (2002) [106] |
83 African-Americans (n/a) 83 African-Americans (n/a) 48 Caucasians (n/a) 43 Caucasians (n/a) 29 Hispanics (n/a) 27 Hispanics (n/a) |
Newborns | WBC Umbilical artery WBC Umbilical artery WBC Umbilical artery |
Telomere dynamics in newborns |
10.98 (SE = 0.09) 10.99 (SE = 0.08) 10.92 (SE = 0.11) 10.89 (SE = 0.09) 11.26 (SE = 0.10) 11.25 (SE = 0.12) |
| Friedrich et al. (2000) [72] |
9 Patients (n/a) Surgery for hip fractures |
73 to 95 | Leukocyte Skin Synovial |
Comparative tissue analysis |
6.54 (SD = 0.52) 7.79 (SD = 0.60) 7.91 (SD = 0.42) |
| Slagboom et al. (1994) [34] |
Young: 16 MZ, 15 DZ Adolescent: 15 MZ, 14 DZ Adult: 28 MZ, 27 DZ |
4.15 (+/−1.4) 17.1 (+/−2.4) 43.7 (+/−5.8) |
WBC | Heritability analysis | 8.3 (SD = 0.64) 7.8 (SD = 0.56) 7.3 (SD = 0.76) |
The implications of studies of telomere length from different types of tissue cells
Another important question concerns the measurement of TL from different types of cells [64]. Because of the ethical difficulties in obtaining blood from children, most studies of children have used buccal cells [32, 33, 50] instead of the peripheral blood cells more commonly used in studies of adults. However, buccal swabs may not only yield buccal cells. Infiltration of immune cells, which have different telomere dynamics than buccal cells, due to poor oral hygiene or infection during sampling can alter the oral cell composition.
In the case of estimating TL from blood cells, different populations of cells may give rise to misleading estimates of TL [57, 65]. For example, telomere erosion with age is more apparent in lymphocytes than granulocytes [66]. In a recent study, the rate of telomere erosion in memory T cells and mature natural killer cells was much higher compared to other leukocyte subpopulations [67]. As the immune system is affected differently by the internal and external environments, and since the performance of the immune system declines with age, estimating TL from blood may give conflicting results. Another problem to consider is that the activity of telomerase varies in different cells of the immune system, which also may affect the length of telomeres [68].
TL may vary among different types of cells due to factors such as cell turnover rates, stem cell capacity to regenerate or differentiate, exposure to oxidative damage or dynamic regulation of telomeres [66, 69]. Given the apparent complexity of telomere dynamics in white blood cells, buccal cells may provide a better estimate of TL since they are more inert and less influenced by regulatory factors. However, the reasons for changes in TL in buccal cells are poorly understood. In addition, TL from different types of cells can be used to answer different types of questions. For example, TL from lymphocytes can indicate the replicative history of hematopoietic stem cell and progenitor cells. Hepatocyte TL may be a marker for liver disease [70] and stem cell telomeres are targets for basic cancer research [27].
There are few studies on comparability of TL between different tissues [71–73]. In the case of buccal and blood cells, positive correlations have been reported between the TL of both cell types [74]; although one report showed no significant correlation [71]. Recent research has suggested that estimates of TL from buccal cells can detect the effects of stress on young children [32, 33, 50]; although validation in broader studies that measure TL from multiple tissues is needed (see Table 2 for all known published data on TL in buccal cells). An impartial evaluation of TL from cells of different tissue types is needed to establish a standard reference for future studies.
Table 2.
Comparison of different studies that have measured TL in human buccal cells
| Reference | N (male/female) | Age in years | Method | Study outcome | Telomere lengtha |
|---|---|---|---|---|---|
| Shalev et al. (2012) [33] |
236 Children (120/116) | 5 (baseline) 10 (follow-up) |
T/S ratio | Exposure to violence | 1.08 (SD = 0.44) range 0.02–3.07 0.96 (SD = 0.28) range 0.06–1.71 |
| Wong et al. (2011) [107] |
27 Cases (23/4) 24 Controls (20/4) 29 Case’s offspring (17/12) 25 Control’s offspring (9/16) |
69 (+/−6.9) 66 (+/−6.6) 40 (+/−6.8) 38 (+/−6.0) |
T/S ratio | Ischemic heart failure | 0.97 (SD = 0.27) 1.04 (SD = 0.19) 1.17 (SD = 0.42) 1.21 (SD = 0.25) |
| Kroenke et al. (2011) [50] |
78 Children (31/47) | 5 to 6 | T/S ratio | Laboratory-based stress challenge |
0.97 (SD = 0.26) range 0.33–1.59 |
| Drury et al. (2011) [32] |
100 Children (59/41) | 6 to 8 8 to 9 9 to 10 |
T/S ratio (modified) |
Social deprivation | Median 9.5 (SD = 6.4) Median 8.8 (SD = 6.0) Median 10.0 (SD = 6.5) |
| Gadalla et al. (2010) [74] |
21 Patients (IBMFS) (15/6) | 21 range 8–43 |
T/S ratio | Comparative tissue analysis |
Median 1.16 range 0.51–6.44 |
| Hewakapuge et al. (2008) [64] |
167 Individuals (71/96) | 1 to 96 | T/S ratio | Age prediction | range 0.54–2.83 (SD = 0.51) |
| Thomas et al. (2008) [108] |
30 Young controls (15/15) 26 Adult controls (11/15) 23 Young cases (8/15) 31 Adult cases (8/23) |
22.5 (+/−2.2) 68.7 (+/−2.6) 70.7 (+/−4.1) 83.1 (+/−4.5) |
T/S ratio (modified) |
Alzheimer’s disease | 41.98 (SD = 32.66) 40.57 (SD = 19.28) 34.41 (SD = 34.05) 20.51 (SD = 18.04) |
| Broberg et al. (2005) [109] |
63 Cases (54/9) 158 Controls (121/37) |
Median 69 range 35–85 Median 68 range 21–90 |
T/S ratio | Bladder cancer | Median 0.95 range 0.53–3.2 Median 1.1 range 0.51–2.4 |
Telomere lengthening: True or false?
Longitudinal reports using repeated measurements of TL have revealed that telomeres can lengthen in some individuals [33, 75–81]. Interestingly, those individuals tended to have shorter baseline TL in blood and buccal cells, raising important questions about the dynamics of TL in living cells [75, 77, 80]. Figure 2 presents several potential routes for this observed lengthening [82, 83]. TL appears to oscillate over short periods of time, but these variations are less pronounced when longer-term follow-up measurements are taken [82, 84]. In one study, blood cell TL was analyzed in samples taken six months apart [82]. The authors reported that TL differed more significantly between sampling events than in a previously reported study that analyzed samples taken ten years apart. Furthermore, others have theorized that there is an upper limit on TL and that TL is maintained in equilibrium in cells that have a telomere elongation mechanism.
One plausible route for observed telomere lengthening involves the enzyme telomerase. Interestingly, a recent study has shown increased activation of telomerase in the peripheral blood mononuclear cells of depressed patients. Higher telomerase activity has also been associated with depression ratings for both cases and controls [85]. Furthermore, a better response to antidepressant treatment has been associated with increased telomerase activity in depressed patients [85]. This increased activation may represent a compensatory mechanism to overcome the increased erosion of TL associated with depression.
Another potential and intriguing pathway for telomere lengthening may involve body mass. In a comparative study of telomere biology in cultured cells from more than 60 mammalian species, telomerase expression was linked with body size [86]. Notably, a longitudinal study of adults with BMI >25 measured leukocyte TL at two time points, 4.5 years apart. The surprising results showed that about two-third of the 343 individuals studied had longer TL at the follow-up measurement [87]. Is it the case that increased body weight up-regulates telomerase activity and, as a consequence, telomeres lengthen? Since lengthening of TL can only be observed by measuring change in TL over time, for obvious reasons there is a need for more longitudinal study designs with repeated measurements of TL. Potentially, the easiest way to investigate the telomere lengthening phenomenon is by analyzing existing longitudinal biobank databases that have collected DNA at different time points. Since TL can predict health outcomes, elucidating the temporal dynamics and the mechanisms that regulate TL are of great interest and clearly demand more investigation.
Childhood stress in the modern world: Telomere biology as a primary target for prevention and intervention
Studies have implicated age-related TL as an important determinant of morbidity and mortality, with most of the information arising from studies of adult or elderly populations. However, although the word “aging” is associated with old age and elderly people, aging in the sense of TL is a life-long phenomenon that begins at birth. There is some evidence to suggest that TL rapidly erodes in infants very soon after birth, and for the first years of life, corresponding to the rapid growth rates and high production and turnover of cells [66, 88]. This telomere erosion then continues at a steadier and more moderate rate into childhood, adulthood, and old age [89]. The manifestation of age-related diseases is mostly seen at old age, but the aging process, at the cellular level, is life-long. Given that children who are victims of violence show faster erosion of buccal cell TL [33], early intervention and prevention strategies can potentially ameliorate the acceleration of physiological aging processes early in life.
There are several indications that a healthy lifestyle and stress-coping strategies can alter the rate of telomere erosion and improve our well-being. Evidence from a genetically modified mouse model has demonstrated that activation of telomerase can reverse many of the hallmarks of aging [18]. Intriguingly, telomerase gene therapy has shown the potential to extend lifespan in adult and old mice. It has also been demonstrated that telomerase therapy does not induce cancer in treated mice [90]. Studies of humans have provided further support for this effect. Longer leukocyte telomeres have been associated with the number of years of healthy life [21], the buffering effect of exercise on stress [91], multivitamin use [92], and higher vitamin D levels [93]. Moreover, in one study, a healthy diet and having strong social support attenuated the relationship between short leukocyte TL and the occurrence of heart disease [94]. In another study, a reduction in psychological distress was associated with increased telomerase activity [95]. Similarly, a 3-month meditation intervention was associated with higher telomerase activity and improved health profiles [96].
There are some indications that counteracting the damaging effect of oxidative stress by antioxidants can attenuate the rapid erosion of telomeres. Higher levels of albumin and uric acid, two endogenous antioxidants, have been associated with longer leukocyte TL in elderly men [97]. Treatment of endothelial cells with the antioxidant ascorbic acid (vitamin C) decelerates the erosion of telomeres [98]. Higher folate and glutathione levels, molecules with antioxidant properties that counteract ROS, have also been associated with preservation of TL [99, 100]. In addition, treatment of fibroblast cells in culture with a free-radical scavenger has been shown to prolong the cells’ replicative lifespan and attenuates telomere erosion compared with cells that have been grown under normal conditions [101]. More research is needed to determine whether antioxidant supplementation can buffer the effects of stress on telomere erosion in children.
In summary, childhood stress is a salient early-life stressor with long-term consequences for well-being and a major public health and social welfare problem. Elucidating the molecular pathways, identifying biological substrates that could serve as potential treatment targets and discovering coping mechanisms that may protect children from the effects of childhood stress on telomere erosion is a primary target for science. Studies of this nature have the potential to identify novel targets for interventions to help children recover from exposure to violence. In a general sense, a healthy lifestyle, psychological support, and reductions in oxidative and lifestyle-related stress all appear to be important factors in reducing cellular aging and the deleterious effects that stress and violence have on telomeres.
Conclusions
Telomere research is at the cutting edge of the science of stress biology. The length of telomeres appears to be an important predictor of health and disease. The literature provides evidence that stress-related accelerated telomere erosion can already be observed in childhood. Although much progress has been achieved recently, many questions remain. For some of these questions, we currently only have partial answers. There is some indication that the link between stress and telomere erosion is mediated by several factors including the immune and endocrine systems, oxidative stress, and mitochondrial dysfunction. It seems that the effect of stress on telomere erosion is cumulative rather than a single-event; although other factors, such as severity of the stressor, should not be excluded. Existing methods to measure TL are highly correlated, but impartial evaluation of these methods is needed to establish an appropriate measurement framework. Measuring TL from different types of tissue may help answer specific research hypotheses. More research is needed to establish standard references for future studies. Finally, there is some evidence to suggest that TL is a dynamic feature and can even lengthen over time, and this should be investigated further.
Lifestyle factors and a healthy environment can help to buffer the deleterious effects of stress on telomere erosion. It is also tempting to speculate that such factors (e.g. diet, physical activity, stress-reduction methods, and antioxidants) are involved in two of the main mechanistic pathways in telomere integrity, i.e. the immune-system and oxidative stress (Fig. 2). It will be important to elucidate the complex cascade leading from stress exposure during early life to cellular aging via telomere biology.
This body of evidence suggests the importance of integrating telomeres as stress markers in research. TL measurement is now being offered to adults as a diagnostic tool to monitor health and predict disease risk [102]. It is conceivable that research may eventually promote TL measurement in clinical pediatrics. However, more research will be needed to uncover the underlying mechanisms that govern TL dynamics before such a clinical application could be deemed reliable. Factors for a healthy lifestyle and therapeutic strategies aimed at the deceleration of telomere erosion are potentially important targets for the treatment of young victims of violence.
Acknowledgments
The author is supported by NICHD grant HD061298 and by the Jacobs Foundation. I am grateful to Avshalom Caspi, Terrie E. Moffit, and Daniel W. Belsky for helpful comments on this paper. I am also grateful to the E-Risk Study Director, Louise Arsenault, and to the study mothers, fathers, and twins. This paper was presented at the 3rd Klaus-Grawe-Think-Tank-Meeting (KGTM2012) and I am grateful to the Klaus Grawe Foundation for support.
Abbreviations
- FISH
fluorescence in situ hybridization
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- STELA
single telomere length analysis
- TL
telomere length
- TRF
terminal restriction fragments
References
- 1.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2011;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom Med. 2009;71:805–812. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- 4.Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatr. 2011;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 5.Meyne J, Ratliff RL, Moyzis RK. Conservation of the human telomere sequence (TTAGGG)N among vertebrates. Proc Natl Acad Sci USA. 1989;86:7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flemming W. Zellsubstanz, Kern und Zelltheilung. Leipzig: Vogel; 1882. [Google Scholar]
- 7.Carrel A. On the permanent life of tissues outside of the organism. J Exp Med. 1912;15:516. doi: 10.1084/jem.15.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller H. The remaking of chromosomes. Collecting Net. 1938;13:181–198. [Google Scholar]
- 9.McClintock B. The stability of broken ends of chromosomes in Zea mays . Genetics. 1941;26:234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 11.Olovnikov A. Principle of marginotomy in template synthesis of polynucleotides. Doklady Akademii Nauk SSSR. 1971;201:1496. [PubMed] [Google Scholar]
- 12.Watson JD. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 13.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 14.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 15.Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978;120:33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- 16.Bodnar AG, Ouellette M, Frolkis M, Holt SE, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 17.Heidinger BJ, Blount JD, Boner W, Griffiths K, et al. Telomere length in early life predicts lifespan. Proc Natl Acad Sci USA. 2012;109:1743–1748. doi: 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaskelioff M, Muller FL, Paik JH, Thomas E, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 20.Atzmon G, Cho M, Cawthon RM, Budagov T, et al. Evolution in health and medicine Sackler colloquium: genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc Natl Acad Sci USA. 2010;107:1710–1717. doi: 10.1073/pnas.0906191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Njajou OT, Hsueh WC, Blackburn EH, Newman AB, et al. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population- based cohort study. J Gerontol A-Biol. 2009;64:860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valdes AM, Andrew T, Gardner JP, Kimura M, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 23.Epel ES, Blackburn EH, Lin J, Dhabhar FS, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, et al. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon NM, Smoller JW, McNamara KL, Maser RS, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatr. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Yu WY, Chang HW, Lin CH, Cho CL. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J Psychiatr Neurosci. 2008;33:244–247. [PMC free article] [PubMed] [Google Scholar]
- 27.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 28.Tyrka AR, Price LH, Kao HT, Porton B, et al. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatr. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kananen L, Surakka I, Pirkola S, Suvisaari J, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Donovan A, Epel E, Lin J, Wolkowitz O, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatr. 2011;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glass D, Parts L, Knowles D, Aviv A, et al. No correlation between childhood maltreatment and telomere length. Biol Psychiatr. 2010;68:e21–e22. doi: 10.1016/j.biopsych.2010.02.026. author reply e3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drury SS, Theall K, Gleason MM, Smyke AT, et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol Psychiatr. 2011;17:719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shalev I, Moffitt TE, Sugden K, Williams B, et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 Years of age: a longitudinal study. Mol Psychiatr. 2012 doi: 10.1038/mp.2012.32. in press, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 35.Benetti R, Garcia-Cao M, Blasco MA. Telomere length regulates the epigenetic status of mammalian telomeres and subtelomeres. Nat Genet. 2007;39:243–250. doi: 10.1038/ng1952. [DOI] [PubMed] [Google Scholar]
- 36.Baur JA, Zou Y, Shay JW, Wright WE. Telomere position effect in human cells. Science. 2001;292:2075–2077. doi: 10.1126/science.1062329. [DOI] [PubMed] [Google Scholar]
- 37.Maicher A, Kastner L, Dees M, Luke B. Deregulated telomere transcription causes replication-dependent telomere shortening and promotes cellular senescence. Nucleic Acids Res. 2012;40:6649–6659. doi: 10.1093/nar/gks358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the health, aging and body composition study. PLoS One. 2011;6:e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 40.Pearson TA, Mensah GA, Alexander RW, Anderson JL, et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice – a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 41.Dantzer R, O’Connor JC, Freund GG, Johnson RW, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danese A, Pariante CM, Caspi A, Taylor A, et al. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pace TW, Mletzko TC, Alagbe O, Musselman DL, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatr. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 44.Goronzy JJ, Fujii H, Weyand CM. Telomeres, immune aging and autoimmunity. Exp Gerontol. 2006;41:246–251. doi: 10.1016/j.exger.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harman D. Aging – a theory based on free-radical and radiationchemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 47.Sahin E, Colla S, Liesa M, Moslehi J, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Behl C, LezoualcH F, Trapp T, Widmann M, et al. Glucocorticoids enhance oxidative stress-induced cell death in hippocampal neurons in vitro . Endocrinology. 1997;138:101–106. doi: 10.1210/endo.138.1.4835. [DOI] [PubMed] [Google Scholar]
- 49.Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22:600–605. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kroenke CH, Epel E, Adler N, Bush NR, et al. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosom Med. 2011;73:533–540. doi: 10.1097/PSY.0b013e318229acfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hewitt G, Jurk D, Marques FD, Correia-Melo C, et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fumagalli M, Rossiello F, Clerici M, Barozzi S, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damageresponse activation. Nat Cell Biol. 2012;14:355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parks CG, Miller DB, McCanlies EC, Cawthon RM, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidem Biomar. 2009;18:551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakagawa S, Gemmell NJ, Burke T. Measuring vertebrate telomeres: applications and limitations. Mol Ecol. 2004;13:2523–2533. doi: 10.1111/j.1365-294X.2004.02291.x. [DOI] [PubMed] [Google Scholar]
- 55.Allshire RC, Dempster M, Hastie ND. Human telomeres contain at least three types of G-rich repeat distributed non-randomly. Nucleic Acids Res. 1989;17:4611–4627. doi: 10.1093/nar/17.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rufer N, Dragowska W, Thornbury G, Roosnek E, et al. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol. 1998;16:743–747. doi: 10.1038/nbt0898-743. [DOI] [PubMed] [Google Scholar]
- 58.Poon SS, Lansdorp PM. Quantitative fluorescence in situ hybridization (Q-FISH). Chapter 18, Unit 18.14. Curr Protoc Cell Biol. 2001 doi: 10.1002/0471143030.cb1804s12. [DOI] [PubMed] [Google Scholar]
- 59.Baird DM, Rowson J, Wynford-Thomas D, Kipling D. Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat Genet. 2003;33:203–207. doi: 10.1038/ng1084. [DOI] [PubMed] [Google Scholar]
- 60.Abdallah P, Luciano P, Runge KW, Lisby M, et al. A two-step model for senescence triggered by a single critically short telomere. Nat Cell Biol. 2010;11:988–993. doi: 10.1038/ncb1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aubert G, Hills M, Lansdorp PM. Telomere length measurement- Caveats and a critical assessment of the available technologies and tools. Mutat Res. 2012;730:59–67. doi: 10.1016/j.mrfmmm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aviv A, Hunt SC, Lin J, Cao X, et al. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39:e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aviv A. Commentary: raising the bar on telomere epidemiology. Int J Epidemiol. 2009;38:1735–1736. doi: 10.1093/ije/dyp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hewakapuge S, van Oorschot RA, Lewandowski P, Baindur-Hudson S. Investigation of telomere lengths measurement by quantitative real-time PCR to predict age. Leg Med (Tokyo) 2008;10:236–242. doi: 10.1016/j.legalmed.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 65.Ouyang Q, Baerlocher G, Vulto I, Lansdorp PM. Telomere length in human natural killer cell subsets. Ann NY Acad Sci. 2007;1106:240–252. doi: 10.1196/annals.1392.001. [DOI] [PubMed] [Google Scholar]
- 66.Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, et al. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aubert G, Baerlocher GM, Vulto I, Poon SS, et al. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012;8:e1002696. doi: 10.1371/journal.pgen.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Autexier C, Greider CW. Telomerase and cancer: revisiting the telomere hypothesis. Trends Biochem Sci. 1996;21:387–391. [PubMed] [Google Scholar]
- 69.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28(−)CD8(+) T cells imply a replicative history that is distinct from their CD28(+)CD8(+) counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 70.Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- 71.Thomas P, OC NJ, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev. 2008;129:183–190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 72.Friedrich U, Griese E, Schwab M, Fritz P, et al. Telomere length in different tissues of elderly patients. Mech Ageing Dev. 2000;119:89–99. doi: 10.1016/s0047-6374(00)00173-1. [DOI] [PubMed] [Google Scholar]
- 73.Butler MG, Tilburt J, DeVries A, Muralidhar B, et al. Comparison of chromosome telomere integrity in multiple tissues from subjects at different ages. Cancer Genet Cytogen. 1998;105:138–144. doi: 10.1016/s0165-4608(98)00029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gadalla SM, Cawthon R, Giri N, Alter BP, et al. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging (Albany NY) 2010;2:867–874. doi: 10.18632/aging.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aviv A, Chen W, Gardner JP, Kimura M, et al. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen W, Kimura M, Kim S, Cao X, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol J Gerontol A-Biol. 2011;66:312–319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ehrlenbach S, Willeit P, Kiechl S, Willeit J, et al. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int J Epidemiol. 2009;38:1725–1734. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- 78.Epel ES, Merkin SS, Cawthon R, Blackburn EH, et al. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY) 2009;1:81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farzaneh-Far R, Lin J, Epel E, Lapham K, et al. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS One. 2010;5:e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nordfjall K, Svenson U, Norrback KF, Adolfsson R, et al. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009;5:e1000375. doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gardner JP, Li SX, Srinivasan SR, Chen W, et al. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 82.Svenson U, Nordfjall K, Baird D, Roger L, et al. Blood cell telomere length is a dynamic feature. PLoS One. 2011;6:e21485. doi: 10.1371/journal.pone.0021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11:319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 84.Chen W, Kimura M, Kim S, Cao X, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A-Biol. 2011;66:312–319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolkowitz OM, Mellon SH, Eper ES, Lin J, et al. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatr. 2012;17:164–172. doi: 10.1038/mp.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gomes NMV, Ryder OA, Houck ML, Charter SJ, et al. Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging cell. 2011;10:761–768. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hovatta I, de Mello VD, Kananen L, Lindstrom J, et al. Leukocyte telomere length in the Finnish diabetes prevention study. PLoS One. 2012;7:e34948. doi: 10.1371/journal.pone.0034948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zeichner SL, Palumbo P, Feng Y, Xiao X, et al. Rapid telomere shortening in children. Blood. 1999;93:2824–2830. [Google Scholar]
- 89.Eisenberg DT. An evolutionary review of human telomere biology: the thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am J Hum Biol. 2011;23:149–167. doi: 10.1002/ajhb.21127. [DOI] [PubMed] [Google Scholar]
- 90.Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, et al. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med. 2012;4:691–704. doi: 10.1002/emmm.201200245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puterman E, Lin J, Blackburn E, O’Donovan A, et al. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. 2010;5:e10837. doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu Q, Parks CG, Deroo LA, Cawthon RM, et al. Multivitamin use and telomere length in women. Am J Clin Nutr. 2009;89:1857–1863. doi: 10.3945/ajcn.2008.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Richards JB, Valdes AM, Gardner JP, Paximadas D, et al. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am J Clin Nutr. 2007;86:1420–1425. doi: 10.1093/ajcn/86.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diaz VA, Samani NJ. Effect of healthy lifestyle behaviors on the association between leukocyte telomere length and coronary artery calcium. Am J Cardiol. 2010;106:659–663. doi: 10.1016/j.amjcard.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 95.Daubenmier J, Lin J, Blackburn E, Hecht FM, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2011;37:917–928. doi: 10.1016/j.psyneuen.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jacobs TL, Epel ES, Lin J, Blackburn EH, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36:664–681. doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 97.de Vos-Houben JM, Ottenheim NR, Kafatos A, Buijsse B, et al. Telomere length, oxidative stress, and antioxidant status in elderly men in Zutphen and Crete. Mech Ageing Dev. 2012;133:373–377. doi: 10.1016/j.mad.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 98.Furumoto K, Inoue E, Nagao N, Hiyama E, et al. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci. 1998;63:935–948. doi: 10.1016/s0024-3205(98)00351-8. [DOI] [PubMed] [Google Scholar]
- 99.Paul L. Diet, nutrition and telomere length. J Nutr Biochem. 2011;22:895–901. doi: 10.1016/j.jnutbio.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 100.Kurz DJ, Decary S, Hong Y, Trivier E, et al. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004;117:2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 101.Von Zglinicki T, Pilger R, Sitte N. Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radical Biol Med. 2000;28:64–74. doi: 10.1016/s0891-5849(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 102.Leslie M. Cell biology. Are telomere tests ready for prime time? Science. 2011;332:414–415. doi: 10.1126/science.332.6028.414. [DOI] [PubMed] [Google Scholar]
- 103.Cherkas LF, Hunkin JL, Kato BS, Richards JB, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 104.Damjanovic AK, Yang YH, Glaser R, Kiecolt-Glaser JK, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Andrew T, Aviv A, Falchi M, Surdulescu GL, et al. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet. 2006;78:480–486. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okuda K, Bardeguez A, Gardner JP, Rodriguez P, et al. Telomere length in the newborn. Pediatr Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 107.Wong LSM, Huzen J, de Boer RA, van Gilst WH, et al. Telomere length of circulating leukocyte subpopulations and buccal cells in patients with ischemic heart failure and their offspring. PLoS One. 2011;6:e23118. doi: 10.1371/journal.pone.0023118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thomas P, O’Callaghan NJ, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev. 2008;129:183–190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 109.Broberg K, Bjork J, Paulsson K, Hoglund M, et al. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis. 2005;26:1263–1271. doi: 10.1093/carcin/bgi063. [DOI] [PubMed] [Google Scholar]