Abstract
Aim
To explore the hypothesis that grafts of exogenous stem cells in the spinal cord of athymic rats or rats with transgenic motor neuron disease can induce endogenous stem cells and initiate intrinsic repair mechanisms that can be exploited in amyotrophic lateral sclerosis therapeutics.
Materials & methods
Human neural stem cells (NSCs) were transplanted into the lower lumbar spinal cord of healthy rats or rats with transgenic motor neuron disease to explore whether signals related to stem cells can initiate intrinsic repair mechanisms in normal and amyotrophic lateral sclerosis subjects. Patterns of migration and differentiation of NSCs in the gray and white matter, with emphasis on the central canal region and ependymal cell-driven neurogenesis, were analyzed.
Results
Findings suggest that there is extensive cross-signaling between transplanted NSCs and a putative neurogenic niche in the ependyma of the lower lumbar cord. The formation of a neuronal cord from NSC-derived cells next to ependyma suggests that this structure may serve a mediating or auxiliary role for ependymal induction.
Conclusion
These findings raise the possibility that NSCs may stimulate endogenous neurogenesis and initiate intrinsic repair mechanisms in the lower spinal cord.
Keywords: amyotrophic lateral sclerosis, ependyma, neural stem cell, neurogenesis, niche, spinal cord, transplantation
Motor neuron disease (MND), exemplified by amyotrophic lateral sclerosis (ALS), is characterized by degeneration and death of ‘upper’ and ‘lower’ motor neurons. Despite significant progress in genetics, which has allowed for the construction of spectacular transgenic (Tg) rodent models, many fundamental aspects of pathogenesis remain unknown and there are no established disease-modifying treatments. Cell death prevention strategies, including the use of trophic factors and small neuroprotective compounds, have had limited success [1-4]. In addition, the generally accepted molecular and cellular alterations associated with familial ALS mutations have not always predicted therapeutic efficacy in clinical trials [5]. While molecular and cellular research should continue to explore ‘rational’ or ‘biological’ therapies for MND [6], existing therapeutic tools must continue to be refined and adjusted to clinical utilization, including further characterization of therapies based on the principle of cell replacement [7]. Cell replacement (i.e., a long-standing approach to experimental therapeutics for illnesses featured by massive death of neuronal or glial cells such as MND or spinal cord injury), has been invigorated with the emergence of stem cells as sources for cell supplementation [8,9]. Reasons for this excitement include the availability of stem cells in theoretically unlimited supplies and our increasing ability to manipulate these cells to specified fates and therapeutic outcomes [10].
One of the early goals of stem cell transplantation for MND has been the differentiation of transplanted cells themselves into motor neurons capable of innervating the appropriate muscle targets. The achievement of this goal depends on the establishment of molecular and/or mechanical means to overcome the observed difficulties in bridging the PNS–CNS frontier and to allow pathfinding to, and the functional innervation of, peripheral muscles. Unfortunately, the field of stem cell transplantation does not have, as a whole, a reproducible method to ensure neuronal network replacement (i.e., restoration of both nerve cells and their principal connections in the neuromuscular system or elsewhere). Therefore, cell replacement with exogenous stem cells remains an overoptimistic goal for ALS treatment at this juncture [10].
Another option in stem cell therapeutics is the induction of regenerative processes in the host spinal cord environment. Based on findings from the early applications of human neural stem cell (NSC) grafts in the spinal cord, graft–host interactions may hold substantial regenerative promise even in the absence of advanced motor neuron differentiation of NSCs. For example, transplanted NSCs express and release trophic peptides, such as BDNF and GDNF, that may become delivered to host motor neurons via classical cellular mechanisms [11,12]. Here we demonstrate another type of regenerative signaling introduced by transplanted NSCs: first, NSCs transplanted in lumbar segments of spinal cord exhibit a targeted migration to the ependyma, where they undergo substantial neuronal differentiation; second, NSCs appear to induce marked biological changes in ependymal cells, including proliferation and differentiation into neural precursors (NPs) and, potentially, neurons. These facts suggest that signaling by differentiated NSCs may induce lumbar cord ependyma in the direction of neurogenesis, thus activating an intrinsic repair mechanism in the host spinal cord.
Materials & methods
Preparation of human NSCs
As described before [13], human NSCs were derived from the cervical-thoracic cord of a single 8-week human fetus. Tissues were donated by the mother in a manner compliant with NIH and US FDA guidelines. Human NSCs were serially expanded in monolayer [14], harvested from various passages and cryopreserved in liquid nitrogen. Cells from passages 10–12 were used in this study. Viability of cells thawed and processed from these passages was greater than 80%. Immediately prior to transplantation, the vast majority of human NSCs expressed nestin, whereas approximately 5% were immunoreactive for polysialylated neuronal cell adhesion molecule (PSA-NCAM). Less than 1% of cells expressed the neuronal class III β-tubulin (TUJ1) and MAP2 or the astroglial marker GFAP [15].
Experimental subjects & surgical procedures
All animal care and surgery procedures were carried out in compliance with protocols approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions. To differentiate between disease-related and graft-related molecular and cellular factors that induce and guide neurogenesis in the lumbar spinal cord, we used both SOD1 G93A Tg rats from Taconic (Germantown, NY, USA) and normal athymic nude rats from Charles River (Wilmington, MA, USA), and compared the effects of live cell grafts in these two types of hosts. Using a Kopf spinal stereotaxic unit, 8-week-old SOD1 G93A Tg rats and nude rats of both sexes (n = 15 per group) were transplanted with live or dead human NSCs in the lumbar protuberance (L4–L6 segments) on the right side or on both sides (1 μl with 2 × 104 NSCs per injection site, four injection sites per side) using pulled-beveled glass micropipettes connected to 10 μl Hamilton microsyringes via silastic tubing under microscopic guidance. Dead cells were prepared by 3× freezing in liquid nitrogen (-70°F) and then thawing at room temperature; cell death was confirmed by a Trypan Blue uptake assay [11]. All SOD1 G93A Tg rats were treated with FK-506 (1 mg/kg intraperitoneally daily) till euthanized to prevent immune rejection.
Tracking of ependyma-derived cells
DiI is a carbocyanine lipophilic tracer that has been used to investigate the proliferation and migration of labeled ependymal cells in various animal models [16,17]. When injected intraventricularly, DiI only labels the ventricular column of ependymal cells (i.e., cells facing the cerebrospinal fluid) [16]. In addition, DiI is only transferred from the labeled cell to its progeny, not from labeled to unlabeled cells [18]. Therefore, by tracking cytoplasmic DiI labeling, the migration of ependymal cells can be disclosed. In order not to directly damage or disturb ependymal cells lining the central canal in the lumbar spinal cord, we delivered DiI into the lateral brain ventricle, expecting that DiI would reach the lumbar ependyma following the cerebrospinal fluid circulation.
DiI in dimethylsulfoxide (20 μl of 0.2% [weight per volume] SP-DiIC18, Molecular Probes® [NY, USA]), was stereotactically injected into the right lateral ventricle 10 days before the anticipated day of sacrifice in SOD1 G93A Tg and nude rats (n = 3 per genotype × live or dead cell transplantation). Injection coordinates were 0.9 mm posterior and 1.5 mm lateral to bregma and 3.5 mm below dura. Cytogenesis in the spinal cord was traced with daily injections of 20 mg/ml 5-bromodeoxyuridine (BrdU; 50 mg/kg intraperitoneally in saline; Sigma, MO, USA) 10 days prior to euthanasia.
Retrograde trans-synaptic tracing
To assess whether NSC-derived neurons that had migrated in the central canal area can elaborate axons and generate mature synapses with host neurons, the widely used retrograde trans-synaptic marker Bartha-pseudorabies virus (PRV, given by P Card, Princeton University, NJ, USA) was used to label lumbar motor neurons and nerve cells that innervate them, as described previously [12]. A total of 50 μl of a Bartha-PRV solution (1 × 109 plaque-forming units/ml) were injected into the right lateral gastrocnemius muscle/sciatic nerve of 90-day-old SOD1 G93A rats with live NSC grafts (n = 3) and of nude rats with live NSC grafts (n = 3). To directly compare between simple retrograde and trans-synaptic transport, after PRV injection, 5 μl of a 0.25% solution of the classical retrograde tracer Cholera toxin B (CTB; diluted with sterile distilled water) were injected with a new syringe into the same gastrocnemius/sciatic nerve site. Five days after tracer injection, rats were euthenized by perfusion-fixation and spinal cord segments were dissected and stored for subsequent sectioning.
Histology, immunocytochemistry & microscopy
Tissues were processed using methods well established in our laboratory. After animals were perfused with fresh 4% neutral-buffered paraformaldehyde, lower thoracic and lumbar spinal cord segments were dissected and post-fixed with the same fixative for an additional 4 h. Blocks containing the entire grafts area plus a 1-mm border above and below were cryoprotected and frozen for further processing. Transverse or sagittal sections (35 μm) through the upper and lower segments of the lumbar protuberance were prepared for the visualization of BrdU, DiI, markers of neuronal and astrocytic lineage, and other markers mentioned below by using a multiple-label immunocytochemistry method as previously described [19]. Briefly, for immunofluorescence staining, sections were first incubated with the primary antibody overnight at 4°C, treated with secondary antibodies labeled with Cy3 or Cy2 (1:200; Jackson ImmunoResearch, PA, USA) for 2–4 h at room temperature, then counterstained with the fluorescent DNA dye 4’,6-diamidino-2-phenylindole (DAPI) and coverslipped with DePeX polystyrene. For immunoperoxidase staining, after incubation with peroxidase-conjugated secondary antibody linked with biotin (1:200; Jackson ImmunoResearch), sections were developed with a 3,3’-diaminobenzidine (DAB) kit (VECTASTAIN Elite ABC Kit, Vector laboratories Inc., CA, USA). Primary antibodies were from Millipore (Billerica, MA, USA) except otherwise noted and included: mouse anti-human nuclear protein antibody (HNu, 1:600); rabbit anti-TUJ1 (1:400; Research Diagnostics Inc., NJ, USA); rabbit anti-GFAP (1:400; Dako, CA, USA); mouse anti-rat nestin (1:200); mouse anti-neurofilament 70 (NF70, 1:100, human and porcine specific); mouse anti-synaptophysin (SYN, 1:200, human and hamster specific); rabbit anti-CTB (1:1000, List Biological Laboratory, CA, USA); rat anti-BrdU (1:200, ABD Serotec, Raleigh, NC, USA), goat anti-doublecortin (DCX,1:1500). For PRV immunocytochemistry, we used a rabbit polyclonal antibody (1:10,000, given by P Card, University of Pittsburgh, PA, USA, and L Enquist, Princeton University, NJ, USA). Immunofluorescent sections were studied with a Zeiss Axiophot microscope equipped for epifluorescence and images were captured with a Spot RT™ Slider digital camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA).
Using Adobe PhotoShop® 6.0 software (Adobe Systems, San Jose, CA, USA), figures were montaged from series of adjacent overlapping frames shot under the exact same conditions, if the size of a region of interest was too big to be captured in one frame. Confocal microscopic images were captured, in most cases, with a Zeiss LSM 510 inverted confocal microscope (Carl Zeiss, Inc., NY, USA). Except in cases of 3D reconstruction by Z-stack scanning through regions of interest, single-plane scanning was acquired using LSM software with pinhole set as 0.8 μm, meaning that only 0.8-μm thickness tissue was processed to eliminate cell overlapping and to ensure true colocalization of multiple labels at the same resolution level corresponding to a semi-thin section.
Electron microscopy of human synapses in the central canal
Human NSC-derived synapses in the central canal zone were studied with pre-embedding immuno-electron microscopy. Tissue sections were first stained with human-selective monoclonal antibodies to synaptophysin with a commercially available avidin-biotin-peroxidase complex (ABC) kit (Elite ABC kit PK6100, Vector, Burlingame, CA, USA) and then developed with nickel-enhanced DAB [12]. The central canal zone was dissected and embedded in BEEM capsules. Serial ultrathin sections were collected on Formvar-coated slotted grids and viewed with a Hitachi H-7600 transmission electron microscope equipped with a 2k × 2k bottom mount AMT-XR-100 CCD camera. Digital images were optimized for brightness/contrast and resolution (set at 600 pixels per inch) with the aid of Adobe PhotoShop 6.0 software.
Cell counts
To study ependyma-derived cell migration and differentiation, confocal microscopic pictures (40×) of area X were captured by random single-plane scanning of five serial sections (with the requirement that the start section must contain the main graft in live-cell transplantation groups; section interval is six in SOD1 G93A Tg and nude rats with dead- or live-cell grafts [n = 3 in each group]). Ependymal cells labeled with DAPI, DiI-positive ependyma-derived cells (outside the ependyma column) and DCX-positive host cells were counted in area X. BrdU-positive ependymal cells (in the ependyma column) were also counted. Numbers of single DAPI-labeled and double-labeled cells with DAPI and DiI or DCX or BrdU were generated and averaged for each group. To evaluate migration of ependyma-derived cells and proliferation of cells in the ependyma column, rates of numbers of DiI-positive ependyma-derived cells versus total ependymal cells and BrdU-positive ependymal cells versus total ependymal cells were calculated. To test the impact of NSC transplantation on potential neurogenic niche under MND or no MND healthy conditions, groups with live- or dead-cell grafts were separately compared in SOD1 G93A Tg rats (Sprague-Dawley background, FK-506 treatment) and in normal nude rats (athymic background, no FK-506 treatment) using student’s t-test.
Results
Migration & neuronal differentiation of transplanted human NSCs in the central canal zone
Transplanted NSCs in the ventral horn of L4–L6 segments migrate from the inoculation site to the host central canal through area X (in some cases, over a distance of 500 μm), both in SOD1 G93A Tg rats and in unlesioned nude rats (Figure 1A). This is a consistent finding with almost no exceptions in all subjects analyzed. Migration of NSCs from transplantation site to the central canal area is evident by the physical separation of the initial graft from NSC colonies at the central canal and also the issuance of processes linking up the initial graft with the central canal region (Figures 1 & 2). At the central canal site, NSCs appear to have differentiated into TUJ1-positive neuronal profiles (Figure 1B). Large numbers of synaptophysin-immunoreactive profiles are present at these sites of migration (Figure 1C) that appear to correspond to mature synapses replete with synaptic vesicles and specializations by immuno-electron microscopy (Figure 1D & E).
Figure 1. Migration of transplanted human neural stem cells from the inoculation site to the central canal zone and differentiation into neurons with mature synapses.
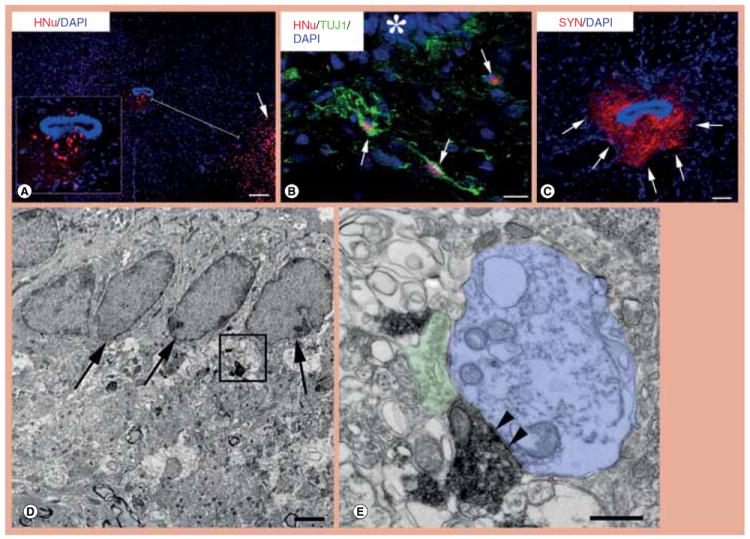
(A) Transplanted neural stem cells (NSCs) were attracted to the central canal by crossing the distance between the inoculation site in the ventral horn (red, lower right, arrow) and central canal. Inset is a magnification of the central canal zone with migrated NSC-derived cells. (B) NSCs that have migrated from the initial transplantation site to the ventral central canal (asterisk) differentiate extensively into TUJ1-positive neurons (green, arrows). (C) In this section that is adjacent to the section photographed for (A), NSCs that had migrated in the central canal zone are shown to generate dense human-specific SYN-positive terminals (arrows). (D & E) These electron photomicrographs taken from a human SYN-labeled section show SYN-positive terminals apparently derived from differentiated human NSCs next to ependymal cells (D, arrows). (E) Enlargement of the framed area in (D) to show the ultrastructure of a human NSC-derived terminal replete with synaptic vesicles, mitochondria and terminal membrane specializations (arrowheads) forming a synapse with an unlabelled, possibly dendritic, postsynaptic structure (blue); the human-derived synaptic terminal is juxtaposed to an unlabeled host terminal (green) on the same postsynaptic structure. Scale bars: (A) 100 μm; (B) 20 μm; (C) 25 μm; (D) 150 nm; (E) 50 nm.
DAPI: 4’,6-diamidino-2-phenylindole; HNu: Human nuclear protein antibody; SYN: Synaptophysin; TUJ1: Neuronal class III β-tubulin.
Figure 2. Axonal projections of neural stem cell-derived neurons in the central canal zone.
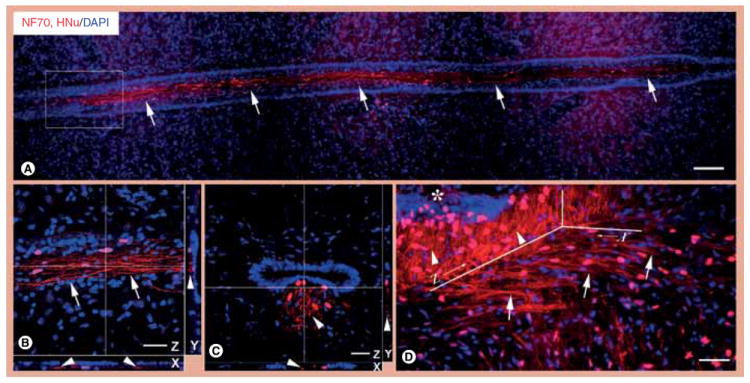
(A) Neurons differentiated from neural stem cells (NSCs; red nucleus, HNu positive) in L4–L6 segments next to the ependyma elaborate long axons (red lines, NF70 positive) parallel and tight to the ependyma axis (arrows). (B) Enlarged from frame area in (A) is a confocal picture with 3D reconstruction to illustrate the fact that axons (arrows in Z plane) from NSC-derived neurons are outside the ependyma, as demonstrated in planes X and Y (arrowheads). (C) This confocal picture with 3D reconstruction in a transversely cut spinal cord section further demonstrates that when newly formed axons (red lines, NF70 positive) reach ependyma, they change projections and course parallel to the ependyma axis, as demonstrated in planes X, Y and Z (arrowheads). (D) NSC-derived neurons in the transplantation site elaborate axon bundles (red lines, NF70 positive) towards the ependyma (thick arrows), possibly functioning as NSC-derived tracts for NSC migration to ependyma (asterisk). Note that the axons in the central canal zone change their directions from horizontal (plane X, thick arrows) into vertical, parallel to the ependyma axis (plane Z, arrowheads).
Scale bars: (A) 100 μm; (B–D) 25 μm.
DAPI: 4’,6-diamidino-2-phenylindole; HNu: Human nuclear protein antibody; NF70: Neurofilament 70.
The study of multiple coronal sections or sections in the transverse plane reveals that the migration of NSC-derived cells towards the ependymal region in the lower lumbar cord results in the formation of a longitudinal cord comprised of neuronally differentiated NSCs and their bundled processes (axons) that run immediately ventral to the central canal (Figure 2A–C). Some axons course initially at the coronal plane but, as soon as they reach the central canal, they switch to a course parallel to that of the central canal, joining in the longitudinal bundle of other axons formed by differentiated NSCs closer to the central canal zone (Figure 2D).
These observations indicate that transplanted NSCs are universally attracted to the central canal region where they differentiate into neurons forming a longitudinal cord just ventral to the central canal.
Proliferation & migration of ependymal cells in the spinal cord of SOD1 G93A Tg & nude rats with & without live NSC grafts
SOD1 G93A Tg rats and nude rats with live- or dead-cell grafts were used to ascertain the impact of transplantation-related signals on cytogenesis in the lumbar cord of subjects with MND or healthy subjects (Figure 3A, C, D & F). DiI was used to selectively label ependyma-derived cells and, as such, to explore the ependymal response under conditions of ALS-like disease on NSC transplantation. Results show that DiI-positive ependyma-derived cells not only migrate out into area X in nude rats with live grafts (Figure 3B) and SOD1 G93A Tg rats with live (Figure 3A) or dead NSC grafts, but can travel all the way to the distal gray matter and even into the spinal white matter (Figure 3C & D). The rates of DiI-positive cells derived from ependymal cells in nude rats with live cell grafts are significantly higher than that of nude rats with dead cell grafts (Figure 3F).
Figure 3. Proliferation and migration of ependymal cells in the spinal cords of SOD1 G93A-transgenic rats and nude rats after neural stem cell transplantation, as demonstrated by DiI labeling of central canal cells and confocal microscopy.
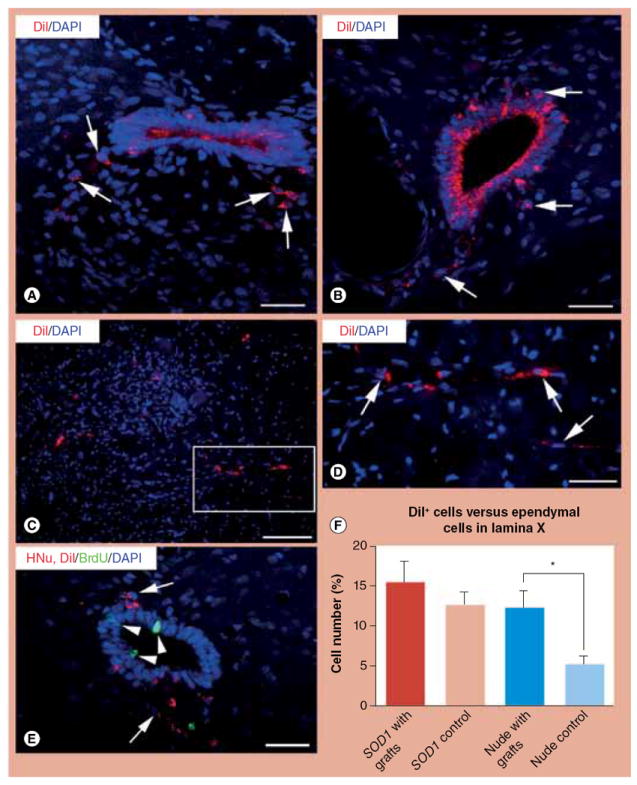
(A) In area X of this spinal cord from a representative SOD1 G93A rat, DiI-positive ependyma-derived cells migrate out from the ependyma column (red, arrows) after neural stem cell (NSC) transplantation. (B) In area X of this spinal cord of a nude rat, migrating ependymal cells (arrows) have the same DiI labeling pattern as in SOD1 G93A rats. (C & D) After NSC transplantation in the spinal cord of SOD1 G93A rats, DiI-positive ependymal cells (red) migrate into the deep ventral horn. (D) Magnification of framed area in (C) to show DiI labeling in detail (arrows). (E) This photograph depicts proliferating ependymal cells (BrdU positive, green, arrowheads) and migrating of DiI-positive ependymal cells (red, arrows) in a representative SOD1 G93A rat spinal cord after NSC transplantation. (F) The bar diagram depicts percentage rates of migrating DiI-positive ependymal cells in area X versus total ependymal cells from the same section in four groups: SOD1 G93A rats with live NSC grafts (‘SOD1 with grafts’); SOD1 G93A rats with dead NSC grafts (‘SOD1 control’); nude rats with live NSC grafts (‘Nude with grafts’); and nude rats with dead NSC grafts (‘Nude control’). Student’s t-test in the SOD1 G93A and nude groups reveals that there is a significantly higher rate of DiI-positive ependyma-derived cells in nude rats with live grafts (12.25 ± 6.57%) compared with nude controls (5.19 ± 2.62%). The absence of significance in the SOD1 G93A groups could be due to a genuine lack of effect of graft or to an independent effect of motor neuron disease on ependymal cell migration.
Scale bars: (A) 25 μm; (B) 25 μm; (C) 50 μm; (D) 25 μm; (E) 25 μm.
BrdU: 5-bromodeoxyuridine; DAPI: 4’,6-diamidino-2-phenylindole; HNu: Human nuclear protein antibody; SOD1: Superoxide dismutase 1.
BrdU incorporation staining shows proliferating ependymal cells (Figure 3E). Counts of BrdU-positive ependymal cells in nude and SOD1 G93A Tg rats demonstrate that NSC transplantation significantly increases ependymal cell proliferation, as evidenced by comparisons between BrdU-positive ependymal cell rates in nude rats with live (0.110 ± 0.032) and dead (0.030 ± 0.036) NSC grafts (p < 0.05) as well as in SOD1 G93A Tg rats with live (0.143 ± 0.036) and dead (0.079 ± 0.041) NSC grafts (p < 0.05).
Taken together, these findings indicate that NSC transplantation promotes ependymal cell proliferation and migration under MND or healthy conditions in the spinal cord.
Ependymal cell-driven neurogenesis in central canal zone after NSC transplantation
Additional staining of lumbar cord sections from nude rats with NSC markers (i. e., nestin) and neuronal markers (i.e., TUJ1), demonstrates that host ependymal cells may also undergo differentiation into NPs (Figure 4A) and, potentially, neurons, in proximity to transplanted NSCs (Figure 4B).
Figure 4. Differentiation of ependymal cells into neural stem cells and neurons in the spinal cord of nude rats after neural stem cell transplantation, as demonstrated by nestin and TUJ1 staining and confocal microscopy.
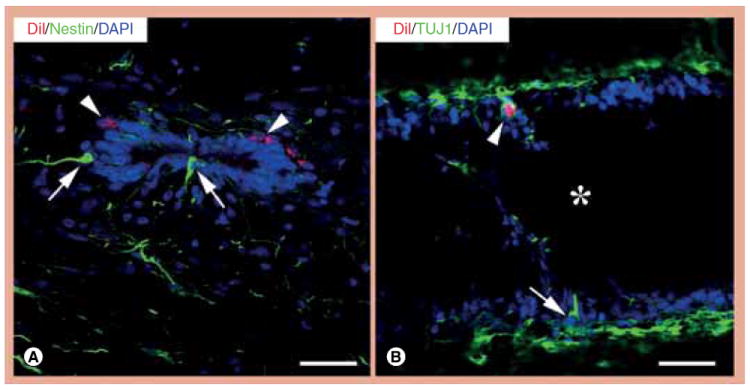
(A) Some ependymal cells in the spinal cord of nude rats are nestin positive (green, arrows) after NSC transplantation. Note that DiI-positive cells are nestin negative (red, arrowheads). (B) Neuronal differentiation of ependyma-derived cells is also confirmed by positive labeling with the neuronal marker TUJ1. This panel shows a TUJ1-positive ependyma-derived cell (green, arrow) outside the central canal (asterisk) in a longitudinally sectioned spinal cord. Note that one NSC-derived cell (red, HNu positive) is TUJ1 positive (green, arrowhead).
Scale bars: (A & B) 25 μm.
DAPI: 4’,6-diamidino-2-phenylindole; TUJ1: Neuronal class III β-tubulin.
Under conditions of NSC transplantation or MND, some DiI-positive host cells in area X and even in areas far from area X, such as the deep ventral horn, are immunoreactive for DCX, a marker of migrating neuronal precursors (Figure 5A & B); this finding indicates the presence of neurogenesis in ependymal cells. Several host cells in area X, especially in areas closer to the central canal, are DCX positive (Figure 5C). In addition, numbers of DCX-positive host cells in area X in SOD1 G93A Tg and nude rats with live-cell grafts are significantly higher than in animals with dead-cell grafts, respectively (Figure 5E), a pattern suggesting that NSC grafts induce or promote the neurogenesis in the central canal zone.
Figure 5. Ependymal cell-driven neurogenesis in the central canal zone after neural stem cell transplantation as demonstrated with confocal microscopy and comparison of doublecortin-positive neuron counts in area X after live or dead neural stem cell transplantation.
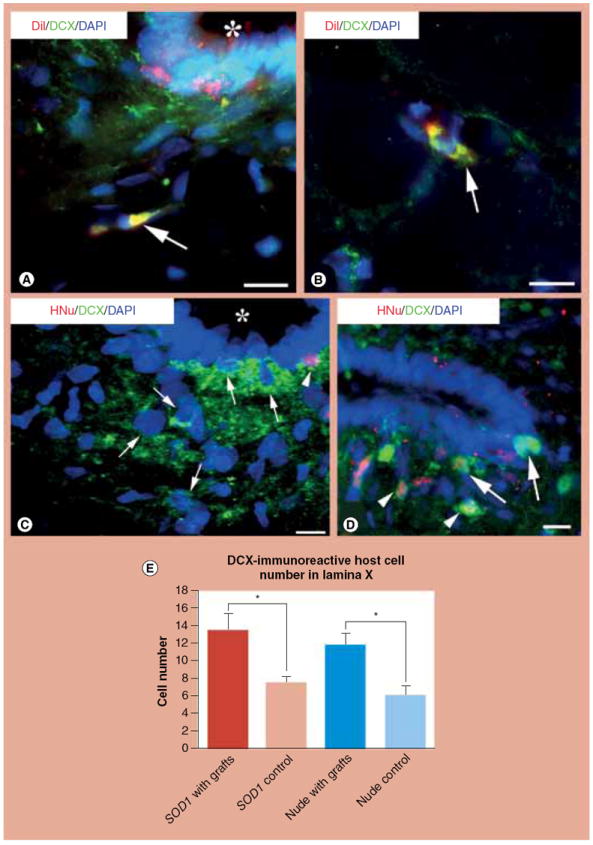
(A & B) Some migrating ependyma-derived cells (red, DiI positive) differentiate into cells with neuronal phenotypes identified by labeling with the migratory neuronal precursor marker DCX (green) in area X (A, arrow) and outside of area X (B, arrow). Note the colocalization of green and red in the cytoplasm (resulting in a yellow composite color). The central canal is indicated by an asterisk in (A). (C) This confocal picture shows that, in the central canal, a neural stem cell (NSC)-derived cell (HNu positive) that had migrated from the transplantation site is DCX positive (arrowhead). A few host cells (HNu negative) are also DCX positive (arrows), a pattern revealing their phenotype as migrating neuronal precursors. (D) Retrograde trans-synaptic tracer pseudorabies virus (PRV) staining in spinal area X of nude rats shows that some NSC-derived cells (HNu positive) are PRV positive (arrowhead). Some ependymal cells are also PRV immunoreactive (arrows). PRV particles can be transferred either directly from primarily labeled motor neurons or indirectly from PRV-positive nerve cells innervating motor neurons, a reasoning that leads to the conclusion that PRV-positive cells in area X are mature neurons. (E) Comparison by Student’s t-test of DCX-positive host cell counts in area X shows that SOD1 G93A rats with live NSC grafts (‘SOD1 with grafts’) or nude rats with live NSC grafts (‘Nude with grafts’) have significantly higher numbers of DCX-positive cells compared with SOD1 G93A rats with dead NSC grafts (‘SOD1 control’) or nude rats with dead NSC grafts (‘Nude control’).
Scale bars: (A) 10 μm; (B) 10 μm; (C & D) 25 μm.
DAPI: 4’,6-diamidino-2-phenylindole; DCX: Doublecortin; HNu: Human nuclear protein antibody; SOD1: Superoxide dismutase 1.
In the trans-synaptic labeling experiment with PRV, many graft-derived cells were PRV positive (Figure 5D), a finding similar to our previous study [12], this demonstrated that these cells are mature neurons that form direct or indirect synaptic connections with host motor neurons. In this study, PRV particles are first transported to α-motor neurons from muscles or nerve; then, viral particles are transferred to higher-order neurons via trans-synaptic means. Direct connections of PRV-positive graft-derived cells other than motor neurons with inoculated muscle are ruled out with the aid of CTB labeling, showing that only host motor neurons are CTB positive (picture not shown). Some ependyma-derived cells are also PRV positive (Figure 5D), a finding that further supports the view that these cells are mature neurons innervating PRV-positive host motor neurons or neurons with PRV particles trans-synaptically infected from PRV-positive host motor neurons. Maturation of ependyma-derived cells at the level of functional synapses further demonstrates the presence of ependymal cell-driven neurogenesis in the central canal zone after NSCs transplantation.
In conclusion, these results suggest that NSC transplantation induces neuronal differentiation of newly generated ependymal cells; these cells can form synapses with host motor neurons.
Discussion
NSC grafts have shown therapeutic effects in transgenic rodent models of ALS [10,11,15,20,21] and ongoing clinical trials of these cells in human ALS appear to be well tolerated [9]. The mechanisms for the therapeutic effects of these grafts have not been fully elucidated, although they do not appear to involve the previously hypothesized ‘cell replacement’ of dying motor neurons with freshly differentiated ones from transplanted NSCs [10]. The expression and release, by transplanted NSCs, of diffusible trophic signals such as GDNF and BDNF, are a likely mechanism for the observed neuroprotective effects of NSC grafts [11,12,13,19]. The findings of this study indicate that, besides trophic signaling from grafted NSCs, another possible mechanism of host motor neuron protection by NSC grafts is the induction of endogenous neurogenesis from ependymal cells in the lumbar region.
One of the main findings in the present paper is that NSC grafts engage in cross-talk with the host ependyma in L4-L6 spinal cord segments. This cross-talk may be accomplished with direct molecular signals but may also involve intermediate tissue structures such as microvessels. These signals play key roles in the targeted migration of transplanted NSCs towards ependyma and their orderly differentiation into a longitudinal cord of neurons running just underneath and parallel to the central canal. In parallel, ependyma-derived cells cued by signals introduced by NSC grafts are induced to undergo mitotic divisions and then migration and neural differentiation, thus initiating a major intrinsic repair mechanism in the host spinal cord. The longitudinal cord of graft-derived neurons ventral to the central canal may serve as an ‘auxiliary niche’, defined as an introduced microenvironment that promotes neurogenesis in host ependyma and may facilitate several steps in endogenous repair mechanisms (Figure 6) [22].
Figure 6. Summary sketch of the hypothesis that exogenous neural stem cells stimulate host endogenous neurogenesis and activate intrinsic repair mechanisms in the lower spinal cord.
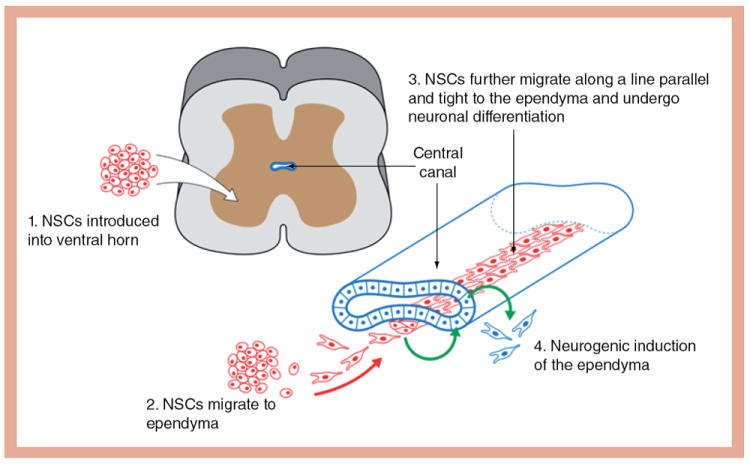
NSC: Neural stem cell.
There is increasing evidence that the ependymal zone of the central canal may serve as a stem cell niche comprised of ependymal cells, sub-ependymal astrocytes and possible endothelial vascular cells. Both stem and progenitor cell types have been identified in this area [23-28]. NP cells and immature neurons at the spinal cord ependymal zone are considered to exist in a ‘standby mode’, awaiting appropriate signals to complete their maturation process all the way to the stage of integrating into spinal cord circuits [23]. For example, newly generated NPs or neurons can migrate to lesion loci and possibly play a role in the structural and functional restoration after lesions [29-34]. In addition, transplanted human mesenchymal stem cells ameliorate the developmental apoptosis of newly generated NPs and young neurons in the host brain [33,34].
In the adult spinal cord, stem cell/progenitor cells in ependymal zone are in a quiescent status but can be activated by physiological signals such as Notch 1, Shh, Numb and BMP4 [28,35], but also via pathological mechanisms operating in diverse conditions such as spinal cord injury, MND, and multiple sclerosis [36-38]. These patterns are consistent with our findings. In the present study, in SOD1 G93A rats and nude rats with live NSC grafts (i.e., under both MND or healthy conditions), the number of new host neurons in lamina X is significantly higher compared with SOD1 G93A rats and nude rats with dead NSCs, a pattern suggesting that NSCs may both induce neurogenesis in the ependymal niche and promote the survival of newly generated host neurons. Similar patterns have been observed in neurogenic niches of the forebrain and may represent a more disseminated property along the adult neuraxis. For example, neurogenesis in the subventricular zone can be induced by NSC and mesenchymal stem cell grafts [33,34,39-41].
The results of retrograde trans-synaptic tracing with PRV in our study show that newly generated host neurons are mature and integrate into motor circuitry. PRV tracing after NSC transplantation has been demonstrated to be an effective method to study integration of mature neurons derived from transplanted NSCs into the host motor circuitry [12]. Other studies show that stem cells from the ependymal zone of central canal may have motor neuron maturation potential [42,43]. Although there is no evidence showing that these newly integrated host neurons differentiate into motor neurons, this result sheds light onto a possible intrinsic repair mechanism via which dead motor neurons are replenished with the host’s own newly generated neurons; such a mechanism, with further work and understanding, may be harnessed to form the basis for novel regenerative therapies for MND.
In short, the findings presented here add to a growing body of literature indicating alternative mechanisms for neural repair using stem cell grafts that transcend the older cell replacement vision using exclusively exogenous cells. That vision anticipated that fully differentiated exogenous cells would integrate into neural circuits and perform the same or similar functions to those of lost cells. However, as noted by our group [10] and others, there are still major hurdles in what can be presently achieved with traditional replacement strategies using stem cells in the nervous system. These hurdles make it necessary that alternative uses of stem cells are considered in regenerative medicine, including uses that initiate intrinsic the neurogenic/restorative potential of the adult nervous system. The idea that, in the future, very small stem cell grafts might be used in patients, enough to stimulate endogenous niches of stem cells and/or to supplement these niches to a particular regenerative task, is enormously appealing: with smaller numbers of transplanted cells, the risks of overgrowth and tumor formation in the spinal cord are reduced [44-47]. In our own work with spinal NSCs used in this study but also human embryonic stem cell-generated NPs, we have observed overgrowth of a high number of grafts [Xu L, Nasonkin I, Koliatsos VE, Unpublished Data]. In addition, regenerative efficacy may be higher if endogenous precursors are induced to differentiate and integrate in the host nervous system. Furthermore, the conceptual move from replacement experiments using stem cells to projects involving physiological signals of growth and differentiation in the adult nervous system may spark discoveries with far-reaching implications in the field of neural regeneration.
Conclusion
Transplanted NSCs appear to initiate cross-signaling with the host ependyma in the lumbar cord of adult rats with or without MND. For example, NSCs from the grafts migrate ventral to the central canal and, at that site, they form a cord of newly differentiated graft-derived neurons. In parallel, ependyma is induced to generate new neurons that migrate out and show signs of integration into the local motor circuitry. We postulate that the cord of NSC-derived neurons serves the role of an auxiliary niche for ependyma induction. Our findings suggest that NSC grafts have the potential to induce intrinsic repair mechanisms of therapeutic significance for the treatment of ALS and other spinal cord conditions, such as spinal cord injury.
Executive summary.
Migration & neuronal differentiation of transplanted human neural stem cells in the central canal zone
-
▪
Cells from human neural stem cell (NSC) grafts into the ventral horn of lumbar spinal cord always migrate to the central canal zone.
-
▪
NSCs migrating to the central canal zone differentiate into neurons with mature synapses that form a cord running parallel to and immediately ventral to the host central canal.
Proliferation & migration of ependymal cells in the spinal cord of SOD1 G93A-transgenic & nude rats in the presence of NSC grafts
-
▪
Exogenous NSCs in the original graft or, more likely, the NSC-derived subependymal neuronal cord induce cell proliferation, migration and neuronal differentiation of host ependymal cells.
Ependymal cell-driven neurogenesis in the central canal zone in the presence of NSC grafts
-
▪
Ependyma-derived neurons in transplanted subjects show signs of synaptic integration into local motor circuitry.
-
▪
Our findings suggest that NSC grafts have the potential of inducing intrinsic repair mechanisms in the spinal cord; this potential can be exploited in novel therapies for amyotrophic lateral sclerosis or spinal cord injury.
Acknowledgments
The authors would like to thank DK Ryugo and T Pongstaporn for their help with electron microscopy work and K Johe and T Hazel of NeuralStem Inc. for providing NSI-566RSC cells for use in this study. PRV and antibody were gifts from P Card, Department of Neuroscience, University of Pittsburgh and L Enquist, Department of Molecular Biology, Princeton University through the service of the National Center for Experimental Neuroanatomy with Neurotropic Viruses (NCRR P40 RRO118604).
This work was supported by National Institutes of Health (NS45140, DC000232, EY01765), the Muscular Dystrophy Association, and the Robert Packard Center for ALS Research at Johns Hopkins.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
-
▪
of interest
-
▪▪
of considerable interest
- 1.A controlled trial of recombinant methionyl human BDNF in ALS: the BDNF Study Group (Phase III) Neurology. 1999;52(7):1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- 2.Ochs G, Penn RD, York M, et al. A Phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(3):201–206. doi: 10.1080/14660820050515197. [DOI] [PubMed] [Google Scholar]
- 3.Miller RG, Petajan JH, Bryan WW, et al. A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. Ann Neurol. 1996;39:256–260. doi: 10.1002/ana.410390215. [DOI] [PubMed] [Google Scholar]
- 4.Barinaga M. Neurotrophic factors enter the clinic. Science. 1994;264:772–774. doi: 10.1126/science.8171331. [DOI] [PubMed] [Google Scholar]
- 5.Rothstein JD. Of mice and men: reconciling preclinical ALS mouse studies and human clinical trials. Ann Neurol. 2003;53(4):423–426. doi: 10.1002/ana.10561. [DOI] [PubMed] [Google Scholar]
- 6.Price DL, Ackerley S, Martin LJ, et al. Motor neuron diseases. In: Brady ST, Siegel GJ, Albers RW, Price DL, editors. Basic Neurochemistry. Elsevier; NY, USA: 2005. pp. 731–743. [Google Scholar]
- 7.Mallet J, Björklund A, Caskey CT, et al. Group report: neuronal replacement and functional modification. In: Price DL, Thoenen H, Aguayo AJ, editors. Neurodegenerative Disorders Mechanisms and Prospects for Therapy. John Wiley & Sons; NY, USA: 1991. pp. 271–290. [Google Scholar]
- 8.Silani V, Cova L, Corbo M, et al. Stem-cell therapy for amyotrophic lateral sclerosis. Lancet. 2004;364(9429):200–202. doi: 10.1016/S0140-6736(04)16634-8. [DOI] [PubMed] [Google Scholar]
- 9.Glass JD, Boulis NM, Johe K, et al. Lumbar intraspinal injection of neural stem cells in patients with ALS: results of a Phase I trial in 12 patients. Stem Cells. 2012;30(6):1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- 10▪.Koliatsos VE, Xu L, Yan J. Human stem cell grafts as therapies for motor neuron disease. Expert Opin Biol Ther. 2008;8(2):137–141. doi: 10.1517/14712598.8.2.137. Summarizes the translational potential of preclinical studies on the effects of neural stem cell (NSC) grafts for amyotrophic lateral sclerosis and raises some important concerns and caveats. [DOI] [PubMed] [Google Scholar]
- 11▪▪.Xu L, Yan J, Chen D, et al. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82(7):865–875. doi: 10.1097/01.tp.0000235532.00920.7a. First paper showing significant therapeutic effects of human NSC grafts in transgenic motor neuron disease in rats and also points to potential therapeutic mechanisms. [DOI] [PubMed] [Google Scholar]
- 12▪.Xu L, Ryugo DK, Pongstaporn T, et al. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J Comp Neurol. 2009;514(4):297–309. doi: 10.1002/cne.22022. Shows the synaptic integration of neurons differentiated from human NSC grafts as inhibitory neurons within the segmental motor circuitry of the host. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan J, Xu L, Welsh AM, et al. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4(2):e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johe KK, Hazel TG, Muller T, et al. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10(24):3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 15.Yan J, Xu L, Welsh AM, et al. Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells. 2006;24(8):1976–1985. doi: 10.1634/stemcells.2005-0518. [DOI] [PubMed] [Google Scholar]
- 16.Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131(1):177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Johansson CB, Momma S, Clarke DL, et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96(1):25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 18.Honig MG, Hume RI. Dil and diO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 1989;12(9):333–331. [PubMed] [Google Scholar]
- 19.Yan J, Welsh AM, Bora SHR, et al. Differentiation and tropic/trophic effects of exogenous neural precursors in the adult spinal cord. J Comp Neurol. 2004;480(1):101–114. doi: 10.1002/cne.20344. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Shen P, Hazel T, et al. Dual transplantation of human neural stem cells into cervical and lumbar cord ameliorates motor neuron disease in SOD1 transgenic rats. Neurosci Lett. 2011;494(3):222–226. doi: 10.1016/j.neulet.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki M, McHugh J, Tork C, et al. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One. 2007;2(1):e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪.Ourednik V, Ourednik J. Plasticity of the central nervous system and formation of “auxiliary niches” after stem cell grafting: an essay. Cell Transplant. 2007;16(3):263–271. doi: 10.3727/000000007783464696. Proposes the concept of ‘auxiliary niches’ formed in the host nervous system by transplanted stem cells. [DOI] [PubMed] [Google Scholar]
- 23.Marichal N, Garcia G, Radmilovich M, et al. Enigmatic central canal contacting cells: immature neurons in “standby mode”? J Neurosci. 2009;29(32):10010–10024. doi: 10.1523/JNEUROSCI.6183-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss S, Dunne C, Hewson J, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16(23):7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulbatski I, Mothe AJ, Keating A, et al. Oligodendrocytes and radial glia derived from adult rat spinal cord progenitors: morphological and immunocytochemical characterization. J Histochem Cytochem. 2007;55(3):209–222. doi: 10.1369/jhc.6A7020.2006. [DOI] [PubMed] [Google Scholar]
- 26.Martens DJ, Seaberg RM, van der Kooy D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and the central canal of the spinal cord. Eur J Neurosci. 2002;16(6):1045–1057. doi: 10.1046/j.1460-9568.2002.02181.x. [DOI] [PubMed] [Google Scholar]
- 27.Horner PJ, Power AE, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20(6):2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto S, Nagao M, Sugimori M, et al. Transcription factor expression and Notch-dependent regulation of neural progenitors in the adult rat spinal cord. J Neurosci. 2001;21(24):9814–9823. doi: 10.1523/JNEUROSCI.21-24-09814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 30.Hou SW, Wang YQ, Xu M, et al. Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke. 2008;39(10):2837–2844. doi: 10.1161/STROKEAHA.107.510982. [DOI] [PubMed] [Google Scholar]
- 31.Parent JM, Vexler ZS, Gong C, et al. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52(6):802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita T, Ninomiya M, Hernandez AP, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26(24):6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cova L, Armentero MT, Zennaro E, et al. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson’s disease. Brain Res. 2010;1311:12–27. doi: 10.1016/j.brainres.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 34▪.Bao X, Wei J, Feng M, et al. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011;1367:103–113. doi: 10.1016/j.brainres.2010.10.063. One of the first demonstrations of the neurogenic induction of an endogenous stem cell niche by stem cell grafts. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Leong SY, Schachner M. Differential expression of cell fate determinants in neurons and glial cells of adult mouse spinal cord after compression injury. Eur J Neurosci. 2005;22(8):1895–1906. doi: 10.1111/j.1460-9568.2005.04348.x. [DOI] [PubMed] [Google Scholar]
- 36.Chi L, Ke Y, Luo C, et al. Motor neuron degeneration promotes neural progenitor cell proliferation, migration, and neurogenesis in the spinal cords of amyotrophic lateral sclerosis mice. Stem Cells. 2006;24(1):34–43. doi: 10.1634/stemcells.2005-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danilov AI, Covacu R, Moe MC, et al. Neurogenesis in the adult spinal cord in an experimental model of multiple sclerosis. Eur J Neurosci. 2006;23(2):394–400. doi: 10.1111/j.1460-9568.2005.04563.x. [DOI] [PubMed] [Google Scholar]
- 38.Ke Y, Chi L, Xu R, et al. Early response of endogenous adult neural progenitor cells to acute spinal cord injury in mice. Stem Cells. 2006;24(4):1011–1019. doi: 10.1634/stemcells.2005-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39▪.Kan I, Barhum Y, Melamed ER, et al. Mesenchymal stem cells stimulate endogenous neurogenesis in the subventricular zone of adult mice. Stem Cell Rev. 2011;7(2):404–412. doi: 10.1007/s12015-010-9190-x. One of the first demonstrations of the neurogenic induction of an endogenous stem cell niche by stem cell grafts. [DOI] [PubMed] [Google Scholar]
- 40.Stroemer P, Patel S, Hope A, et al. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil Neural Repair. 2009;23(9):895–909. doi: 10.1177/1545968309335978. [DOI] [PubMed] [Google Scholar]
- 41.Jin K, Xie L, Mao X, et al. Effect of human neural precursor cell transplantation on endogenous neurogenesis after focal cerebral ischemia in the rat. Brain Res. 2011;1374:56–62. doi: 10.1016/j.brainres.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42▪▪.Moreno-Manzano V, Rodriguez-Jimenez FJ, Garcia-Rosello M, et al. Activated spinal cord ependymal stem cells rescue neurological function. Stem Cells. 2009;27(3):733–743. doi: 10.1002/stem.24. One of the first demonstrations that ependymal cells in the spinal cord can become activated by disease and, after culturing, serve as cell grafts for the treatment of animals with spinal cord contusions. [DOI] [PubMed] [Google Scholar]
- 43.Corti S, Locatelli F, Papadimitriou D, et al. Transplanted ALDHhiSSClo neural stem cells generate motor neurons and delay disease progression of nmd mice, an animal model of SMARD1. Hum Mol Genet. 2006;15(2):167–187. doi: 10.1093/hmg/ddi446. [DOI] [PubMed] [Google Scholar]
- 44.Burns TC, Steinberg GK. Stem cells and stroke: opportunities, challenges, and strategies. Expert Opin Biol Ther. 2011;11(4):447–461. doi: 10.1517/14712598.2011.552883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aubry L, Bugi A, Lefort N, et al. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc Natl Acad Sci USA. 2008;105(43):16707–16712. doi: 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dressel R. Effects of histocompatibility and host immune responses on the tumorigenicity of pluripotent stem cells. Semin Immunopathol. 2011;33:573–591. doi: 10.1007/s00281-011-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amariglio N, Hirshberg A, Scheithauer B, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6(2):e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]


