Abstract
Chronic ingestion of caffeine by mice caused a marked reduction in locomotor exploratory activity. At least 4 days of withdrawal were required to restore activity to normal levels. Stimulatory effects of injected caffeine were lower in chronically treated mice and the biphasic dose-response (stimulatory followed by depressant) curve for injected caffeine was left shifted. Seven days of withdrawal were required before the dose-response curve to caffeine was identical to that of control mice. The depressant effects of a potent xanthine phosphodiesterase inhibitor, 1,3-dipropyl-7-methylxanthine, were blunted in caffeine-treated mice. The depressant effects of A1- and A2-selective adenosine analogs were enhanced after chronic caffeine. There was little or no effect of chronic caffeine on the stimulatory effects of dopaminergic agents (amphetamine, caffeine), while both depressant and stimulatory effects of chollnergic agents (nicotine, oxotremorine, scopolamine) were reduced. The results indicate that chronic caffeine affects functions of adenosine and chollnergic receptors related to regulation of locomotor exploratory activity.
Keywords: Caffeine, Adenosine receptors, Cocaine, Amphetamine, Chollnergic receptors, Nicotine, Locomotor activity
THE behavioral stimulant activity of caffeine is widely recognized, but the underlying mechanism of action remains poorly defined. At present, it is in general accepted that caffeine, at least in part, owes its behavioral stimulant activity to blockade of adenosine receptors (2). Caffeine and other stimulant xanthines block adenosine receptors and reverse the depressant effects of adenosine analogs (4,19,27,29,39,42). Adenosine analogs appear capable of eliciting behavioral depression through activation of either A1 or A2 adenosine receptors (37,38). Indeed, chronic ingestion of caffeine results in an upregulation in levels of adenosine receptors (7,16,20,21,35). Remarkably, caffeine and other xanthines are more potent in reversing the depressant effects of adenosine analogs in vivo than in causing behavioral stimulation of locomotor exploratory activity when administered alone (27). Further, caffeine can become a behavioral depressant at higher doses. It has been suggested that the behavioral depressant effects of caffeine and other xanthines are due to inhibition of a cyclic adenosine monophosphate (cAMP) phosphodiesterase (9). Finally, the well-known ability of caffeine to mobilize intracellular calcium [cf. (5,44) and ref. therein], leading perhaps to enhanced release of neurotransmitters, cannot be completely ignored in seeking the mechanisms underlying the central actions of caffeine.
Chronic effects of caffeine, leading to tolerance, are well known in man and rodents (1,10,22-24,32). The mechanism(s) underlying such tolerance, as well as the withdrawal syndrome, are not well understood. The increase of levels of adenosine receptors after chronic caffeine (7,16,20,21,35) suggests upregulation of adenosine receptor-mediated functions as a basis for caffeine tolerance. A number of points argue against such a simple explanation: First, in rats the tolerance to caffeine can appear insurmountable (16); second, there are no clear precedents for loss of antagonist potency after chronic administration of a competitive receptor antagonist. Thus, tolerance suggests that caffeine may be enhancing activity in certain central pathways through blockade of adenosine receptors normally inhibitory to neurotransrnitter release and that such an enhanced activity may result in the downregulation of the neurotransmitter receptors subserving such pathways, resulting in an “insurmountable” tolerance to caffeine. Caffeine does appear to have effects on function of dopaminergic (10,15,18,43) and chollnergic systems (12,36) in rodents. The present study focused on the effects of chronic ingestion of caffeine and then withdrawal on locomotor exploratory activity in mice and on the effects of caffeine, adenosine analogs, and dopaminergic and cholinergic agents on locomotor activity in control and chronically caffeine-treated animals. Chronic caffeine enhanced behavioral responses to adenosine analogs, reduced behavioral responses to cholinergic agents, and had little effect on behavioral responses to dopaminergic agents.
METHOD
Animals
Adult, male mice of the NIH Swiss strain, weighing 25–30 g, were used. Mice were 5 weeks old when received. Mice were kept for 5–7 days at NIH before the start of any experiment. The groups of 12–15 mice per cage were housed and kept on a 12 dim lighting:12 dark cycle in the animal holding room. Mice were given free access to standard food pellets and water. In chronic caffeine-treated groups, a caffeine solution (1 g/l water) was provided instead of drinking water during the time prescribed by the experiment. Before testing, mice were habituated for 24 h in the laboratory monitoring room on a 12 dim lighting:12 dark cycle. Each mouse was used only once in the activity monitor except for the chronic caffeine time curve experiments, where the same animals were monitored for the locomotor activity on successive days. Each experimental group of caffeine-treated mice had a control group of water-treated mice. The liquid intake in both groups were comparable: 27.1 ± 1.7 ml/day/10 animals (n = 8) for controls and 26.7 ± 1.1 ml/day/10 animals (n = 8) for caffeine treatment over the first 7 days of treatment. This corresponds to about 100 mg/kg/day caffeine. Caffeine-treated animals appeared normal and healthy. Weights were 24.4 ± 1.3 (n = 14) and 25.1 ± 1.3 (n = 10) for caffeine-treated and control mice after 7 days of treatment.
Drugs
Compounds were obtained from the following sources: caffeine (free base, Matheson, Coleman and Bell, Cincinnati, OH); N6-cyclohexyladenosine (CHA), N-ethylcarboxamidoadenosine (NECA), (–)nicotine tartrate, mecamylamine HCl, 3-isobutyl-1-methylxanthine (IBMX), and oxotremorine sesquifumarate (Research Biochemicals, Inc., Natick, MA); (–)scopolamine HCl (Sigma Chemical Co., St. Louis, MO); (–)amphetamine sulfate (Smith, Kline and French Labs, Philadelphia, PA); cocaine HCl (Nederlandsche Cocalnefabriek, The Netherlands). APEC and 1,3-dipropyl-7-methylxanthine was synthesized as described (14,25).
Drug Administration
Drugs were dissolved in a 1 : 4 v/v mixture of Emulphor EL-620 (Rhone-Poulenc Chemicals Corp., Wayne, NJ) and phosphate-buffered saline. The same mixture was used as a vehicle for the injection in control animals. All drugs were administered intrapedtoneally in a volume of 5 ml/kg body weight. Immediately after injection, the mouse was placed in the middle of the activity monitor cage (see below).
Behavioral Evaluation
Effects of chronic caffeine treatment and withdrawal either alone or in combination with injected drugs on locomotor exploratory activity were investigated as follows: Individual mice were placed in a Digiscan Activity Monitor (Omnitech Electronics Inc., Columbus, OH) connected with a Digiscan Analyzer and an IBM-compatible computer. The activity monitor cages (40 × 40 × 30.5 cm) were surrounded by horizontal and vertical sensors not detectable by mice. Data collection was begun 10 min after the mouse was placed in the middle of the open-field activity monitor except for experiments with 1,3-dipropyl-7-methylxanthine and IBMX, where data collection was begun after 5 min.
Multivariant locomotor data were collected over a specified period of time. Two nonequivalent parameters were analyzed, namely, horizontal activity, which represents the total number of beam interruptions in the horizontal direction, and total distance traveled, which represents the distance in centimeters traveled by the mouse, in a particular period of time. There was a high degree of correlation between horizontal activity and total distance traveled; therefore, only the data for total distance are presented. The testing of mice was performed individually in a sound- and light-controlled area between 7:00 a.m. and 1:00 p.m. Data collected over three consecutive intervals of 10 min were analyzed for the overall 30-min period and are presented as mean ± SEM. The number of mice for each experimental point varied from 6–49. Statistical analysis was performed by Student's t-test.
The details of caffeine treatment and withdrawal periods are provided in the legends to each figure. All groups of caffeine-treated mice had a corresponding control group from the same shipment of mice and were tested at the same time of day and the same experimental day as caffeine-treated mice. In experiments involving IP injections, all mice were used in only one such experiment and controls consisted of injection with vehicle.
RESULTS
Locomotor Activity During Chronic Caffeine Treatment of Mice and Withdrawal
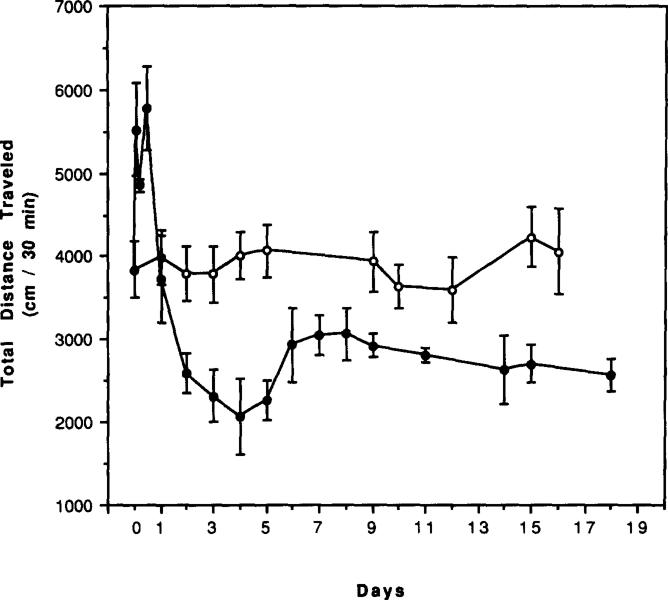
The locomotor activity of mice measured for a 30-min test period between 7:00 a.m. and 1:00 p.m. during 18 days of caffeine treatment (1 g/l drinking water) is presented in Fig. 1. There was an increase in total distance traveled during the first 12 h of caffeine ingestion. At 2 h, there was a 44% increase, at 6 h a 26% increase, and at 12 h a 50% increase. By 24 h, locomotor activity had returned to control levels. Thereafter, there was a gradual decrease in locomotor activity during days 2–4, followed by an increase of locomotor activity during days 5–6, and then stabilization at levels significantly below the controls activity from days 7–18 (p < 0.02–0.001).
FIG. 1.
Effects of chronic caffeine ingestion on locomotor activity in mice. (○), control; (●), chronic caffeine. Locomotor activity was determined at 2, 6, 12, 24 h and 2–18 days after initiation of chronic caffeine ingestion. Four separate groups of mice were used for the first 24 h, while only one group was monitored consecutively on each of the following days. Control groups were monitored at the same time of day and at the same number of hours or days of the experiment with chronic caffeine. Values are means ± SEM (n = 6–15).
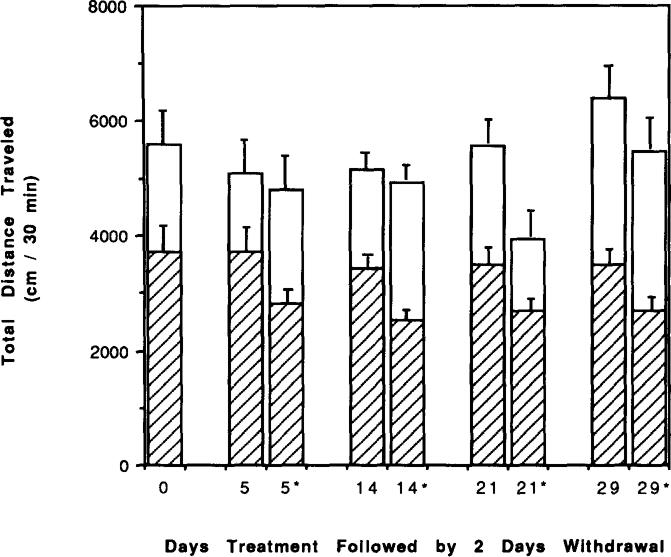
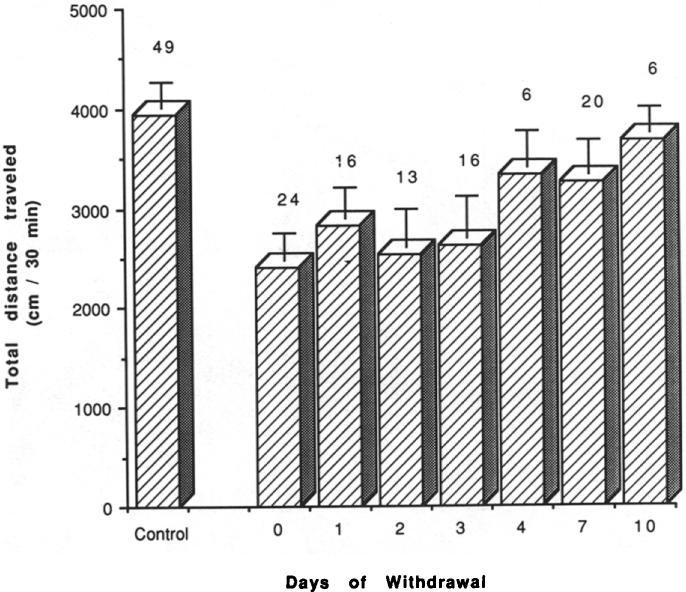
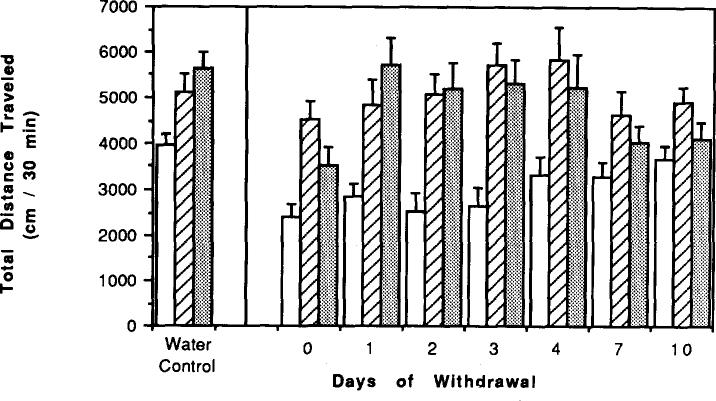
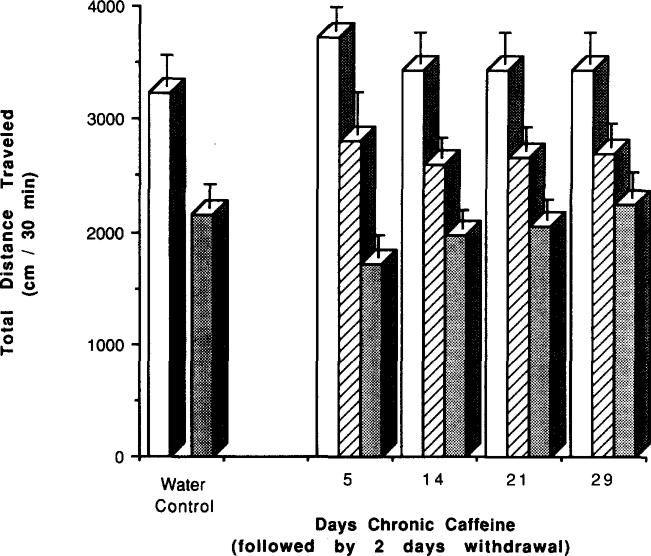
Two days of withdrawal after 5, 14, 21, and 29 days of chronic caffeine treatment had no significant effect on locomotor activity compared to mice with no withdrawal. This is based upon a comparison of activity in mice with no withdrawal (see Fig. 1) vs. activity in mice after 2 days of withdrawal (see Fig. 4 below). After 7 days of chronic caffeine treatment, 4 days of withdrawal was required before locomotor activity approached control levels (Fig. 2). The activity was now not significantly lower than control levels (p < 0.2).
FIG. 4.
Effects of caffeine injections on locomotor activity of mice after chronic caffeine ingestion followed by withdrawal. Locomotor activity was determined after IP injection of vehicle (hatched bars) or 20 mg/kg caffeine (increase over vehicle indicated by open bars) to control mice or chronic caffeiue-treated mice. Chronic ingestion of caffeine was for 5, 14, 21, and 29 days, followed by 2 days of withdrawal. Bars corresponding to chronic caffeine-treated mice are indicated by a star on the x-axis. Each day represents a separate group of mice. Values are means ± SEM. (n = 6–17).
FIG. 2.
Locomotor activity in mice after chronic ingestion of caffeine followed by withdrawal. Effects of 1–10 days of withdrawal on locomotor activity of mice that had chronically ingested caffeine for 7 days. Each day represents a separate group of mice and each had a control group monitored at the same time of day and same number of experimental days. Values are means ± SEM (n = 6–24 for chronic caffeine and n = 49 for controls).
Effect of Ingestion of Caffeine on Locomotor Activity of Mice During Chronic Caffeine Treatment and Withdrawal
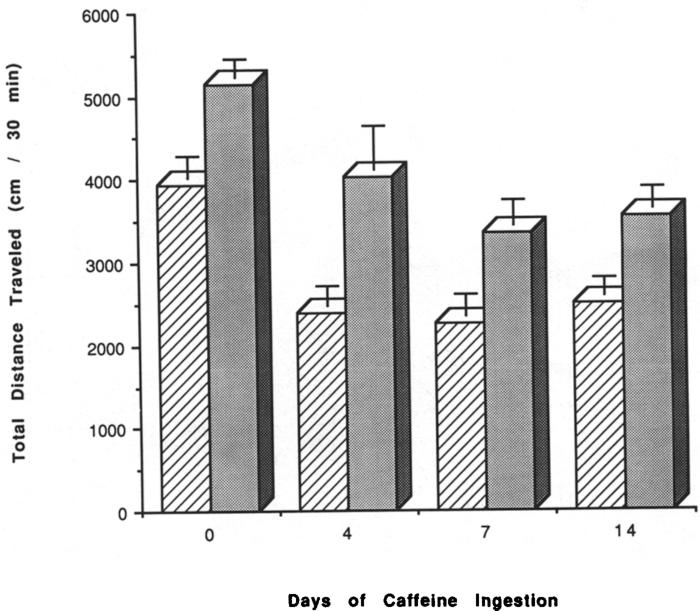
IP injection of caffeine (20 mg/kg) to mice that had been chronically treated with caffeine for 4, 7, or 14 days, followed by a 2-h withdrawal period, caused significantly less stimulation of locomotor activity (p < 0.05–p < 0.001) than in control mice (Fig. 3). In mice chronically treated with caffeine for 5–29 days, followed by 2 days of withdrawal, IP injection of caffeine elicited a stimulation of locomotor activity similar to that in control mice (Fig. 4). The effects of injected caffeine on locomotor activity in mice chronically treated with caffeine for 7 days, followed by 1–7 days of withdrawal, are presented in Fig. 5. There was significantly less response to injected caffeine after 7 days of chronic caffeine and no withdrawal, but after 1–4 days of withdrawal the stimulatory effects of injected caffeine were similar to those in control mice. After 7 or 10 days of withdrawal, the response to injected caffeine appeared reduced compared to control.
FIG. 3.
Effects of caffeine injections on locomotor activity of mice after chronic caffeine ingestion. Locomotor activity was determined after IP injection of vehicle (hatched bars) or 20 mg/kg caffeine (solid bars) IP in control mice and in mice after chronic ingestion of caffeine for 4, 7, and 14 days followed by 2 h of withdrawal. Each day represents a separate group of mice with an appropriate control group. Values are means ± SEM (n = 6–39).
FIG. 5.
Effects of caffeine injections on locomotor activity in mice during withdrawal from chronic caffeine. Locomotor activity was determined afer IP injection of vehicle (open bars) or either 10 mg/kg caffeine (hatched bars) or 20 mg/kg caffeine (solid bars) to control mice and to mice after chronic ingestion of caffeine for 7 days followed by O–10 days of withdrawal. Each day represents a separate group of mice with an appropriate control group. Values are means ± SEM. (n = 4–49).
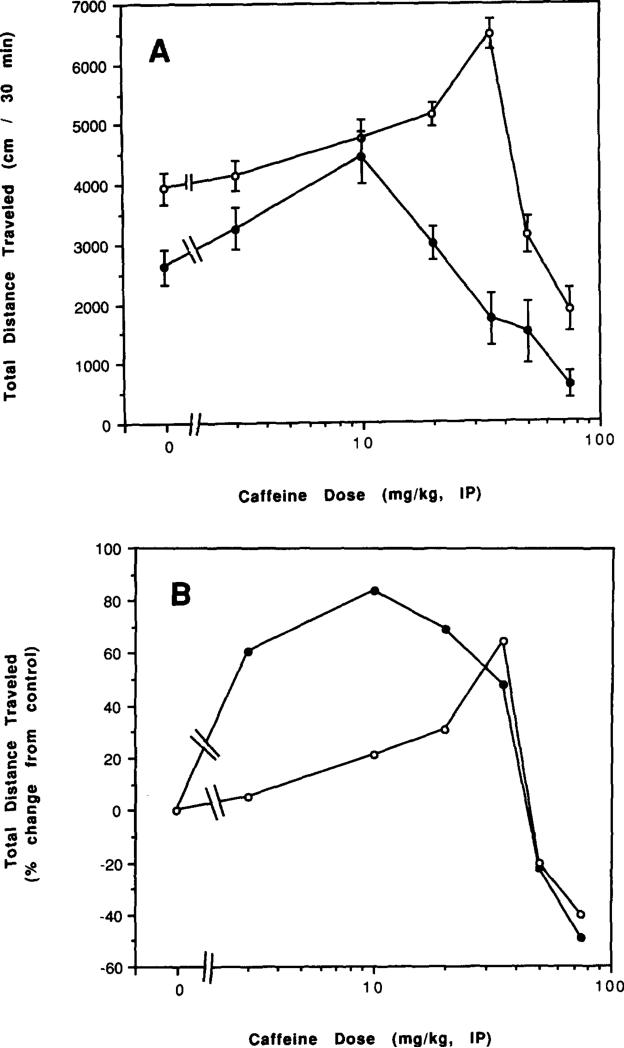
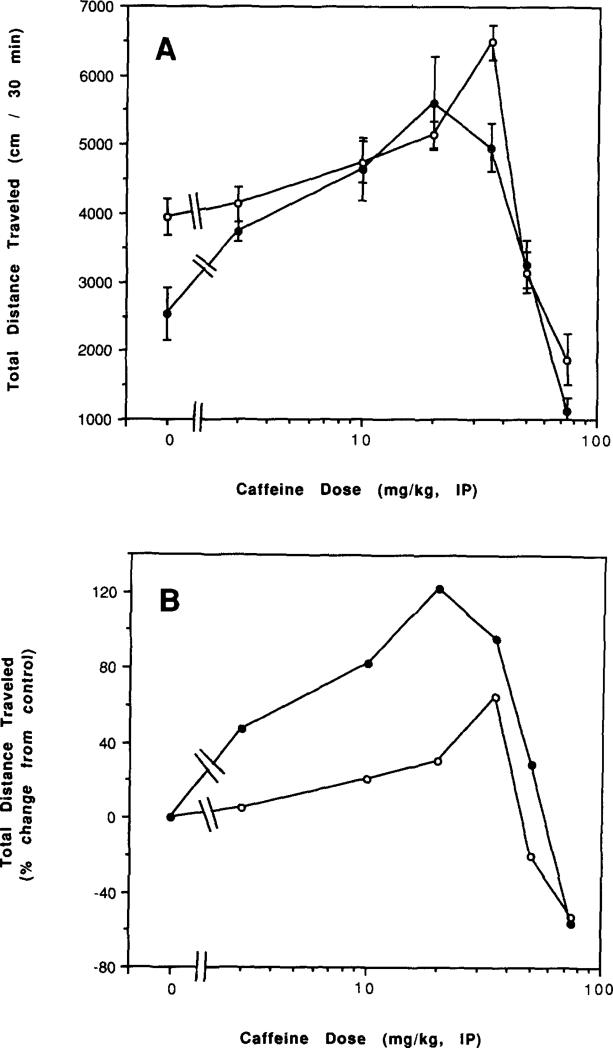
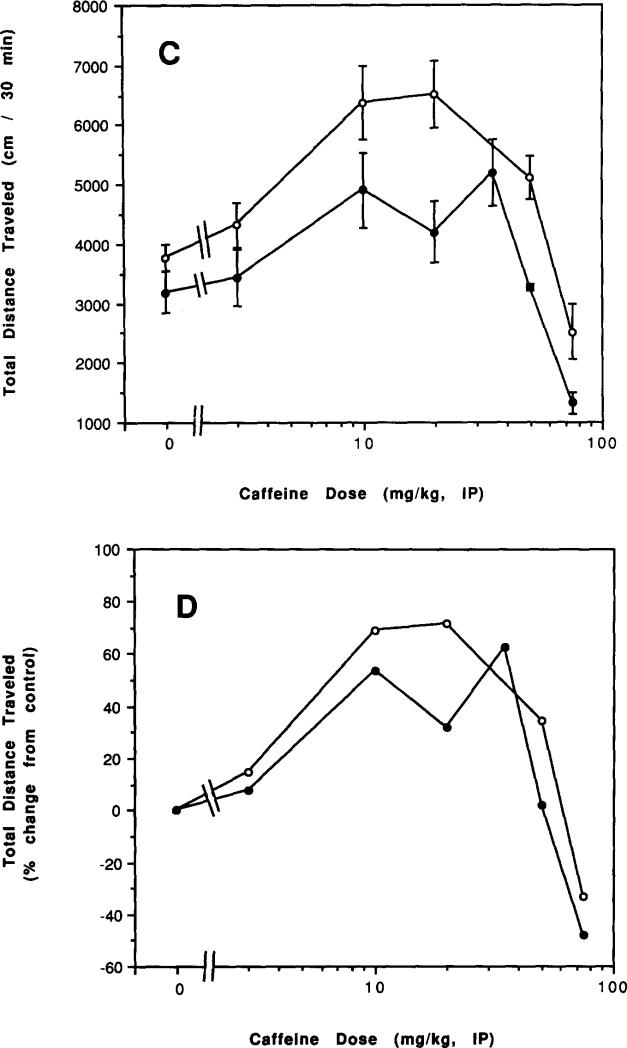
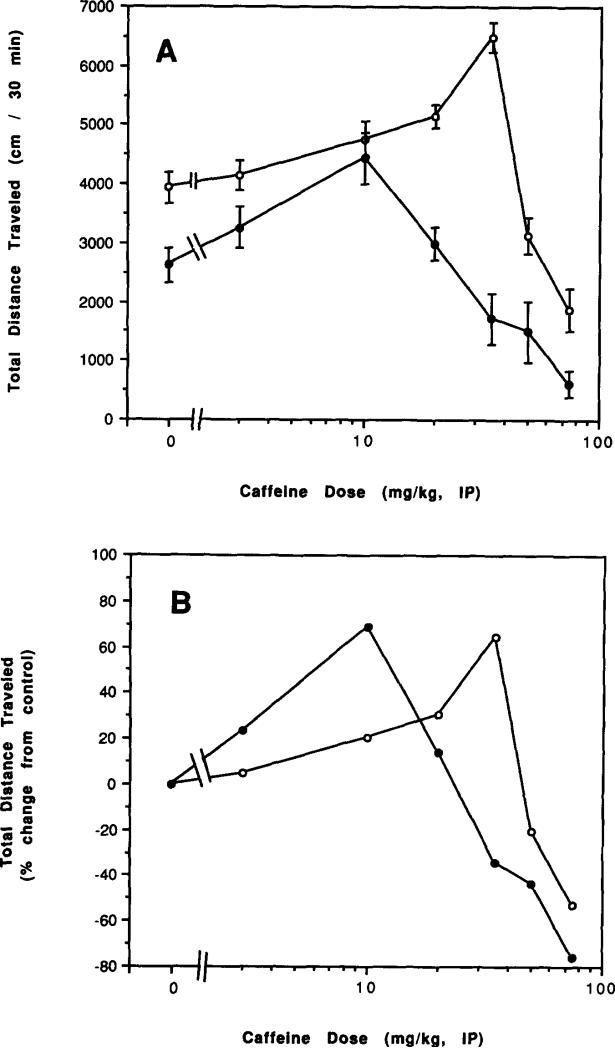
Dose-response relationships for acute administration of caffeine to control mice and to mice after chronic caffeine treatment and during withdrawal are presented in Figs. 6–8. There was an apparent left shift in the overall dose–response curves for caffeine both after chronic treatment with caffeine (Figs. 6A and 6B) and after 2 h of withdrawal (Figs. 7A and 7B). After 2 days of withdrawal, there was a less pronounced left shift in the dose–response curve (Figs. 8A and 8B). Only after 7 days of withdrawal was the dose–response curve to injected caffeine similar to that of controls (Figs. 8C and 8D).
FIG. 6.
Dose–response curve for effects of caffeine injections on locomotor activity of mice after chronic caffeine ingestion for 4 days. Locomotor activity was determined after intraperitoneal injection of various doses of caffeine to control mice and mice after chronic ingestion of caffeine for 4 days with no withdrawal. Each point represents a separate group of mice with an appropriate control group. (A) Total distance traveled. (B) Percent of locomotor activity relative to mice injected with vehicle. Each point represents a separate group of mice with an appropriate control group. Values are means ± SEM (n = 6–39). An ED50 for stimulation of locomotor activity in control mice can be estimated at about 20 mg/kg, while an ED50 for chronic caffeine-treated mice can be estimated at about 2 mg/kg.
FIG. 8.
Dose–response curve for effects of caffeine injections on locomotor activity of mice after chronic caffeine ingestion and withdrawal. Locomotor activity was determined as described in the legend to Fig. 6 in control mice and in mice after injestion of caffeine for 7 days followed by either (A, B) 2 days of withdrawal (C, D) 7 days of withdrawal. (A, C) Total distance traveled. (B, D) Percent of locomotor activity relative to mice injected with vehicle. Values are means ± SEM (n = 6–25).
FIG. 7.
Dose–response curve for effects of caffeine injections on locomotor activity of mice after chronic caffeine ingestion for 7 days. Locomotor activity was determined as described in the legend to Fig. 6 in control mice and in mice after ingestion of caffeine for 7 days. (A) Total distance traveled. (B) Percent of locomotor activity relative to mice injected with vehicle. Values are means ± SEM (n = 6–25).
Effects of Xanthine Phosphodiesterase Inhibitors on Locomotor Activity of Mice
The dose-dependent depressant effects of IBMX and 1,3-dipropyl-7-methylxanthine on locomotor activity in mice are shown in Fig. 9. Chronic caffeine treatment appeared to significantly reduce the depressant effects of 1,3-dipropyl-7-methylxanthine, in particular at a low dose of 1 mg/kg (p < 0.05). 1,3-Dipropyl-7-methylxanthine, a homolog of caffeine, was more potent than IBMX.
FIG. 9.
Dose–response curves for depression of locomotor activity by xanthine phosphodiesterase inhibitors in mice: Effect of chronic ingestion of caffeine on the response to 1,3-dipropyl-7-methylxanthine. Locomotor activity was determined after IP injection of various doses of IBMX (X) to control mice or of 1,3-dipropyl-7-methylxanthine to (○)control mice or (●) mice after ingestion of caffeine for 7 days. Values are means ± SEM (n = 6–25).
Effect of Adenosine Analogs on Locomotor Activity Before and After Chronic Treatment of Mice With Caffeine
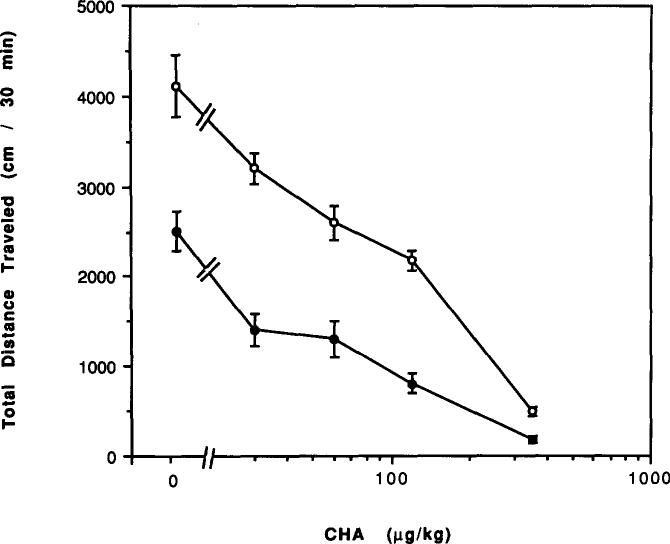
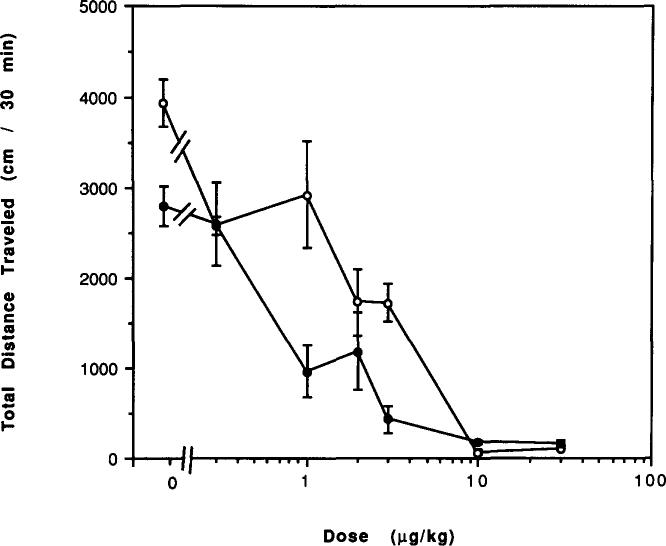
The A1-selective adenosine analog N6-cyclohexyladenosine elicited a greater percentage reduction in locomotor activity in mice chronically treated with caffeine for 7 days as compared to control mice (Fig. 10). For example, a dose of 30 μg/kg N6-cyclohexyladenosine caused a 22% depression in control mice (p < 0.05) and a 44% depression in mice chronically treated with caffeine (p < 0.001). However, the potency of N6-cyclohexyladenosine was not markedly altered. After 2 days of withdrawal from caffeine, N6-cyclohexyladenosine at 60 μg/kg appeared to cause less reduction in locomotor activity except in mice chronically treated for only 5 days with caffeine (Fig. 11). For example, in control mice this dose caused a 33% depression of locomotor activity (p < 0.01), while in mice treated with caffeine for 29 days followed by 2 days of withdrawal the same dose caused a 16% depression, not statistically significant.
FIG. 10.
Dose–response curves for effects of N6-cyclohexyladenosine (CHA) on locomotor activity in mice. Locomotor activity was determined after IP injection of various doses of CHA to (○) control mice or (●) mice after chronic ingestion of caffeine for 7 days. Values are means ± SEM. (n = 4–10 for control mice and 12–16 for caffeine-treated mice).
FIG. 11.
Effects of chronic caffeine ingestion and withdrawal on locomotor depression elicited by N6-cyclohexyladenosine (CHA) in mice. Locomotor activity was determined after IP injection of vehicle to control mice (open bars) or of vehicle (hatched bars) or 60 μg/kg CHA (solid bars) to mice after chronic ingestion of caffeine for 5, 14, 21, and 29 days followed by 2 days of withdrawal. Each bar represents mean ± SEM (n = 6–31 for control mice and 6–8 for caffeine-treated mice).
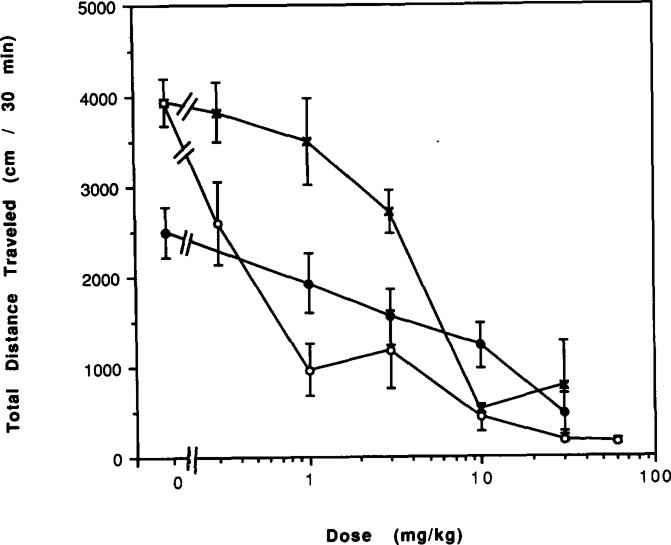
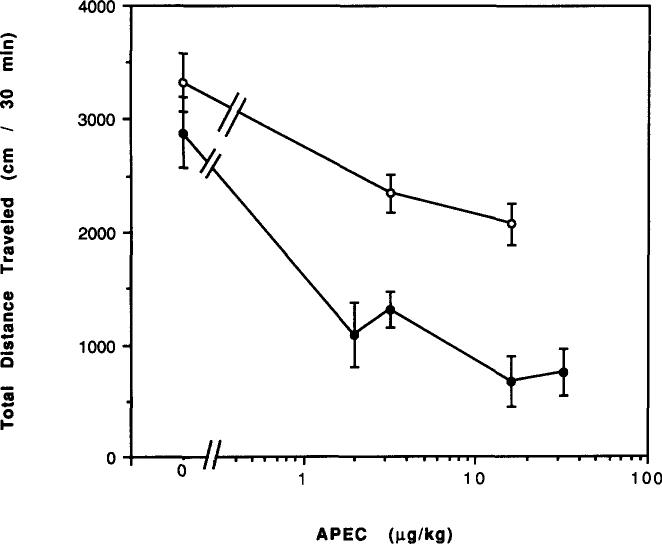
The A2-selective adenosine analog APEC elicited a greater reduction of locomotor activity in mice chronically treated with caffeine, followed by 2 h of withdrawal, as compared to control mice (Fig. 12). For example, a dose of 3 μg/kg APEC caused a 30% depression in control mice and a 55% depression in mice chronically treated with caffeine (p < 0.001). APEC appeared more potent in caffeine-treated mice. APEC at 16 μg/kg also caused a greater reduction in locomotor activity as compared to control mice in mice chronically treated with caffeine for 14 and 60 days followed by 1 day of withdrawal (Fig. 13).
FIG. 12.
Dose–response curves for effects of APEC on locomotor activity in mice. Locomotor activity was determined after IP injection of various doses of APEC to (○) control mice or (●) mice after chronic ingestion of caffeine for 7 days followed by 2 h of withdrawal. Values are means ± SEM. (n = 6–15).
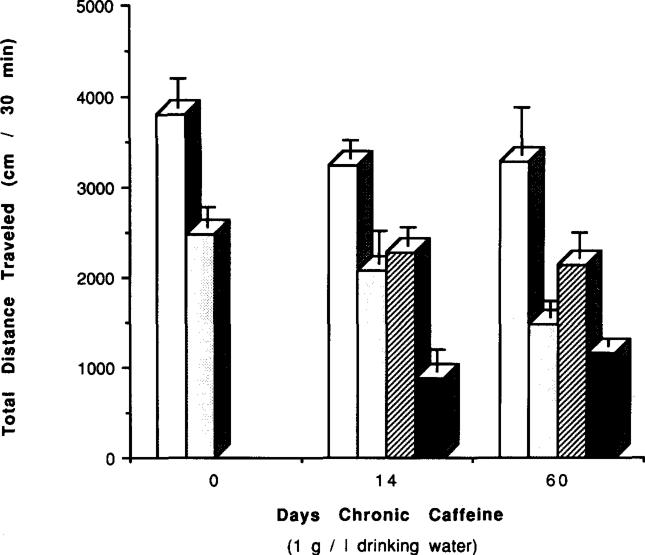
FIG. 13.
Effects of chronic caffeine ingestion and withdrawal on the locomotor depression elicited by APEC in mice. Locomotor activity was determined after IP injection of vehicle (control, open bars; caffeine treated, hatched bars) or 16 μg/kg APEC (control, dotted bars; caffeine treated, solid bars) to control mice or to mice after chronic ingestion of caffeine for 14 or 60 days followed by 1 day of withdrawal. Control mice were the same age as chronic caffeine mice. Values are means ± SEM (n = 5–12).
The potent but nonselective adenosine analog NECA elicited a greater reduction of locomotor activity in mice chronically treated with caffeine for 7 days (Fig. 14). This was most evident at a dose of 1 μg/kg, where NECA caused a 27% reduction in control mice (p < 0.05) and a 64% reduction in caffeine-treated mice (p < 0.001).
FIG. 14.
Dose–response curves for effects of N-ethylcarboxamidoadenosine (NECA) on locomotor activity in mice. Locomotor activity was determined after IP injection of various doses of NECA to (○) control mice or (●) mice after chronic ingestion of caffeine for 7 days followed by 2 h of withdrawal. Values are means ± SEM (n = 5–32).
Effect of Amphetamine and Cocaine, Agents Affecting Central Dopaminergic Function, on Locomotor Activity Before and After Chronic Treatment of Mice with Caffeine
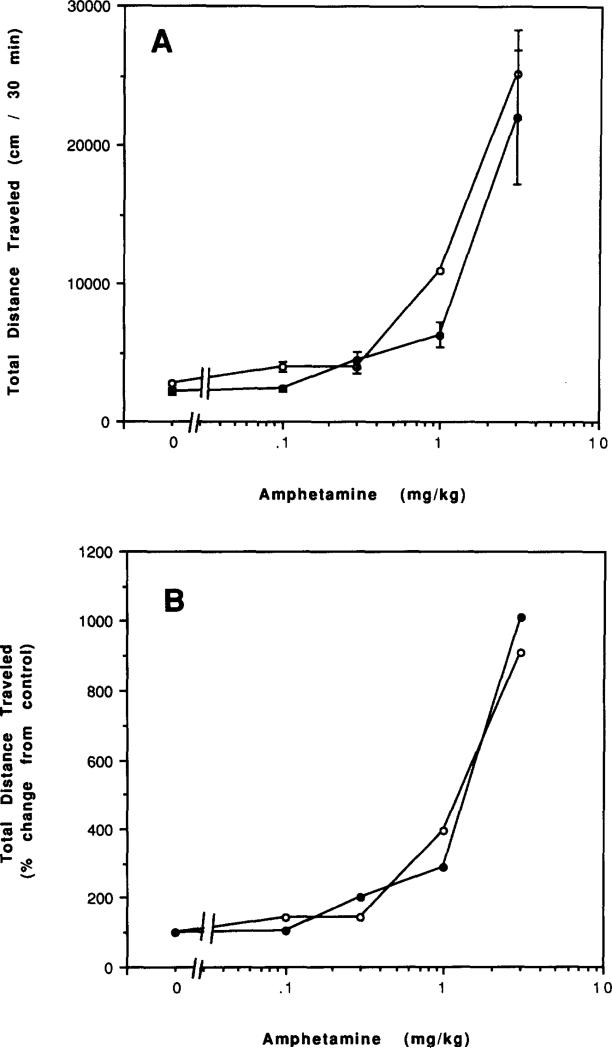
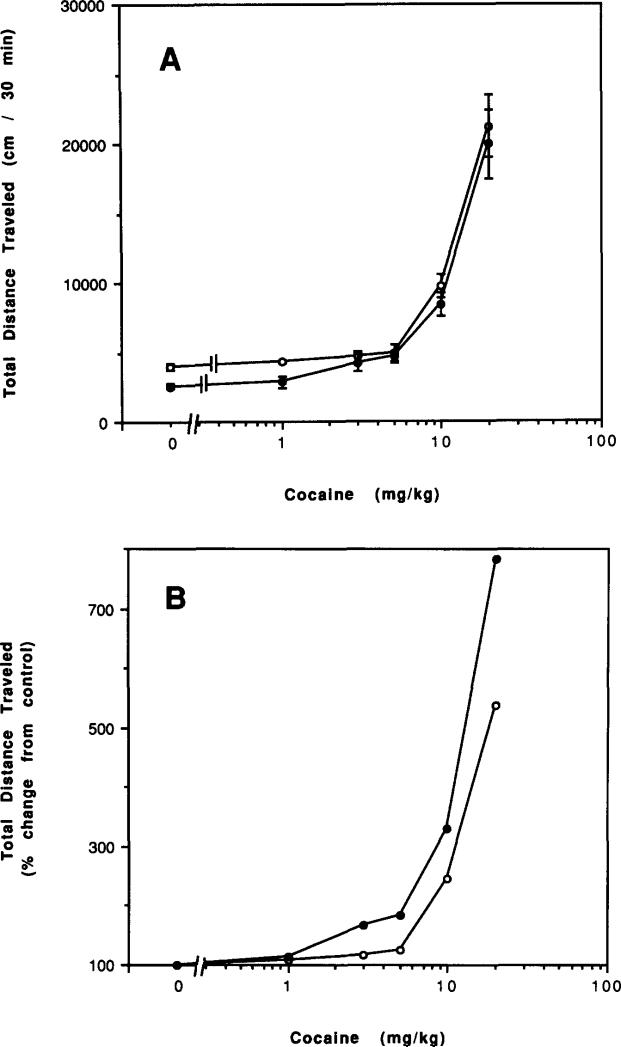
Both amphetamine and cocaine had similar stimulatory effects on locomotor activity in caffeine-treated and control mice (Figs. 15 and 16). At low doses, the effects of amphetamine were somewhat reduced and the effects of cocaine somewhat enhanced in mice chronically treated with caffeine. Amphetamine at 0.1 mg/kg caused a 45% stimulation in control mice (p < 0.001) and an insignificant 8% stimulation in mice chronically treated with caffeine. Cocaine at 3.0 mg/kg caused an insignificant 18% stimulation in control mice and a 66% stimulation in mice chronically treated with caffeine (p < 0.001).
FIG. 15.
Dose–response curves for effects of amphetamine on locomotor activity of mice. Locomotor activity was determined after IP injetions of various doses of amphetamine to (○) control mice or (●) mice after chronic ingestion of caffeine for 4 days followed by 2 h of withdrawal. (A) Total distance traveled. (B) Percent of locomotor activity relative to mice injected with vehicle. Values are means ± SEM (n = 6–11).
FIG. 16.
Dose–response curves for effects of cocaine on locomotor activity of mice. Locomotor activity was determined after IP injections of various doses of cocaine to (○) control mice or (●) mice after chronic ingestion of caffeine for 4 days followd by 2 h of withdrawal. (A) Total distance traveled. (B) Percent of locomotor activity relative to mice injected with vehicle. Values are means ± SEM (n = 5–25).
Effects of Nicotine, Oxotremorine, and Scopolamine, Agents Affecting Central Cholinergic Function, on Locomotor Activity Before and After Chronic Treatment of Mice With Caffeine
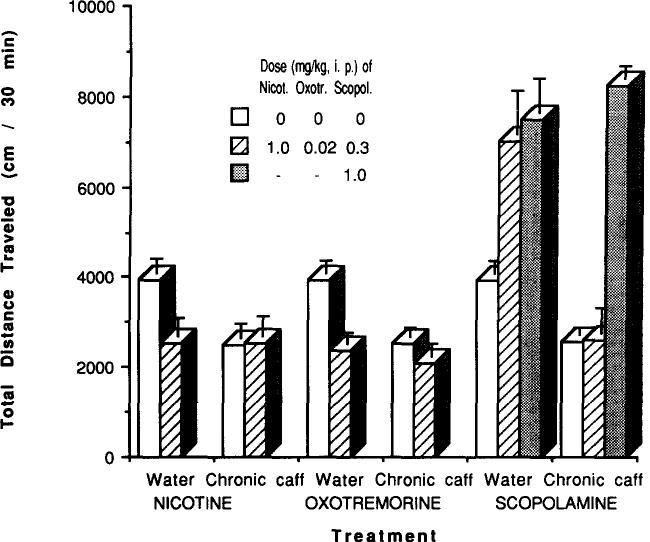
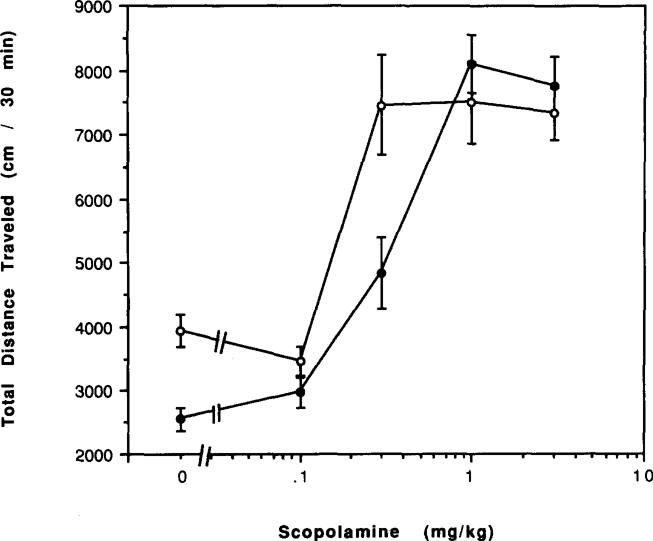
The nicotinic agonist nicotine at 1 mg/kg caused a marked reduction in locomotor activity in control mice (p < 0.01) but not in mice chronically treated with caffeine (Fig. 17). Similarly, the muscarinic agonist oxotremorine at 0.02 mg/kg caused a marked reduction in locomotor activity only in control mice (Fig. 17). Scopolamine, a nonselective muscarinic antagonist, had a stimulatory effect on locomotor activity at 1 mg/kg in both control and chronically caffeine-treated mice (Fig. 17). But, there was a clear decrease in potency of scopolamine in chronically treated mice (Fig. 18). The EC50 of scopolamine as a stimulant was estimated as 0.19 mg/kg in control mice and 0.35 mg/kg in mice chronically treated with caffeine.
FIG. 17.
Effects of cholinergic agents on locomotor activity of mice after chronic caffeine ingestion. Locomotor activity was determined after IP injection of vehicle (open bars) or indicated dose of nicotine, oxotremorine, or scopolamine (hatched and solid bars) to control mice (Water) or to mice after chronic ingestion of caffeine (Chronic caff) for 4 days followed by 2 h of withdrawal. Each bar represents mean ± SEM (n = 5–25).
FIG. 18.
Dose–response curves for effects of scopolamine on locomotor activity of mice. Locomotor activity was determined after IP injections of various doses of scopolamine to (○) control mice or (●) mice after chronic ingestion of caffeine for 4 days. Values are means ± SEM (n = 6–32).
DISCUSSION
Tolerance to the behavioral stimulant effects of caffeine after chronic ingestion of caffeine is a well-known phenomenon in rats (10,22–24,32,40). In most such studies, there have not been significant changes in basal locomotor activity in chronically caffeine-treated animals prior to challenge by acute administration of caffeine. In contrast, in the present study chronic ingestion of caffeine was found to cause a significant reduction in locomotor activity in NIH Swiss strain male mice (Fig. 1). The depression became maximal during the first 5 days of caffeine ingestion (48% reduction at 4 days), then partially reversed, but never recovered to control levels during the period from 6–18 days (30% reduction at 10 days). It is unknown whether this significant and reproducible depression is entirely a species- or strain-specific phenomenon or whether it also is linked to caffeine dosage levels or assay paradigms. The depression was relatively long lasting during withdrawal, suggesting that long-lasting changes in central function had occurred rather than a simple drug-related effect, which should reverse rapidly through clearance of caffeine. Indeed, about 4 days were required before locomotor activity approached control levels (Fig. 2). Such a depression complicates assessment of the degree of tolerance to caffeine (see below) but does provide an opportumty to assess possible central functions or pathways involved in such a depression. Agents affecting adenosine-, dopamine- and acetylcholine-dependent functions and/or pathways have been probed because there is evidence that caffeine does affect such pathways (see below).
Chronically caffeine-treated mice did exhibit a lower locomotor activity compared to controls when injected with caffeine (Fig. 3). However, in terms of percent stimulation of basal activity there was no tolerance (Figs. 6B and 7B). Indeed, the most remarkable effect was the pronounced left shift in the caffeine dose–response curves, that is, caffeine-treated animals were more, not less, sensitive to the stimulatory effects of caffeine. The second (depressant) phase of the dose–response curve to caffeine also seemed to begin at somewhat lower doses in caffeine-treated mice.
Unlike the 4 days required for recovery from the chronic effects of caffeine on basal locomotor activity (Fig. 2), reinstatement of a normal response to injected caffeine appeared nearly complete after 2 days (Fig. 8A). However, comparison of the percentage response suggests that recovery is not fully complete after 2 days of withdrawal (Fig. 8B) but is complete only after 7 days of withdrawal (Fig. 8D).
The left shift in the dose–response curves for caffeine after chronic treatment with caffeine can complicate measurements of tolerance based upon a single injected dose of caffeine. For example, in NIH Swiss strain mice tolerance to caffeine might have been judged to be complete and insurmountable only if doses of 20 or 30 mg/kg or greater were studied (see Fig. 7). Interpretation of the marked left shift of the caffeine dose–response curve suggests that the pathways underlying the stimulatory effects of caffeine are more readily blocked by caffeine in chronically treated animals and that rather than becoming tolerant mice are now “sensitized.” To probe whether or not the second depressant phase of the caffeine dose–response curve also might be affected, a xanthine, 1,3-dipropyl-7-methylxanthine, that has only depressant effects on locomotor activity was studied. It has been proposed that the depressant effects of this and other depressant xanthines are due to potent inhibition of a cAMP phosphodiesterase (9). The depressant effects of IBMX also were determined. There appeared to be a significant reduction in the depressant activity of the 1,3-dipropyl-7-methylxanthine in caffeine-treated mice (Fig. 9), although the initial depression in basal locomotor activity complicates any interpretation. Thus, any conclusions as to altered levels or roles of cAMP phosphodiesterases after chronic caffeine treatment would be premature. The 1,3-dipropyl-7-methylxanthine was 10-fold more potent than IBMX as a behavioral depressant in mice (Fig. 9) but is only 2-fold more potent as an inhibitor of the rat brain cAMP phosphodiesterase (9). Differing degrees of penetration into the brain, with IBMX presumably penetrating less well than the trisubstituted homolog of caffeine 1,3-dipropyl-7-methylxanthine [cf. (42)], probably are the basis for such a difference in relative potencies in vitro compared to in vivo. IBMX is well known as a behavioral depressant in rodents (9,27,39,42,45). Remarkably, IBMX becomes a behavioral stimulant when administered with a depressant dose of an adenosine analog (27,42). IBMX also becomes a stimulant when administered with a depressant dose of caffeine (39). Such interactions are not understood and require additional study. It is of interest that IBMX alone has stimulant properties in monkeys (11).
The depressant effects of adenosine analogs were enhanced in mice chronically treated with caffeine. These included an A1-selective agonist, N6-cyclohexyladenosine (Fig. 10), an A2-selective agonist, APEC (Fig. 12), and an A1/A2-nonselective agonist, NECA (Fig. 14). Such a heightened response to adenosine analogs is consonant with the known upregulation of A1 and A2 adenosine receptors in the brain of caffeine-treated rodents (7,13,16,20,21,35) and in the ability of either A1 or A2 receptors to subserve behavioral depression in mice (38). In only one study (24) did chronic treatment of rats with caffeine not cause an increase in brain A1 receptors. The increase in depressant activity of N6-cyclohexyladenosine or APEC appeared to be reversed after, respectively, <2 or 1 days of withdrawal (Figs. 11 and 13). This rapid reversal is in contrast to a reported sustained elevation of A1 receptors in rats for at least 15 days after caffeine withdrawal (6,47).
Dopaminergic systems have been implicated in the central actions of caffeine (10,15,26,43) and, therefore, effects of two agents, amphetamine and cocaine, known to act via dopamine pathways were investigated in control and caffeine-treated mice. Amphetamine is a well-known behavioral stimulant acting through direct release of dopamine from central dopaminergic neurons (31). Caffeine can potentiate behavioral stimulant effects of amphetamine (28,41,46) and apomorphine (28), a directly acting dopamine agonist. In the present study, chronic treatment of mice with caffeine had little effect on the stimulation of locomotor activity elicited by various doses of amphetamine (Fig. 15A). There was, however, a somewhat lessened response to a low dose of amphetamine (Fig. 15B). Cocaine, like amphetamine, causes behavioral stimulation through interaction with the dopaminergic system. Cocaine does so by potentiating the effects of endogenous dopamine through inhibition of reuptake of dopamine (31). Caffeine can potentiate the behavioral stimulant responses to cocaine (34). In mice chronically treated with caffeine, there was no significant increase in the potency of cocaine as a behavioral stimulant (Fig. 16A) although there was a greater percentage increase in locomotor activity by cocaine in chronically caffeine-treated mice, in particular at low doses of cocaine (Fig. 16B). Further studies are required before any conclusions are warranted. However, the data with both amphetamine and cocaine suggest some changes in dopaminergic function in mice chronically treated with caffeine.
There is limited evidence that cholingeric systems are involved in the action of caffeine (12,36). Nonetheless, adenosine anaiogs inhibit central release of acetylcholine (8,17,36) and cholinergic agonists and antagonists have effects on locomotor activity (3,18,30,33). Therefore, both nicotinic and muscarinic agents were investigated in control and caffeine-treated mice.
In mice chronically treated with caffeine, the depressant effects of a nicotine agonist, nicotine, at 1 mg/kg and of a muscarinic agonist, oxotremorine, at 0.02 mg/kg were abolished (Fig. 17). The results suggest that heightened cholinergic activity in the brain might underlie the behavioral depression of mice chronically treated with caffeine. If so, then a competitive cholinergic antagonist should be less potent in causing behavioral stimulation. This prediction was confirmed with the muscarinic antagonist scopolamine, which although causing the same maximal stimulation of locomotor activity in control and caffeine-treated mice (Fig. 17) was significantly less potent in the latter group (Fig. 18).
The present data suggest that chronic treatment of mice results in a behavioral depression partially due to an increase in cholinergic activity in brain. The relationship of the behaviorai depression to heightened sensitivity to low doses of caffeine and alterations in adenosine receptor functions is unclear. The results provide the basis for further delineation of the mechanism(s) and pathways affected by chronic administration of caffeine.
ACKNOWLEDGEMENTS
Research on caffeine in the Laboratory of Bioorganic Chemistry has been supported in part by a series of grants from the International Life Science Institute.
REFERENCES
- 1.Ahlijanian MK, Takemori AE. Cross-tolerance studies between caffeine and (–)-N6(phenylisopropyl)adenosine (PIA) in mice. Life Sci. 1986;88:577–588. doi: 10.1016/0024-3205(86)90051-2. [DOI] [PubMed] [Google Scholar]
- 2.Arnaud MJ. The pharmacology of caffeine. Prog. Drug Res. 1987;31:273–313. doi: 10.1007/978-3-0348-9289-6_9. [DOI] [PubMed] [Google Scholar]
- 3.Austin MC, Kalivas PW. The effect of cholinergic stimulation in the nucleus accumbens on locomotor behavior. Brain Res. 1988;441:209–214. doi: 10.1016/0006-8993(88)91400-x. [DOI] [PubMed] [Google Scholar]
- 4.Barraco RA, Coffin VL, Altman HJ, Phillis JW. Central effects on adenosine analogs on locomotor activity in mice and antagonism of caffeine. Brain Res. 1983;272:392–395. doi: 10.1016/0006-8993(83)90591-7. [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ. Caffeine inhibits inositol-trisphosphate-induced membrane potential oscillations in Xenopus oocytes. Proc. Royal Soc. Lond. B. 1991;244:57–62. doi: 10.1098/rspb.1991.0051. [DOI] [PubMed] [Google Scholar]
- 6.Boulenger J-P, Marangos PJ. Caffeine withdrawal affects central adenosine receptors but not benzodiazepine receptors. J. Neural. Trans. 1989;78:9–19. doi: 10.1007/BF01247109. [DOI] [PubMed] [Google Scholar]
- 7.Boulenger J-P, Patel J, Post RM, Parma AM, Marangos PJ. Chronic caffeine consumption increases the number of brain adenosine receptors. Life Sci. 1983;32:1135–1342. doi: 10.1016/0024-3205(83)90119-4. [DOI] [PubMed] [Google Scholar]
- 8.Brown SJ, James S, Reddington M, Richardson PJ. Both A1 and A2a purine receptors regulate striatal acetylcholine release. J. Neurochem. 1990;55:31–38. doi: 10.1111/j.1471-4159.1990.tb08817.x. [DOI] [PubMed] [Google Scholar]
- 9.Choi OH, Shamim MT, Padgett WL, Daly JW. Caffeine and theophylline analogues: Correlation of behavioral effects with activity as adenosine receptor agonists and as phosphodiesterase inlfibitors. Life Sci. 1988;43:387–398. doi: 10.1016/0024-3205(88)90517-6. [DOI] [PubMed] [Google Scholar]
- 10.Chou DT, Khan S, Forde J, Hirsh KR. Caffeine tolerance: Behavioral, electrophysiological and neurochemical evidence. Life Sci. 1985;36:2347–2358. doi: 10.1016/0024-3205(85)90325-x. [DOI] [PubMed] [Google Scholar]
- 11.Coffin VL, Spealman RD. Psychomotor-stimulant effects of 3-isobutyl-1-methylxanthine; comparison with caffeine and 7-(2-chloroethyl)-theophylline. Eur. J. Pharmacol. 1989;170:35–40. doi: 10.1016/0014-2999(89)90130-1. [DOI] [PubMed] [Google Scholar]
- 12.Corradetti R, Pedata F, Pepeu G, Vannuchi MG. Chronic caffeine treatment reduces caffeine but not adenosine effects on cortical acetylcholine release. BR. J. Pharmacol. 1986;88:671–676. doi: 10.1111/j.1476-5381.1986.tb10249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daval JL, Deckert J, Weiss SRB, Post RM, Marangos PJ. Upregulation of adenosine A1 receptors and forskofin binding sites following chronic treatment with caffeine or carbamazepine: A quantitative autoradiographic study. Epllepsia. 1989;30:26–33. doi: 10.1111/j.1528-1157.1989.tb05276.x. [DOI] [PubMed] [Google Scholar]
- 14.Daly JW, Padgett WL, Shamim MT. Analogues of caffeine and theophylline: Effect of structural alterations on affinity of adenosine receptors. J. Med. Chem. 1986;29:1305–1308. doi: 10.1021/jm00157a035. [DOI] [PubMed] [Google Scholar]
- 15.Foote WE, Holmes P, Pritchard A, Hatcher C, Mordes J. Neurophysiological and pharmacodynamic studies on caffeine and on interactions between caffeine and nicotinic acid in the rat. Neuropharmacology. 1978;17:7–12. doi: 10.1016/0028-3908(78)90167-3. [DOI] [PubMed] [Google Scholar]
- 16.Fredholm BB. Adenosine actions and adenosine receptors after 1 week treatment with caffeine. Acta Physiol. Scand. 1982;115:283–286. doi: 10.1111/j.1748-1716.1982.tb07078.x. [DOI] [PubMed] [Google Scholar]
- 17.Fredholm BB. Adenosine A1-receptor-mediated inhibition of evoked acetylcholine release in rat hippocampus does not depend on protein kinase C. Acta Physiol. Scand. 1990;140:245–255. doi: 10.1111/j.1748-1716.1990.tb08996.x. [DOI] [PubMed] [Google Scholar]
- 18.Fujii W, Kuribara H, Tadokoro S. Cross interaction between methamphetamine and scopolamine by means of ambulatory activity in mice. Jpn. J. Pharmacol. 1990;52:533–539. doi: 10.1254/jjp.52.533. [DOI] [PubMed] [Google Scholar]
- 19.Glowa JR, Sobel E, Malaspina S, Dews PB. Behavioral effects of caffeine, (–)-N-[(R)-1-methyl-2-phenylethyl]adenosine (PIA), and their combination in the mouse. Psychopharmacolngy (Berl.) 1985;87:421–424. doi: 10.1007/BF00432506. [DOI] [PubMed] [Google Scholar]
- 20.Green RM, Stiles GL. Chronic caffeine ingestion sensitizes the A1 adenosine receptor-adenylate cyclase system in rat cerebral cortex. J. Clin. Invest. 1986;77:222–227. doi: 10.1172/JCI112280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawkins M, Dugich MM, Porter NM, Urbancic M, Raduiovacki M. Effects of chronic administration of caffeine on adenosine A2 and A2 receptors in rat brain. Brain Res. Bull. 1988;21:479–482. doi: 10.1016/0361-9230(88)90162-1. [DOI] [PubMed] [Google Scholar]
- 22.Holtzman SG. Complete, reversible drug-specific tolerance to stimulation of locomotor activity by caffeine. Life Sci. 1983;33:779–787. doi: 10.1016/0024-3205(83)90784-1. [DOI] [PubMed] [Google Scholar]
- 23.Holtzman SG, Finn IB. Tolerance to behavioral effects of caffeine in rats. Pharmacol. Biochem. Behav. 1988;29:411–418. doi: 10.1016/0091-3057(88)90179-7. [DOI] [PubMed] [Google Scholar]
- 24.Holtzman SG, Mante S, Minneman KP. Role of adenosine receptors in caffeine tolerance. J. Pharmacol. Exp. Ther. 1991;256:62–68. [PubMed] [Google Scholar]
- 25.Jacobson KA, Barrington WW, Pannell LK, Jarvis MF, Ji X-D, Hutchison AJ, Stiles GL. Agonist-derived molecular probes for A1-adenosine receptors. J. Mol. Recogn. 1989;2:170–178. doi: 10.1002/jmr.300020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Josselyn SA, Beninger RJ. Behavioral effects of intrastriatal caffeine mediated by adenosinergic modulation of dopamine. Pharmacol. Biochem. Behav. 1991;39:97–103. doi: 10.1016/0091-3057(91)90403-o. [DOI] [PubMed] [Google Scholar]
- 27.Katims JJ, Annau Z, Snyder SH. Interactions in the behavioral effects of methylxanthines and adenosine derivatives. J. Pharmacol. Exp. Ther. 1983;227:167–173. [PubMed] [Google Scholar]
- 28.Klawans HL, Moses H, III, Beaulieu DM. The influence of caffeine on d-amphetamine- and apomorphine-induced stereotyped behavior. Life Sci. 1974;14:1493–1500. doi: 10.1016/0024-3205(74)90160-x. [DOI] [PubMed] [Google Scholar]
- 29.Logan L, Carney JM. Antagonism of the behavioral effects of l-phenyl-isopropyladenosine (L-PIA) by caffeine and its metabofites. Pharmacol. Biochem. Behav. 1984;21:375–379. doi: 10.1016/s0091-3057(84)80098-2. [DOI] [PubMed] [Google Scholar]
- 30.Martin TJ, Suchocki J, May EL, Martin BR. Pharmacological evaluation of the antagonism of nicotine's central effects by mecamylamine and pempidine. J. Pharmacol. Exp. Ther. 1990;254:45–51. [PubMed] [Google Scholar]
- 31.McMillen BA. CNS stimulants: Two distinct mechanisms of action of amphetamine-like drugs. Trends Pharmacol. Sci. 1983;4:429–432. [Google Scholar]
- 32.Meliska CJ, Landram RE, Landrum TA. Tolerance and sensitization to chronic and subchronic oral caffeine: Effects on wheelranning in rats. Pharmaeol. Biochem. Behav. 35:477--479. 1990 doi: 10.1016/0091-3057(90)90189-o. [DOI] [PubMed] [Google Scholar]
- 33.Meyers B, Roberts KH, Riciputi RH, Domino EF. Some effects of muscarinic blocking drugs on behavior and the electrocorticngram. Psychopharmacologia. 1964;5:289–300. doi: 10.1007/BF02341261. [DOI] [PubMed] [Google Scholar]
- 34.Misra AL, Vadlamani NL, Pontani RB. Effect of caffeine on cocaine locomotor stimulant activity in rats. Pharmacol. Biochem. Behav. 1986;24:761–764. doi: 10.1016/0091-3057(86)90587-3. [DOI] [PubMed] [Google Scholar]
- 35.Murray TF. Up-regulation of rat cortical adenosine receptors following chronic administration of theophylline. Eur. J. Pharmacol. 1982;82:113–114. doi: 10.1016/0014-2999(82)90563-5. [DOI] [PubMed] [Google Scholar]
- 36.Murray TF, Blaker WD, Cheney DL, Costa E. Inhibition of acetylcholine turnover rate in rat hippocampus and cortex by intraventricular injection of adenosine analogs. J. Pharmacol. Exp. Ther. 1982;222:550–554. [PubMed] [Google Scholar]
- 37.Nikodijevic O, Daly JW, Jacobson KA. Characterization of the locomotor depression produced by an A1-selective adenosine agonist. FEBS Lett. 1990;261:67–70. doi: 10.1016/0014-5793(90)80638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikodijevic O, Sarges R, Daly JW, Jacobson KA. Behavioral effects of A1- and A2-selective adenosine agonists and antagonists: Evidence for synergism and antagonism. J. Pharmacol. Exp. Ther. 1991;259:286–294. [PMC free article] [PubMed] [Google Scholar]
- 39.Phillis JW, Barraco RA, Deiong RE, Washington DO. Behavioral characteristics of centrally administered adenosine analogs. Pharmacol. Biochem. Behav. 1986;24:263–270. doi: 10.1016/0091-3057(86)90349-7. [DOI] [PubMed] [Google Scholar]
- 40.Ray SK, Poddar MK. Role of central serotonin in caffeine-induced stimulation of locomotor activity in rat. Biogen. Amines. 1990;7:153–164. [Google Scholar]
- 41.Schechter MD. Caffeine potentiation of amphetamine: Implications for hyperkinesis therapy. Pharmacol. Biochem. Behav. 1977;6:359–361. doi: 10.1016/0091-3057(77)90038-7. [DOI] [PubMed] [Google Scholar]
- 42.Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW. Adenosine receptors and behavioral actions of methylxanthines. Proc. Natl. Acad. Sci. USA. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoner GR, Skirboll LR, Werkraan S, Hommer DW. Preferential effects of caffeine on limbic and cortical dopamine systems. Biol. Psychiatry. 1988;23:761–768. doi: 10.1016/0006-3223(88)90064-9. [DOI] [PubMed] [Google Scholar]
- 44.Vigne P, Breittmayer J-P, Marsault R, Frelin C. Endothelin mobilizes CA2+ from a caffeine- and ryanodine-insensitive intracellular pool in rat atrial cells. J. Biol. Chem. 1990;265:6782–6787. [PubMed] [Google Scholar]
- 45.Wachtel H. Characteristic behavioral alterations in rats induced by rolipram and other selective adenosine cyclic 3′, 5′-monophasphate phosphodiesterase inhibitors. Psychopharmacology (Berl.) 1982;77:309–316. doi: 10.1007/BF00432761. [DOI] [PubMed] [Google Scholar]
- 46.White BC, Keller GE., III Caffeine pretreatment: Enhancement and attenuation of d-amphetamine-induced activity. Pharmacol. Biochem. Behav. 1984;20:383–386. doi: 10.1016/0091-3057(84)90275-2. [DOI] [PubMed] [Google Scholar]
- 47.Wu PH, Coffin VL. Up-regulation of brain [3H]diazepam binding sites in chronic caffeine-treated rats. Brain Res. 1984;294:186–189. doi: 10.1016/0006-8993(84)91329-5. [DOI] [PubMed] [Google Scholar]