Abstract
Purpose
Prostate cancer treatment has the potential to lead to posterior urethral stricture. These strictures are sometimes recalcitrant to dilation and urethrotomy alone. We present our experience with the Urolume® stent for prostate cancer treatment related stricture.
Materials and Methods
A total of 38 men with posterior urethral stricture secondary to prostate cancer treatment were treated with Urolume stenting. Stents were placed in all men after aggressive urethrotomy over the entire stricture. A successfully managed stricture was defined as open and stable for greater than 6 months after any necessary secondary procedures.
Results
The initial success rate was 47%. After a total of 31 secondary procedures in 19 men, including additional stent placement in 8 (18%), the final success rate was 89% at a mean ± SD followup of 2.3 ± 2.5 years. Four cases (11%) in which treatment failed ultimately requiring urinary diversion (3) or salvage prostatectomy (1). Incontinence was noted in 30 men (82%), of whom 19 (63%) received an artificial urinary sphincter a mean of 7.2 ± 2.4 months after the stent. Subanalysis revealed that irradiated men had longer strictures (3.6 vs 2.0 cm, p = 0.003) and a higher post-stent incontinence rate (96% vs 50%, p <0.001) than men who underwent prostatectomy alone but the initial failure rate was similar (54% vs 50%, p = 0.4).
Conclusions
Urolume stenting is a reasonable option for severe post-prostate cancer treatment stricture when patients are unwilling or unable to undergo open reconstructive surgery. Incontinence should be expected. The need for additional procedures is common and in some men may be required periodically for the lifetime of the stent.
Keywords: prostate, prostatic neoplasms, urethral stricture, stents, urinary bladder neck obstruction
Of the almost 200,000 men diagnosed with PCa in the United States annually1 all those electing definitive treatment are at risk for posterior urethral stricture.2,3 Radical prostatectomy is complicated by BNC in 1 to 25% of cases4 and radiation therapy can lead to stricture along the entire length of the posterior urethra.5 Fortunately many strictures are amenable to simple endoscopic procedures.6 However, a small percent in which conservative measures fail require more radical intervention if a permanent solution is sought.
Traditionally 2 options have been available in these men. One generally involves open perineal and/or abdominal surgery, in which the strictured segment is excised and an anastomosis is then formed.7 While these operations often successfully remove the stricture, they may lead to postoperative incontinence and are often technically challenging.8 The other option is urinary diversion, which patients and urologists usually think is an option of last resort.9,10
In 2001 we began using the Urolume urethral stent for recalcitrant PCa treatment related strictures. We hoped that the stent would provide a reasonable alternative in men who elected definitive stricture management but were not interested in complex surgical options and those in whom we thought surgery would not be successful or advisable. Initial reports of stent use were encouraging with an overall success rate of greater than 80%.11 We discuss our updated experience with the stents, focusing specifically on management for PCa therapy related posterior urethral stricture.
MATERIALS AND METHODS
Patient Population
We reviewed our large, single surgeon, prospective, institutional review board approved stricture database on all men in whom Urolume stents were placed for posterior urethral stricture. We included only those in whom stricture was a result of PCa treatment and then only if the stent had been placed at our institution with greater than 6 months of followup.
Procedure Characteristics
The Urolume stent is a biocompatible, corrosion resistant, super alloy mesh that self-expands to almost 30Fr in the urethra after deployment from a 24Fr disposable delivery tool. All stents were placed by a single surgeon (JWM). The surgical technique for stent placement has been described previously11,12 but a few important technical considerations/modifications are worth mentioning. 1) The entire length of the urethral stricture must be stented. In our experience if a previously strictured segment is opened but not stented, the stricture recurs in the nonstented area despite aggressive urethrotomy. Also, if the stricture is greater than 3 cm, which is the currently the length of the largest stent provided by the Urolume manufacturer, overlapping stents of at least 5 mm must be placed and one should always work from proximal to distal if multiple stents are needed. 2) We prefer to have 5 mm of the stent protruding into the bladder in men with pure BNC, which we have found can help prevent proximal intraluminal recurrence with little morbidity. 3) We prefer to place all patients with a Urolume stent on suppressive, prophylactic antimicrobial therapy, generally nitrofurantoin, for the lifetime of the stent.
Patient Followup
Patients are followed with uroflowmetry and post-void residual urine measurement at 3-month intervals. If large post-void residual urine is noted, or there is an obstructed voiding curve and/or low flow rate on uroflowmetry, retrograde urethrogram and/or cystoscopy is done. If stricture recurrence or stone encrustation is noted within the existing stent, the tissue is excised as previously described with a holmium laser or resectoscope.13 If recurrence is proximal or distal to the existing stent, it is managed by another stent. If incontinence is noted after stenting, we prefer to wait at least 6 months before AUS placement to ensure a stable stricture. If recurrence or obstruction due to stone formation is noted after AUS placement, resection is done with a pediatric resectoscope or ureteroscope with the cuff deactivated.
Statistical Analysis
Initial success was defined as stent placement that achieved a patent urethra for greater than 6 months without the need for secondary procedures. Overall treatment success was defined as a currently stable stricture for greater than 6 months regardless of the number of secondary procedures required. Incontinence was defined as the need for more than 1 pad daily.
We used descriptive statistics to characterize the study population. For all categorical variables we used the chi-square test. The unpaired t test was used to assess differences among continuous variables. We developed multivariate logistic regression models with predictor variables selected a priori. The final model included only variables associated with progression to surgery at p ≤0.20. Statistical significance was considered at p <0.05 and all tests were 2 sided. Stata® 11 was used for all analysis.
RESULTS
A total of 38 men met study inclusion criteria (table 1). in 24 men (63%) radiation therapy was the primary treatment (16) or adjuvant therapy after radical prostatectomy for PCa (8). A total of 14 men (37%) underwent radical prostatectomy alone. Comparison of irradiated to nonirradiated men revealed that radiation induced strictures developed later (3.8 vs 1.3 years, p = 0.008) but were longer (3.6 vs 2.0 cm, p = 0.003, table 1). All nonirradiated men had anastomotic BNC, which in 2 (14%) extended into the bulbar urethra. Radiation induced strictures involved the proximal bulbar urethra in 3 patients (13%), the membranous urethra in 15 (63%), the prostatic urethra in 14 (58%) and BNC in 18 (75%) with many strictures spanning multiple anatomical sites.
Table 1.
Demographics in men with posterior urethral strictures after prostate cancer treatment managed by stents
| No. Pts |
Mean ± SD Followup (yrs) |
Mean ± SD Age |
Mean ± SD Time to Stricture (yrs) |
Mean ± SD Stricture Length (cm) |
Mean ± SD No. Stents |
|
|---|---|---|---|---|---|---|
| Overall | 38 | 2.3 ± 2.5 | 67.2 ± 6.9 | 2.9 ± 3.2 | 3.0 ± 1.7 | 1.4 ± 0.6 |
| Radical prostatectomy, no radiation | 14 | 2.6 ± 3.1 | 64.2 ± 7.6 | 1.3 ± 2.3 | 2.0 ± 0.7 | 1.1 ± 0.4 |
| Radiation: | 24 | 2.0 ± 2.0 | 68.9 ± 6.0 | 3.8 ± 3.3 | 3.6 ± 1.9 | 1.6 ± 0.7 |
| Adjuvant external beam radiotherapy | 8 | 1.9 ± 1.1 | 68.7 ± 6.1 | 3.7 ± 3.2 | 3.25 ± 1.9 | 1.3 ± 0.5 |
| External beam radiotherapy + salvage prostatectomy | 2 | 3.9 ± 3.7 | 67.8 ± 6.1 | 0.3 ± 0.12 | 3.75 ± 1.8 | 1.5 ± 0.7 |
| Brachytherapy | 8 | 1.2 ± 0.9 | 66.6 ± 6.8 | 3.4 ± 3.3 | 3.5 ± 1.6 | 1.6 ± 0.7 |
| Brachytherapy + external beam radiotherapy | 6 | 2.4 ± 3.1 | 72.8 ± 3.2 | 5.6 ± 3.4 | 4.4 ± 2.5 | 2.3 ± 0.8 |
| p Value (unpaired t test) | 0.4 | 0.02 | 0.008 | 0.003 | 0.05 |
At an average 2.3 ± 2.5-year followup the primary success rate of Urolume stent placement was 47% (table 2). After a total of 33 secondary endoscopic procedures in 19 men the overall success rate was 89%. In 4 men (11%) treatment ultimately failed. All had received radiation therapy. Three of the 4 patients elected urinary diversion while 1 underwent salvage prostatectomy.
Table 2.
Outcomes in men with posterior urethral stricture after PCa treatment managed by stents
| No. Pts | No. Postop Recurrence (%) |
Mean ± Time to Restenosis (mos) |
No. Incontinence (%) | |||
|---|---|---|---|---|---|---|
| Overall | 38 | 19 | (50) | 18.6 ± 26.5 | 30 | (79) |
| Radical prostatectomy, no radiation | 14 | 7 | (50) | 31.5 ± 36.1 | 7 | (50) |
| Radiation: | 24 | 13 | (54) | 10.2 ± 11.2 | 23 | (96) |
| Adjuvant external beam radiotherapy | 8 | 4 | (50) | 7.5 ± 3.9 | 7 | (88) |
| External beam radiotherapy + salvage prostatectomy | 2 | 0 | Not applicable | 2 | (100) | |
| Brachytherapy | 8 | 5 | (63) | 5.8 ± 3.0 | 6 | (75) |
| Brachytherapy + external beam radiotherapy | 6 | 4 | (67) | 24.7 ± 31.8 | 5 | (83) |
| p Value | 0.4 (chi-square test) | 0.05 (unpaired t test) | <0.001 (chi-square test) | |||
Overall median time to stricture recurrence after stent placement was 7.4 months (range 3.5 to 91.0). Men with recurrence had longer initial strictures than those without recurrence (3.6 vs 2.1 cm p = 0.04) but were equally as likely to have received radiation (54% vs 50%, p = 0.5, table 2). However, men with radiation experienced recurrence sooner (mean 10.2 ± 11.2 vs 21.1 ± 20.1 months, p <0.001) and required more secondary procedures (mean 1.9 ± 0.8 vs 1.1 ± 0.4) at a similar mean followup (2.0 ± 2.0 vs 2.6 ± 3.1 years, p = 0.4). On multivariate analysis neither stricture length (HR 1.4, 95% CI 0.8 –2.3, p = 0.4) nor radiation exposure (HR 1.2, 95% CI 0.6–11.4, p = 0.5) was an independent risk factors for failure. The site of recurrent obstruction varied but was proximal to the stent in 10 men (53%), in the stent in 6 (32%) and distal to the stent in 5 (26%) while in 2 it was proximal to as well as in the stent. In 8 men (42%), including 6 (75%) with prior radiation therapy, the additional procedure included placement of a second Urolume stent. In 6 of the men obstructing calcification was present in the stent, which was treated with laser ablation. Stone analysis done in 5 of these men revealed a stone composition of 100% CaHPO4.
Perioperative complications included perineal pain in 6 patients (16%), urinary tract infection in 7 (18%) and clot retention in 3 (8%). All men with perineal pain had membranous strictures and had received radiation. Median time to pain resolution was 4 months (range 1 to 13). One man still had improved but persistent pain 15 months postoperatively. In 1 patient with prior sigmoid resection for carcinoid tumor a postoperative rectourethral fistula developed at 3 weeks, which was treated with diverting ileostomy. This was reversed after spontaneous fistula closure at 1.5 months.
The overall incontinence rate was 82% with a higher rate in men who did vs did not receive radiation (96% vs 50%, p <0.001, table 2). Incontinent men had longer strictures (3.3 ± 0.6 vs 2.4 ± 1.9 cm, p = 0.05). All men with strictures involving the bulbar or membranous urethra were incontinent. Only 1 of the 7 nonirradiated patients with incontinence postoperatively had been continent preoperatively. All 7 men who remained continent after stent placement had strictures less than 2 cm (mean 1.5) and all had pure BNC that required a single 2 cm or less stent. Of the 14 men who had received radiation and reported continence preoperatively only 1, who had BNC, remained continent after stent placement.
An AUS was inserted in 19 men with incontinence after stent placement at a mean of 7.2 ± 2.4 months after stenting. At a median followup of 42.3 months (range 3 to 129) the AUS was removed in 3 men (19%) due to infection in 2 and erosion in 1. Two of these men received prior radiation therapy. A transcorporeal sphincter technique was used in 7 cases, of which none had failed to date. One patient with incontinence underwent successful InVance® placement 9.5 months after Urolume placement. In 6 men an AUS was in place at the time of a secondary procedure, of which all were done with the sphincter deactivated. One patient required an additional stent placed through the sphincter using the 24Fr insertion device. Otherwise all procedures were done using a flexible cystoscope and/or pediatric resectoscope. None showed any perioperative complication involving the sphincter.
DISCUSSION
We evaluated our use of Urolume stents for recalcitrant posterior urethral stricture secondary to PCa treatment. Results show that Urolume stenting is a reasonable option for this difficult urological problem. However, multiple procedures are often required before the stricture can be considered stable and they may be necessary for the lifetime of the stent in some individuals. Previously published results show a 52% to 75% intermediate term success rate for stenting with a reoperation rate of almost 25%.11,12,14,15 Our initial and final success rates of 48% and 89%, respectively, are in accordance with these previous studies but our 53% stent specific reoperation rate was slightly higher than previously reported.
In our previously published experience with Urolume stent placement for posterior stricture disease in 13 men, which was the result of PCa therapy in 11 (85%), we cautiously recommended Urolume stenting for posterior stricture.11 In most men we continue to advocate open reconstructive procedures as first line treatment for PCa treatment related stricture7,8 and our previously reported outcomes of reconstructive surgery have been favorable. In the series by Elliott et al 22 of the 32 men with post-PCa therapy urethral strictures had stricture in the posterior urethra.7 We managed 13 of these cases with open reconstructive procedures and achieved an 86% success rate.
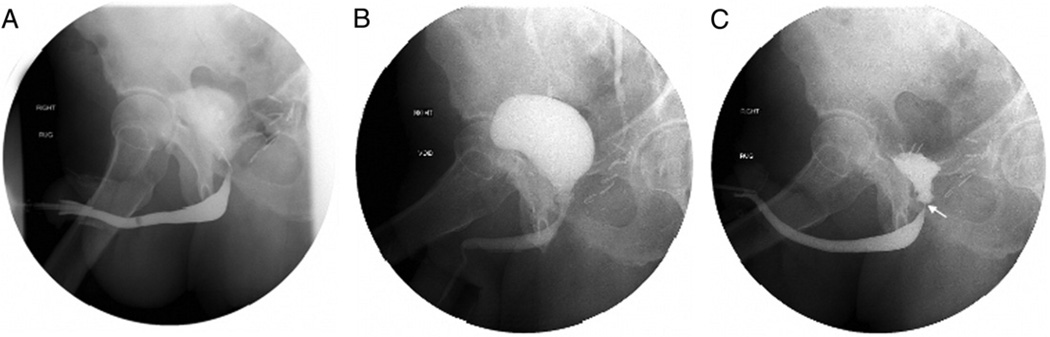
However, in select men we have found Urolume stenting to be a reasonable option for posterior urethral stricture. While these stents have a poor track record for anterior urethral strictures,16,17 for which many other superior reconstructive options are available, they have a place in our reconstructive armamentarium for posterior strictures. Obvious instances in which they would be recommended would be in men unable to tolerate major operations who nonetheless desire urethral patency. Also, in nonirradiated men with shorter anastomotic strictures in the setting of a functional external sphincter we found that a carefully placed stent proximal to the external sphincter can often manage the stricture without compromising postoperative continence while open repair would almost certainly have damaged the continence mechanism (fig. 1). In this series 8 men with recalcitrant BNC after radical prostatectomy were continent with residual external sphincter activity, of whom 7 remained continent after the stent was carefully placed proximal to the sphincter.
Figure 1.
A, patient with anastomotic BNC after radical retropubic prostatectomy. B, stricture successfully managed by stent. C, intact external sphincter function after stent placement.
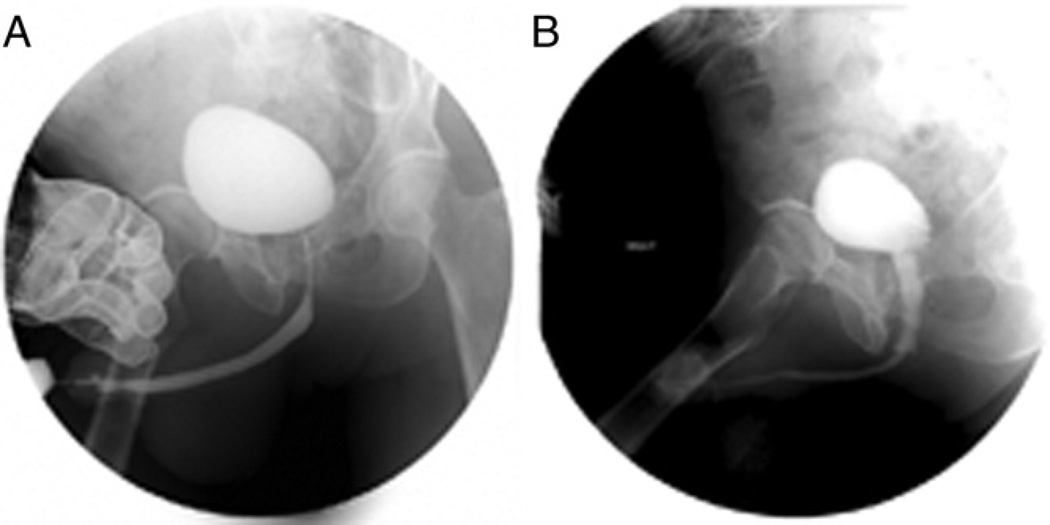
However, the group in which we have most commonly placed the stents comprises men with complex, radiation induced strictures (fig. 2). While we always prefer to surgically excise these strictures, most irradiated men in this series had strictures that were extremely long at an average of 3.6 cm, which is almost 1.5 cm longer than strictures caused by surgery alone. Surgical excision in these cases would likely have required pubectomy and/or salvage prostatectomy, procedures that introduce significant patient morbidity. Also, postoperative healing of the anastomosis is always a concern.3,5,18,19 In these situations despite the risk of failure many men were willing to try the this stent if it avoided a major operation. The initial success rate was unfortunately poor with more than half of the men requiring secondary operations. Almost all were incontinent, which likely had a multifactorial etiology involving prior radiation damage to the sphincter, and stricture length and site. However, with relatively easy and well tolerated secondary procedures almost 83% of irradiated men have avoided major operations or urinary diversion at almost 2 years followup. Another man who underwent combined brachytherapy and external beam radiation underwent successful salvage prostatectomy with the stent still in place, highlighting the fact that stent placement does not preclude future posterior urethral reconstruction.
Figure 2.
A, patient with posterior urethral stricture after combined brachytherapy and external beam radiotherapy. B, stricture successfully managed by stents with total length of 7.5 cm, that is 3 × 2 and 1.5 × 1 cm.
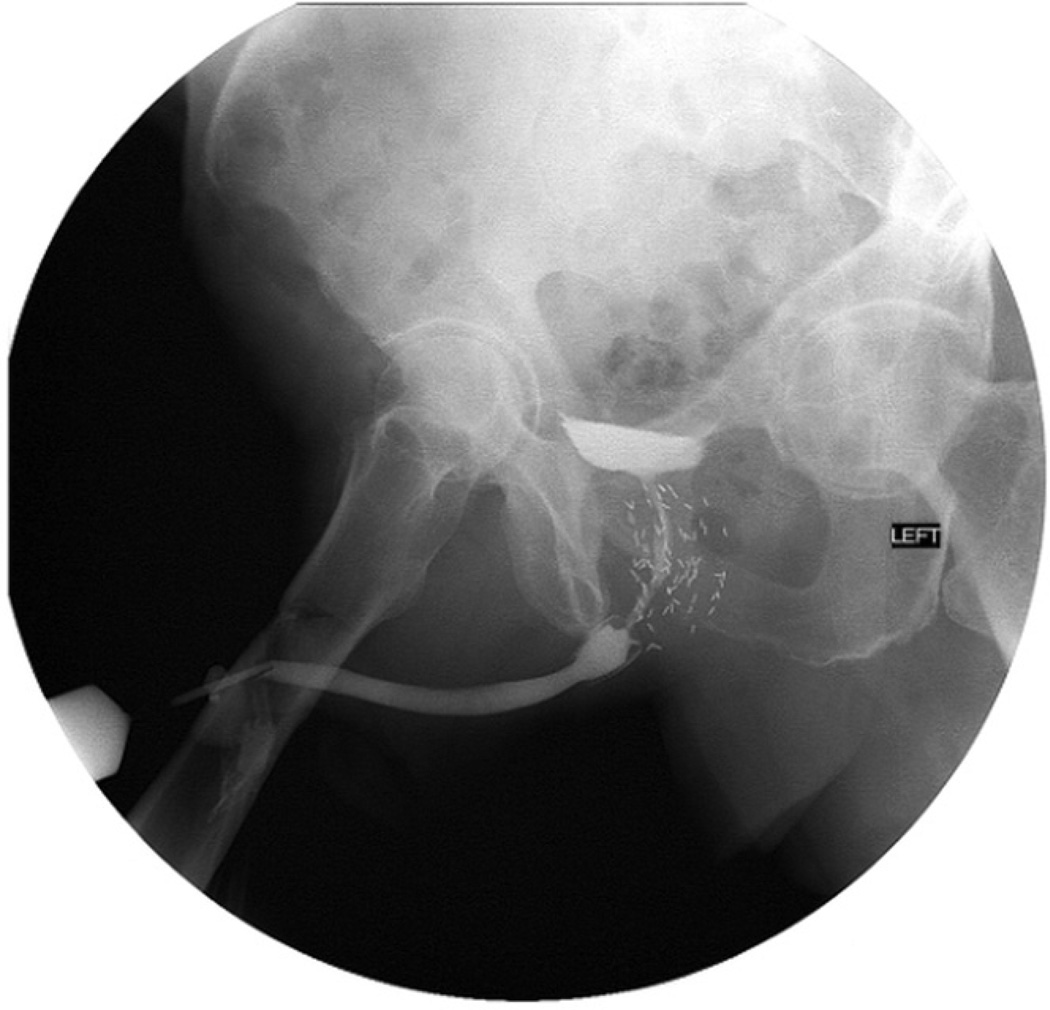
Still, the need for secondary procedures in this series was extremely high, which can be discouraging to the patient and surgeon seeking a permanent solution (fig. 3). Clearly the Urolume stent is not a perfect solution to this difficult problem and design improvements that would more effectively prevent tissue ingrowth and stent encrustation would be worth investigating. However, what this study unfortunately cannot answer is whether, despite the need for periodic secondary procedures in most men, having the stent in place is better than 1 of the alternatives. An anecdotal advantage of having the stent in place that we have noted is that the lateral borders of the urethra are better defined, making subsequent endoscopic procedures for recurrence much easier and safer than they would be without the stent. Also, by placing the stent we seem not to have eliminated the possibility of future reconstructive or diversion options. However, it appears that regardless of the predicted surgical outcome stent placement in the posterior urethra for PCa treatment induced stricture should only be done in patients and by surgeons willing to take on the responsibility of caring for a device that will possibly require a lifetime of maintenance.
Figure 3.
Recurrent stricture proximal to existing stent.
CONCLUSIONS
Urolume stenting is a reasonable option for severe post-PCa treatment stricture in motivated men who are unwilling or unable to undergo open reconstructive surgery. Incontinence and the need for additional procedures should be expected in most men, especially those with prior radiation exposure.
Abbreviations and Acronyms
- AUS
artificial urinary sphincter
- BNC
bladder neck contracture
- PCa
prostate cancer
REFERENCES
- 1.American Cancer Society. [Accessed February 10, 2009]; Available at http://www.cancer.org.
- 2.Elliott SP, Meng MV, Elkin EP, et al. Incidence of urethral stricture after primary treatment for prostate cancer: data from CaPSURE. J Urol. 2007;178:529. doi: 10.1016/j.juro.2007.03.126. [DOI] [PubMed] [Google Scholar]
- 3.Merrick GS, Butler WM, Wallner KE, et al. Risk factors for the development of prostate brachytherapy related urethral strictures. J Urol. 2006;175:1376. doi: 10.1016/S0022-5347(05)00681-6. [DOI] [PubMed] [Google Scholar]
- 4.Erickson BA, Meeks JJ, Roehl KA, et al. Bladder neck contracture after retropubic radical prostatectomy: incidence and risk factors from a large single-surgeon experience. BJU Int. 2009;104:1615. doi: 10.1111/j.1464-410X.2009.08700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen ZA, Merrick GS, Butler WM, et al. Detailed urethral dosimetry in the evaluation of prostate brachytherapy-related urinary morbidity. Int J Radiat Oncol Biol Phys. 2005;62:981. doi: 10.1016/j.ijrobp.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 6.Dalkin BL. Endoscopic evaluation and treatment of anastomotic strictures after radical retropubic prostatectomy. J Urol. 1996;155:206. [PubMed] [Google Scholar]
- 7.Elliott SP, McAninch JW, Chi T, et al. Management of severe urethral complications of prostate cancer therapy. J Urol. 2006;176:2508. doi: 10.1016/j.juro.2006.07.152. [DOI] [PubMed] [Google Scholar]
- 8.Wessells H, Morey AF, McAninch JW. Obliterative vesicourethral strictures following radical prostatectomy for prostate cancer: reconstructive armamentarium. J Urol. 1998;160:1373. [PubMed] [Google Scholar]
- 9.Ullrich NF, Wessells H. A technique of bladder neck closure combining prostatectomy and intestinal interposition for unsalvageable urethral disease. J Urol. 2002;167:634. doi: 10.1016/S0022-5347(01)69101-8. [DOI] [PubMed] [Google Scholar]
- 10.Westney OL. Salvage surgery for bladder outlet obstruction after prostatectomy or cystectomy. Curr Opin Urol. 2008;18:570. doi: 10.1097/MOU.0b013e328311c9de. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg ML, Elliott SP, McAninch JW. Preservation of lower urinary tract function in posterior urethral stenosis: selection of appropriate patients for urethral stents. J Urol. 2007;178:2456. doi: 10.1016/j.juro.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Magera JS, Jr, Inman BA, Elliott DS. Outcome analysis of urethral wall stent insertion with artificial urinary sphincter placement for severe recurrent bladder neck contracture following radical prostatectomy. J Urol. 2009;181:1236. doi: 10.1016/j.juro.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg ML, Elliott SP, McAninch JW. Management of restenosis after urethral stent placement. J Urol. 2008;179:991. doi: 10.1016/j.juro.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 14.Anger JT, Raj GV, Delvecchio FC, et al. Anastomotic contracture and incontinence after radical prostatectomy: a graded approach to management. J Urol. 2005;173:1143. doi: 10.1097/01.ju.0000155624.48337.a5. [DOI] [PubMed] [Google Scholar]
- 15.Elliott DS, Boone TB. Combined stent and artificial urinary sphincter for management of severe recurrent bladder neck contracture and stress incontinence after prostatectomy: a long-term evaluation. J Urol. 2001;165:413. doi: 10.1097/00005392-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Armitage JN, Cathcart PJ, Rashidian A, et al. Epithelializing stent for benign prostatic hyperplasia: a systematic review of the literature. J Urol. 2007;177:1619. doi: 10.1016/j.juro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Parikh AM, Milroy EJ. Precautions and complications in the use of the Urolume wallstent. Eur Urol. 1995;27:1. doi: 10.1159/000475113. [DOI] [PubMed] [Google Scholar]
- 18.Dearnaley DP, Sydes MR, Langley RE, et al. The early toxicity of escalated versus standard dose conformal radiotherapy with neo-adjuvant androgen suppression for patients with localised prostate cancer: results from the MRC RT01 trial (ISRCTN47772397) Radiother Oncol. 2007;83:31. doi: 10.1016/j.radonc.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Merrick GS, Butler WM, Tollenaar BG, et al. The dosimetry of prostate brachytherapy-induced urethral strictures. Int J Radiat Oncol Biol Phys. 2002;52:461. doi: 10.1016/s0360-3016(01)01811-9. [DOI] [PubMed] [Google Scholar]