Abstract
Rolling adhesion on vascular surfaces is the first step in recruiting circulating leukocytes, hematopoietic progenitors, or platelets to specific organs or to sites of infection or injury. Rolling requires the rapid yet balanced formation and dissociation of adhesive bonds in the challenging environment of blood flow. This review explores how structurally distinct adhesion receptors interact through mechanically regulated kinetics with their ligands to meet these challenges. Remarkably, increasing force applied to adhesive bonds first prolongs their lifetimes (catch bonds) and then shortens their lifetimes (slip bonds). Catch bonds mediate the counterintuitive phenomenon of flow-enhanced rolling adhesion. Force-regulated disruptions of receptor interdomain or intradomain interactions remote from the ligand-binding surface generate catch bonds. Adhesion receptor dimerization, clustering in membrane domains, and interactions with the cytoskeleton modulate the forces applied to bonds. Both inside-out and outside-in cell signals regulate these processes.
Keywords: selectin, integrin, glycoprotein Ib, von Willebrand factor, PSGL-1, catch bond, leukocyte, platelet, endothelial cell
Introduction
Adhesion of cells to other cells or to extracellular matrix is rarely static. Cells alter adhesion during morphogenesis, tissue remodeling, and other responses to environmental cues. Perhaps the most dynamic regulation of cell adhesion occurs under blood flow. Hematopoietic cells use a multistep process in which they initially tether to and roll along the vessel wall, then decelerate and arrest, and finally aggregate or emigrate into the underlying tissues. For cells to tether, interactions between adhesion molecules must form rapidly. For cells to roll, these interactions must break rapidly. Rolling adhesion provides an important checkpoint for cells to encounter tissue-specific signals before committing to enter into a particular organ. It is the initial step in recruitment of hematopoietic stem cells to bone marrow niches, naïve lymphocytes to secondary lymphoid organs, myeloid leukocytes and effector lymphocytes to sites of inflammation, and platelets to sites of hemorrhage. Furthermore, tumor cells usurp rolling adhesion mechanisms to metastasize through the blood to distant organs.
Kinetic and mechanical parameters of cell tethering and rolling under flow
Cell adhesion is mediated through reversible interactions, or “bonds,” between cell-surface receptors and their ligands, or counterreceptors, on other cell surfaces or in extracellular matrix. Here, an adhesive bond is defined as the sum of noncovalent interactions, e.g. hydrogen bonds, electrostatic interactions, van der Waals forces, dipole-dipole interactions, between two macromolecules. As for other biochemical reactions, the equilibrium affinity of an adhesion receptor for its ligand is the ratio of the on-rate (kon) to the off-rate (koff). Proteins in solution may interact after they collide during diffusion in three-dimensional (3D) space. In contrast, adhesion receptors diffuse laterally in the cell membrane–a two-dimensional (2D) space–and bind ligands on another membrane or in extracellular matrix. A moving cell carries adhesion receptors to ligands on the surface of another cell or in matrix in both normal and tangential directions. Blood flow imposes additional transport and mechanical constraints on interacting molecules as one of them resides on a moving cell.
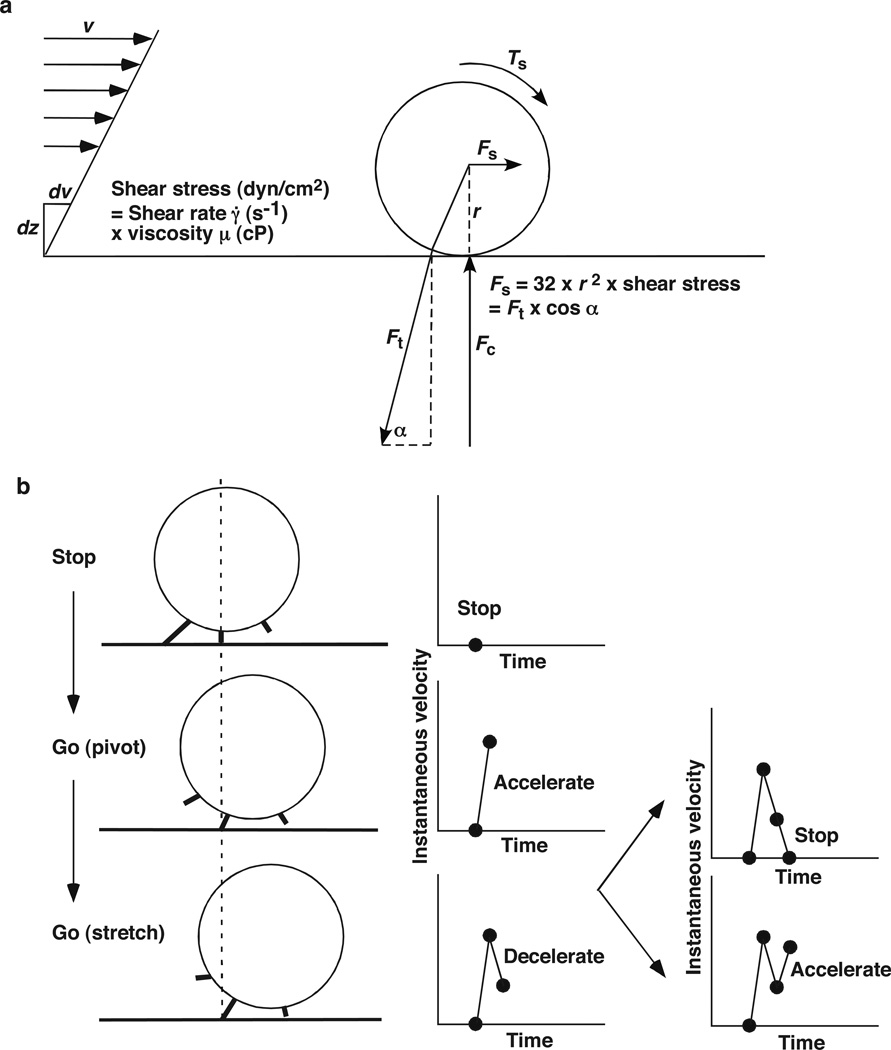
In steady laminar flow, the velocity of fluid (and cells freely flowing with it) increases with its distance from the vessel wall. The change in velocity per unit distance is the shear rate, usually expressed in a unit of s−1 (Fig. 1a). At a given shear rate, larger cells have higher velocities because they tend to extend further from the wall. A cell flowing near the vessel wall may be able to tether if its adhesion receptors contact ligands on the wall. Bond formation, however, involves two steps: transport, which brings two molecules into close proximity, and reaction, during which the molecules dock. Faster cell velocity produces more frequent collisions (Fig. 1b) but also shortens the contact time between adhesion molecules (Fig. 1c). Thus, the relative timescales for transport and docking affect the efficiency of tethering a flowing cell to the surface.
Figure 1.
Parameters of tethering under flow. (a) The fluid velocity v of a Couette flow field bordered with a solid surface (x-y plane) is parallel to the surface and increases linearly with the distance away from the surface (z direction). The shear rate γ̇ = dv/dz is reciprocal to the slope of the velocity profile. Fluid mechanics theory predicts that the translational velocity V and angular velocity Ω of a sphere (or cell) of radius r freely moving above a surface in an otherwise Couette flow are proportional to rγ̇ and r, respectively (Goldman et al 1967). The sphere bottom has a sliding velocity Vs ≡ V − rΩ ∝ r relative to the surface (Chang & Hammer 1999). Adapted with permission from (Yago et al 2007). (b) Faster sliding velocity increases the number of surface ligands that an adhesion receptor on the flowing cell contacts per unit time. (c) Faster sliding velocity reduces the time that the receptor contacts the ligand before it moves away.
The product of shear rate and viscosity is shear stress, a measure of tangential force per unit area, usually expressed in a unit of dyne/cm2 (Fig. 2a). A cell rolls by forming new adhesive bonds at the leading edge to replace bonds that dissociate at the trailing edge. Shear stress imposes a force Fs and a torque Ts to the rolling cell, which reach maximum when the cell stops. Fs and Ts must be balanced by a tensile force Ft on the adhesive bonds at the trailing edge and a compressive force Fc on the cell bottom. Fc and Ft affect the on- and off-rates of the bonds, respectively. A rolling cell stops when the adhesive bond (or bond cluster) can withstand the force required to balance the maximal force and torque applied to the cell (Fig. 2b). After dissociation of this bond (or the last bond in a bond cluster), the cell accelerates as it pivots on a newly formed bond downstream and then decelerates as force develops in the bond. The cell stops again if the new bond persists long enough with sufficient strength to counter the maximal Fs and Ts. If the bond dissociates prematurely, the cell accelerates again before it can stop. The velocity of a rolling cell is primarily determined by the off-rates of adhesive bonds at the trailing edge of the cell. In turn, the mechanical regulation of these bonds, that is, how their off-rates respond to force, critically determines whether and if so, how cells roll under flow.
Figure 2.
Parameters of rolling adhesion under flow. (a) The rolling motions of a sphere or cell of radius r are governed by the balance of the resultant force Fs and torque Ts exerted by the flowing fluid, the tether force Ft applied through the receptor-ligand bond, and the contact force Fc. The conversion of wall shear stress to Ft using the indicated variables is described in (Yago et al 2004). (b) A rolling sphere stops when the adhesive bond sustains the full load required to balance the maximum Ft and Ts. After the bond dissociates, the sphere accelerates as it pivots on a newly formed bond downstream and then decelerates as force develops in the bond. The sphere stops again if the new bond has sufficient strength to withstand the full load and lives long enough to survive loading, or it accelerates if the bond dissociates prematurely. Both panels adapted with permission from (Yago et al 2004).
Leukocyte rolling
Rolling of leukocytes or hematopoietic progenitors on vascular surfaces is the first step in a multistep cascade of adhesion and signaling events (Fig. 3). This cascade, which has been reviewed in detail (Ley et al 2007), begins as cells tether to and roll on the vessel wall. The rolling cells transduce signals from adhesion receptors and chemokine receptors that cause the cells to roll slower and then to arrest, a prerequisite for emigration through the vasculature into underlying tissues. The expression of specific combinations of adhesion and chemokine receptors determines which leukocyte subsets enter a particular tissue during immune surveillance or in response to infection or injury. Interactions of selectins with their ligands mediate most tethering and rolling. Interactions of integrins with their ligands mediate arrest (firm adhesion) and migration but, as described later, can also support rolling.
Figure 3.
Multistep leukocyte adhesion cascade. Selectins initiate tethering and rolling of leukocytes. Depending on their activation state, integrins mediate slower rolling or cause the cells to arrest. Integrins also mediate spreading, crawling, and migration between or through endothelial cells into the underlying tissues.
Selectin-mediated leukocyte rolling
Selectins
Each of the three selectins has an N-terminal carbohydrate-recognition domain characteristic of Ca2+-dependent (C-type) lectins, followed by an epidermal growth factor (EGF)-like domain, a series of short consensus repeats like those in complement regulatory proteins, a transmembrane domain, and a short cytoplasmic tail (McEver 2002, McEver et al 1995, Vestweber & Blanks 1999) (Fig. 4). L-selectin is constitutively expressed on leukocytes. E-selectin is constitutively expressed on endothelial cells of skin and bone marrow. In most other tissues, inflammatory cytokines such as TNF-α transiently induce its expression in endothelial cells of postcapillary venules. P-selectin is constitutively synthesized by megakaryocytes, where it is incorporated into platelets, and by endothelial cells, mostly in postcapillary venules. It is sorted to membranes of α granules of platelets and Weibel-Palade bodies of endothelial cells. Upon stimulation by secretagogues such as thrombin or histamine, the membranes of these organelles rapidly fuse with the plasma membrane and P-selectin redistributes to the cell surface.
Figure 4.
Selectins and their major glycoprotein ligands. The upper inset depicts the N-terminal glycosulfopeptide region of human PSGL-1 that binds to P-selectin (and L-selectin). The lower insert depicts an example of a sialylated, fucosylated, and sulfated O-glycan on PNAd mucins that binds to L-selectin.
Selectin ligands
Each selectin mediates adhesion in part through interactions of its N-terminal lectin domain with a sialyl Lewis x (sLex) capping structure (NeuAcα2-3Galβ1-3[Fucα1-3]GlcNAcβ1-R) on cell-surface glycoconjugates (McEver 2002, McEver et al 1995, Vestweber & Blanks 1999). Crystal structures of sLex bound to the lectin domains of P- and E-selectin reveal multiple interactions between the fucose, a Ca2+ ion, and several amino acids, including those that coordinate the Ca2+ ion (Somers et al 2000). This explains the Ca2+-dependent binding to fucosylated glycans. Other lectin-domain residues contact sialic acid and galactose. P- and L-selectin, but not E-selectin, preferentially bind to specific glycoproteins that must be sulfated as well as sialylated and fucosylated.
P-selectin glycoprotein ligand-1 (PSGL-1) is a transmembrane, homodimeric mucin on leukocytes and some activated endothelial cells (McEver 2002, McEver et al 1995, Rivera-Nieves et al 2006, Vestweber & Blanks 1999) (Fig. 4). The extracellular region of each subunit bears multiple O-glycans on serine and threonine residues that cause it to extend above the membrane. PSGL-1 is the dominant ligand for P- and L-selectin on leukocytes. This is due to stereospecific binding of P- and L-selectin to sulfated tyrosines, adjacent peptide determinants, and fucose, sialic acid, and galactose on a single core 2 O-glycan near the N-terminus of PSGL-1 (Leppänen et al 2000, Leppänen et al 2003, Somers et al 2000) (Fig. 4). These additional contacts explain why P- and L-selectin bind with higher affinity to PSGL-1 than to sLex alone.
L-selectin also binds to mucins expressed on endothelial cells in lymph node high endothelial venules and at some sites of inflammation (Fig. 4). These mucins, collectively called peripheral node addressin (PNAd), include CD34, glycosylated cell adhesion molecule-1 (GlyCAM-1), and podocalyxin (Rosen 2004). Unlike sulfation of tyrosines on PSGL-1, PNAd mucins are sulfated at the C6 position of galactose and N-acetylglucosamine (GlcNAc) residues on multiple O-glycans and on some N-glycans. Targeted disruption of genes encoding specific glycosyltransferases and sulfotransferases reveal that a combination of N-glycans, branched core 2 O-glycans, and extended core 1 O-glycans capped with 6-sulfo-sLex (sLex modified with a sulfate ester attached to the C6 position of GlcNAc) confer optimal binding of PNAd mucins to L-selectin (Kawashima et al 2005, Mitoma et al 2007, Uchimura et al 2005, Yeh et al 2001) (Fig. 4). To date there is no crystal structure of 6-sulfo-sLex bound to L-selectin to indicate where the sulfate docks to the lectin domain. The available evidence suggests that L-selectin binds to 6-sulfo-sLex but not to amino acids on PNAd, which contrasts with the cooperative binding to sLex, sulfated tyrosines, and other amino acids at the N-terminus of PSGL-1.
E-selectin was originally thought to mediate leukocyte rolling through interactions with multiple glycoproteins that bear sLex-capped glycans. However, gene knockout studies demonstrate that PSGL-1 and CD44 are the major glycoprotein ligands for E-selectin on murine leukocytes (Katayama et al 2005, Xia et al 2002) (Fig. 4). Why these glycoproteins serve as preferential E-selectin ligands is not known. Because it has no affinity for sulfate, E-selectin binding is probably not limited to the N-terminus of PSGL-1. siRNA knockdown of transcripts for a glycoprotein termed E-selectin ligand-1 (ESL-1) suggests that it comprises the remaining E-selectin ligand activity on murine leukocytes (Hidalgo et al 2007). Unlike murine leukocytes, human neutrophils express sialylated glycosphingolipids with repeating Galβ1-4GlcNAc units, some of which are modified with α1-3 fucose (Nimrichter et al 2008). The immobilized glycolipids support rolling of E-selectin-expressing cells. An inhibitor of glycolipid synthesis partially blocks rolling of human neutrophils on E-selectin, but this could be an indirect effect of altered cell deformability (see below). Glycolipids are much shorter than most glycoproteins. Proteolytic digestion of human neutrophil surfaces may expose glycolipids to interact with E-selectin under flow (Kobzdej et al 2002), but it is unclear whether they do so within an intact glycocalyx.
Force-free kinetics and affinity selectin-ligand interactions
Measurements of selectin-ligand interactions with surface plasmon resonance where selectin or ligand moves in 3D space confirm rapid on- and off-rates, as seems intuitively necessary for cell tethering and rolling. The measured koff for P-selectin dissociating from PSGL-1 is ~1.4 s−1 (Klopocki et al 2008, Mehta et al 1998). Despite the rapid off-rate, the measured equilibrium affinity (Kd ~0.3–1.5 µM) is unexpectedly high because the calculated kon is remarkably fast: ~1–4 × 106 M−1s−1. The affinity of L-selectin for PSGL-1 is lower (Kd ~47 µM) due to both a faster off-rate and a slower on-rate (Klopocki et al 2008). The koff measured by surface plasmon resonance is at least 10 s−1, the upper limit of resolution for this method. Measurements of 2D interactions of L-selectin with PSGL-1 by thermal fluctuations at high temporal resolution establish the koff to be 10.2 s−1 and confirm that L-selectin binds to PSGL-1 with a faster off-rate but a slower on-rate than P-selectin (Chen et al 2007). As determined by surface plasmon resonance, L-selectin binds with equivalent affinity (Kd ~108 µM) to GlyCAM-1 and to 6-sulfo-sLex, suggesting that L-selectin binds only to glycan determinants on PNAd (Klopocki et al 2008, Nicholson et al 1998). E-selectin binds to ESL-1 with a Kd of 62 µM, a measured koff of 4.6 s−1, and a calculated kon of 7.4 × 104 M−1s−1 (Wild et al 2001). Thus, all three selectins dissociate rapidly from their ligands, with the fastest off-rate observed for L-selectin. Compared to E-selectin, P- and L-selectin have higher on-rates, perhaps driven by electrostatic interactions with sulfate on their ligands. The fast on-rate of P-selectin for PSGL-1 is consistent with its dominant role in the initial capture, or tethering, of leukocytes to vascular surfaces (Smith et al 2004, Xia et al 2002). Measurements of 2D selectin-ligand interactions with a micropipette adhesion frequency assay are consistent with the 3D measurements (Huang et al 2004, Long et al 2001).
Mechanical regulation of selectin-ligand interactions
Blood flow exerts force on the selectin-ligand bonds that anchor rolling cells, which affects their lifetimes by altering their off-rates. Indeed, the first demonstration that force affects adhesion receptor bond lifetimes was for interactions of P-selectin with PSGL-1 (Alon et al 1995a). Measuring force-dependent off-rates requires low densities of receptor and ligand to favor single-molecule interactions, sufficient temporal resolution to detect even short-lived bonds, and application of a range of physiologically relevant forces. Initially, leukocytes perfused over low densities of an immobilized selectin or selectin ligand were observed by video microscopy (Alon et al 1997, Alon et al 1995a, Ramachandran et al 1999). The lifetimes of transient leukocyte tethers, each assumed to represent a single selectin-ligand bond, were measured as a function of wall shear stress, which was used to estimate the force applied to the bond anchoring the cell. Bond lifetimes obey first-order dissociation kinetics, as required by single-state, single-molecular interactions. More recently, atomic force microscopy or a biomembrane force probe was used to apply tensile force to the bonds via a bent cantilever or a stretched red blood cell. These methods measure bond lifetimes over a range of constant forces (Lou et al 2006, Marshall et al 2003, Sarangapani et al 2004) or rupture forces over a range of ramp rates (Evans et al 2001).
Early theories suggested that force might shorten bond lifetimes, e.g., accelerate dissociation, by lowering the energy barrier between the bound and free states (Bell 1978). These are termed slip bonds. Conversely, force might prolong bond lifetimes, e.g., decelerate dissociation, by deforming the molecules such that they lock more tightly. These are termed catch bonds (Dembo et al 1988). Initial studies detected only slip bonds between selectins and their ligands (Alon et al 1997, Alon et al 1995a, Ramachandran et al 1999). Later studies also identified catch bonds (Marshall et al 2003, Sarangapani et al 2004). Earlier reports failed to detect catch bonds because the forces studied were too high, because video frame speeds were too slow to measure the shortest bond lifetimes, or because rupture forces from simple constant-ramp experiments missed the catch bond regime (Evans et al 2004). Thus, force exerts a biphasic effect on selectin-ligand interactions, first prolonging lifetimes (catch bonds) until a maximal value is reached and then shortening lifetimes (slip bonds) as force continues to increase (Fig. 5). In contrast, selectin-antibody interactions exhibit only slip bonds in response to force, suggesting a specific role for catch bonds with physiological selectin ligands (Marshall et al 2003, Sarangapani et al 2004).
Figure 5.
Force-dependent lifetimes of single bonds between PSGL-1 and P-selectin or L-selectin. Lifetimes of transient neutrophil tethers to low-density P-selectin or L-selectin at different wall shear stresses were measured by video microscopy. Adapted with permission from (Marshall et al 2003, Sarangapani et al 2004).
Two major models for catch bonds have been proposed. The first invokes allosteric change in the ligand-binding surface of the lectin domain (Springer 2009, Waldron & Springer 2009). The second invokes a sliding-rebinding mechanism (Lou et al 2006, Lou & Zhu 2007). The models are not mutually exclusive; indeed, the second is a form of allostery. Both models rely on distinct crystal structures of P-selectin-ligand complexes (Somers et al 2000). In one, crystals of the lectin and EGF domains of P-selectin were soaked with sLex. In the other, the lectin and EGF domains of P-selectin were co-crystallized with an N-terminal glycosulfopeptide from PSGL-1. Fig. 6a shows a ribbon overlay of the P-selectin structures without their bound ligands. A striking difference is the relative orientations of the lectin and EGF domains. In the P-selectin-sLex complex, the angle between the domains is more closed or bent. This bent conformation is also observed in crystal structures of all three selectins without ligand (Graves et al 1994, Klopocki et al 2008, Lou et al 2006, Somers et al 2000). In the P-selectin-PSGL-1 complex, the angle between the lectin and EGF domains is more open or extended. Straightening of the interdomain hinge is associated with movement of several loops along one face of the lectin domain (Fig. 6a), including a loop near the Ca2+ coordination site that introduces a new contact for fucose (Fig. 6b) and optimizes docking to a sulfated tyrosine on PSGL-1. Both models assume equilibrium between the interdomain orientations. Force applied to bound ligand shifts the equilibrium to the more straight or extended orientation. In the allosteric model, force, by opening the interdomain hinge, propagates structural changes to the interface with ligand that decrease off-rate and prolong bond lifetime (Fig. 7a). The sliding-rebinding model neither requires nor excludes loop movement at the interface with ligand (Fig. 7b). In this model, force, by opening the interdomain hinge, causes bound ligand to slide across the interface instead of pulling directly away. During sliding, existing interactions break but new interactions also form, decreasing off-rate and prolonging lifetime.
Figure 6.
Bent and extended selectin structures. (a) Overlay of the lectin and EGF domains of P-selectin in its bent and extended forms. The magenta and blue spheres at the top represent the respective Ca2+ ion on each structure. (b) Binding of fucose (part of the sLex binding determinant) to the bent and extended forms of P-selectin. The dashed black lines represent interactions of the Ca2+ ion with residues on P-selectin. The dashed red lines represent interactions of the fucose with the Ca2+ ion or residues on P-selectin. (c) Relative orientations of Y37 in the lectin domain and G138 in the EGF domain in the bent and extended forms of P-selectin. (d) Relative orientations of Y37 in the lectin domain and N138 in the EGF domain in the bent and extended forms of L-selectin. The black dashed line indicates a hydrogen bond. [From Protein Data Bank (PDB) ID codes 1G1Q, 1G1R, 1G1S, and 3CFW (Klopocki et al 2008, Somers et al 2000).] The extended structure of L-selectin was derived by molecular modeling (Lou et al 2006).
Figure 7.
Models for catch bonds. (a) Allosteric model. (b) Sliding-rebinding model.
Two mutants of P-selectin increase the force-free affinity for PSGL-1, in part by reducing off-rate. One mutant introduces a glycan “wedge” between the lectin and EGF domains to force the interdomain angle open (Phan et al 2006). The other substitutes a bulky residue (A28H) to shift a loop in the lectin domain along the proposed allosteric pathway (Waldron & Springer 2009). The effects of force on bond lifetimes were not examined. The force-free affinity data support the allosteric model, although the mutations might alter structure differently than does force.
Molecular dynamics (MD) simulations of force applied to the P-selectin-PSGL-1 complex support the sliding-rebinding model (Lou & Zhu 2007). Transitions from catch to slip bonds occur at a higher force range for L-selectin than P-selectin (Sarangapani et al 2004) (Fig. 5), which is due in part to different interdomain contacts. EGF-domain residue 138 is Gly in P-selectin but Asn in L-selectin. In the bent P-selectin structure, Gly138 is close to but does not interact with Tyr37 in the lectin domain (Fig. 6c). In the extended P-selectin structure, Tyr37 pivots about Gly138 without steric clashes. In the bent L-selectin structure, Asn138 forms a hydrogen bond with Tyr37 in the lectin domain (Lou et al 2006) (Fig. 6d). Molecular modeling indicates that this hydrogen bond must break and steric clashes must be overcome for the interdomain angle to open. An N138G substitution in L-selectin increases hinge flexibility and elicits catch bonds at lower forces with longer lifetimes with both 6-sulfo-sLex and PSGL-1 (Lou et al 2006). Sliding-rebinding readily explains this effect because it can act on ligands with different structures as force is applied.
In summary, there is evidence supporting both allosteric and sliding-rebinding models. Further studies are required to determine whether either or both of these models, or perhaps another model, explain the mechanistic basis for catch bonds. Indeed, an L-selectin substitution in the lectin domain (A108H) predicted to create a contact with PSGL-1 peptide but not glycan eliminates catch bonds with PSGL-1 but not with 6-sulfo-sLex (Klopocki et al 2008), indicating that selectins might use different mechanisms for different ligands to generate catch bonds.
Flow-enhanced adhesion: the shear threshold phenomenon
Selectins require a minimal shear to support cell adhesion (Finger et al 1996, Lawrence et al 1997). This counterintuitive phenomenon is particularly striking for L-selectin. Below the shear threshold, few cells tether. As shear rises above the threshold, the tether rate increases, reaches a maximum at an optimal shear, and then declines as shear increases further (Fig. 8a). In parallel, cells roll more slowly and more regularly until an optimal shear is reached where rolling velocity is minimal; they then roll faster as shear increases further (Fig. 8b). Thus, flow-enhanced adhesion results from both increased tethering and slower rolling (Fig. 8c).
Figure 8.
Flow-enhanced rolling adhesion. Neutrophils were perfused over immobilized PSGL-1 at the indicated wall shear stress. The tethering rate (a), mean rolling velocity (b), and number of cells rolling per field (c) were measured. Adapted with permission from (Yago et al 2004, Yago et al 2007).
Transport governs flow-enhanced tethering through three mechanisms (Yago et al 2007). The first is the sliding velocity of the cell bottom near the vascular surface (Fig. 1a), which is controlled by the product of the shear rate and the radius of the cell. The second is Brownian motion of the cell, which modulates the gap distance above and below the threshold where a selectin and its ligand can contact. The third is molecular diffusion, which allows a selectin and its ligand to orient their binding sites for docking. The N138G substitution in L-selectin increases its rotational diffusivity and augments tethering (Lou et al 2006). As flow increases, transport increases encounters between L-selectin and its ligands; this favors productive interactions because the docking rate (a function of the kon) is rapid (Yago et al 2007). Above the flow optimum, the tethering rate declines as the encounter times become shorter than the time for docking, and thus become limiting (Fig. 1c).
Force governs flow-enhanced rolling by eliciting catch bonds (Yago et al 2004). As flow increases, rolling becomes slower and more regular as force lengthens the lifetimes of L-selectin bonds. Above the flow optimum, rolling becomes faster and less regular as higher forces shorten bond lifetimes (slip bonds). The N138G substitution in L-selectin reduces the shear threshold for rolling on both PSGL-1 and 6-sulfo-sLex by prolonging bond lifetimes at lower forces (Lou et al 2006). The A108H substitution in L-selectin predicted to enhance binding to peptide but not glycan components of PSGL-1 increases the force-free affinity for PSGL-1 but not for 6-sulfo-sLex (Klopocki et al 2008). Bond lifetimes with PSGL-1, but not with 6-sulfo-sLex, are longer at low forces and decrease as force increases. The A108H substitution eliminates the shear threshold for rolling on PSGL-1 but does not affect rolling on 6-sulfo-sLex, consistent with its selective prolongation of bond lifetimes with PSGL-1 at low forces (Klopocki et al 2008).
Flow-enhanced adhesion may have important biological functions. Circulating leukocytes do not aggregate even though they express both L-selectin and its ligand PSGL-1. Although colliding leukocytes might form bonds between L-selectin and PSGL-1, the force applied to these bonds is small because of the minor velocity differences between circulating cells (Fig. 9). The bonds are therefore short-lived and the cells rapidly dissociate. Consistent with this hypothesis, microspheres bearing L-selectin N138G, but not L-selectin, form doublets and larger aggregates with neutrophils in a shear field (Lou et al 2006). The same shear stress exerts larger forces on the bonds that anchor a cell to the vessel wall, prolonging their lifetimes and enabling rolling (Fig. 9). Circulating platelets express P-selectin but do not mobilize it to the surface until they are activated (McEver 2002, McEver et al 1995, Vestweber & Blanks 1999). PSGL-1 bonds with P-selectin have longer lifetimes than those with L-selectin even at low forces (Marshall et al 2003, Sarangapani et al 2004) (Fig. 5). The combination of high P-selectin densities and longer bond lifetimes initiates adhesion of circulating activated platelets to leukocytes. Subsequent signaling activates β2 integrins that stabilize adhesion (Evangelista et al 2007). Thus, circulating platelet-leukocyte aggregates are observed in disorders that increase platelet activation (Michelson et al 2001). The shear threshold for leukocyte rolling through P-selectin is less evident than through L-selectin because P-selectin bonds last longer than L-selectin bonds at all force levels. Similar principles likely explain the less evident shear threshold for rolling through E-selectin, which may also form catch bonds with its ligands (Piper 1997).
Figure 9.
Schematic showing tensile forces (Ft) between cells in a flowing doublet as well as between a cell and the wall under a simple shear field. <Ft> indicates average force over a cycle of tumbling.
Cellular features that modulate selectin-mediated leukocyte rolling
Microspheres bearing a selectin (or selectin ligand) roll on an immobilized selectin ligand (or selectin), establishing that the molecular features of selectins and their ligands are sufficient to support rolling (Brunk et al 1996, Greenberg et al 2000, Yago et al 2002). However, cell activation regulates the densities of receptors and ligands on the plasma membrane (McEver 2002, McEver et al 1995, Vestweber & Blanks 1999). Furthermore, the physical features of cells and the manner in which they present selectins and their ligands influence rolling behavior. The surfaces of leukocytes are highly irregular because of multiple microvilli that extend ~ 1 µm from the cell body (Fig. 10). L-selectin and PSGL-1 are concentrated on the tips of microvilli; this enhances tethering by increasing the contacts with ligands on endothelial cells (Moore et al 1995, Von Andrian et al 1995, Yago et al 2002). Cells are deformable. At higher wall shear stresses, the compressive force Fc acting on the cell bottom enlarges the contact area so that more selectin-ligand bonds can form (Lei et al 1999). The tether force Ft also rapidly extrudes long membrane tethers at the trailing edge of the cell (Schmidtke & Diamond 2000) (Fig. 10a). This process is dynamic, increasing at higher shears and decreasing at lower shears (Ramachandran et al 2004). Tethers extend by stretching microvilli and by separating membrane around adhesion receptors from the cytoskeleton (Evans et al 2005, Shao et al 1998). Most tethers retract after selectin-ligand bonds dissociate (Ramachandran et al 2004, Schmidtke & Diamond 2000), suggesting that the cytoskeleton maintains some interactions with the membrane. Endothelial cells also extrude tethers that connect to tethers extended from rolling leukocytes (Girdhar & Shao 2007). By altering the geometry of the structures that anchor cells under flow, long membrane tethers reduce force on adhesive bonds. This explains why leukocytes roll only slightly faster as wall shear stress increases to levels that generate slip bonds, whereas microspheres and fixed cells roll much faster until they detach (Yago et al 2002).
Figure 10.
Effects of cell-surface organization on selectin-ligand interactions under flow. (a) Extension of a long membrane tether from a microvillus after disruption of cytoskeletal connections with the membrane. (b) P-selectin and PSGL-1 form dimers. P-selectin clusters in clathrin-coated pits through interactions of its cytoplasmic domain. PSGL-1 associates with lipid rafts and clusters in microvilli, perhaps indirectly through interactions of other raft components with the cytoskeleton. Note that the tip of a microvillus is actually larger than a clathrin-coated pit, and PSGL-1 molecules in different regions of the tip may interact with P-selectin molecules in two or more clustered pits. (c) L-selectin clusters in microvilli through direct interactions of its cytoplasmic domain with α-actinin and ERM proteins, which connect to actin filaments.
Both P-selectin and PSGL-1 are extended molecules with membrane-distal binding domains (Li et al 1996, Ushiyama et al 1993) (Fig. 10b). This architecture enhances tethering and rolling by increasing encounters between molecules (Huang et al 2004, Patel et al 1995, Yago et al 2002). PSGL-1 forms noncovalent dimers through interactions of the transmembrane and cytoplasmic domains that are stabilized by a juxtamembrane disulfide bond (Epperson et al 2000, Miner et al 2008). P-selectin forms noncovalent dimers through interactions of the transmembrane domains (Barkalow et al 2000, Ushiyama et al 1993). Dimeric binding of P-selectin to PSGL-1 causes slower rolling in the catch bond regime because force is distributed between both subunits of dimeric bonds (Marshall et al 2003, Ramachandran et al 2001). Furthermore, the cell remains anchored when one pair of subunits dissociates, providing an opportunity for the pair to rebind. L-selectin may not dimerize (Sarangapani et al 2004) (Fig. 10c), and no publication has reported whether E-selectin forms dimers.
Clustering of selectins and their ligands in membrane domains provides another mechanism to stabilize rolling by increasing bond number and reducing the force on individual bonds. The cytoplasmic domain links L-selectin to the cytoskeleton through a membrane-distal binding site for α-actinin (Pavalko et al 1995) and a membrane-proximal site for ezrin/radixin/moesin (ERM) proteins (Ivetic et al 2002) (Fig. 10c). In transfected cells, mutating the ERM-binding site shifts L-selectin from microvilli to the cell body; this reduces tethering but does not impair rolling (Ivetic et al 2004). Deleting the α-actinin-binding site destabilizes rolling, and deleting both α-actinin- and ERM-binding sites virtually eliminates rolling (Dwir et al 2001). Thus, direct cytoskeletal anchorage of the cytoplasmic tail of L-selectin is essential to support rolling. The cytoplasmic domain of PSGL-1 also has a membrane-proximal binding site for ERM proteins (Serrador et al 2002). However, contrary to an earlier report (Snapp et al 2002), deleting the cytoplasmic domain of PSGL-1 does not impair rolling of transfected cells on P-selectin (Miner et al 2008). Leukocytes from knock-in mice that express a truncated form of PSGL-1 without the cytoplasmic domain still concentrate PSGL-1 in microvilli, roll normally on P-selectin, and extend and retract long membrane tethers at the trailing edge. Like WT PSGL-1, the truncated PSGL-1 associates with lipid rafts (Miner et al 2008), which may anchor it indirectly to the cytoskeleton through interactions with raft-enriched proteins (Rossy et al 2009) (Fig. 10c). L-selectin, by contrast, is not associated with lipid rafts in leukocytes (Dwir et al 2007) (Fig. 10c). The cytoplasmic domains of P- and E-selectin interact with clathrin-coated pits (Chuang et al 1997, Kluger et al 2002, Setiadi et al 1995) (Fig. 10b). Clustering of P- and E-selectin in clathrin-coated pits supports slower rolling by forming bond clusters with PSGL-1 and other ligands (Setiadi & McEver 2003, Setiadi & McEver 2008, Setiadi et al 1998). Clustering of E-selectin in lipid rafts of endothelial cells further slows leukocyte rolling (Setiadi & McEver 2008). Interactions of clathrin-coated pits and lipid rafts with the cytoskeleton may prevent force-induced extraction of P- and E-selectin from the membrane as leukocytes roll on endothelial cells.
CD44-mediated leukocyte rolling
The membrane-distal domain of CD44 is structurally related to C-type lectins (Kohda et al 1996). Activated T cells express a form of CD44 that binds to hyaluronan, a linear polymer of glucuronic acid/N-acetylglucosamine repeats (DeGrendele et al 1996, DeGrendele et al 1997). In vitro, CD44 mediates rolling of activated T cells on immobilized hyaluronan or on hyaluronan expressed on activated endothelial cells (DeGrendele et al 1996). In vivo, T helper-1 and T helper-2 cells roll on hyaluronan in TNF-α-activated postcapillary venules (Bonder et al 2006). The biochemical and biophysical mechanisms that underlie CD44-dependent rolling on hyaluronan have not been studied. It is intriguing that CD44 can serve as both a glycoconjugate ligand for E-selectin and a lectin receptor for hyaluronan.
Integrin-mediated leukocyte rolling
Integrins are transmembrane heterodimers of noncovalently paired α and β subunits (Luo et al 2007a). Integrins αLβ2, αMβ2, α4β1, and α4β7 primarily mediate leukocyte adhesion to vascular surfaces. In the original multistep paradigm for leukocyte recruitment, selectin-mediated rolling on endothelial cells enables leukocytes to encounter immobilized chemokines that activate integrins, leading to arrest, or firm adhesion. Later studies demonstrated that α4 and β2 integrins mediate leukocyte rolling as well as arrest (Ley et al 2007). α4 integrins support both leukocyte tethering and rolling, although less efficiently than selectins (Alon et al 1995b, Berlin et al 1995). Flowing leukocytes require selectins to tether to the endothelium before αLβ2 and αMβ2 can mediate rolling (Dunne et al 2002). In the past decade, enormous progress has been made in characterizing the structural basis for regulating integrin function (Luo et al 2007a). Integrin conformations in dynamic equilibrium are modulated by both “inside-out” and “outside-in” mechanisms. Here, we focus on how signals cause integrins to mediate leukocyte rolling rather than arrest under flow.
Structures of integrins and their endothelial cell ligands
Integrins are multidomain proteins that undergo large-scale rearrangements coupled to changes in affinity for ligands (Luo et al 2007a). Three main integrin conformations have been described: a bent form with a closed headpiece that has low affinity for ligand, an extended form with a closed headpiece that has low affinity for ligand, and an extended form with an open headpiece that has high affinity for ligand (Fig. 11). Transitional conformations likely exist. The headpiece comprises the β-propeller domain of the α subunit and the β I (or A) and hybrid domains of the β subunit. The headpiece of some integrins, including β2 integrins, contains an α I domain inserted in the β-propeller domain, and ligand binds to the α I domain (Fig. 11b). For integrins without an α I domain, including the α4 integrins, ligand binds to the β-propeller and β I domains (Fig. 11a). Extension enhances access of the headpiece to cell-surface ligands and permits the hybrid domain to “swing out,” opening the angle between the hybrid and β I domains. This propagates allosteric changes to the ligand-binding surface that increase affinity for ligand. A “downward” displacement of the α7-helix of the β I domain is coupled to loop rearrangements that move residues in and around the metal ion-dependent adhesion site (MIDAS). For integrins that lack an α I domain, including α4 integrins, the MIDAS in the β I domain is a critical component of the ligand-binding surface. For integrins that have an α I domain, including β2 integrins, rearrangements of the β I MIDAS enhance interactions with the base of the α I domain. This causes an analogous downward displacement of the α7-helix of the α I domain, inducing loop rearrangements that move residues in and around its MIDAS to bind ligand (Fig. 12). Allostery is bidirectional; ligand binding to a low-affinity site initiates MIDAS-associated loop rearrangements and displacement of the α7-helix.
Figure 11.
Domain organization of integrins and ligands that mediate leukocyte rolling or arrest. (a, b) Rearrangement of domains during activation of integrin α4β1, which lacks an α I domain (a) or during activation of integrin αLβ2, which has an α I domain (b). The domains of each headpiece are indicated. Binding of the N-terminal Ig domain of VCAM-1 (a) or ICAM-1 (b) to the respective headpiece is shown; for clarity, the other Ig domains of VCAM-1 or ICAM-1 are shown only in panel d. (c) Binding of the small-molecule antagonist XVA143 to the β I domain of αLβ2.
Figure 12.
Overlay of the structures of low-affinity, intermediate-affinity, and high-affinity conformations of the αL I domain. Regions of minimal conformational changes in all three conformations are shown in gray. The introduced disulfide bonds that stabilize each conformation and the movements of the MIDAS metal ion (sphere), the α1-loop, and the α7-helix are color coded as shown. The directions of movement are depicted with arrows. [From PDB ID codes 1ZOO, 1MJN, and 1TOP (Qu & Leahy 1996, Shimaoka et al 2003, Song et al 2005).]
The major endothelial cell ligands for leukocyte integrins are transmembrane glycoproteins with multiple domains that belong to the immunoglobulin superfamily (Luo et al 2007a) (Fig. 11d). αLβ2 binds to intercellular adhesion molecule-1 (ICAM-1), α4β1 binds to vascular cell adhesion molecule-1 (VCAM-1), and α4β7 binds to VCAM-1 and to mucosal addressin cell adhesion molecule-1. Interactions of αLβ2 with ICAM-1 have been extensively studied. The αL I domain binds through multiple contacts to the membrane-distal D1 domain of ICAM-1. Among these contacts, a glutamic acid in D1 contributes a negatively charged oxygen to the Mg2+ in the primary coordination sphere of the αL I domain MIDAS. An analogous glutamic acid at the base of the αL I domain helps coordinate the Mg2+ in the β2 I domain MIDAS. The small molecule allosteric antagonist, XVA143, binds to the β2 I domain MIDAS, inducing extension of the integrin but also blocking interactions with the αL I domain (Fig. 11c). XVA143 enhances αLβ2-mediated rolling of leukocytes or transfected cells on ICAM-1 but prevents firm adhesion (Salas et al 2004). Isolated αL I domains with low, intermediate, or high affinity for ICAM-1 have been created by introducing disulfide bonds that lock the α7-helix at different positions to differentially rearrange the MIDAS and surrounding loops. When treated with XVA143, transfected cells expressing αLβ2 with a locked, low-affinity αL I domain roll on ICAM-1, whereas cells expressing αLβ2 with a locked, intermediate- or high-affinity αLI domain arrest (Salas et al 2002). Thus, extension of αLβ2 is sufficient for rolling, whereas transition of the αL I domain to an intermediate- or high-affinity conformation is required for arrest.
Inside-out signaling to activate integrins
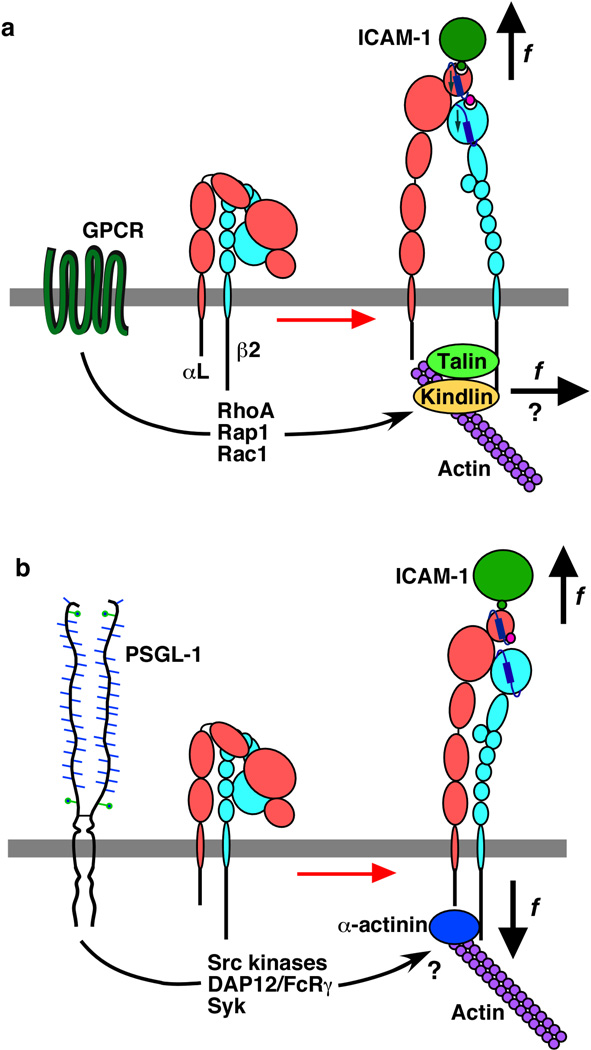
Integrins are maintained in the bent, low-affinity state through weak interactions of the transmembrane and cytoplasmic domains of α and β subunits (Luo et al 2007a). Signals disrupting these interactions cause the legs of the α and β ectodomains to move apart so that they can unbend (Kim et al 2003). A final step in activating integrins to their high-affinity conformations involves binding of the β cytoplasmic tail to talin and kindlins, which connect to actin filaments (Larjava et al 2008, Malinin et al 2009, Moser et al 2009, Ratnikov et al 2005) (Fig. 13a). Cytoskeletal regulation couples different αLβ2 conformers to distinct diffusion rates in the membrane (Cairo et al 2006). Inside-out signals may activate only a subset of these integrins. Chemokine signaling through G protein-coupled receptors on leukocytes activates αLβ2 to an extended, high-affinity state that causes firm adhesion to endothelial cells (Constantin et al 2000). Key signaling effectors include the small GTPases RhoA, Rap1, and Rac1 (Bolomini-Vittori et al 2009). Neutrophils rolling on E-selectin or P-selectin propagate a distinct signaling pathway by engaging PSGL-1, which partially activates αLβ2 to cause rolling but not arrest on ICAM-1 (Zarbock et al 2007). Signaling is rapidly reversible, requires the cytoplasmic domain of PSGL-1, Src family kinases, the immunoreceptor tyrosine activation motif-containing proteins DAP12 and FcRγ, spleen tyrosine kinase (Syk), and p38 mitogen-activated protein kinase (Green et al 2004, Miner et al 2008, Zarbock et al 2008, Zarbock et al 2007) (Fig. 13b). Whether and if so, how these signals affect interactions with integrin cytoplasmic tails has not been determined. One possibility is that the Syk pathway causes α-actinin-1 to bind to the β2 tail (Pavalko & LaRoche 1993) (Fig. 13b). T cells migrating on ICAM-1 express an extended, “intermediate-affinity” form of αLβ2 (Stanley et al 2008). The extended integrin associates with α-actinin-1, although this may be a consequence rather than a cause of integrin extension.
Figure 13.
Differential activation of integrin αLβ2 to mediate leukocyte arrest or rolling. (a) Chemokine binding to its G protein-coupled receptor (GPCR) activates Rho-family GTPases and effectors that recruit talin and kindlins to the β2 tail, which in turn bind to actin filaments. Lateral forces exerted by the cytoskeleton may separate the integrin legs, favoring integrin extension, swing-out of the hybrid domain, and allosteric conversion of the αL I domain to its high-affinity conformation. Tensile force applied to ICAM-1 bound to the αL I domain may further stabilize this conformation, causing leukocyte arrest. (b) Selectin engagement of PSGL-1 on rolling leukocytes propagates signals upstream and downstream of Syk. These signals might permit binding of α-actinin to the β2 tail. Incomplete leg separation may cause the integrin to extend in a conformation that resists force-induced conversion of the αL I domain to its high-affinity state.
Mechanical regulation of integrin-ligand interactions
Force affects conformational transitions of integrins and therefore their interactions with ligands. Steered MD simulations of tensile force applied to the C-terminal α7-helix of the αL I domain reveal ratcheted movements of the α7-helix linked to rearrangements at and around the MIDAS that correspond to low-, intermediate-, and high-affinity states for ligand (Jin et al 2004). C-terminal but not N-terminal anchorage of the αL I domain to a linker on transfected cells or microspheres supports rolling on ICAM-1 under flow (Astrof et al 2006). However, the failure of shear-imposed force to cause the cells or microspheres to arrest on ICAM-1 implies that pulling only the α7-helix is not sufficient to convert the ligand-binding surface of the isolated αL I domain to its high-affinity state. The force vector may be critical because the α7-helix is highly flexible (Zhang et al 2008). Steered MD simulations of a pulling force applied to ligand bound to the αvβ3 headpiece accelerates opening of the angle between the hybrid and β3 I domains and transition of the MIDAS and surrounding residues of the β3 I domain to the high-affinity conformation (Puklin-Faucher et al 2006, Puklin-Faucher & Vogel 2009). On the other hand, simulations of tensile force applied to ligand bound to the extended complete ectodomain of αIIbβ3 stabilize the low-affinity conformation, whereas simulations of lateral force to mimic attachment to moving actin filaments stabilize the high-affinity conformation (Zhu et al 2008).
As noted earlier, integrin conformations in the absence of force are in equilibrium and therefore reversible. Reporter mAbs reveal that flowing T cells that contact immobilized chemokine CCL21 rapidly extend αLβ2, but the cells do not arrest unless they immediately encounter co-immobilized ICAM-1 (Shamri et al 2005). Conversely, T cells in contact with co-immobilized CCL21 and ICAM-1 in stasis do not adhere stably until flow is applied (Woolf et al 2007). These data suggest that force imposed by flow converts a transient, extended but low-affinity conformation of αLβ2 to a high-affinity conformation, resulting in rapid cell arrest on ICAM-1 (Fig. 13a). Yet force imposed by flow does not cause αLβ2 activated by Syk to transition neutrophils from rolling to arrest on ICAM-1 (Zarbock et al 2007) (Fig. 13b). Thus, signals transduced by chemokine receptors and selectin ligands may convert αLβ2 into distinct conformations that respond differently to force. The allosteric antagonist XVA143 does not alter slow rolling on E-selectin and ICAM-1 (Zarbock et al 2007). But unlike XVA143, signaling through Syk might induce an extended state that retains force-insensitive interactions between the hybrid and β2 I domains. Alternatively, signaling might not completely separate the integrin legs, making them resistant to force-induced conversion to the fully extended state.
Atomic force microscopy was used to directly measure the force-dependent lifetimes of bonds between the integrin α5β1 and a fibronectin fragment (Kong et al 2009). Force prolongs bond lifetimes in the 10–30-pN range, establishing that integrin-ligand interactions also exhibit catch bonds. Furthermore, an α5β1-Fc chimera containing only the integrin headpiece forms catch bonds, indicating that leg extension is not required for force-induced transitions to a high-affinity state. However, divalent cation-regulated leg extension affects overall bond lifetime in response to force. It seems likely that α4 and β2 integrins also exhibit force-regulated transitions from catch to slip bonds with their ligands. The extent to which force prolongs catch bond lifetimes may determine whether integrins mediate leukocyte rolling or arrest. Whether β2 or α4 integrins exhibit flow-enhanced rolling, particularly at low shear stresses, has not been examined. There are striking parallels between force-induced opening of the hinge between the lectin and EGF domains of selectins and between the β I and hybrid domains of integrins. The evidence that hinge opening propagates allosteric rearrangements of the ligand-binding surface is particularly strong for integrins. However, further studies are required to fully understand how force affects integrin conformation and function.
Cellular features that modulate integrin-mediated leukocyte rolling
The α4 integrins are concentrated on microvilli, which may explain why they more effectively tether flowing leukocytes to endothelial cells than does αLβ2, which is distributed primarily on the cell body (Abitorabi et al 1997). However, subsets of αLβ2 in microvilli might better support rolling by dissipating force as the microvillus extends. Anchorage of integrin subsets to the cytoskeleton may stabilize αLβ2-ICAM-1 or α4β1-VCAM-1 bonds (Alon et al 2005, Cairo et al 2006). ICAM-1 forms dimers that favor dimeric interactions with two αLβ2 integrins (Miller et al 1995), and arrays of ICAM-1 dimers linked to the cytoskeleton may promote bond clusters that lower rolling velocities (Yang et al 2004).
Platelet rolling
As for leukocytes, rolling of platelets on vascular surfaces is the first step in a cascade of adhesion and signaling events (Chen & Lopez 2005, Varga-Szabo et al 2008). To stem hemorrhage from disrupted blood vessels, particularly in the arterial system, platelets tether to and roll on exposed subendothelial cell matrix (Fig. 14a). They then arrest, form aggregates, and catalyze blood coagulation. Interactions of the platelet glycoprotein (GP) Ib/IX/V complex with immobilized von Willebrand factor (VWF) mediate tethering and rolling. Interactions of platelet integrins with plasma and matrix glycoproteins mediate arrest and aggregation. During inflammation, platelets also use selectins to roll on intact endothelial cells in veins and venules (Fig. 14b).
Figure 14.
Rolling of platelets on vascular surfaces. (a) After vascular injury, platelets tether to and roll (translocate) on subendothelial matrices through interactions of platelet GPIbα with VWF. Signaling during rolling activates platelet β1 and β3 integrins, which mediate arrest and aggregation. (b) Unstimulated platelets roll on P-selectin on activated endothelial cells, probably through interactions with GPIbα. Activated platelets use P-selectin to roll on PNAd or PSGL-1 expressed on some endothelial cells and on PSGL-1 expressed on leukocyte fragments bound to the endothelial cell surface. Activated platelets bound to leukocytes roll together on endothelial cells.
GPIbα-mediated platelet rolling
Platelet GPIb/IX/V complex
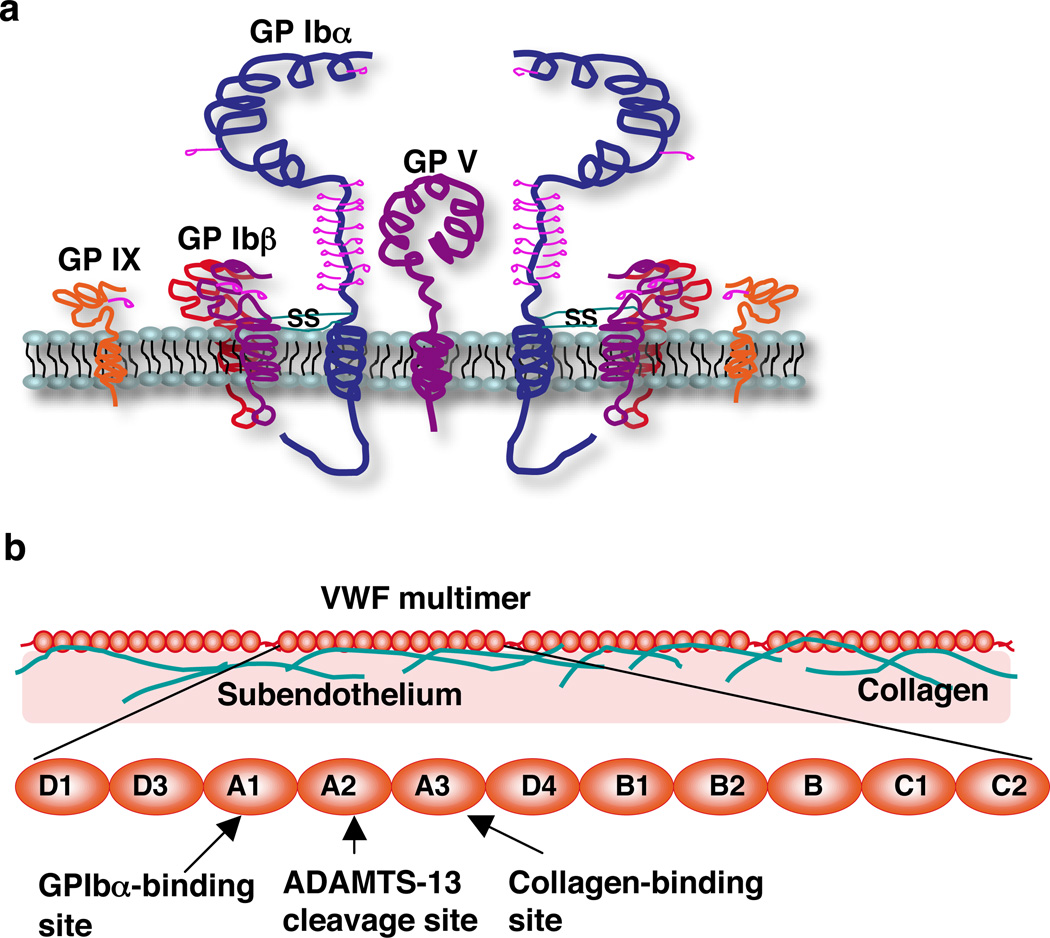
The platelet GPIb/IX/V complex comprises four subunits: GPIbα, GPIbβ, GPIX, and GPV in a 2:4:2:1 stoichiometry (Luo et al 2007b) (Fig. 15a). GPIbα interacts noncovalently with one GPIX subunit and forms disulfide bonds with two GPIbβ subunits. GPV interacts noncovalently with two GPIbα subunits (Modderman et al 1992). The ectodomains of all four subunits have one or more leucine-rich repeats (Chen & Lopez 2005). VWF binds to the largest subunit, GPIbα, which has a 289-residue N-terminal domain with eight tandem leucine-rich repeats (GPIbαN), followed by a long O-glycosylated stalk, a transmembrane domain, and a cytoplasmic tail.
Figure 15.
Schematic of the platelet GPIb/IX/V complex (a) and VWF (b).
VWF
VWF is a multimeric plasma glycoprotein with up to 80 subunits (Sadler 1998) (Fig. 15b). The mature VWF subunit has 2,050 residues with multiple A-, B-, C-, and D-type domains. Endothelial cells secrete ultralarge VWF multimers that are cleaved at the A2 domains into smaller multimers by a disintegrin and metalloproteinase with a thrombospondin type 1 motif (ADAMTS)-13 (Dong et al 2002, Shim et al 2008). VWF multimers attach to exposed subendothelial matrices through interactions of the A3 domains with collagen (Cruz et al 1995). The A1 domain interacts with platelet GPIbα (Cruz et al 1993). Crystallographic studies reveal that the leucine-rich repeats of GPIbαN wrap around one side of the A1 domain but that only the β-finger region on the N-terminal side and the β-switch region on the C-terminal side contact A1 (Dumas et al 2004, Huizinga et al 2002).
Mechanical regulation of GPIbα-VWF interactions and flow-enhanced rolling
Like selectins, GPIbα requires a minimum shear to support adhesion (Savage et al 1996). Selectins mediate optimal leukocyte rolling at the shear stresses found in postcapillary venules, whereas GPIbα mediates platelet rolling at the higher shear stresses found in arterioles and arteries. Because platelets are smaller than leukocytes, the forces applied to GPIbα-VWF bonds on rolling platelets are similar to those applied to selectin-ligand bonds on rolling leukocytes. GPIbα dissociates from VWF with rapid off-rates like those of L-selectin dissociating from its ligands and likely has similarly rapid on-rates (Yago et al 2008). Flow-enhanced tethering of platelets to VWF is probably governed by the same three transport parameters used by selectins: sliding velocity, Brownian motion, and molecular diffusion. In addition, flow may induce conformational changes in the β-switch of GPIbαN. This region is a β-hairpin that forms a major contact with the VWF A1 domain in co-crystal structures (Dumas et al 2004, Huizinga et al 2002). In unliganded GPIbαN, however, it is a disordered loop (Varughese et al 2004). Mutations in the β-switch region (e.g., M239V) that occur in some patients with platelet-type von Willebrand disease cause paradoxical bleeding and remove the flow requirement for platelet tethering (Doggett et al 2003). MD simulations suggest that flow induces a loop-to-β-hairpin transition on the β-switch, thereby promoting VWF binding (Lou & Zhu 2008). This transition is easier to induce in M239V than in WT GPIbαN, providing a structural explanation for the gain-of-function phenotype of the mutant.
As for selectin-ligand dissociation, force exerts a biphasic effect on GPIbα-VWF dissociation, first prolonging lifetimes (catch bonds) until a maximal value is reached and then shortening lifetimes (slip bonds) as force continues to increase (Yago et al 2008). Earlier studies detected only slip bonds because the measured forces were too high or because video frame speeds were too slow to measure the shortest bond lifetimes (Doggett et al 2002, Kumar et al 2003). Catch bonds cause platelets and GPIbα-coated microspheres to roll slower and more regularly on VWF multimers or isolated A1 domains as flow increases from suboptimal levels, explaining flow-enhancing rolling (Yago et al 2008). Above the flow optimum, rolling becomes faster and less regular as higher forces shorten bond lifetimes (slip bonds).
MD simulations of force applied to the GPIbαN-VWF complex support a sliding-rebinding model for catch bonds (Yago et al 2008) (Fig. 16). In the crystal structure of WT GPIbαN bound to WT A1, A1 residue D1269 forms a salt bridge with R1306 and possibly with R1450. GPIbαN contacts A1 via the β-switch and the β-finger (Dumas et al 2004). In the simulations, force applied to the complex ruptures the salt bridge between D1269 and R1306/R1450, which extends the A1 N-terminal sequence and rotates A1 counterclockwise (Yago et al 2008). Further pulling forces the GPIbα β-finger to slide over A1, enabling formation of new interactions, e.g., a new salt bridge between GPIbα E14 and A1 R1334. The GPIbα β-switch then dissociates from the A1 central β-sheet, but the E14-R1334 interaction and a few other hydrophobic interactions remain intact. Continued pulling finally disrupts the E14-R1334 interactions, causing complete dissociation. The sliding-rebinding model for GPIbαN-VWF catch bonds is similar to that for P-selectin-PSGL-1 catch bonds, although the structures of the interacting molecules are very different. Force disrupts interactions between the lectin and EGF domains of selectins, whereas force disrupts interactions within the A1 domain of VWF. Both alterations, however, change the orientation of receptor relative to ligand during dissociation, which enable new interactions to replace those that break. Contacts of A1 residues 1268 and 1269 with the adjacent D3 domain of VWF may also regulate forced dissociation of A1 from GPIbα (Ulrichts et al 2006).
Figure 16.
Structural model for catch bonds between GPIbα and VWF. (a–e) Sequential snapshots of steered MD-simulated structures showing pathways of the sliding-rebinding mechanism. Mauve, A1; gray, GPIbαN; red spheres, D1269; blue spheres, R1306; green spheres, R1450; blue β-strands, β-switch of GPIbα; red β-strands, central β-sheet of A1; red sticks, E14; blue sticks, R1334. The equilibrated structure (a) was used as a starting point of steered MD simulations to generate the simulated structures in b–e. (f) Overlay of the structures in a (colors same as in a) and d (green, A1; cyan, GPIbα), showing the rotation of A1 and sliding of the GPIbα β-finger over A1. (g) Sliding-rebinding mechanism for GPIbα/VWF A1 catch bonds. Adapted with permission from (Yago et al 2008).
Catch bonds provide a mechanism to support platelet rolling on VWF immobilized on vascular surfaces but prevent inappropriate agglutination of circulating platelets by binding to VWF multimers in plasma. Mutations in the A1 domain that occur in some patients with type 2B von Willebrand disease cause paradoxical bleeding and depletion of large VWF multimers despite enhanced force-free interactions of mutant VWF with GPIbα on platelets (Sadler 2005, Sadler et al 2006). Two type 2B von Willebrand disease A1 domain mutants, R1306Q and R1450E, convert catch bonds to slip bonds by prolonging bond lifetimes at low forces (Yago et al 2008). MD simulations suggest that these mutations weaken or disrupt the salt bridge with D1269, allowing the E14-R1334 salt bridge to form at zero force or low force. Flowing platelets agglutinate with microspheres bearing R1306Q or R1450E A1 but not WT A1 (Yago et al 2008). Force applied to GPIbα-VWF bonds also regulates the exposure of the ADAMTS-13 cleavage site in the A2 domain (Dong et al 2002, Shim et al 2008, Wu et al 2010, Zhang et al 2009). At arterial shear rates, ADAMTS-13 reduces platelet agglutination with microspheres bearing a tridomain A1A2A3 VWF fragment with the R1450E mutation (Yago et al 2008). Thus, prolonged lifetimes of mutant VWF bonds with GPIbα on circulating platelets may allow ADAMTS-13 to cleave and therefore deplete large VWF multimers, causing bleeding.
Cellular features that modulate GPIbα-mediated platelet rolling
The high surface density of the GPIb/IX/V complex on platelets favors rapid interactions with immobilized VWF multimers (Chen & Lopez 2005, Varga-Szabo et al 2008). Association of GPV with two GPIbα molecules may promote dimeric interactions with A1 domains on VWF multimers (Modderman et al 1992). Clustering of GPIb/IX/V complexes in lipid rafts enhances rolling (Shrimpton et al 2002). The cytoplasmic domain of GPIbα interacts with filamin, which anchors the GPIb/IX/V complex to the membrane cytoskeleton (Williamson et al 2002). Whereas leukocytes are round and have multiple microvilli, platelets are discoid-shaped and smooth. After tethering to VWF, translocating platelets rapidly mobilize Ca2+, which signals cytoskeletal rearrangements that convert platelets from discs to small spheres (Yuan et al 1999); this may cause more regular rolling. Furthermore, rolling platelets extend and retract membrane tethers that may stabilize rolling velocities at higher shear stresses (Dopheide et al 2002). Engagement of the GPIb/IX/V complex propagates signals that activate integrin αIIbβ3 to intermediate- or high-affinity conformations (Chen & Lopez 2005, Mazzucato et al 2002, Varga-Szabo et al 2008). In turn, binding of activated αIIbβ3 to fibrinogen, VWF, and fibronectin promotes both arrest and aggregation of platelets. Clustering of the GPIb/IX/V complex activates Syk (Kasirer-Friede et al 2002). Signals downstream from Syk might partially activate αIIbβ3 to mediate rolling rather than arrest (Mazzucato et al 2002).
P-selectin-mediated platelet rolling
Unstimulated platelets roll on endothelial cells of veins and postcapillary venules at sites of inflammation in mice (Frenette et al 1995) (Fig. 14b). Platelets roll faster than leukocytes. In larger venules, platelet rolling is mediated by interactions of GPIbα on platelets with VWF on the endothelial cell surface (André et al 2000). In smaller venules, platelets roll on P-selectin on activated endothelial cells (Frenette et al 1995). Disruption of the genes encoding the α1-3-fucosyltransferases that construct sLex eliminates selectin-mediated leukocyte rolling but does not affect platelet rolling in inflamed venules (Frenette et al 1998). Therefore, the selectin ligands on platelets do not require fucosylation. PSGL-1 and GPIbα have been proposed as ligands for P-selectin on platelets (Frenette et al 2000, Romo et al 1999). Without α1-3-fucosylation, PSGL-1 binds to P-selectin with very low affinity (Leppänen et al 1999, Leppänen et al 2000). The density of PSGL-1 on platelets appears to be low, challenging its importance as a P-selectin ligand. By contrast, the high surface densities of GPIbα may support rolling on P-selectin even if the interaction is weak. The N-terminal region of GPIbα has sulfated tyrosines that, like those in PSGL-1, might interact with P-selectin, although this has not been examined (Dong et al 2001). Rolling of unstimulated platelets on endothelial cells may position them to detect early vascular injury (Frenette et al 1995).
In some circumstances, activated platelets expressing P-selectin roll on P-selectin ligands on endothelial cells (Fig. 14b). P-selectin binds to PNAd mucins, although less well than L-selectin. However, its high density on activated platelets supports rolling in high endothelial venules (Diacovo et al 1996). Additional interactions of P-selectin on activated platelets with PSGL-1 augment delivery of lymphocytes to lymph nodes. Platelet-neutrophil aggregates roll on and stimulate endothelial cells to mobilize P-selectin, thus attracting more rolling leukocytes (Dole et al 2007) (Fig. 14b). At sites of inflammation, rolling leukocytes deposit membrane fragments on endothelial cells, probably by scission of membrane tethers (Sperandio et al 2003). These PSGL-1-containing fragments support L-selectin-dependent rolling of leukocytes but might also mediate P-selectin-dependent rolling of activated platelets.
Conclusions
The remarkable ability of circulating leukocytes and platelets to tether to and roll on vascular surfaces requires specialized, mechanically regulated kinetic properties of the interacting adhesion receptors and ligands. Tethering requires fast on-rates that make transport the limiting factor at lower flow rates, providing the opportunity for flow to enhance tethering. Rolling requires a delicate balance between rapid formation and rapid breakage of adhesive bonds. Although both on-rates and off-rates contribute to this balance, the off-rates of bonds that anchor rolling cells at the trailing edge are the principal regulators of rolling velocities. These bonds are subjected to forces that must balance the force and torque imposed by shear stress on the adherent cell. They must have sufficient tensile strength so that force does not accelerate dissociation so rapidly that the cells detach into the fluid stream. On the other hand, they must dissociate sufficiently quickly so that cells pivot about new bonds and continue to roll. Force-regulated transitions from catch bonds to slip bonds provide a mechanism to optimize rolling dynamics and explain the counterintuitive requirement for a shear threshold to support rolling. Catch bonds provide a mechanism to prevent inappropriate leukocyte and platelet aggregation in flowing blood, yet enable cells to roll on the vessel wall. Although structurally diverse, selectins, integrins, and GPIbα undergo transitions from catch bonds to slip bonds with their equally diverse ligands. This diversity may require different structural mechanisms for catch bonds. The allosteric and sliding-rebinding models have received most attention; they need not be mutually exclusive, and other mechanisms deserve consideration (Sokurenko et al 2008, Zhu et al 2005). Although this review has emphasized rolling of blood cells in the vasculature, flow-enhanced adhesion can occur in other sites. The best-documented example is the flow-enhanced attachment of bacteria to enteric surfaces through interactions of the FimH adhesin with mannosylated ligands on epithelial cells (Thomas et al 2002). Force imposed by shear stress generates catch bonds through allosteric conversion of the FimH binding pocket from low to high affinity (Yakovenko et al 2008). Increasing force converts unstable rolling to firm adhesion, underscoring the balance between bond association and dissociation required for rolling (Thomas 2008).
The structural features of receptors and ligands are sufficient to support rolling in cell-free systems, but how cells organize receptors and ligands on their surfaces is equally important. Common themes are self-association into dimers, clustering in membrane domains, and direct or indirect anchorage to the cytoskeleton. These presentations favor bond clusters that distribute force among individual bonds. Bond clusters, extension of microvilli, and pulling of long membrane tethers from the trailing edge cooperate to stabilize rolling velocities, particularly at higher wall shear stresses where slip bonds are dominant. Rolling cells also transduce signals that prime other responses, including the activation of other adhesion receptors.
Although enormous progress has been made in the past 20 years, much remains to be learned about the molecular and cellular requirements for rolling cell adhesion. Further study of the mechanisms for catch bonds is required, with attention to the relative contributions of interactions within and between domains in regulating bond lifetime. We need to understand why force promotes rolling rather than arrest or, in the case of integrins, either rolling or arrest. How partitioning of adhesion receptors into membrane domains regulates both rolling and signaling is ripe for further investigation. Finally, the mechanisms that regulate rolling of leukocytes and platelets in vitro must be studied in vivo to clarify their biological consequences in health and disease.
Summary points.
Rolling on vascular surfaces provides a checkpoint for hematopoietic cells before they commit to arrest, aggregate, or migrate into particular tissues.
Rolling requires rapid and balanced formation and dissociation of adhesive bonds that are subjected to forces applied by shear stress in flowing blood.
Increasing force applied to adhesive bonds first prolongs their lifetimes (catch bonds) and then shortens their lifetimes (slip bonds).
Force-regulated disruptions of receptor interdomain or intradomain interactions remote from the ligand-binding surface generate catch bonds.
Catch bonds mediate the counterintuitive shear threshold phenomenon in which increasing flow augments rolling despite higher dislodging forces.
Catch bonds provide a mechanism to prevent agglutination of flowing leukocytes and platelets.
Interactions of adhesion receptors with membrane domains and the cytoskeleton modulate forces applied to adhesive bonds and thus affect rolling.
Cell signaling influences rolling by regulating the cell-surface density, distribution, and/or conformation of adhesion receptors.
Acknowledgments
The authors are supported by NIH grants HL34363, HL085607, and HL090923 to R.P.M. and AI44902, HL093723, and HL091020 to C.Z. We thank Tadayuki Yago, Lijun Xia, and Wei Chen for assistance with figures.
Acronyms and brief definitions
- Adhesive bond
a reversible interaction between a cell-surface receptor and a ligand on another cell or in extracellular matrix.
- Catch bond
a bond that prolongs its lifetime in response to tensile force
- Slip bond
a bond that shortens its lifetime in response to tensile force
- PSGL-1
P-selectin glycoprotein ligand-1
- Flow-enhanced adhesion
a counterintuitive phenomenon where increasing flow augments cell adhesion despite the higher dislodging forces
- Integrin headpiece
the ligand-binding region comprising the β-propeller, α I (when present), β I, and hybrid domains
Literature cited
- Abitorabi MA, Pachynski RK, Ferrando RE, Tidswell M, Erle DJ. Presentation of integrins on leukocyte microvilli: a role for the extracellular domain in determining membrane localization. J Cell Biol. 1997;139:563–571. doi: 10.1083/jcb.139.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon R, Chen SQ, Puri KD, Finger EB, Springer TA. The kinetics of L-selectin tethers and the mechanics of selectin-mediated rolling. J Cell Biol. 1997;138:1169–1180. doi: 10.1083/jcb.138.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon R, Feigelson SW, Manevich E, Rose DM, Schmitz J, et al. alpha4-beta1-dependent adhesion strengthening under mechanical strain is regulated by paxillin association with the alpha4-cytoplasmic domain. J Cell Biol. 2005;171:1073–1084. doi: 10.1083/jcb.200503155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin: carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995a;374:539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995b;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André P, Denis CV, Ware J, Saffaripour S, Hynes RO, et al. Platelets adhere to and translocate on von Willebrand factor presented by endothelium in stimulated veins. Blood. 2000;96:3322–3328. [PubMed] [Google Scholar]
- Astrof NS, Salas A, Shimaoka M, Chen J, Springer TA. Importance of force linkage in mechanochemistry of adhesion receptors. Biochemistry. 2006;45:15020–15028. doi: 10.1021/bi061566o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkalow FJ, Barkalow KL, Mayadas TN. Dimerization of P-selectin in platelets and endothelial cells. Blood. 2000;96:3070–3077. [PubMed] [Google Scholar]
- Bell GI. Models for the specific adhesion of cells to cells: A theoretical framework for adhesion mediated by reversible bonds between cell surface molecules. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Berlin C, Bargatze RF, Campbell JJ, Von Andrian UH, Szabo MC, et al. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- Bolomini-Vittori M, Montresor A, Giagulli C, Staunton D, Rossi B, et al. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat Immunol. 2009;10:185–194. doi: 10.1038/ni.1691. [DOI] [PubMed] [Google Scholar]
- Bonder CS, Clark SR, Norman MU, Johnson P, Kubes P. Use of CD44 by CD4+ Th1 and Th2 lymphocytes to roll and adhere. Blood. 2006;107:4798–4806. doi: 10.1182/blood-2005-09-3581. [DOI] [PubMed] [Google Scholar]
- Brunk DK, Goetz DJ, Hammer DA. Sialyl Lewis x/E-selectin-mediated rolling in a cell-free system. Biophys J. 1996;71:2902–2907. doi: 10.1016/S0006-3495(96)79487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo CW, Mirchev R, Golan DE. Cytoskeletal regulation couples LFA-1 conformational changes to receptor lateral mobility and clustering. Immunity. 2006;25:297–308. doi: 10.1016/j.immuni.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Chang K-C, Hammer DA. The forward rate of binding of surface-tethered reactants: effect of relative motion between two surfaces. Biophys J. 1999;76:1280–1292. doi: 10.1016/S0006-3495(99)77291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lopez JA. Interactions of platelets with subendothelium and endothelium. Microcirculation. 2005;12:235–246. doi: 10.1080/10739680590925484. [DOI] [PubMed] [Google Scholar]
- Chen W, Evans EA, McEver RP, Zhu C. Monitoring receptor-ligand interactions between surfaces by thermal fluctuations. Biophys J. 2007;94:694–701. doi: 10.1529/biophysj.107.117895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang PI, Young BA, Thiagarajan RR, Cornejo C, Winn RK, Harlan JM. Cytoplasmic domain of E-selectin contains a non-tyrosine endocytosis signal. J Biol Chem. 1997;272:24813–24818. doi: 10.1074/jbc.272.40.24813. [DOI] [PubMed] [Google Scholar]
- Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, et al. Chemokines trigger immediate b2 integrin affinity and mobility changes: Differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759–769. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- Cruz MA, Handin RI, Wise RJ. The interaction of the von Willebrand factor-A1 domain with platelet glycoprotein Ib/IX. The role of glycosylation and disulfide bonding in a monomeric recombinant A1 domain protein. J Biol Chem. 1993;268:21238–21245. [PubMed] [Google Scholar]
- Cruz MA, Yuan H, Lee JR, Wise RJ, Handin RI. Interaction of the von Willebrand factor (vWF) with collagen. J Biol Chem. 1995;270:10822–10827. doi: 10.1074/jbc.270.18.10822. [DOI] [PubMed] [Google Scholar]
- DeGrendele HC, Estess P, Picker LJ, Siegelman MH. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: A novel lymphocyte-endothelial cell primary adhesion pathway. J Exp Med. 1996;183:1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrendele HC, Kosfiszer M, Estess P, Siegelman MH. CD44 activation and associated primary adhesion is inducible via T cell receptor stimulation. J Immunol. 1997;159:2549–2553. [PubMed] [Google Scholar]
- Dembo M, Torney DC, Saxman K, Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B Biol Sci. 1988;234:55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- Diacovo TG, Puri KD, Warnock RA, Springer TA, Von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 1996;273:252–255. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- Doggett TA, Girdhar G, Lawshe A, Miller JL, Laurenzi IJ, et al. Alterations in the intrinsic properties of the GPIbα-VWF tether bond define the kinetics of the platelet-type von Willebrand disease mutation, Gly233Val. Blood. 2003;102:152–160. doi: 10.1182/blood-2003-01-0072. [DOI] [PubMed] [Google Scholar]
- Doggett TA, Girdhar G, Lawshe A, Schmidtke DW, Laurenzi IJ, et al. Selectin-like kinetics and biomechanics promote rapid platelet adhesion in flow: the GPIbα-vWF tether bond. Biophys J. 2002;83:194–205. doi: 10.1016/S0006-3495(02)75161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VS, Bergmeier W, Patten IS, Hirahashi J, Mayadas TN, Wagner DD. PSGL-1 regulates platelet P-selectin-mediated endothelial activation and shedding of P-selectin from activated platelets. Thromb Haemost. 2007;98:806–812. [PubMed] [Google Scholar]
- Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- Dong JF, Ye P, Schade AJ, Gao S, Romo GM, et al. Tyrosine sulfation of glycoprotein Ibα. J Biol Chem. 2001;276:16690–16694. doi: 10.1074/jbc.M101035200. [DOI] [PubMed] [Google Scholar]
- Dopheide SM, Maxwell MJ, Jackson SP. Shear-dependent tether formation during platelet translocation on von Willebrand factor. Blood. 2002;99:159–167. doi: 10.1182/blood.v99.1.159. [DOI] [PubMed] [Google Scholar]
- Dumas JJ, Kumar R, McDonagh T, Sullivan F, Stahl ML, et al. Crystal structure of the wild-type von Willebrand factor A1-glycoprotein Ibα complex reveals conformation differences with a complex bearing von Willebrand disease mutations. J Biol Chem. 2004;279:23327–23334. doi: 10.1074/jbc.M401659200. [DOI] [PubMed] [Google Scholar]
- Dunne JL, Ballantyne CM, Beaud AL, Ley K. Control of leukocyte rolling velocity in TNF-α-induced inflammation by LFA-1 and Mac-1. Blood. 2002;99:336–341. doi: 10.1182/blood.v99.1.336. [DOI] [PubMed] [Google Scholar]
- Dwir O, Grabovsky V, Pasvolsky R, Manevich E, Shamri R, et al. Membranal cholesterol is not required for L-selectin adhesiveness in primary lymphocytes but controls a chemokine-induced destabilization of L-selectin rolling adhesions. J Immunol. 2007;179:1030–1038. doi: 10.4049/jimmunol.179.2.1030. [DOI] [PubMed] [Google Scholar]
- Dwir O, Kansas GS, Alon R. Cytoplasmic anchorage of L-selectin controls leukocyte capture and rolling by increasing the mechanical stability of the selectin tether. J Cell Biol. 2001;155:145–156. doi: 10.1083/jcb.200103042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson TK, Patel KD, McEver RP, Cummings RD. Noncovalent association of P-selectin glycoprotein ligand-1 and minimal determinants for binding to P-selectin. J Biol Chem. 2000;275:7839–7853. doi: 10.1074/jbc.275.11.7839. [DOI] [PubMed] [Google Scholar]
- Evangelista V, Pamuklar Z, Piccoli A, Manarini S, Dell'elba G, et al. Src family kinases mediate neutrophil adhesion to adherent platelets. Blood. 2007;109:2461–2469. doi: 10.1182/blood-2006-06-029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Heinrich V, Leung A, Kinoshita K. Nano- to microscale dynamics of P-selectin detachment from leukocyte interfaces. I. Membrane separation from the cytoskeleton. Biophys J. 2005;88:2288–2298. doi: 10.1529/biophysj.104.051698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Leung A, Hammer D, Simon S. Chemically distinct transition states govern rapid dissociation of single L-selectin bonds under force. Proc Natl Acad Sci USA. 2001;98:3784–3789. doi: 10.1073/pnas.061324998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Leung A, Heinrich V, Zhu C. Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond. Proc Natl Acad Sci U S A. 2004;101:11281–11286. doi: 10.1073/pnas.0401870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EB, Puri KD, Alon R, Lawrence MB, Von Andrian UH, Springer TA. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379:266–269. doi: 10.1038/379266a0. First experimental evidence for flow-enhanced leukocyte adhesion mediated by selectins.
- Frenette PS, Denis CV, Weiss L, Jurk K, Subbarao S, et al. P-selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J Exp Med. 2000;191:1413–1422. doi: 10.1084/jem.191.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette PS, Johnson RC, Hynes RO, Wagner DD. Platelets roll on stimulated endothelium in vivo: An interaction mediated by endothelial P-selectin. Proc Natl Acad Sci USA. 1995;92:7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette PS, Moyna C, Hartwell DW, Lowe JB, Hynes RO, Wagner DD. Platelet-endothelial interactions in inflamed mesenteric venules. Blood. 1998;91:1318–1324. [PubMed] [Google Scholar]
- Girdhar G, Shao JY. Simultaneous tether extraction from endothelial cells and leukocytes: observation, mechanics, and significance. Biophys J. 2007;93:4041–4052. doi: 10.1529/biophysj.107.109298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AJ, Cox RG, Brenner H. Slow viscous motion of a sphere parallel to a plane wall--Couette flow. Chem Eng Sci. 1967;22:653–660. [Google Scholar]
- Graves BJ, Crowther RL, Chandran C, Rumberger JM, Li S, et al. Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains. Nature. 1994;367:532–538. doi: 10.1038/367532a0. [DOI] [PubMed] [Google Scholar]
- Green CE, Pearson DN, Camphausen RT, Staunton DE, Simon SI. Shear-dependent capping of L-selectin and P-selectin glycoprotein ligand 1 by E-selectin signals activation of high-avidity β2-integrin on neutrophils. J Immunol. 2004;172:7780–7790. doi: 10.4049/jimmunol.172.12.7780. [DOI] [PubMed] [Google Scholar]
- Greenberg AW, Brunk DK, Hammer DA. Cell-free rolling mediated by L-selectin and sialyl Lewis x reveals the shear threshold effect. Biophys J. 2000;79:2391–2402. doi: 10.1016/S0006-3495(00)76484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Chen J, Chesla SE, Yago T, Mehta P, et al. Quantifying the effects of molecular orientation and length on two-dimensional receptor-ligand binding kinetics. J Biol Chem. 2004;279:44915–22923. doi: 10.1074/jbc.M407039200. [DOI] [PubMed] [Google Scholar]
- Huizinga EG, Tsuji S, Romijn RA, Schiphorst ME, de Groot PG, et al. Structures of glycoprotein Ibα and its complex with von Willebrand factor A1 domain. Science. 2002;297:1176–1179. doi: 10.1126/science.107355. [DOI] [PubMed] [Google Scholar]
- Ivetic A, Deka J, Ridley A, Ager A. The cytoplasmic tail of L-selectin interacts with members of the ezrin-radixin-moesin (ERM) family of proteins. J Biol Chem. 2002;277:2321–2329. doi: 10.1074/jbc.M109460200. [DOI] [PubMed] [Google Scholar]
- Ivetic A, Florey O, Deka J, Haskard DO, Ager A, Ridley AJ. Mutagenesis of the ezrinradixin- moesin binding domain of L-selectin tail affects shedding, microvillar positioning, and leukocyte tethering. J Biol Chem. 2004;279:33263–33272. doi: 10.1074/jbc.M312212200. [DOI] [PubMed] [Google Scholar]
- Jin M, Andricioaei I, Springer TA. Conversion between three conformational states of integrin I domains with a C-terminal pull spring studied with molecular dynamics. Structure. 2004;12:2137–2147. doi: 10.1016/j.str.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Kasirer-Friede A, Ware J, Leng L, Marchese P, Ruggeri ZM, Shattil SJ. Lateral clustering of platelet GP Ib-IX complexes leads to up-regulation of the adhesive function of integrin alpha IIbbeta 3. J Biol Chem. 2002;277:11949–11956. doi: 10.1074/jbc.M108727200. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS. CD44 is a physiological E-selectin ligand on neutrophils. J Exp Med. 2005;201:1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima H, Petryniak B, Hiraoka N, Mitoma J, Huckaby V, et al. N-acetylglucosamine- 6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat Immunol. 2005;6:1096–1104. doi: 10.1038/ni1259. [DOI] [PubMed] [Google Scholar]
- Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- Klopocki AG, Yago T, Mehta P, Yang J, Wu T, et al. Replacing a lectin domain residue in L-selectin enhances binding to P-selectin glycoprotein ligand-1 but not to 6-sulfo-sialyl Lewis x. J Biol Chem. 2008;283:11493–11500. doi: 10.1074/jbc.M709785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MS, Shiao SL, Bothwell ALM, Pober JS. Cutting edge: Internalization of transduced E-selectin by cultured human endothelial cells: Comparison of dermal microvascular and umbilical vein cells and identification of a phosphoserine-type dileucine motif. J Immunol. 2002;168:2091–2095. doi: 10.4049/jimmunol.168.5.2091. [DOI] [PubMed] [Google Scholar]
- Kobzdej MMA, Leppänen A, Ramachandran V, Cummings RD, McEver RP. Discordant expression of selectin ligands and sialyl Lewis x-related epitopes on murine myeloid cells. Blood. 2002;100:485–494. doi: 10.1182/blood-2002-06-1799. [DOI] [PubMed] [Google Scholar]
- Kohda D, Morton CJ, Parkar AA, Hatanaka H, Inagaki FM, et al. Solution structure of the link module: a hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell. 1996;86:767–775. doi: 10.1016/s0092-8674(00)80151-8. [DOI] [PubMed] [Google Scholar]
- Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. Used atomic force microscopy to document force-dependent transitions from catch bonds to slip bonds of integrin α5β1 with a fibronectin fragment.
- Kumar RA, Dong JF, Thaggard JA, Cruz MA, Lopez JA, McIntire LV. Kinetics of GPIbα-vWF-A1 tether bond under flow: effect of GPIbα mutations on the association and dissociation rates. Biophys J. 2003;85:4099–4109. doi: 10.1016/S0006-3495(03)74822-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H, Plow EF, Wu C. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 2008;9:1203–1208. doi: 10.1038/embor.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MB, Kansas GS, Kunkel EJ, Ley K. Threshold levels of fluid shear promote leukocyte adhesion through selectins (CD62L,P,E) J Cell Biol. 1997;136:717–727. doi: 10.1083/jcb.136.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Lawrence MB, Dong C. Influence of cell deformation on leukocyte rolling adhesion in shear flow. J Biomech Eng. 1999;121:636–643. doi: 10.1115/1.2800866. [DOI] [PubMed] [Google Scholar]
- Leppänen A, Mehta P, Ouyang YB, Ju T, Helin J, et al. A novel glycosulfopeptide binds to P-selectin and inhibits leukocyte adhesion to P-selectin. J Biol Chem. 1999;274:24838–24848. doi: 10.1074/jbc.274.35.24838. [DOI] [PubMed] [Google Scholar]
- Leppänen A, White SP, Helin J, McEver RP, Cummings RD. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J Biol Chem. 2000;275:39569–39578. doi: 10.1074/jbc.M005005200. [DOI] [PubMed] [Google Scholar]
- Leppänen A, Yago T, Otto VI, McEver RP, Cummings RD. Model glycosulfopeptides from P-selectin glycoprotein ligand-1 require tyrosine sulfation and a core 2-branched O-glycan to bind to L-selectin. J Biol Chem. 2003;278:26391–26400. doi: 10.1074/jbc.M303551200. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Li F, Erickson HP, James JA, Moore KL, Cummings RD, McEver RP. Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J Biol Chem. 1996;271:6342–6348. doi: 10.1074/jbc.271.11.6342. [DOI] [PubMed] [Google Scholar]
- Long M, Zhao H, Huang KS, Zhu C. Kinetic measurements of cell surface E-selectin/carbohydrate ligand interactions. Ann Biomed Eng. 2001;29:935–946. doi: 10.1114/1.1415529. [DOI] [PubMed] [Google Scholar]
- Lou J, Yago T, Klopocki AG, Mehta P, Chen W, et al. Flow-enhanced adhesion regulated by a selectin interdomain hinge. J Cell Biol. 2006;174:1107–1117. doi: 10.1083/jcb.200606056. Showed that the hinge between the lectin and EGF domains of L-selectin regulates force-dependent bond lifetimes, the shear threshold for tethering and rolling, and the agglutination of L-selectin-bearing microspheres with neutrophils in a flow field. Tested the sliding-rebinding mechanism for catch bonds using these data.
- Lou J, Zhu C. A structure-based sliding-rebinding mechanism for catch bonds. Biophys J. 2007;92:1471–1485. doi: 10.1529/biophysj.106.097048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou J, Zhu C. Flow induces loop-to-beta-hairpin transition on the beta-switch of platelet glycoprotein Ib alpha. Proc Natl Acad Sci U S A. 2008;105:13847–13852. doi: 10.1073/pnas.0801965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007a;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo SZ, Mo X, Afshar-Kharghan V, Srinivasan S, Lopez JA, Li R. Glycoprotein Ibalpha forms disulfide bonds with 2 glycoprotein Ibbeta subunits in the resting platelet. Blood. 2007b;109:603–609. doi: 10.1182/blood-2006-05-024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinin NL, Zhang L, Choi J, Ciocea A, Razorenova O, et al. A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat Med. 2009;15:313–318. doi: 10.1038/nm.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190–193. doi: 10.1038/nature01605. First demonstration of catch bonds between an adhesion receptor and its ligand (P-selectin and PSGL-1).
- Mazzucato M, Pradella P, Cozzi MR, De Marco L, Ruggeri ZM. Sequential cytoplasmic calcium signals in a 2-stage platelet activation process induced by the glycoprotein Ibalpha mechanoreceptor. Blood. 2002;100:2793–2800. doi: 10.1182/blood-2002-02-0514. [DOI] [PubMed] [Google Scholar]
- McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- McEver RP, Moore KL, Cummings RD. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. 1995;270:11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- Mehta P, Cummings RD, McEver RP. Affinity and kinetic analysis of P-selectin binding to P-selectin glycoprotein ligand-1. J Biol Chem. 1998;273:32506–32513. doi: 10.1074/jbc.273.49.32506. [DOI] [PubMed] [Google Scholar]
- Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104:1533–1537. doi: 10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- Miller J, Knorr R, Ferrone M, Houdei R, Carron CP, Dustin ML. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. J Exp Med. 1995;182:1231–1241. doi: 10.1084/jem.182.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Xia L, Yago T, Kappelmayer J, Liu Z, et al. Separable requirements for cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling under flow. Blood. 2008;112:2035–2045. doi: 10.1182/blood-2008-04-149468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitoma J, Bao X, Petryanik B, Schaerli P, Gauguet JM, et al. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat Immunol. 2007;8:409–418. doi: 10.1038/ni1442. [DOI] [PubMed] [Google Scholar]
- Modderman PW, Admiraal LG, Sonnenberg A, von dem Borne AE. Glycoproteins V and Ib-IX form a noncovalent complex in the platelet membrane. J Biol Chem. 1992;267:364–369. [PubMed] [Google Scholar]
- Moore KL, Patel KD, Bruehl RE, Fugang L, Johnson DA, et al. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, et al. Kindlin-3 is required for [beta]2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med. 2009;15:300–305. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- Nicholson MW, Barclay AN, Singer MS, Rosen SD, Van der Merwe PA. Affinity and kinetic analysis of L-selectin (CD62L) binding to glycosylation-dependent cell-adhesion molecule-1. J Biol Chem. 1998;273:763–770. doi: 10.1074/jbc.273.2.763. [DOI] [PubMed] [Google Scholar]
- Nimrichter L, Burdick MM, Aoki K, Laroy W, Fierro MA, et al. E-selectin receptors on human leukocytes. Blood. 2008;112:3744–3752. doi: 10.1182/blood-2008-04-149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KD, Nollert MU, McEver RP. P-selectin must extend a sufficient length from the plasma membrane to mediate rolling of neutrophils. J Cell Biol. 1995;131:1893–1902. doi: 10.1083/jcb.131.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, LaRoche SM. Activation of human neutrophils induces an interaction between the integrin beta 2-subunit (CD18) and the actin binding protein alpha-actinin. J Immunol. 1993;151:3795–3807. [PubMed] [Google Scholar]
- Pavalko FM, Walker DM, Graham L, Goheen M, Doerschuk CM, Kansas GS. The cytoplasmic domain of L-selectin interacts with cytoskeletal proteins via α-actinin: Receptor positioning in microvilli does not require interaction with α-actinin. J Cell Biol. 1995;129:1155–1164. doi: 10.1083/jcb.129.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan UT, Waldron TT, Springer TA. Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nat Immunol. 2006;7:883–889. doi: 10.1038/ni1366. Showed that substituting residues at the lectin-EGF domain interface influences rolling and alters the force-free affinity of P-selectin for PSGL-1.
- Piper JW. Ph.D. dissertation. Atlanta: School of Mechanical Engineering, Georgia Institute of Technology; 1997. Force dependence of cell bound E-selectin/carbohydrate ligand binding characteristics. [Google Scholar]
- Puklin-Faucher E, Gao M, Schulten K, Vogel V. How the headpiece hinge angle is opened: New insights into the dynamics of integrin activation. J Cell Biol. 2006;175:349–360. doi: 10.1083/jcb.200602071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puklin-Faucher E, Vogel V. Integrin activation dynamics between the RGD-binding site and the headpiece hinge. J Biol Chem. 2009 doi: 10.1074/jbc.M109.041194. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu A, Leahy DJ. The role of the divalent cation in the structure of the I domain from the CD11a/CD18 integrin. Structure. 1996;4:931–942. doi: 10.1016/s0969-2126(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Nollert MU, Qiu H, Liu W-j, Cummings RD, et al. Tyrosine replacement in P-selectin glycoprotein ligand-1 affects distinct kinetic and mechanical properties of bonds with P- and L-selectin. Proc Natl Acad Sci U S A. 1999;96:13771–13776. doi: 10.1073/pnas.96.24.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V, Williams M, Yago T, Schmidtke DW, McEver RP. Dynamic alterations of membrane tethers stabilize leukocyte rolling on P-selectin. Proc Natl Acad Sci U S A. 2004;101:13519–13524. doi: 10.1073/pnas.0403608101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V, Yago T, Epperson TK, Kobzdej MMA, Nollert MU, et al. Dimerization of a selectin and its ligand stabilizes cell rolling and enhances tether strength in shear flow. Proc Natl Acad Sci USA. 2001;98:10166–10171. doi: 10.1073/pnas.171248098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnikov BI, Partridge AW, Ginsberg MH. Integrin activation by talin. J Thromb Haemost. 2005;3:1783–1790. doi: 10.1111/j.1538-7836.2005.01362.x. [DOI] [PubMed] [Google Scholar]
- Rivera-Nieves J, Burcin TL, Olson TS, Morris MA, McDuffie M, et al. Critical role of endothelial P-selectin glycoprotein ligand 1 in chronic murine ileitis. J Exp Med. 2006;203:907–917. doi: 10.1084/jem.20052530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo GM, Dong JF, Schade AJ, Gardiner EE, Kansas GS, et al. The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. J Exp Med. 1999;190:803–813. doi: 10.1084/jem.190.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Rossy J, Schlicht D, Engelhardt B, Niggli V. Flotillins interact with PSGL-1 in neutrophils and, upon stimulation, rapidly organize into membrane domains subsequently accumulating in the uropod. PLoS ONE. 2009;4:e5403. doi: 10.1371/journal.pone.0005403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- Sadler JE. New concepts in von Willebrand disease. Annu Rev Med. 2005;56:173–191. doi: 10.1146/annurev.med.56.082103.104713. [DOI] [PubMed] [Google Scholar]
- Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4:2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- Salas A, Shimaoka M, Chen S, Carman CV, Springer T. Transition from rolling to firm adhesion is regulated by the conformation of the I domain of the integrin lymphocyte function-associated antigen-1. J Biol Chem. 2002;277:50255–50262. doi: 10.1074/jbc.M209822200. [DOI] [PubMed] [Google Scholar]
- Salas A, Shimaoka M, Kogan AN, Harwood C, von Andrian UH, Springer TA. Rolling adhesion through an extended conformation of integrin alphaLbeta2 and relation to alpha I and beta I-like domain interaction. Immunity. 2004;20:393–406. doi: 10.1016/s1074-7613(04)00082-2. [DOI] [PubMed] [Google Scholar]
- Sarangapani KK, Yago T, Klopocki AG, Lawrence MB, Fieger CB, et al. Low force decelerates L-selectin dissociation from P-selectin glycoprotein ligand-1 and endoglycan. J Biol Chem. 2004;279:2291–2298. doi: 10.1074/jbc.M310396200. [DOI] [PubMed] [Google Scholar]
- Savage B, Saldívar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. First experimental evidence for flow-enhanced platelet adhesion mediated by GPIbα.
- Schmidtke DW, Diamond SL. Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow. J Cell Biol. 2000;149:719–729. doi: 10.1083/jcb.149.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrador JM, Urzainqui A, Alonso-Lebrero JL, Cabrero JR, Montoya MC, et al. A juxta-membrane amino acid sequence of P-selectin glycoprotein ligand-1 is involved in moesin binding and ezrin/radixin/moesin-directed targeting at the trailing edge of migrating lymphocytes. Eur J Immunol. 2002;32:1560–1566. doi: 10.1002/1521-4141(200206)32:6<1560::AID-IMMU1560>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Setiadi H, Disdier M, Green SA, Canfield WM, McEver RP. Residues throughout the cytoplasmic domain affect the internalization efficiency of P-selectin. J Biol Chem. 1995;270:26818–26826. doi: 10.1074/jbc.270.45.26818. [DOI] [PubMed] [Google Scholar]
- Setiadi H, McEver RP. Signal-dependent distribution of cell surface P-selectin in clathrin-coated pits affects leukocyte rolling under flow. J Cell Biol. 2003;163:1385–1395. doi: 10.1083/jcb.200307178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiadi H, McEver RP. Clustering endothelial E-selectin in clathrin-coated pits and lipid rafts enhances leukocyte adhesion under flow. Blood. 2008;111:1989–1998. doi: 10.1182/blood-2007-09-113423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiadi H, Sedgewick G, Erlandsen SL, McEver RP. Interactions of the cytoplasmic domain of P-selectin with clathrin-coated pits enhance leukocyte adhesion under flow. J Cell Biol. 1998;142:859–871. doi: 10.1083/jcb.142.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamri R, Grabovsky V, Gauguet JM, Feigelson S, Manevich E, et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- Shao JY, Ting-Beall HP, Hochmuth RM. Static and dynamic lengths of neutrophil microvilli. Proc Natl Acad Sci USA. 1998;95:6797–6802. doi: 10.1073/pnas.95.12.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim K, Anderson PJ, Tuley EA, Wiswall E, Sadler JE. Platelet-VWF complexes are preferred substrates of ADAMTS13 under fluid shear stress. Blood. 2008;111:651–657. doi: 10.1182/blood-2007-05-093021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka M, Xiao T, Liu JH, Yang Y, Dong Y, et al. Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell. 2003;112:99–111. doi: 10.1016/s0092-8674(02)01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrimpton CN, Borthakur G, Larrucea S, Cruz MA, Dong JF, Lopez JA. Localization of the adhesion receptor glycoprotein Ib-IX-V complex to lipid rafts is required for platelet adhesion and activation. J Exp Med. 2002;196:1057–1066. doi: 10.1084/jem.20020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Sperandio M, Galkina EV, Ley K. Autoperfused mouse flow chamber reveals synergistic neutrophil accumulation through P-selectin and E-selectin. J Leukoc Biol. 2004;76:985–993. doi: 10.1189/jlb.1003483. [DOI] [PubMed] [Google Scholar]
- Snapp KR, Heitzig CE, Kansas GS. Attachment of the PSGL-1 cytoplasmic domain to the actin cytoskeleton is essential for leukocyte rolling on P-selectin. Blood. 2002;99:4494–4502. doi: 10.1182/blood.v99.12.4494. [DOI] [PubMed] [Google Scholar]
- Sokurenko EV, Vogel V, Thomas WE. Catch-bond mechanism of force-enhanced adhesion: counterintuitive, elusive, but … widespread? Cell Host Microbe. 2008;4:314–323. doi: 10.1016/j.chom.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000;103:467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- Song G, Yang Y, Liu JH, Casasnovas JM, Shimaoka M, et al. An atomic resolution view of ICAM recognition in a complex between the binding domains of ICAM-3 and integrin alphaLbeta2. Proc Natl Acad Sci U S A. 2005;102:3366–3371. doi: 10.1073/pnas.0500200102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio M, Smith ML, Forlow SB, Olson TS, Xia L, et al. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J Exp Med. 2003;197:1355–1363. doi: 10.1084/jem.20021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Structural basis for selectin mechanochemistry. Proc Natl Acad Sci U S A. 2009;106:91–96. doi: 10.1073/pnas.0810784105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P, Smith A, McDowall A, Nicol A, Zicha D, Hogg N. Intermediate-affinity LFA-1 binds alpha-actinin-1 to control migration at the leading edge of the T cell. EMBO J. 2008;27:62–75. doi: 10.1038/sj.emboj.7601959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. Catch bonds in adhesion. Annu Rev Biomed Eng. 2008;10:39–57. doi: 10.1146/annurev.bioeng.10.061807.160427. [DOI] [PubMed] [Google Scholar]
- Thomas WE, Trintchina E, Forero M, Vogel V, Sokurenko EV. Bacterial adhesion to target cells enhanced by shear force. Cell. 2002;109:913–923. doi: 10.1016/s0092-8674(02)00796-1. [DOI] [PubMed] [Google Scholar]
- Uchimura K, Gauguet JM, Singer MS, Tsay D, Kannagi R, et al. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat Immunol. 2005;6:1105–1113. doi: 10.1038/ni1258. [DOI] [PubMed] [Google Scholar]
- Ulrichts H, Udvardy M, Lenting PJ, Pareyn I, Vandeputte N, et al. Shielding of the A1 domain by the D'D3 domains of von Willebrand factor modulates its interaction with platelet glycoprotein Ib-IX-V. J Biol Chem. 2006;281:4699–4707. doi: 10.1074/jbc.M513314200. [DOI] [PubMed] [Google Scholar]
- Ushiyama S, Laue TM, Moore KL, Erickson HP, McEver RP. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J Biol Chem. 1993;268:15229–15237. [PubMed] [Google Scholar]
- Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol. 2008;28:403–412. doi: 10.1161/ATVBAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- Varughese KI, Ruggeri ZM, Celikel R. Platinum-induced space-group transformation in crystals of the platelet glycoprotein Ib alpha N-terminal domain. Acta Crystallogr D Biol Crystallogr. 2004;60:405–411. doi: 10.1107/S0907444903026805. [DOI] [PubMed] [Google Scholar]
- Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- Von Andrian UH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell. 1995;82:989–999. doi: 10.1016/0092-8674(95)90278-3. [DOI] [PubMed] [Google Scholar]
- Waldron TT, Springer TA. Transmission of allostery through the lectin domain in selectin-mediated cell adhesion. Proc Natl Acad Sci U S A. 2009;106:85–90. doi: 10.1073/pnas.0810620105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild MK, Huang MC, Schulze-Horsel U, van Der Merwe PA, Vestweber D. Affinity, kinetics, and thermodynamics of E-selectin binding to E-selectin ligand-1. J Biol Chem. 2001;276:31602–31612. doi: 10.1074/jbc.M104844200. [DOI] [PubMed] [Google Scholar]
- Williamson D, Pikovski I, Cranmer SL, Mangin P, Mistry N, et al. Interaction between platelet glycoprotein Ibalpha and filamin-1 is essential for glycoprotein Ib/IX receptor anchorage at high shear. J Biol Chem. 2002;277:2151–2159. doi: 10.1074/jbc.M109384200. [DOI] [PubMed] [Google Scholar]
- Woolf E, Grigorova I, Sagiv A, Grabowsky V, Feigelson S, et al. Immobilized lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8:1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- Wu T, Lin J, Cruz MA, Dong JF, Zhu C. Force-induced cleavage of single VWF A1A2A3-tridomains by ADAMTS-13. Blood. 2010 doi: 10.1182/blood-2009-03-210369. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Sperandio M, Yago T, McDaniel JM, Cummings RD, et al. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109:939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yago T, Leppänen A, Qiu H, Marcus WD, Nollert MU, et al. Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow. J Cell Biol. 2002;158:787–799. doi: 10.1083/jcb.200204041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yago T, Lou J, Wu T, Yang J, Miner JJ, et al. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest. 2008;118:3195–3207. doi: 10.1172/JCI35754. Demonstrated that catch bonds between GPIbα and VWF govern flow-enhanced platelet rolling and may prevent platelet agglutination, provided evidence for the sliding-rebinding mechanism for catch bonds, and showed that eliminating catch bonds in type 2B von Willebrand disease may cause platelet agglutination and increased VWF cleavage by ADAMTS-13.
- Yago T, Wu J, Wey CD, Klopocki AG, Zhu C, McEver RP. Catch bonds govern adhesion through L-selectin at threshold shear. J Cell Biol. 2004;166:913–923. doi: 10.1083/jcb.200403144. First demonstration that catch bonds mediate flow-enhanced adhesion.
- Yago T, Zarnitsyna VI, Klopocki AG, McEver RP, Zhu C. Transport governs flow-enhanced cell tethering through L-selectin at threshold shear. Biophys J. 2007;92:330–342. doi: 10.1529/biophysj.106.090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovenko O, Sharma S, Forero M, Tchesnokova V, Aprikian P, et al. FimH forms catch bonds that are enhanced by mechanical force due to allosteric regulation. J Biol Chem. 2008;283:11596–11605. doi: 10.1074/jbc.M707815200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Jun CD, Liu JH, Zhang R, Joachimiak A, et al. Structural basis for dimerization of ICAM-1 on the cell surface. Mol Cell. 2004;14:269–276. doi: 10.1016/s1097-2765(04)00204-7. [DOI] [PubMed] [Google Scholar]
- Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, et al. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension β1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Kulkarni S, Ulsemer P, Cranmer SL, Yap CL, et al. The von Willebrand factor-glycoprotein Ib/V/IX interaction induces actin polymerization and cytoskeletal reorganization in rolling platelets and glycoprotein Ib/V/IX-transfected cells. J Biol Chem. 1999;274:36241–36251. doi: 10.1074/jbc.274.51.36241. [DOI] [PubMed] [Google Scholar]
- Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA, Ley K. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J Exp Med. 2008;205:2339–2347. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced αLβ2 integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26:773–783. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Casasnovas JM, Jin M, Liu JH, Gahmberg CG, et al. An unusual allosteric mobility of the C-terminal helix of a high-affinity alphaL integrin I domain variant bound to ICAM-5. Mol Cell. 2008;31:432–437. doi: 10.1016/j.molcel.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Halvorsen K, Zhang CZ, Wong WP, Springer TA. Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science. 2009;324:1330–1334. doi: 10.1126/science.1170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Lou J, McEver RP. Catch bonds: physical models, structural bases, biological function and rheological relevance. Biorheology. 2005;42:443–462. [PubMed] [Google Scholar]
- Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell. 2008;32:849–861. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]