Abstract
Fourteen vertebrate species (10 mammals and 4 birds) were assessed for their ability to transmit Anaplasma phagocytophilum, the bacterium that causes human granulocytic anaplasmosis, to uninfected feeding ixodid ticks. Small mammals were most likely to infect ticks but all species assessed were capable of transmitting the bacterium, in contrast to previous findings.
Keywords: Anaplasma phagocytophilum, bacteria, reservoir competence, vertebrate hosts, bacteria, infectivity, prevalence, tick-borne diseases, ticks, zoonoses
Human granulocytic anaplasmosis (HGA), formerly known as human granulocytic ehrlichiosis, is an emerging infectious disease in the United States, Europe, and Asia (1,2). In the United States, most reported cases are concentrated in north-central and northeastern states. Patients with HGA typically have nonspecific febrile symptoms, including fever, chills, headache, and myalgia (1). Most patients with HGA respond well to antimicrobial drug treatment, but complications are not uncommon and some cases are fatal (2). Because of difficulties in diagnosis and lack of awareness of HGA by physicians and the public, many cases are misdiagnosed, and national statistics likely dramatically underreport this disease (1).
HGA is caused by a rickettsial bacterium, Anaplasma phagocytophilum (1), groups of which form dense aggregations in granulocytes (3). The bacterium is passed from host to host through the bite of an infected ixodid tick: Ixodes scapularis in the eastern and central United States and Ix. pacificus in the western United States (4–6). Serosurveys and molecular diagnostics within disease-endemic zones show that many ground-dwelling vertebrate species are exposed to or infected with A. phagocytophilum (2). These data indicate that tick-to-host transmission rates are high and that infection is widespread in host communities.
However, few studies have examined rates of transmission from infected hosts to uninfected ticks, a trait known as the reservoir competence of these hosts. Quantification of host species–specific reservoir competence can identify animals most responsible for producing infected ticks and therefore increasing risk for human exposure. Overall, robust quantitative information on reservoir competence is scarce and key hosts remain unstudied. We determined the reservoir competence for A. phagocytophilum of 14 species (10 mammals and 4 birds) in a disease-endemic region of the eastern United States.
The Study
All procedures were conducted after approval from the Cary Institute of Ecosystem Studies Institutional Animal Care and Use Committee. We conducted our research in Dutchess County, New York, a region where human cases of anaplasmosis are rapidly increasing. We trapped hosts on the property of the Cary Institute of Ecosystem Studies (Millbrook, NY, USA) during the peak abundance of larval blacklegged ticks (Ix. scapularis) during July–September in 2008, 2009, and 2010. Detailed methods have been reported (7).
We held members of 10 mammal and 4 bird species (Table 1) for 3 days in cages with wire mesh floors suspended over pans lined with wet paper towels. Ticks feeding on hosts were allowed to feed to repletion and drop from hosts into the pans, from which they were collected. In some cases, if hosts did not drop >10 ticks within 3 days, we infested them with unfed larval ticks following methods described (8). Because no evidence has been found for transovarial transmission of A. phagocytophilum (9) or of infection in larval ticks, these infestations likely did not affect host exposure to the pathogen. Hosts that had been infested were held for an additional 4 days, and engorged ticks were collected each day. All engorged larval ticks were held in moistened glass vials at constant temperature and humidity until they molted into the nymphal stage. Newly molted nymphs were flash-frozen in liquid nitrogen and stored at −80°C.
Table 1. Host species tested for Anaplasma phagocytophiluum reservoir competence, southeastern New York, USA, 2008–2010*.
| Host species | Common name | No. hosts tested | No. ticks tested | Mean no. ticks sampled per host (range) |
|---|---|---|---|---|
| Mammals | ||||
| Blarina brevicauda | Northern short-tailed shrew | 28 | 529 | 18.9 (11–25) |
| Didelphis virginiana | Virginia opossum | 25 | 501 | 20.0 (11–25) |
| Glaucomys volans | Southern flying squirrel | 4 | 59 | 14.8 (6–25) |
| Mephitis mephitis | Striped skunk | 1 | 21 | 21.0 (21–21) |
| Peromyscus leucopus | White-footed mouse | 30 | 571 | 19.0 (10–25) |
| Procyon lotor | Raccoon | 25 | 484 | 19.4 (10–25) |
| Sciurus carolinensis | Eastern gray squirrel | 20 | 358 | 17.9 (10–25) |
| Sorex cinereus | Masked shrew | 6 | 41 | 6.8 (4–10) |
| Tamias striatus | Eastern chipmunk | 19 | 300 | 15.8 (9–25) |
| Tamiasciurus hudsonicus | Eastern red squirrel | 15 | 297 | 19.8 (11–25) |
| Birds | ||||
| Catharus fuscescens | Veery | 21 | 427 | 20.3 (10–25) |
| Dumetella carolinensis | Gray catbird | 14 | 235 | 16.8 (9–24) |
| Hylocichla mustelina | Wood thrush | 28 | 496 | 17.7 (10–24) |
| Turdus migratorius | American robin | 18 | 321 | 17.8 (8–24) |
*Number of ticks tested per host can include samples from either natural body loads or experimental infestations, as described in the text, and is not representative of mean total body loads.
DNA extraction was conducted as described (7). To amplify extracted DNA, we used protocols reported by Courtney et al. (10). Briefly, we used primers ApMSP2f and ApMSP2r and probe ApMSP2p, which are specific for the msp2 gene of A. phagocytophilum and generate a 77-bp fragment. Real-time PCR was performed by using a CFX96 Real-Time PCR System (Bio-Rad, Hercules, CA, USA) . We used extracted DNA from unfed larval ticks and ultrapure water as negative controls to account for potential contamination during the extraction and PCR processes, respectively. The cloned 77-bp fragment was used as a positive control. Barrier pipette tips were used throughout the process to prevent contamination. We conducted 3 replicate PCRs per tick.
Ticks were considered positive for A. phagocytophilum if any 1 of 3 replicate samples showed amplified DNA for A. phagocytophilum relative to negative controls. Ticks with marginal results (i.e., moderate fluorescence) were tested a second time with the same primers and SYBR green dye. For these confirmatory tests, we included a melt curve analysis in which we determined the temperature at which half of the PCR products had denatured. PCR products were heated from 70°C through 85°C, raising the temperature by 0.5°C every 10 s. Positive controls consistently had melting point maxima of 80.5°C. Using a TOPO-TA Cloning Kit (Invitrogen, Carlsbad, CA, USA), we cloned and sequenced 140 fragments that had a melting point of 80.5°C. Identity of sequences was confirmed by conducting BLAST searches (National Center for Biotechnology Information, Bethesda, MD, USA) of GenBank using the blastn algorithm (11). One hundred thirty-one of 140 fragments were identified as A. phagocytophilum; the remaining 9 fragments either had poor-quality sequences or did not have the cloning vector inserted. If any replicate was positive in the confirmatory test, ticks were considered positive for A. phagocytophilum. If all 3 replicates in the confirmatory test showed marginal or negative results, the ticks were considered negative. Reservoir competence for each host species was calculated as the average percentage of ticks infected per individual host.
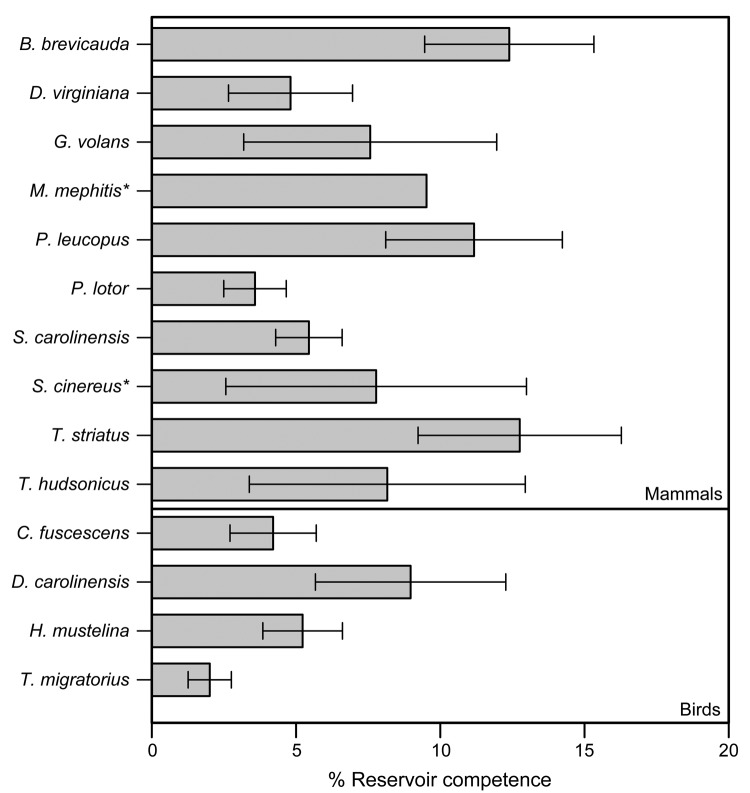
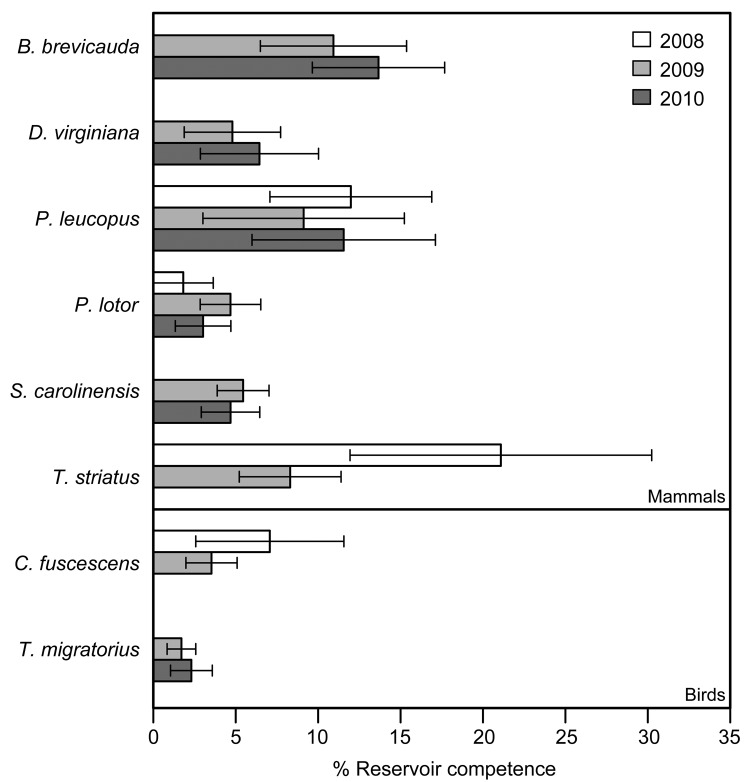
Using data for 4,640 ticks collected from 254 animals over 3 years, we assessed levels of reservoir competence for 14 host species (10 mammals and 4 birds) (Table 1). Short-tailed shrews, white-footed mice, and eastern chipmunks had mean levels of reservoir competence >10% (Figure 1). All other hosts, including opossums, gray and red squirrels, and all 4 species of birds, had mean levels of reservoir competence ranging from 2% to 10%. Reservoir competence differed significantly among these 11 species (F = 2.294, df = 10,232, p = 0.014, by 2-way analysis of variance). Southern flying squirrels, striped skunks, and masked shrews all transmitted A. phagocytophilum to ticks, but our sample sizes were too small to draw strong conclusions about reservoir competence. For species that we collected in abundant numbers in multiple years (>4 animals in >2 years), reservoir competence of each species did not vary significantly from year to year (p>0.10 for all species tested, by analysis of variance or Kruskal-Wallis tests as appropriate) (Figure 2).
Figure 1.
Mean reservoir competence of 14 host species (10 mammals and 4 birds) for Anaplasma phagocytophilum, southeastern New York, USA, 2008–2010. Error bars indicate SE. Reservoir competence is defined as the mean percentage of ticks infected by any individual host of a given species. Host species with <10 individual hosts sampled are indicated by an asterisk. See Table 1 for sample sizes. Single-letter abbreviations for genera along the left indicate Blarina, Didelphis, Glaucomys, Mephitis, Peromyscus, Procyon, Sciurus, Sorex, Tamias, Tamiasciurus, Catharus, Dumetella, Hylocichla, and Turdus, respectively.
Figure 2.
Mean reservoir competence of 14 host species (10 mammals and 4 birds) for Anaplasma phagocytophilum, southeastern New York, USA, 2008–2010. Error bars indicate SE. Reservoir competence is defined as the mean percentage of ticks infected by any individual host of a given species. For inclusion, sample sizes for a species had to be >4 in >2 years. No species showed significant variation in reservoir competence across years (p>0.10, by 2-way analysis of variance or Kruskal-Wallis test as appropriate, for all species tested). Single-letter abbreviations for genera along the left indicate Blarina, Didelphis, Peromyscus, Procyon, Sciurus, Tamias, Catharus, and Turdus, respectively.
Conclusions
Our data contradict several assumptions about the role of hosts in infecting ticks with A. phagocytophilum. First, the role of the white-footed mouse in infecting ticks has been controversial (2). Our data suggest that although the mouse is a major reservoir, short-tailed shrews and eastern chipmunks have comparable levels of reservoir competence. In addition, previous work has suggested that chipmunks, skunks, and opossums do not infect feeding ticks (12). At our sites, all of these species infected feeding ticks (Table 2). Thus, the potential for these hosts to contribute to human risk for HGA should not be ignored.
Table 2. Host species infected with Anaplasma phagocytophilum southeastern New York, USA, 2008–2010* .
| Host species | No. hosts infected/no. tested (%) | No. (%) ticks infected | Mean % infected ticks per infected host (range) |
|---|---|---|---|
| Mammals | |||
| Blarina brevicauda | 17/28 (61) | 67 (13) | 20 (4–56) |
| Didelphis virginiana | 9/25 (36) | 20 (4) | 13 (4–50) |
| Glaucomys volans† | 2/4 (50) | 5 (8) | 15 (14–16) |
| Mephitis mephitis† | 1/1 (100) | 2 (10) | 10 |
| Peromyscus leucopus | 15/30 (50) | 63 (11) | 22 (4–50) |
| Procyon lotor | 10/25 (40) | 17 (4) | 9 (4–20) |
| Sciurus carolinensis | 14/20 (70) | 19 (5) | 8 (4–20) |
| Sorex cinereus† | 2/6 (33) | 4 (10) | 23 (17–30) |
| Tamias striatus | 10/19 (53) | 40 (13) | 24 (6–46) |
| Tamiasciurus hudsonicus | 7/15 (47) | 17 (6) | 17 (4–73) |
| Birds | |||
| Catharus fuscescens | 9/21 (43) | 19 (4) | 10 (4–25) |
| Dumetella carolinensis | 7/14 (50) | 20 (9) | 18 (4–33) |
| Hylocichla mustelina | 14/28 (50) | 27 (5) | 10 (4–25) |
| Turdus migratorius | 6/18 (33) | 7 (2) | 6 (4–11) |
*Infected hosts are those that transmitted A. phagocytophilum to >1 Ixodes scapularis tick larva. †Host species with <10 individual hosts sampled.
Because hosts are capable of clearing A. phagocytophilum infections (13), surveys of host exposure might not represent species-specific probabilities of transmitting the pathogen to uninfected ticks. Instead, the role of particular species in contributing to the pool of infected ticks is best assessed by determining host reservoir competence using field-captured animals that usually carry ticks. On the basis of the community of hosts we sampled, small mammals are most responsible for infecting uninfected larval ticks in nature, and this result is consistent across years.
Acknowledgments
We thank Mitch Le Sage, Kelly Oggenfuss, Laura Cheney, Micah Strauss, and the 2008–2011 research assistants for laboratory and field assistance.
This study was supported by National Sciences Foundation Emerging Infectious Diseases grant 0813041.
Biography
Dr Keesing is an educator and scientist in the Program in Biology at Bard College in Annandale, NY, and an adjunct scientist at the Cary Institute of Ecosystem Studies in Millbrook, NY. Her major research interest is the ecology of infectious diseases.
Footnotes
Suggested citation for this article: Keesing F, Hersh MH, Tibbetts M, McHenry DJ, Duerr S, Brunner J, et al. Reservoir competence of vertebrate hosts for Anaplasma phagocytophilum. Emerg Infect Dis [Internet]. 2012 Dec [date cited]. http://dx.doi.org/10.3201/eid1812.120919
References
- 1.Demma LJ, Holman RC, McQuiston JH, Krebs JW, Swerdlow DL. Epidemiology of human ehrlichiosis and anaplasmosis in the United States, 2001–2002. Am J Trop Med Hyg. 2005;73:400–9. [PubMed] [Google Scholar]
- 2.Jin H, Feng W, Qian J. Epidemiology and control of human granulocytic anaplasmosis: a systematic review. Vector Borne Zoonotic Dis. 2012;12:269–74. 10.1089/vbz.2011.0753 [DOI] [PubMed] [Google Scholar]
- 3.Dumler JS, Choi K, Garcia-Garcia J, Barat N, Scorpio D, Garyu J, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828–34. 10.3201/eid1112.050898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley JE, Foley P, Brown RN, Lane RS, Dumler JS, Madigan JE. Ecology of granulocytic ehrlichiosis and Lyme disease in the western United States. J Vector Ecol. 2004;29:41–50. [PubMed] [Google Scholar]
- 5.Hodzic E, Fish D, Maretzki CM, De Silva AM, Feng S, Barthold SW. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J Clin Microbiol. 1998;36:3574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer VL, Randolph MP, Hui LT, Irwin WE, Gutierrez AG, Vugia DJ. Detection of the agents of human ehrlichioses in ixodid ticks from California. Am J Trop Med Hyg. 1999;60:62–5. [DOI] [PubMed] [Google Scholar]
- 7.Hersh MH, Tibbetts M, Strauss M, Ostfeld RS, Keesing F. Reservoir competence of wildlife host species for Babesia microti. 2012;18:1951–7. 10.3201/eid1812.111392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keesing F, Brunner J, Duerr S, Killilea M, LoGiudice K, Schmidt K, et al. Hosts as ecological traps for the vector of Lyme disease. Proc Biol Sci. 2009;276:3911–9. 10.1098/rspb.2009.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunning Hotopp JC, Lin M, Madupu R, Crabtree J, Angiuoli SV, Eisen J, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2:e21. 10.1371/journal.pgen.0020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courtney JW, Kostelnik LM, Zeidner NS, Massung RF. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol. 2004;42:3164–8. 10.1128/JCM.42.7.3164-3168.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. [DOI] [PubMed] [Google Scholar]
- 12.Levin ML, Nicholson WL, Massung RF, Sumner JW, Fish D. Comparision of the reservoir competence of medium-sized mammals and Peromyscus leucopus for Anaplasma phagocytophilum in Connecticut. Vector Borne Zoonotic Dis. 2002;2:125–36. 10.1089/15303660260613693 [DOI] [PubMed] [Google Scholar]
- 13.Levin ML, Fish D. Immunity reduces reservoir host competence of Peromyscus leucopus for Ehrlichia phagocytophila. Infect Immun. 2000;68:1514–8. 10.1128/IAI.68.3.1514-1518.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]