Abstract
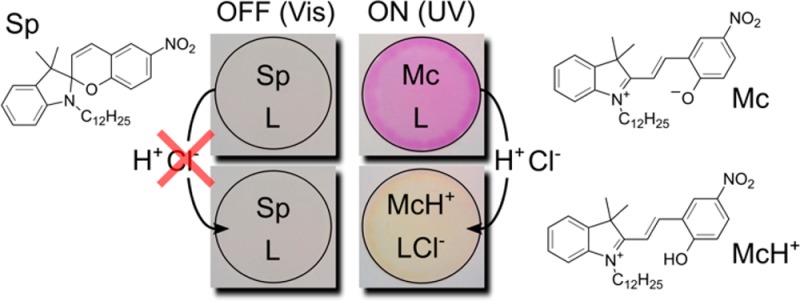
We report here for the first time on a reversible photodynamic bulk optode sensor based on the photoswitching of a spiropyran derivative (Sp). The photoswitching of Sp induces a large basicity increase in the polymeric phase, which triggers the extraction of Cl– and H+. Cl– is stabilized by a lipophilic chloride-selective ionophore inside the membrane, while H+ binds with the open form of Sp and induces a spectral change, hence providing the sensor signal. The system was studied with spectroscopic and electrochemical methods.
Modern optical ion sensors that benefit from commercially available ionophores within a polymeric system such as plasticized poly(vinyl chloride) (PVC) have been introduced and applied for over two decades.1−4 Such sensors often use a lipophilic pH indicator (also called chromoionophore) to monitor the level of hydrogen ion which functions as a reference ion. Most of these sensors work on the basis of a competitive ion-exchange or an electrolyte co-extraction equilibrium between the hydrophobic sensing phase and the contacting aqueous phase. Consequently, they work only in a passive measurement mode. Optical sensors that work in an active mode, that is, so that they can be switched on or off at defined times, would allow one to modulate the sensor signal and, consequently, correct for a nonmodulated background signal. Moreover, analyte exchange between sample and sensing phase may be blocked during sensor delivery or storage in the off-state, for example. Finally, in case of a fast switching sensor, the kinetics of the sensor response will reveal additional information about the speciation and total concentration of the analyte.
Recent progress in the development of active sensors includes the use of a photoacid generator that releases acid upon UV light illumination.5 However, the photolysis of the photoacid generator is an irreversible process, and thus can only be used once. Our group recently proposed a photodynamic sensing concept based on a light induced pKa change of the chromoionophore.6 We report here on a reversible photodynamic sensor that utilizes the pKa change of a spiropyran derivative (Sp) upon UV and visible light irradiation. Spiropyran has been extensively studied because of its pronounced photoswitching capability. Chelators for metal ions and amino acids based on a modified spiropyran or the copolymerization of spiropyran within hydrogels have been reported.7−9 Poly(terthiophene) membranes bearing spiropyran functionalities can be switched both photo- and electrochemically.10 However, robust photoswitchable ion sensors based on bulk optode principles have, to the best of our knowledge, not been reported.
Chloride is chosen here as a model ion for this “proof of concept” study. It is the major extracellular anion and is principally responsible for maintaining proper hydration, osmotic pressure, and a normal cation–anion balance in the vascular and intestinal fluid compartments. Chloride imbalance causes either hypochloremia or hyperchloremia.
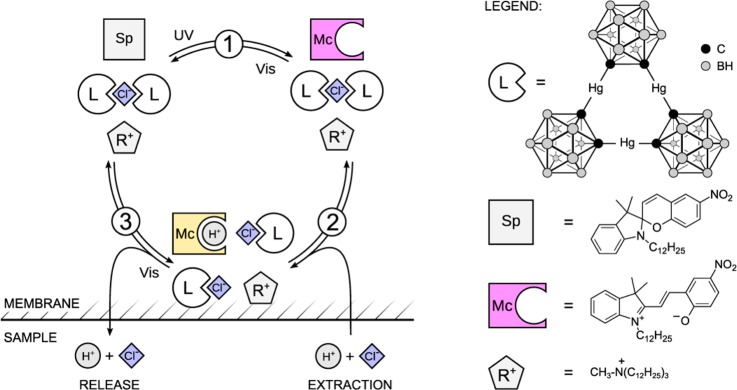
A plasticized PVC film contains a lipophilic spiropyran derivative, a chloride selective ionophore (L) and anion exchanger (R+Cl–), see Scheme 1. Under visible light, spiropyran exists in a stable ring-closed form (Sp) with very low basicity (pKa = 2.3 ± 0.1).11 When illuminated by UV light, it transforms into a strongly colored ring-opened merocyanine (Mc) form. The exposed phenolic group in this form tremendously increases the basicity of the molecule compared to the Sp form.11 This transformation is designed here to encourage the co-extraction of H+ and Cl– from the contacting aqueous solution into the sensing film where Cl– is stabilized by the ionophore and H+ will protonate Mc to form McH+. The spectral difference between Mc and McH+ helps to visualize the co-extraction process using optical techniques. Visible light will reverse the process by promoting the ring closing reaction. H+ and Cl–, being highly hydrophilic species, will leave the sensing film to the contacting aqueous phase. In addition to the switching capability of optical sensors, the light induced ion flux may also be used for local ion perturbation in biological systems such as cells.
Scheme 1. Photodynamic Sensing Mechanism and Chemical Structures of the Compounds Used in This Work.
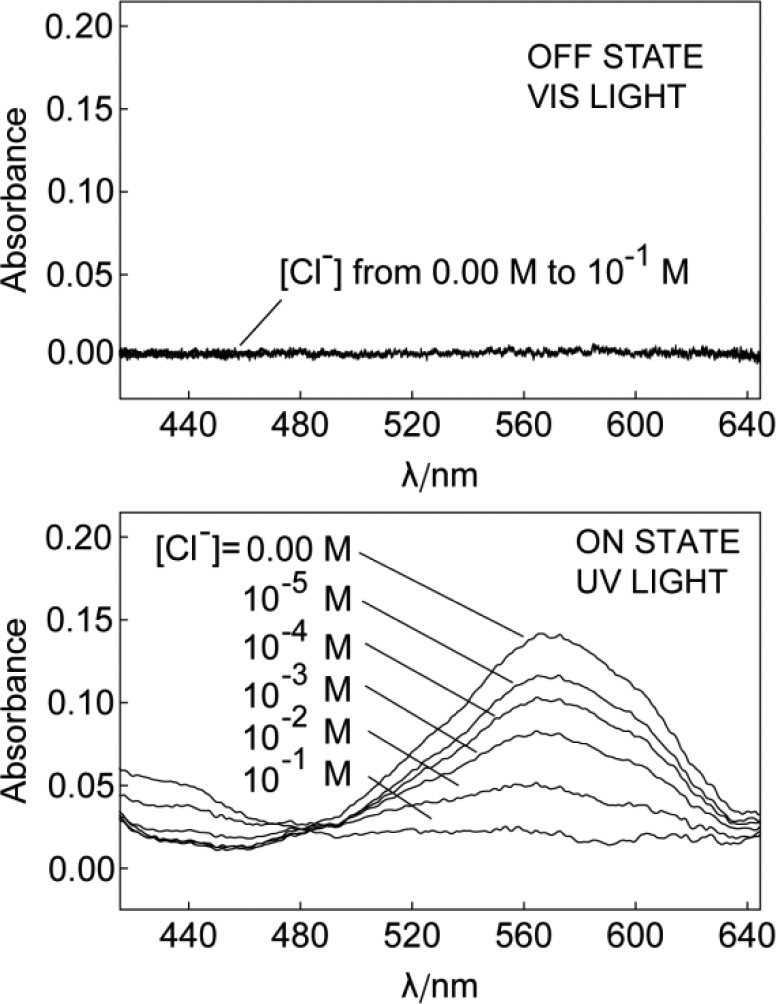
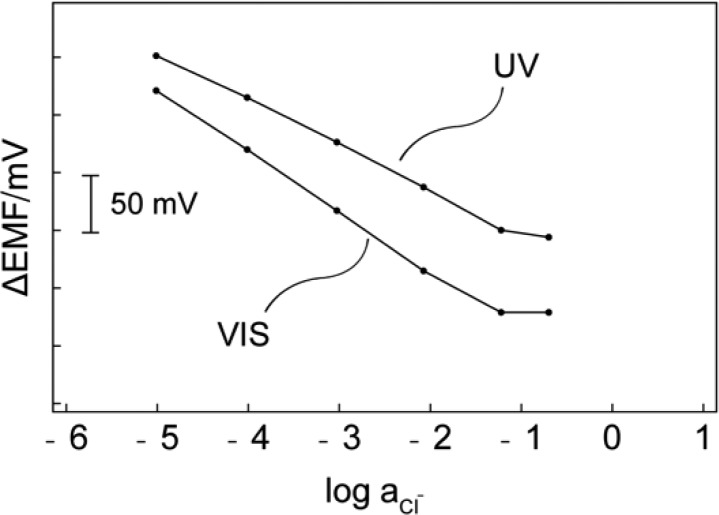
As shown in Figure 1, under visible light (>410 nm), the Sp form exhibits no absorbance throughout the visible range. Because of the small pKa value of Sp mentioned above, increasing the chloride concentration up to 0.1 M in the buffered (pH 7.4) sample is still incapable of initiating the co-extraction. The potentiometric response of the membrane under visible light further confirms the absence of co-extraction. For this purpose, membranes with the same composition as for the optical measurements were prepared by solvent casting and mounted in commercial electrode bodies. The potential of the electrode is measured in the same buffer solution with different Cl– levels against a double junction Ag/AgCl reference electrode. The introduction of an anion exchanger allows one to evaluate the system electrochemically using potentiometric methods. Without anion-exchanger R+, the potential of the electrode is not well-defined because of the lack of permselectivity. The membrane with additional R+ behaves as chloride selective membrane under visible light illumination, because the pKa of Sp is too low to cause any interference at pH 7.4. Under these conditions, one should observe a Nernstian response slope for chloride.12 Figure 2 demonstrates a slope of −53.8 mV per 10-fold concentration change of Cl– for the electrode measured under visible light. Upon UV irradiation in the absence of Cl–, the mole fraction of the Mc form increases. This change is visible by a color change from colorless to purple which is also manifested in a strong absorption band at 570 nm (Figure 1). The increase of dye basicity triggers HCl uptake and the formation of McH+ in the sensing film with increasing concentration in the sample. This process results in a color change from purple (Mc) to yellow (McH+), which can be quantified by measuring the absorbance spectra of the membrane. The co-extraction should also result in a change in potentiometric response for Cl–. Since the electrode membrane is no longer permselective, a sub-Nernstian response is expected (see Supporting Information). As shown in Figure 2, exposing the electrode membrane to UV light indeed causes the slope of the response curve to decrease. The leveling off at high concentration (>0.1 M) is explained with the co-extraction of NaCl.13
Figure 1.
Absorption spectra of the sensing membrane under illumination of visible (upper) and UV light (lower) in pH 7.4 MOPS buffer solution with different concentrations of Cl–.
Figure 2.
Potentiometric response for a membrane containing R+, L and Sp under UV and visible light illumination.
Previous work on this chloride ionophore established that it is able to form both 1:1 and 2:1 complexes with Cl–.14,15 In the initial state, that is, without co-extraction, the ionophore forms a 2:1 complex with Cl– since the amount of ligand in the membrane is 40 mmol/kg and the anion-exchanger salt R+Cl– is 20 mmol/kg. Co-extraction of Cl– and H+ forces to form a 1:1 complex (Scheme 1). The process under UV light illumination can be expressed with the following equation:
| 1 |
The response function can be expressed with the following equation according to the optode theory:16
| 2 |
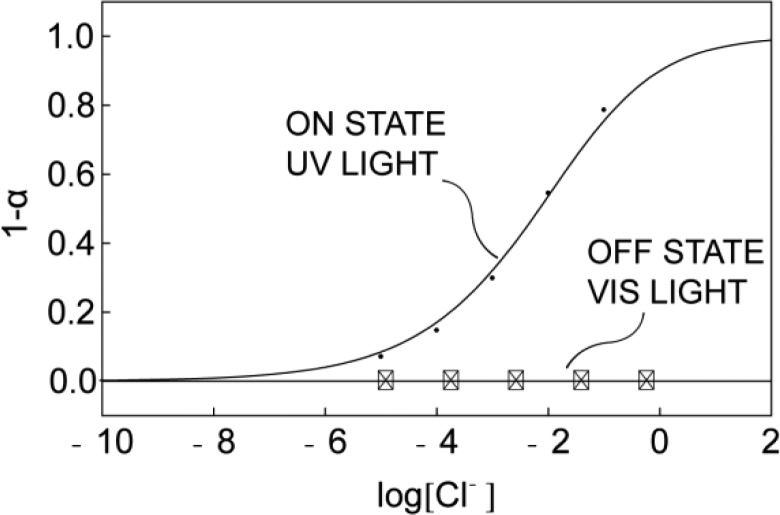
where aCl– and aH+ are the sample activities of Cl– and H+, respectively, Kcoex is the co-extraction constant for eq 1, α is the mole fraction of deprotonated Mc, which can be calculated from the absorption spectrum,1 and RT+, IndT and LT are the total concentrations of R+, Sp, and L, respectively. The theoretical response curve (Figure 3) shows satisfactory correlation with the experimental calibration data.
Figure 3.
Calibration curves for the response of the membrane to Cl– with UV and visible light illumination.
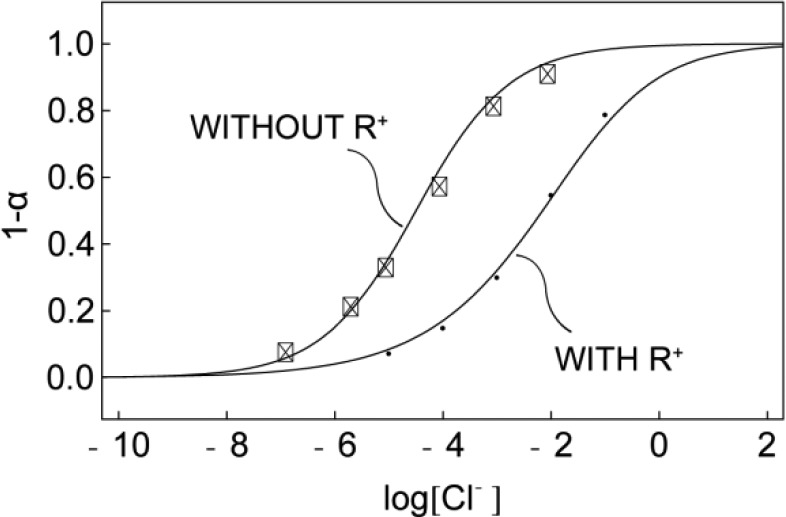
Besides its contribution to potentiometric characterization, the presence of a lipophilic anion exchanger in the membrane may seem redundant because it is not required to ensure HCl uptake. Indeed, the membrane without additional anion exchanger, shown in Figure 4, gives a response to chloride from 10–7 to 10–2 M at pH 7.4. However, the addition of R+ shifts the response window to higher concentrations of Cl– which is more physiologically relevant. This can be used to make sensors with a tunable detection range. When anion exchanger is present, a 2:1 complex dominates at low levels of Cl–. With increasing Cl– sample concentrations, the extraction forces the decomplexation of the 2:1 complex and the formation of 1:1 complex in the membrane. Since the stability constant for the 1:1 complex is smaller than that for the 2:1 complex, the presence of anion exchanger will consequently result in a shift of the response to higher Cl– concentrations .15,17
Figure 4.
Cl– response for membranes with and without anion exchanger in pH 7.4 MOPS buffer solution under UV light.
The selectivity of the photoswitchable membrane was evaluated and the selectivity coefficients for chloride against common anions are presented in Table 1. In agreement with previous reports, anions such as perchlorate, sulfate, nitrate and salicylate are highly suppressed while SCN– shows significant interference.15 This also indicates that the Mc form has no profound interaction with the incoming anions.
Table 1. Selectivity Coefficients Log KCl,JOpt at pH 7.4 for Chloride.
| ion J | log KCl,JOpt |
|---|---|
| ClO4– | –3.6 |
| NO3– | –3.8 |
| Sal– | –2.9 |
| SCN– | +0.6 |
| SO42- | –9.3 |
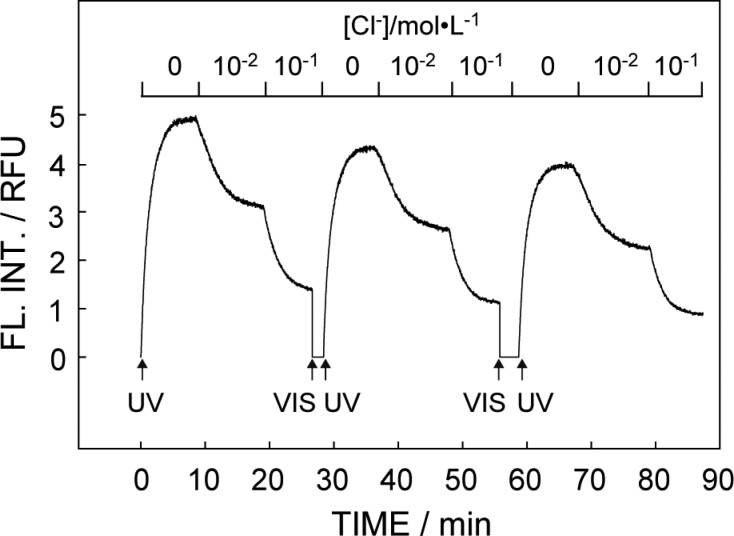
The dynamic photoactivated sensor response behavior is illustrated in Figure 5. The fluorescence intensity at 650 nm was used as signal output. When illuminated with UV, the emission intensity started to respond to different Cl– concentrations while the response time was likely limited by the diffusion controlled mass transport within the membrane. The ring-opening process upon UV irradiation is thought to follow first order reaction kinetics, in agreement with previously reported models.18,19 Afterward, visible light was introduced to deactivate the sensor and release Cl–, preparing it for the next photoactivated sensing step. After each on–off step, an emission intensity drop was observed that is ascribed to the photofatigue of spiropyran.20−22 The influence of photofatigue may be reduced by increasing the concentration of ionophore and anion exchanger in the membrane, as predicted by optode theory and confirmed experimentally (see Figure S2). Increased photo stability can also be achieved by the replacement of spiropyran with spirooxazine, or by the covalent attachment of spiropyran to a polymeric backbone.23,24 The latter reduces intermolecular interactions, which were reported to cause photobleaching of spiropyran in its Mc form.24
Figure 5.
Reversible photochromism and switching with alternative UV and visible light in pH 7.4 MOPS buffer with different Cl– concentrations.
To conclude, an active Cl–-selective optical sensor with tunable measuring range based on a photochromic spiropyran derivative was presented. Spectroscopic and electrochemical measurements confirmed the anticipated mechanism for the system. This work forms a new platform for the realization of a toolbox of dynamic optical ion sensors and selective systems for triggered, localized ion perturbation.
Acknowledgments
The authors thank the Swiss National Science Foundation (SNF) for financial support. G. Mistlberger gratefully acknowledges the support by the Austrian Science Fund (FWF): J3343.
Supporting Information Available
Experimental details, additional experimental data (fluorescence mode, kinetics of sensor deactivation), and simulation data concerning photofatigue and potentiometric response. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Bakker E.; Buhlmann P.; Pretsch E. Chem. Rev. 1997, 97, 3083. [DOI] [PubMed] [Google Scholar]
- Buhlmann P.; Pretsch E.; Bakker E. Chem. Rev. 1998, 98. [DOI] [PubMed] [Google Scholar]
- Morf W. E.; Seiler K.; Rusterholz B.; Simon W. Anal. Chem. 1990, 62, 738. [Google Scholar]
- Wygladacz K.; Radu A.; Xu C.; Qin Y.; Bakker E. Anal. Chem. 2005, 77, 4706. [DOI] [PubMed] [Google Scholar]
- Shvarev A. J. Am. Chem. Soc. 2006, 128, 7138. [DOI] [PubMed] [Google Scholar]
- Bakker E.; Crespo G.; Grygolowicz-Pawlak E.; Mistlberger G.; Pawlak M.; Xie X. Chimia 2011, 65, 141. [DOI] [PubMed] [Google Scholar]
- Byrne R.; Ventura C.; Lopez F. B.; Walther A.; Heise A.; Diamond D. Biosens. Bioelectron. 2010, 26, 1392. [DOI] [PubMed] [Google Scholar]
- Fries K. H.; Driskell J. D.; Samanta S.; Locklin J. Anal. Chem. 2010, 82, 3306. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Fan M.; Zhang S.; Sheng X.; Yao J. New J. Chem. 2007, 31, 1878. [Google Scholar]
- Wagner K.; Byrne R.; Zanoni M.; Gambhir S.; Dennany L.; Breukers R.; Higgins M.; Wagner P.; Diamond D.; Wallace G. G.; Officer D. L. J. Am. Chem. Soc. 2011, 133, 5453. [DOI] [PubMed] [Google Scholar]
- Mistlberger G.; Crespo G. A.; Xie X.; Bakker E. Chem. Commun. 2012, 48, 5662. [DOI] [PubMed] [Google Scholar]
- Bakker E.; Pretsch E. Angew. Chem., Int. Ed. 2007, 46, 5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y.; Bakker E. Anal. Chem. 2002, 74, 3134. [DOI] [PubMed] [Google Scholar]
- Badr I. H. A.; Diaz M.; Hawthorne M. F.; Bachas L. G. Anal. Chem. 1999, 71, 1371. [DOI] [PubMed] [Google Scholar]
- Ceresa A.; Qin Y.; Peper S.; Bakker E. Anal. Chem. 2003, 75, 133. [DOI] [PubMed] [Google Scholar]
- Barker S. L. R.; Shortreed M. R.; Kopelman R. Anal. Chem. 1997, 69, 990. [DOI] [PubMed] [Google Scholar]
- Xu C.; Qin Y.; Bakker E. Talanta 2004, 63, 180. [DOI] [PubMed] [Google Scholar]
- Levitus M.; Talhavini M.; Negri R. M.; Atvars T. D. Z.; Aramendia P. F. J. Phys. Chem. B 1997, 101, 7680. [Google Scholar]
- Poisson L.; Raffael K. D.; Soep B.; Mestdagh J.-M.; Buntinx G. J. Am. Chem. Soc. 2006, 128, 3169. [DOI] [PubMed] [Google Scholar]
- Tong R.; Hemmati H. D.; Langer R.; Kohane D. S. J. Am. Chem. Soc. 2012, 134, 8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen G.; Yan J.; Zhou Y.; Zhang D.; Mao L.; Zhu D. Chem. Commun. 2006, 3016. [DOI] [PubMed] [Google Scholar]
- Minkin V. I. Chem. Rev. 2004, 104, 2751. [DOI] [PubMed] [Google Scholar]
- Deniz E.; Tomasulo M.; Cusido J.; Sortino S.; Raymo F. M. Langmuir 2011, 27, 11773. [DOI] [PubMed] [Google Scholar]
- Radu A.; Byrne R.; Alhashimy N.; Fusaro M.; Scarmagnani S.; Diamond D. J. Photochem. Photobiol., A 2009, 206, 109. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.