Abstract
Reactive oxygen species (ROS) generate a type of DNA damage called tandem lesions, two adjacent nucleotides both modified. A subcategory of tandem lesions consists of adjacent nucleotides linked by a covalent bond. Covalently linked tandem lesions generate highly characteristic liquid chromotography-tandem mass spectrometry (LC-MS/MS) elution profiles. We have used this property to comprehensively survey X-irradiated DNA for covalently linked tandem lesions. A total of 15 tandem lesions were detected in DNA irradiated in deoxygenated aqueous solution, five tandem lesions were detected in DNA that was irradiated in oxygenated solution.
INTRODUCTION
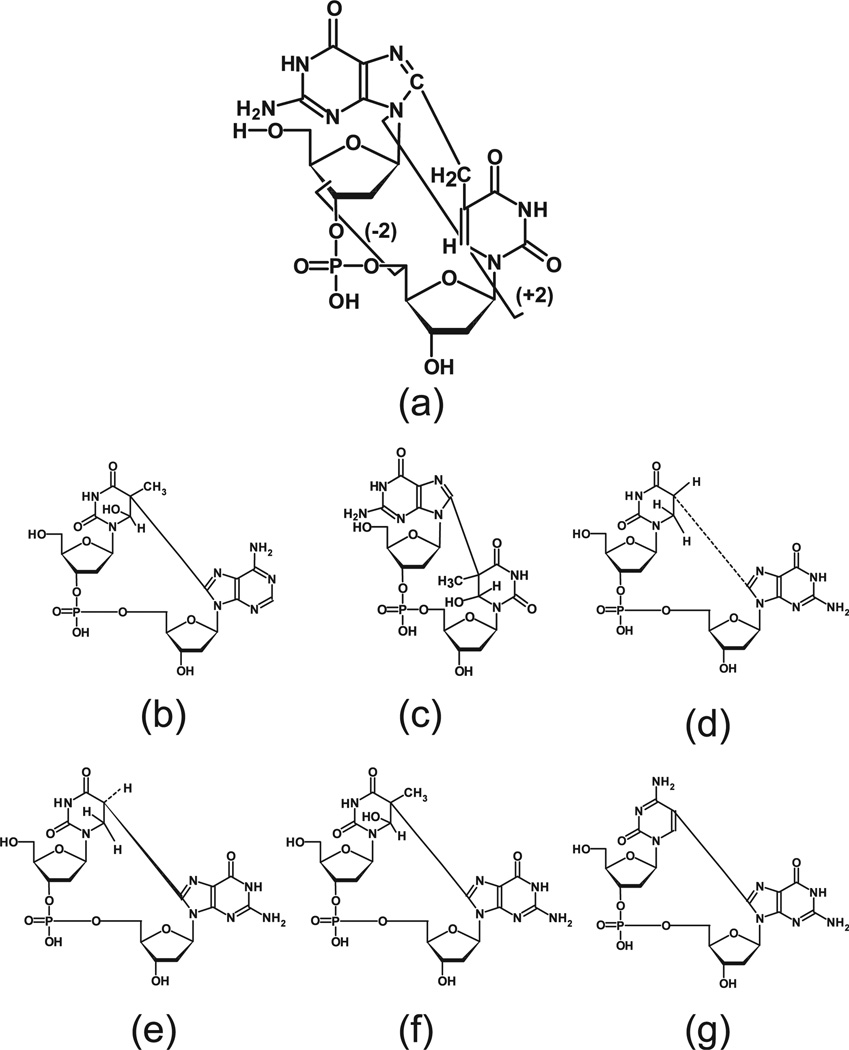
Tandem lesions are modifications of DNA in which two adjacent nucleotides on the same strand are modified. The exemplar displayed in Fig. 1a, denoted as d(G^T), has adjacent nucleotides linked by a covalent bond between C8 of guanine and the methyl carbon atom of thymine. This type of tandem lesion was initially observed in DNA oligomers X-irradiated in solution under hypoxic conditions (1). Detection of d(C^G) in X-irradiated DNA oligomers by liquid chromatography-tandem mass spectrometry (LC-MS/MS) revealed a unique fragmentation pattern of four products that elute simultaneously (2). We reasoned that analogous fragmentation would take place in tandem lesions regardless of how the adjacent bases were linked. Based on this assumption a comprehensive in vitro assessment was made of covalently linked tandem lesions in X-irradiated DNA. We observed 15 tandem lesions in DNA X-irradiated in vitro in a hypoxic environment and 5 tandem lesions in DNA X-irradiated in an oxygenated environment.
FIG. 1.
Prototype tandem lesion showing breakpoints (a). Other tandem lesions previously reported in the literature (b)–(g).
MATERIALS AND METHODS
Double-stranded calf thymus DNA was X irradiated at 480 Gy in aqueous solution. For the hypoxic study, the solution was continuously flushed by an N2 stream that had passed through an OMI gas purifier (Supelco). The experiment was repeated with the DNA solution continuously flushed with oxygen. The DNA was fractionated into aliquots of 100 µg and was enzymatically hydrolyzed as follows: 100 µg DNA was lyophilized to dryness, denatured by adding 25 µl H2O, and heated to 95°C for 5 min, then cooled in dry ice for 5 min. Enzymatic hydrolysis was accomplished by adding 25 µl ZnCl2, 3.0 mM, 7.5 µl 0.25 M sodium acetate, pH 5.2, 1.0 µl nuclease P1 equivalent to 1.0 unit, followed by incubation at 37°C for 2 h. Next, 12.5 µl Tris HCl, 1.0 M, pH 9.0, and 75 units of alkaline phosphatase from bovine intestinal mucosa were added and the solution incubated an additional 2 h at 37°C. The sample was filtered using a Spin-X HPLC micro centrifuge filter, 0.22 µm nylon, and was centrifuged at 9000g for 2 min. The filtrate was lyophilized to dryness and transferred to a MS/MS vial in 25 µl H2O. LC-MS/MS measurements were made on an AB Sciex Qtrap 5500 instrument operating as a triple quadrupole in the negative ion mode.
RESULTS
Detection of Tandem Lesions
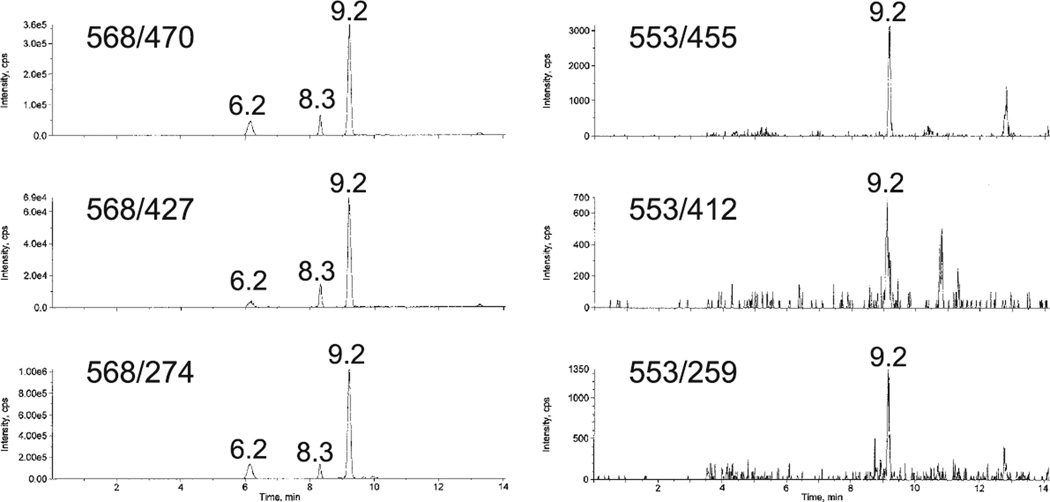
Tandem lesions linked by a covalent bond can be released from DNA by sequential treatment with nuclease P1 and alkaline phosphatase (2). For detection of d(G^T) by LC-MS/MS, the spectrometer is programmed for the MS/MS values 568/470 and 568/274 consistent with the double breakpoints shown in Fig. 1a. Two additional transitions can be observed for MS/MS values 568/427 and 568/231 resulting from a further breakdown of the primary products with the loss of 43 mass units. The four fragments, generated simultaneously from the same precursor in the Q2 collision chamber of the spectrometer, appear in their respective elution profiles at identical elution times, as shown in Fig. 2. This method of detection serves to uniquely distinguish covalently linked tandem lesions from other forms of ROS-induced DNA damage.
FIG. 2.
Examples of sets of LC-MS/MS elution profiles that demonstrate the presence of tandem lesions in X-irradiated calf thymus DNA. The left side profiles are for the tandem lesion, d(G^T), produced in DNA irradiated in the absence of oxygen. The right side profiles are for the tandem lesion produced in DNA irradiated in presence of oxygen, and having a molecular weight consistent with the structure shown in Fig. 1g.
A reasonable hypothesis is that dinucleoside mono-phosphates linked by a covalent bond will fragment analogously to d(G^T) irrespective of the chemical form of the linkage. Based on this hypothesis a comprehensive in vitro search for tandem lesions in X-irradiated calf thymus DNA was undertaken. The DNA was irradiated in deoxygenated solution. For a tandem lesion of a given precursor ion molecular weight (first MS value), the molecular weight of its four fragment ions (second MS value) can be calculated. Thus, the fragmentation generated signals for MS/MS values W/(W-98), W/(W-294), W/(W-141) and W/(W-337) where W is the molecular weight of the parent tandem ion. The first MS value was scanned in increments of one mass unit over the mass range from 510–630 Da. Twelve DNA samples were prepared. Each sample was analyzed for 10 sets of three MS/MS values (the predictably weakest transition was omitted) for a total of 360 LC-MS/MS profiles. Altogether, 15 covalently linked tandem lesions were revealed as was evidenced by 15 sets of LC-MS/MS elution profiles having three peaks exactly coincident. The prototype tandem lesion, d(G^T), serves as an example (Fig. 2). MS/MS values, elution times, and relative intensities of the largest peak in each set of profiles are tabulated in Table 1. Several of these results could have been anticipated because the ROS-induced covalently linked tandem lesions shown in Fig. 1 have already been reported in the literature (3–5). Molecular weights that were compatible with all of the tandem lesions anticipated from the literature are, in fact, observed. Structures that are compatible with the observed molecular weights are referenced in Table 1. A further observation is that if MS/MS values are calculated for the structures shown in Fig. 1, except replacing a guanine base with adenine or vice versa, these calculated values are also found in Table 1. Light-induced tandem lesions are not included in Fig.1.
TABLE 1.
MS/MS Values, Elution Times and Relative Intensity of Peaks Observed for Tandem Lesions in X-Irradiated DNA in a Hypoxic Environment
| Tandem lesion no. |
MS/MS values |
Elution time* (m) |
Relative intensity (cps) |
Compatible structure |
|---|---|---|---|---|
| 1 | 529/431, 529/388, 529/235 | 6.4 | 2,800 | |
| 2 | 531/433, 531/390, 531/237 | 7.6 | 7,300 | |
| 3 | 537/439, 537/396, 537/243 | 19.0 | 6,000 | |
| 4 | 540/442, 540/399, 540/246 | 11.2 | 4,000 | |
| 5 | 543/445, 543/402, 543/249 | 8.3 | 1,700 | |
| 6 | 544/446, 544/403, 544/250 | 8.9 | 2,500 | |
| 7 | 551/453, 551/410, 551/257 | 16.0 | 40,000 | |
| 8 | 552/454, 552/411, 552/258 | 12.8 | 100,000 | |
| 9 | 553/455, 553/412, 553/259 | 9.7 | 20,000 | Fig. 1g |
| 10 | 556/458, 556/415, 556/262 | 6.9 | 40,000 | Fig. 1d and e |
| 11 | 567/469, 567/426, 567/273 | 13.2 | 45,000 | |
| 12 | 568/470, 568/427, 568/274 | 9.2 | 360,000 | Fig. 1a |
| 13 | 570/472, 570/429, 570/276 | 8.8 | 20,000 | Fig. 1b |
| 14 | 586/488, 586/445, 486/292 | 10.9 | 12,000 | Fig. 1c and f |
| 15 | 590/492, 590/449, 590/296 | 5.6 | 4,500 |
Largest peak.
Some additional observations are of interest. The tandem lesion that served as the prototype for this study (Fig. 1a), generated the largest signal in the comprehensive assessment. This finding comports with the observation that the yield of this product is the largest of all products in d(CGTA) X irradiated in deoxygenated solution (1). Some sets of profiles evidenced additional sets of weaker signals, e.g., products eluting identically but at a different elution time from the main product (Fig. 2). Evidently, structurally different tandem lesions are produced having the same molecular weight as the main product. This finding is likely due to isomers, perhaps even sequence isomers (6).
The foregoing experiment was repeated using DNA X-irradiated in an oxygenated environment. Under this condition, 5 sets of MS/MS values revealed the formation of tandem lesions. Table 2 lists the MS/MS values, relative intensities and elution times for these tandem lesions. Two of the entries in Table 1 have the same MS/MS values as an entry in Table 1 and may well be the same product.
TABLE 2.
MS/MS Values, Elution Times and Relative Intensity of Peaks Observed for Tandem Lesions in X-Irradiated DNA in an Oxygenated Environment
| Tandem lesion no. |
MS/MS values | Elution time* | Relative intensity (cps) |
Compatible structure |
|---|---|---|---|---|
| 1 | 526/428, 526/385, 526/232 | 5.1 | 900 | |
| 2 | 553/455, 553/412, 553/259 | 9.1 | 3,150 | Fig. 1g |
| 3 | 568/470, 568/427, 568/274 | 7.8 | 1,600 | Fig. 1a |
| 4 | 601/503, 601/460, 601/307 | 10.2 | 350 | |
| 5 | 624/526, 624/483, 624/330 | 8.2 | 300 |
Largest peak.
Structures of Tandem Lesions
For some purposes it may suffice to characterize a DNA radiation product simply by its mass, chromatographic properties and as a tandem lesion formed by a covalent link between nucleotides. However, a more complete structural identification is always preferable. In this section we illustrate how a more complete structural characterization of a tandem lesion can be ascertained employing an oligomer that contains the lesion in question. For example, our prototype tandem lesion, d(G^T), was originally identified in d(CGTA) after X irradiation in deoxygenated solution (1). The product was characterized in the modified tetramer by mass spectrometry and proton NMR spectroscopy. Our task here is to demonstrate that a product obtained from irradiated DNA is identical to the identified product in d(CGTA).
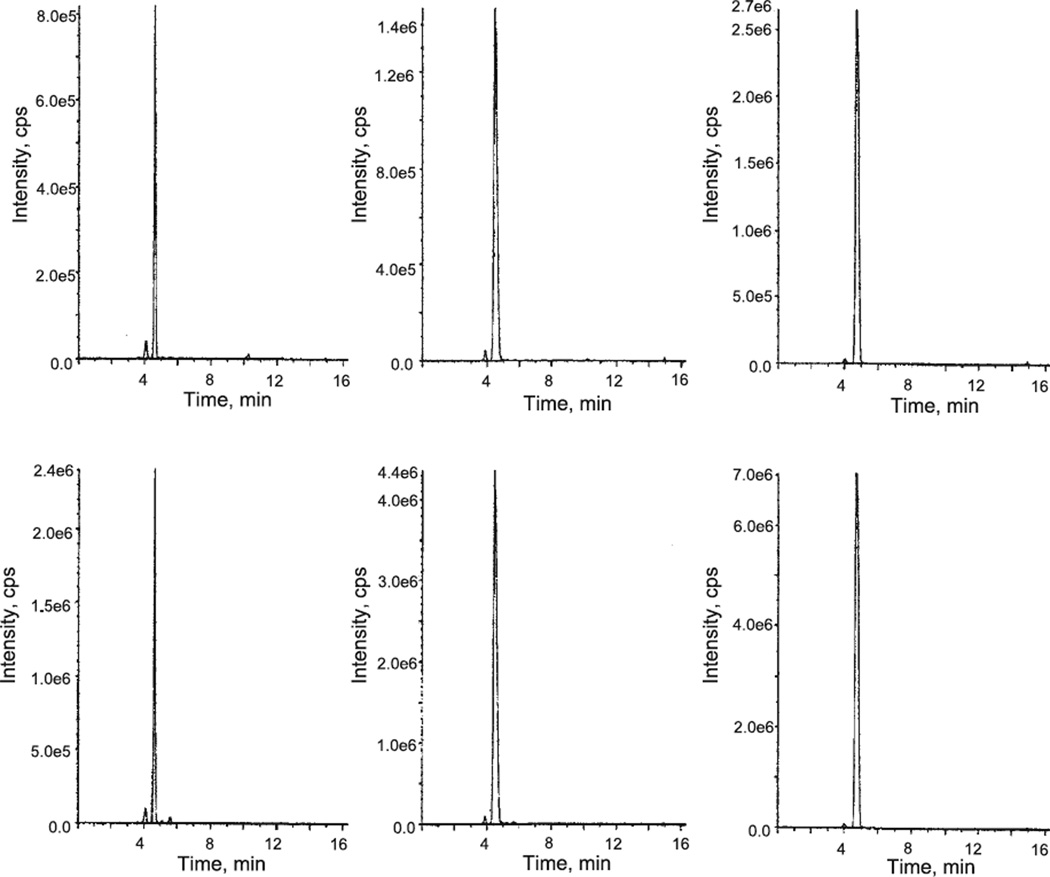
Calf thymus DNA was X irradiated in deoxygenated solution as described in Materials and Methods. The LC-MS/MS profiles, shown in Fig. 3, left side, were obtained from samples of X-irradiated DNA prepared in standard fashion as described in Materials and Methods. The two profiles in Fig. 3, left side, were obtained with the spectrometer programmed for the distinctive MS/MS values appropriate for the two main transitions expected for the tandem lesion d(G^T). Remember that these products must elute identically. The two profiles demonstrate clearly that the irradiated DNA contains a lesion of the expected molecular weight that has the peculiar properties of a tandem lesion. The next question is whether this product is the same tandem lesion identified in d(CGTA). Figure 3, right side, shows the corresponding LC-MS/MS profiles obtained from samples prepared from d(CGTA) X irradiated in deoxygenated solution. Programming for the same MS/MS values and the presence of a tandem lesion of the requisite molecular weight is demonstrated. Finally, it can be shown that the tandem lesion from irradiated d(CGTA) and the tandem lesion from irradiated calf thymus DNA have identical elution times. The middle profiles in Fig. 3 that are derived from a sample of a half and half mixture of the two DNAs, manifest a single product.
FIG. 3.
The left side column of LC-MS/MS elution profiles are derived from calf thymus DNA X-irradiated in deoxygenated solution. The right side column of LC-MS/MS elution profiles are derived from d(CGTA) X-irradiated in deoxygenated solution. The middle column LC-MS/MS profiles are derived from an equal mixture of the calf thymus and oligomer samples. The upper row of profiles were obtained for MS/MS values 568/470, and the second row of profiles were obtained for MS/MS values 568/274 (Table 1, tandem lesion no.12).
The tandem lesion Fig. 1f was identified previously by mass spectrometry and proton NMR spectroscopy as a radiation-induced modification of d(CATG) (4). The experiment illustrated in Fig. 3 was repeated using the MS/MS values shown in Table 1 for tandem lesion no. 14. Results completely analogous to those shown in Fig.3 were obtained, except that each profile exhibited two main peaks attributable to stereoisomers. Thus, d(C^G) identified in irradiated oligomer is also found in irradiated calf thymus DNA.
CONCLUSION
We are interested in ROS-induced DNA damage as it relates to cancer (7). Tandem lesions are increasingly recognized as an important aspect of oxidative DNA damage (8–12). A subcategory of tandem lesions, adjacent nucleosides that are covalently linked, is the subject of this study. The fragmentation of covalently linked tandem lesions produced in the collision chamber of the mass spectrometer generates distinctive LC-MS/MS profiles. Since the two nucleotides are doubly linked, enzymatic hydrolysis of the DNA does not dissociate the nucleotides. Fragmentation products are generated by double breaks causing loss of the phosphate group or loss of the dideoxyribophosphate group. The residual ions are reduced in molecular weight from that of the precursor ion by 98 and 294 Da, respectively. Two other ions, the genesis are less certain, have molecular weights equal to those of the major fragment ions less 43 Da. The LC-MS/MS elution profiles are highly characteristic for covalently linked tandem lesions since all four products elute at identical times.
An LC-MS/MS search was carried out for covalently linked tandem lesions in calf thymus DNA X-irradiated in a deoxygenated environment. The same experiment was repeated using DNA X-irradiated in an oxygenated environment. A total of 15 tandem lesions were detected in DNA X-irradiated in the absence of oxygen and 5 were detected in DNA X-irradiated in the presence of oxygen. Each lesion was characterized by its molecular weight, relative yield and LC elution time. Based on a consideration of molecular weights, fewer than half of the tandem lesions detected herein have been described in the literature.
The repair and potential mutational consequences of tandem lesions are being elucidated (13–16). Recently, a particularly noteworthy endeavor has demonstrated the presence of the prototype lesion (Fig. 1a) in the DNA of tissues from healthy humans (18).
LC-MS/MS technology provides a definitive and sensitive method for detecting a category of ROS-induced DNA damage that is otherwise difficult to characterize. Although we cannot be sure all covalently linked tandem lesions are detected by this method (17), our research provides an enlarged perspective of an aspect of ROS-induced DNA damage that has been largely overlooked until recently.
ACKNOWLEDGMENTS
The work was supported by grant RO3 CA139513 from the National Cancer Institute. Mass spectrometer facilities are shared resources supported by NCI grant CA 016056.
REFERENCES
- 1.Box HC, BudzinskI EE, Dawidzik JD, Wallace JC, Evans MS, Gobey JS. Radiation-induced formation of a crosslink between base moieties of deoxyguanosine and thymidine in deoxygenated solutions of d(CpGpTpA) Radiat Res. 1996;145:641–643. [PubMed] [Google Scholar]
- 2.Box HC, Patrzyc HB, Dawidzik JB, Wallace JC, Freund HG, Iijima H, et al. Double base lesions in DNA X-irradiated in the presence and absence of oxygen. Radiat Res. 2000;153:442–446. doi: 10.1667/0033-7587(2000)153[0442:dblidx]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Box HC, Dawidzik JB, Budzinski EE. Free radical-induced double lesions in DNA. Free Radic Biol Med. 2001;31:856–868. doi: 10.1016/s0891-5849(01)00653-0. [DOI] [PubMed] [Google Scholar]
- 4.Box HC, Budzinski EE, Dawidzik JB, Wallace JC, Iijima H. Tandem lesions and other products in X-irradiated DNA oligomers. Radiat Res. 1998;149:433–439. [PubMed] [Google Scholar]
- 5.Hong H, Cao H, Wang Y. Formation and genotoxicity of a guanine-cytosine intrastrand cross-link lesion in vivo. Nucleic Acids Res. 2007;35:7118–7127. doi: 10.1093/nar/gkm851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colis LC, Raychaudhury P, Basu AK. Mutational specificity of γ-radiation-induced guanine-thymine and thymine-guanine intra-strand cross-links in mammalian cells and translesion synthesis past the guanine-thymine lesion by human DNA polymerase η. Biochemistry. 2008;47:8070–8079. doi: 10.1021/bi800529f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iijima H, Patrzyc HB, Budzinski EE, Freund HG, Dawidzik JB, Rodabaugh HJ, et al. A study of pyrimidine base damage in relation to oxidaive stress and cancer. Br J Cancer. 2009;101:452–456. doi: 10.1038/sj.bjc.6605176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Box HC, Budzinski EE, Freund HG, Evans MS, Patrzyc HB, Wallace JC, et al. Vicinal lesions in X-irradiated DNA? Int J Radiat Biol. 1993;64:261–263. doi: 10.1080/09553009314551411. [DOI] [PubMed] [Google Scholar]
- 9.Box HC, Freund HG, Budzinski EE, Wallace JC, Maccubbin AE. Free radical-induced double base lesions. Radiat Res. 1995;141:91–94. [PubMed] [Google Scholar]
- 10.Yang Z, Colis LC, Basu AK, Zou Y. Recognition and incision of γ-radiation-induced cross-linked guanine-thymine tandem lesion G[8,5-Me]T by UvrABC nuclease. Chem Res Toxicol. 2005;18:1339–1346. doi: 10.1021/tx050147+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellon S, Gasparutto D, Saint-Pierre C, Cadet J. Guanine-thymine intrastrand cross-linked lesion containing oligonucleotides: from chemical synthesis to in vitro enzymatic replication. Org Biomol Chem. 2006;4:3831–3837. doi: 10.1039/b609460k. [DOI] [PubMed] [Google Scholar]
- 12.Bellon S, Ravanat JL, Gasparutto D, Cadet J. Cross-linked thymine-purine base tandem lesions: synthesis, characterization, and measurement in γ-irradiated isolated DNA. Chem Res Toxicol. 2002;15:598–606. doi: 10.1021/tx015594d. [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Imoto S, Greenberg MM. The mutagenicity of thymine glycol in Escherichia coli is increased when it is part of a tandem lesion. Biochemistry. 2009;48:7833–7841. doi: 10.1021/bi900927d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Y, Hong H, Cao H, Wang Y. In vivo formation and in vitro replication of a guanine-thymine intrasrand cross-link lesion. Biochemistry. 2007;46:12757–12763. doi: 10.1021/bi7012195. [DOI] [PubMed] [Google Scholar]
- 15.Gentil A, Le Page F, Cadet J, Sarasin A. Mutational spectra induced by replication to two vicinal oxidative DNA lesions in mammalian cells. Mutat Res. 2010;452:51–56. doi: 10.1016/s0027-5107(00)00034-8. [DOI] [PubMed] [Google Scholar]
- 16.Yuan B, Jiang Y, Wang Y, Wang Y. Efficient formation of the tandem thymine glycol/8-oxo-7,8-dihydroguanine lesion in isolated DNA and the mutagenic and cytotoxic properties of the tandem lesions in Escherichia coli cells. Chem Res Toxicol. 2009;23:11–19. doi: 10.1021/tx9004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong H, Wang Y. Formation of intrastrand cross-link products between cytosine and adenine from UV irradiation of d(BrCA) and duplex DNA containing a 5-bromocytosine. J Am Chem Soc. 2005;127:13969–13977. doi: 10.1021/ja0531677. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Cao H, You C, Yuan B, Bahde R, Gupta S, et al. Endogenous formation and repair of oxidatively induced G[8-5m]T intrastrand cross-link lesion. Nucl Acids Res. 2012 doi: 10.1093/nar/gks357. [DOI] [PMC free article] [PubMed] [Google Scholar]