Abstract
This study was undertaken to examine gender sensitivity and chemopreventive responsiveness of celecoxib on intestinal stem-like cells as biomarker of colon carcinogenesis, using the MIN mouse model. Male and female MIN mice (6–7 weeks old) were randomized to either control diet or to one supplemented with celecoxib (1500 ppm). The animals were euthanized ten weeks later and the intestines were flushed and opened longitudinally to assess tumor count. Small intestinal segments were formalin fixed and tissue sections were subjected to immunohistochemical evaluation of DCAMKL1, a known marker of stem-like cells. We found that in animals receiving control (AIN 76A diet) alone, female MIN mice had higher polyp count than that of males (52.32±13.89 vs. 35.43±16.05; p<0.0005). However, compared to control diet groups, celecoxib supplementation caused a larger reduction in the number of polyps in females than their male cohorts (6.38±1.43 vs. 12.83±6.74; a reduction of 88% in females to 64% in males). Significant differences (p=0.013) were observed in the number of DCAMKL1 stained cells in the crypts of the wildtype (WT) (10.01 ± 1.07 stem cells per high powered field; HPF) compared to the MIN mice (24.15 ± 8.08 stem cells per HPF), illustrating increased stem-like cells in animals that are more prone to neoplasia. DCAMKL1 labeled stem-like cells were equal in number in the male and female groups receiving the control AIN 76A diet alone (females= 25.73 stem-like cells/HPF); males= 24.15 stem-like cells/HPF). However females showed a greater reduction in the number of DCAMKL1 labeled stem like cells with the celecoxib supplementation than the males (16.63±4.23 vs. 21.56±9.06; a reduction of 35.4% in females to 10.7% in males). We conclude that a higher number of stem-like cells in the uninvolved mucosa paralleled tumorigenesis and mirrored greater chemopreventive responsiveness of female MIN mice to males.
Keywords: Gender, Chemoprevention, Intestine, Celelcoxib, DCAMKL1, Stem-like Cells
Introduction
Colorectal malignancies remain the third leading cause of cancer deaths in both men and women, underscoring the need for more effective cancer prevention efforts (1). The cornerstone of these endeavors has been precise population screening with an emerging component of risk factor modification/chemoprevention. Colonoscopy has been particularly promising given that it not only can diagnose early stage colorectal cancers, but also helps prevent it through precise selection and extraction of the precursor lesions, the adenomatous polyps. However, from a screening perspective, colonoscopy is usually inadequate for CRC prevention in women, potentially due to higher proximal distribution of lesions that appear to be less revealed by colonoscopy (2). Emerging evidence has revealed a significant disparity in the biology and epidemiology of CRC between men and women. However, despite these significant differences the clinical recommendations have, by and large, remained gender neutral. Furthermore, the issue of gender and chemoprevention has largely been unexplored except for the documented role of estrogens in CRC prevention, although with numerous untoward effects that make it untenable for clinical implementation.
There have been a myriad of agents purported to have chemopreventive benefits against colorectal cancer. Of these, nonsteroidal anti-inflammatory drugs (NSAIDs) have been utilized in the majority of studies. For instance, several recent large scale multi-center trials have shown a profound reduction in advanced adenomas (50–60% reduction) (3). Although the cardiac toxicity would prevent widespread adoption, this still provides a significant proof of concept that chemoprevention may herald a new generation of safer and effective pharmaceutical or neutraceutical chemopreventive agents. This could precisely be achieved by targeting the population with novel agents to maximize their chemopreventive sensitivity while minimizing the toxicity (by not treating patients who are unlikely to achieve antineoplastic benefit).
One approach to recognize the appropriate subgroup population for NSAIDS responsiveness could be achieved by selectively gauging unambiguous molecular target of carcinogenesis. However, this has been complicated by the existence of a large number of putative targets, including COX2, β-catenin, AKT etc, leading to incompatible recommendations (4). Therefore, in order to evaluate the potential efficacy, a precise and more fundamental marker is warranted. One approach would be to assess specific lineage of cells that may directly be involved in the initiation of colon carcinogenesis. Recently, there has been a consensus that intestinal stem cells may represent the fundamental precursor cells (5) that can survive long enough to endure serial genetic/epigenetic changes that symbolizes colon carcinogenesis as opposed to normal colonocytes which survive a short lifespan of only 3–7 days.
Intestinal stem cells have a regenerating component as well as the capability to differentiate into functionally competent and specific cells. These cells are critical in renewing the long-term intestinal epithelium. There is emerging data to suggest that during the earliest known stages of carcinogenesis, stem cell numbers may go up in the microscopically normal mucosa of patients at risk for colon carcinogenesis. For instance, in familial adenomatous polyposis (FAP), which engenders a 90% lifetime risk of CRC, the number of stem cells present in intestinal crypts have been shown to increase (6). The stem cells, being the precursors to every colorectal cancer (7), may thus represent an outstanding fundamental biomarker regardless of specific cancer biology(5). However, to date, the modulation of stem cell number in colon carcinogenesis and chemoprevention or the impact of gender on stem cells has largely remained unexplored.
In this study we explore the issue of gender sensitivity to NSAIDs on intestinal stem-like cells using the MIN mouse model, which has a germline mutation in the adenomatous polyposis coli (APC) and thus recapitulates the innate features of genetic initiation of most sporadic colonic neoplasia. For these studies, we used celecoxib, as it is one of the best established chemopreventive agents to date (8). In order to quantify the changes in the intestinal epithelium we immuno-stained the intestinal stem-like cells with antibody against a known biomarker, DCAMKL1 (doublecortin and calcium/calmodulin-dependent protein kinase-like- 1), as a surrogate for quantifying future adenoma formation and cancer risk (9). Moreover, we examined the changes in the number of stem-like cells present in the intestinal epithelia, as evidenced by cells positive for DCAMKL1 staining, and investigated how chemoprevention and gender modulated these changes.
Materials and methods
Animals
All animal studies were done in accordance with the Institutional Animal Care and Use Committee (IACUC) of NorthShore University Healthcare, Evanston, IL. Eight wildtype (WT) C57BL6 male mice (5 to 6 weeks old) were obtained from Jackson Laboratory (Bar Harbor, ME). Animals were given a standard AIN 76A diet (Teklad Labs, Madison, WI). The mice were euthanized at 11–13 weeks of age. A small distal segment from the resected small intestine was formalin fixed, paraffin embedded, sectioned (4 µm) and mounted on glass slides.
Chemoprevention study protocol
Apcmin mice (10 male and 10 female mice; 5 to 6 weeks old) were procured from Jackson Laboratory (Bar Harbor, ME). Starting at ages 6–7 weeks, each gender group was randomized to either AIN 76A diet alone or the one supplemented with celecoxib 1,500 ppm (Teklad Labs, Indianapolis, IN). After 10 weeks, the animals were euthanized and the resected small intestines and colons were flushed with cold PBS and sliced longitudinally to expose the lumen. The length of each section of the intestine was measured and the segments (with mucosal surface facing upwards) were gently spread on a strip of moist 3M Whatman paper. These paper strips were then placed on top of dry-ice-cooled steel plates for few minutes to assist easy and accurate identification of the polyps (10). The polyps were then manually counted under illuminated magnifiers (All-Spec Industries; Wilmington, NC) by 3 independent observers that were blinded to the treatment design. For additional microscopic re-evaluation of the assessed tumors, the intestinal sections were prepared into “Swiss rolls” which were formalin fixed, paraffin embedded, sectioned for H&E staining and subjected to independent pathological verification for the adenomatous nature of the tissue. For immunohistochemical analysis, a small segment from the distal small intestine was separately fixed in a 10% buffered formalin solution, paraffin embedded in blocks and cut into 4 µm sections and mounted on slides.
Immunohistochemistry staining and quantification
The tissue slides were baked at 60° C for 60 minutes, deparaffinized in xylene for 15 minutes, and rehydrated through decreasing concentrations of ethanol washes. Quenching of endogenous peroxidase was accomplished by incubating sections in a 3% hydrogen peroxide solution in methanol (Sigma, St Louis, MO) for 30 minutes. Slides were then given three washes each in PBS and distilled water. The sections were incubated in goat normal serum at room temperature for 30 minutes, using the Rabbit Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). After incubation with primary antibody DCAMKL-1 (ab31704 at 1:200 dilutions, Abcam Inc., Cambridge, MA) for 30 minutes at room temperature, the slides were washed three times in PBS and subjected to secondary anti-rabbit antibody incubation for 30 minutes, followed by another set of PBS washes. The sections were then incubated in streptavidin horseradish peroxidase for 30 minutes. After a final wash in PBS, chromogenic development was performed using 3, 3- diaminobenzidine tetrahydrochloride (DAB; Vector Laboratories, Burlingame, CA). The sections were counterstained using Gill (# 3) hematoxylin (Sigma Aldrich, St Louis, MO), washed in distilled water and followed by color enrichment in a saturated lithium carbonate bluing solution (Sigma Aldrich, St Louis, MO). The slides were washed in water, dehydrated in graded alcohols, cleared in xylene, and the cover slip permanently mounted with Permaslip mounting medium (Alban Scientific, St Louis, MO).
The DCAMKL1 stained slides were viewed on a Nikon Eclipse E800 microscope. Each prepared tissue section was evaluated by viewing longitudinal full length crypts and scoring them by counting the number of positive cells as described previously (11). In order to account for variations in tissue specimen sizes, the area of intestine was calculated by counting the total number of high power fields (HPF; at 20× magnification), along with the total number of the DCAMKL1 labeled stem-like cells. A ratio of the number of DCAMKL1 labeled stemlike cells per high power field was then calculated.
Results
Gender based tumor count
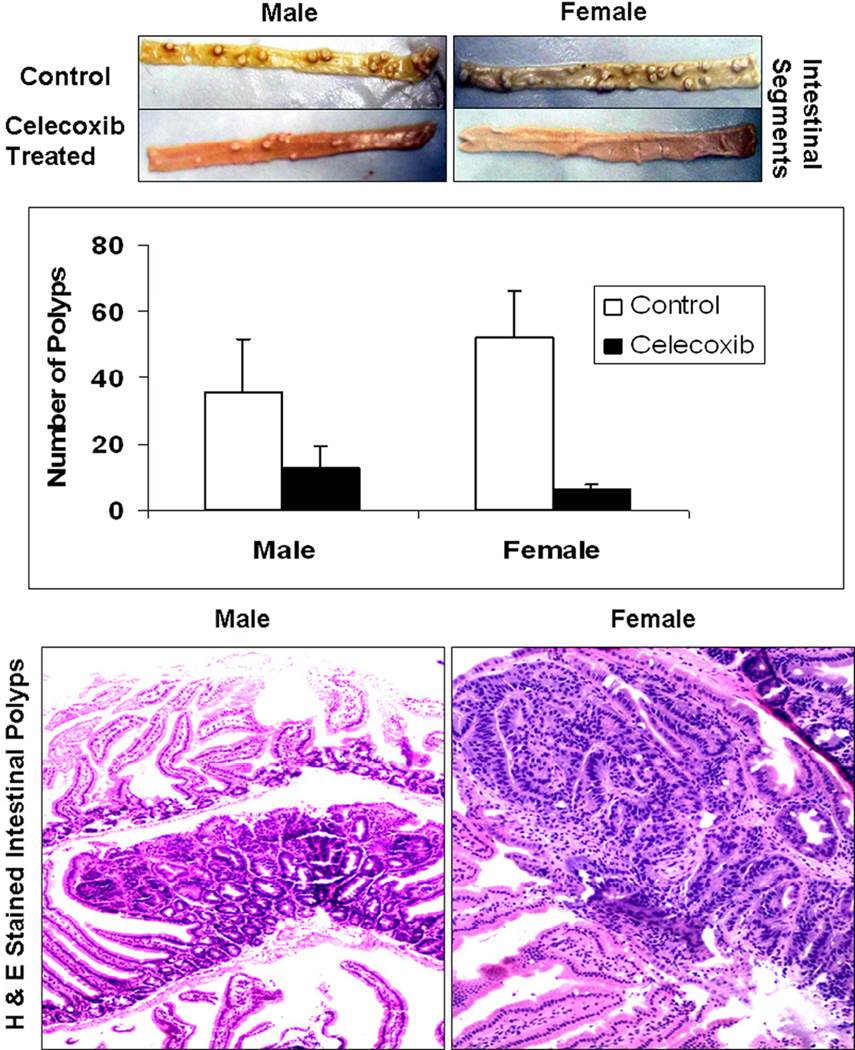
Three independent scorers, blinded to the group classification, separately counted the total number of tumors in the small intestine (proximal and distal) and the colon for each animal sample. After calculating the mean of the total number of tumors counted, it was observed that without celecoxib treatment females developed more polyps than males (52.32±13.89 vs. 35.43±16.05). Celecoxib treated females developed fewer number of tumors than their celecoxib treated males counterparts (6.38±1.43 vs. 12.83±6.74), which reflects that celecoxib treatment resulted in 87.8% decrease (p=2×10−14) in tumor count in females as compared to only 63.7% decrease (p=7×10−7) in their males counterparts (Figure 1A). Furthermore, the histological analysis of the H&E stained sections of the “Swiss rolls” positively confirmed the adenomatous nature of the intestinal polyps (Figure 1B).
Figure 1. Female MIN mice demonstrated higher celecoxib responsiveness to intestinal polyp reduction than males.
Total number of tumors in the small intestine (proximal and distal) and the colon for each animal were independently scored by three individuals blinded to the gender group. For easy identification, the tissue samples were cooled on dry-ice as discussed in the “Materials and Methods” section. After calculating the mean of the total number of tumors counted, it was observed that without celecoxib treatment, females developed more polyps than males (52.32±13.89 vs. 35.43±16.05). Celecoxib treated females developed lesser number of tumors than their celecoxib treated males counterparts (6.38±1.43 vs. 12.83±6.74) which reflects that celecoxib treatment resulted in 87.8% decrease (p=2×10−14) in tumor count in females as compared to only 63.7% decrease (p=7×10−7) in their males counterparts (Figure 1A). Furthermore, the histological analysis of the H&E stained sections of the “Swiss rolls” positively confirmed the adenomatous nature of the intestinal polyps (Figure 1B).
DCAMKL1 staining of the stem-like cells
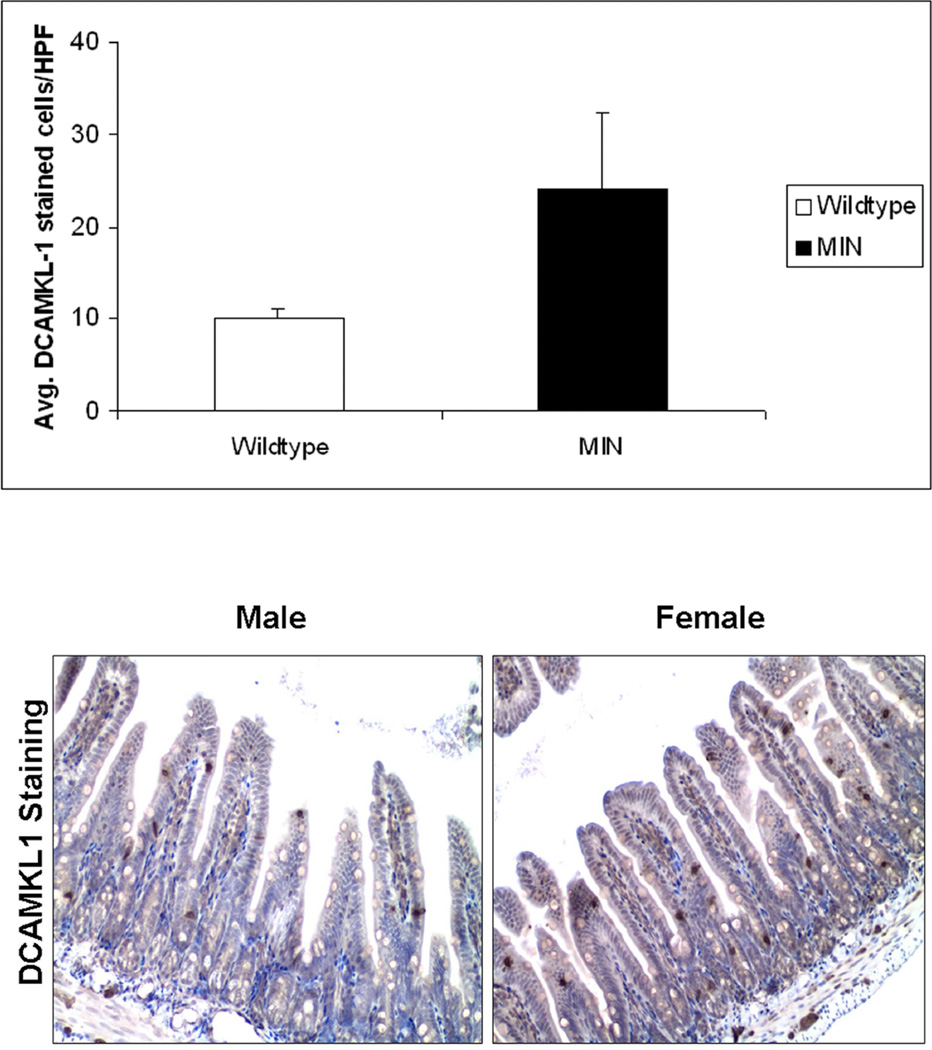
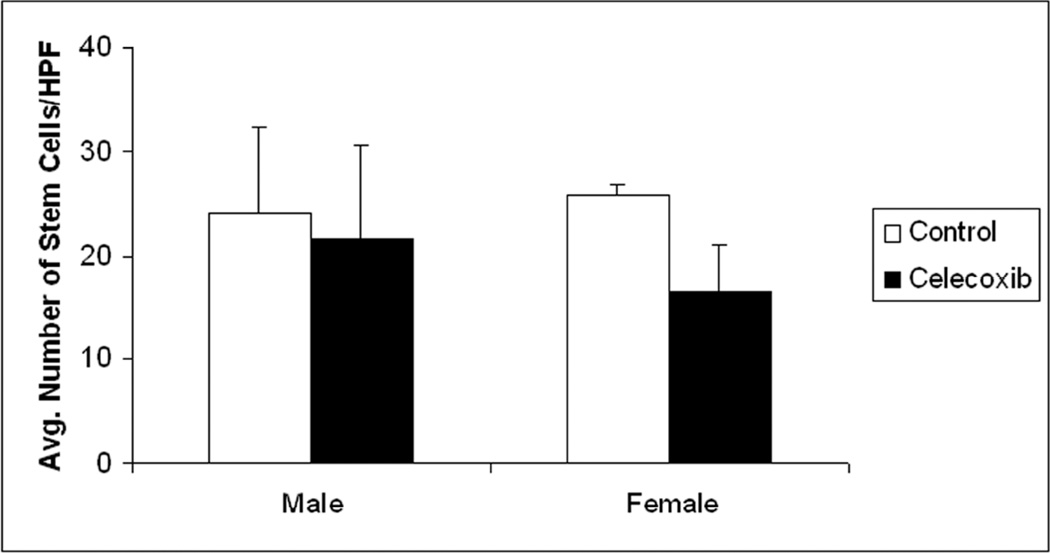
Since DCAMKL1 has both nuclear and cytoplasmic expression, the antigen-antibody staining was very apparent to discern positively stained stem-like cells from an amalgam of intestinal cells (Figure 2). Significant differences in the number of DCAMKL1 stained cells was observed in the crypts of the wildtype mice (WT) compared to the MIN mice on control AIN-76A diet. The MIN mice on average had 24.15 ± 8.08 stem cells per HPF compared to the WT mice which had 10.01 ± 1.07 stem cells per HPF, p=0.013. These studies were carried out in males simply to illustrate that animals prone to develop neoplasia demonstrated increased number of stem-like cells compared to normal WT controls (Figure 2A). Staining of the distal small intestinal mucosa for the male and female MIN mice yielded results that showed a clear gender-specific trend. On an average, the number of DCAMKL1 labeled stem-like cells were equal in the male and female groups receiving the standard AIN 76A diet alone, (females= 25.73±1.13 stem cells/HPF, males= 24.15±8.08 stem cells/HPF; Figure 2B). However females had a greater reduction with the celecoxib supplemented diet than males (16.63±4.23 Vs 21.56±9.06; a reduction of 35.4% in females to 10.7% in males; Figure 2C).
Figure 2. Larger reduction in DCAMKL1 stained intestinal stem-like cells in Female MIN mice in response to chemopreventive celecoxib compared to males.
The intestinal tissue sections were subjected to immunohistochemical staining for stem-cell marker DCAMKL1 as described in “Materials and Methods”. The MIN mice (male) on average had 24.15 ± 8.08 stem cells per HPF compared to the wildtype mice which had 10.01 ± 1.07 stem cells per HPF, p=0.013, thus illustrating the increase in stem cells seen in animals more prone to neoplasia (Figure 2A. Furthermore, the number of DCAMKL1 labeled stem-like cells were equal in the male and female groups receiving the control (AIN 76A) diet alone, (females= 25.73 stem cells/HPF, males= 24.15 stem cells/HPF) (Figure 2B). As shown (by intense brown staining), DCAMKL1 had distinct nuclear and cytoplasmic expression pattern. The females that received celecoxib enriched diet however, had a greater reduction in stem-like cells than their male cohorts (16.63±4.23 vs. 21.56±9.06; a reduction of 35.4% in females to 10.7% in males) (Figure 2C).
Discussion
We herein demonstrate that the efficiency of chemopreventive responsiveness of celecoxib against colon cancer was at least, in part, gender specific. The use of a well-controlled preclinical CRC model enabled us to discern this specificity without being confounded by difficult to avoid factors typically associated with epidemiological studies (e.g. explicit gender differences in lifestyle/medical utilization) related to gender involvement in cancer. Importantly, we observed that a presence of higher number of stem-like cells in the uninvolved mucosa paralleled tumorigenesis and mirrored greater chemopreventive responsiveness of female MIN mice compared to males. Moreover, to our knowledge, this is the first evidence that a chemopreventive agent, such as celecoxib, can modulate stem-like cells within the intestinal crypts of the microscopically normal mucosa thus providing a potential mechanism for altered tumorigenesis.
A major challenge in the field of chemoprevention is the unpredictability of the success of cancer prevention strategy. This has been a major practical issue as chemoprevention does not work equivalently in the entire population so a priori targeting the appropriate subset is critical. For instance, while both aspirin and NSAIDs provide an estimated range of efficacy of 30–50%, all the treated patients (including the majority of patients who may never achieve any neoplasia reduction) are subjected to the GI toxicity associated with this strategy (12, 13). These potential side effects have led the US Preventive Service Task Force not to recommend aspirin for chemoprevention since the potential harms outweigh the benefits (14, 15).
Predicting the chemopreventive responsiveness is, therefore, an important strategy for achieving maximal benefits in a less harmful setting. One recent area of investigation has been “pharmacogenomics” that emphasizes the need for personalized chemopreventive approach. For instance, polymorphisms in putative targets (COX2), metabolizing enzymes (e.g. UGT1A6 etc) and pro-neoplastic pathways (e.g. ornithine decarboxylase) have been shown to impact NSAID responsiveness differently (16). Other approaches, such as proteomic analysis have been less focused although this requires treatment rather than pre-treatment examination and moreover it still is in its infancy (17).
Since it is estimated that the CRC risk is considerably determined by exogenous factors (diet, weight etc), it is logical to postulate that chemopreventive strategies may be influenced by these factors. Gender, in particular, is a very attractive putative modulator of chemoprevention given the striking biological differences of CRC in men and women (18). For instance, compared to men, CRC in females have a late age of onset as well as a predominant predilection to the proximal colon. Clinically, it is well accepted that because of these known gender-specific predispositions to the location of colorectal cancer, women are less likely to benefit from flexible sigmoidoscopy (2). Moreover, our analyses of the literature suggest that cancer prevention efforts based on interrupting the adenoma-carcinoma sequence may be less relevant in women. In addition, CRC in females is more likely to have microsatellite instability, which may be one reason why women tend to have a better prognosis but worse response to chemotherapy (19). Indeed, while the current screening and treatment recommendations are gender-neutral, vital gender based biological differences appear to have been overlooked.
With regards to chemoprevention, while pharmacogenomics has received the most attention, the impact of non-genomic factors is being increasingly recognized. The epidemiology, biology and clinical manifestations of colorectal cancer are all distinctly different in women and men. The examination of gender as a component in cancer biology is particularly intriguing, given that the emerging data reveals powerful chemopreventive efficacy of estrogens (20). However, the interactions of gender with other exogenous factors are only beginning to be explored. For instance, there is evidence that patients with a higher BMI have an elevated CRC risk (21) but may be more responsive to aspirin chemoprevention (15). With regards to NSAIDS chemopreventive activity, a recent lung cancer study showed that the chemoprevention benefit was only limited to males (22). The meta-analysis of aspirin showed that for advanced adenomas, aspirin tended to work better in women than men but failed to reach statistical significance (RR 0.78 males vs. 0.62 females) (23). In another North Carolina Colon Cancer Study, it was shown that regular users of NSAIDs had a risk of CRC of 0.78 in males versus 0.28 in women (24). Taken together, these reports support the notion that gender is a key factor in NSAIDs responsiveness. However, the potential for confounding with other lifestyle/genetic issues in humans underscore the importance of animal models to elucidate this vital issue.
With regards to mechanisms involved in differential responsiveness of NSAIDS in females and males, the data has been complicated by the myriad of putative molecular targets used for end-point analyses. Intriguingly, there is data that celecoxib may alter methylation and hence gene expression of putative-gender related CRC genes such as estrogen receptor alpha (25, 26). Our approach to understand the differential chemopreventive efficacy of NSAIDS was to assess the putative cells of initiation (stem cells) in the microscopically normal mucosa. Stem cells have an established role in intestinal cryptal homeostasis (27). Stem cells retain the ability to produce all epithelial cell types populating the mucosa. These cells are slowly cycling, capable of self-renewal and long-lived, thus being logical targets for the multistep carcinogenesis process as they have a greater propensity to accumulate mutations compared to short lived progressively differentiated cells (as previously discussed, typically colonocytes life-span is only 3–7 days). Indeed, Barker and colleagues have demonstrated that knocking out APC (the initiating mutation in most colon carcinogenesis) selectively in stem cells was sufficient to induce intestinal tumorigenesis (7). Clinically, in patients with FAP, there is an increased population of stem-like cells in the microscopically-normal mucosa which is further accentuated in dysplastic tissue. Thus, while our finding of altered stem-like cells with gender and reversal with chemoprevention are novel, there is considerable biological precedence.
The critically limiting factor when working with stem cells is identification. There have been numerous putative markers of stem cells identified recently, including Mushashi-1 (an RNA binding protein), Aldehyde dehydrogenase 1(28), Lgr5 (GPR49)(29), β-catenin phosphorylated on serine 552 (p-β-catenin-S552), phosphorylated PTEN, BMI-1 and most recently prominin1 CD 133 (30). DCAMKL1, double cortin and calcium/calmodulindependent protein kinase-like-1, a microtubule-associate protein expressed at high levels in the developing brain, has recently been found to be a putative intestinal stem cell biomarker in the Apc/min mouse model (18). Using a panel of markers could be a possible approach, as it has been demonstrated that these markers do not label the same stem-like cells in the intestinal crypts.
Thus, while it is clear that DCAMKL1 is a marker for progenitor, it is a matter of considerable debate as to whether these are true stem cells or simply stem-like cells. This is true of many of the putative stem-cell marker. Moreover, these markers maybe identifying different facets of the progenitor cell population. For instance, it has been reported DCAMKL1 is predominantly expressed in quiescent cells in the intestinal crypt epithelium, while Lgr5 may be labeling more rapidly cycling proliferative cells that are limited to the lowest regions of the crypt. Furthermore, there is emerging evidence to suggest that these biomarkers may be labeling two distinct and functionally different stem-like cells. Previous publications have demonstrated that the position in the crypt could be helpful in identifying which stem cell is more rapidly cycling or is quiescent. While the initial reports on intestinal stem cells were in position +4 in the crypt, it is clear that stem-like cells can occur at any location within the crypt, but have a tendency to be closer to the base of the crypt. There is some evidence to suggest that certain stem-like cells are niche specific; cells closer to the base of the crypt being responsible for gut homeostasis and injury response, with others higher in the crypt are responsible for Paneth cell repopulation in response to bacterial mediated injury (11). On the other hand, it is clear that DCAMKL1 appears to be functionally significant as it is a potent regulator of the microRNA Let-7a, thereby controls the powerful proto-oncogene c-Myc (18). Taken together, this provides robust evidence for the role of DCAMKL1 as a marker for stemlike cells thus underscoring its important role in early carcinogenesis.
Aside from issues regarding identification of stem cells, other limitations of this study revolved around the choice of experimental model and chemopreventive agent. With regards to the model, the MIN mouse is a standard model with the APC mutation and is widely used in chemoprevention studies. The translation from the MIN mouse to humans has been well established. However, the differences between the two reveal that the MIN mouse is predominantly a small bowel adenoma model rather than frank CRC model. Moreover, we noted that females had more tumors than males consistent with previous MIN mouse reports but contrary to epidemiological studies (which suggest men have a slightly higher risk of colonic neoplasia than women). On the other hand, a modeling study suggests that initiation in the proximal colon (the region women have a predilection to tumorigenesis) was 2–3 fold greater than in the distal colon, albeit progression was slower. This supports the translatability of our MIN mice findings to humans. Moreover, the success of the MIN mouse represents a “cleaner” model in order to study the effect of gender without confounding of lifestyle or other biological factors (women tend to have more proximal and microsatellite unstable disease). From an agent perspective, Celecoxib is a standard chemopreventive agent that is shown to prevent adenomas FAP (the human equivalent of the MIN mouse) (8) and is effective against sporadic neoplasia (advanced adenomas) in large scale randomized trials (15). It should be pointed out that our current data on the anti-tumor effects of celecoxib and alterations in stem cell dynamics is only correlative and does not prove any causal relation. Additionally, the generalizability of celecoxib to other NSAIDS, especially agents without COX 2 selectivity is less clear. Furthermore, we did not observe any celecoxib related tumor resistance or inflammation as has been reported by Carothers et al (31, 32). It should be emphasized that in their studies this phenomenon was seen only after 5 months of chronic celecoxib treatment, which is twice the duration of the present studies. In another study, comparable to the duration of the current investigation, no tumor resistance was observed (33). Finally, we need to consider whether stem-like cells are a target of NSAIDs or simply represent a biomarker. The notion of stem cells as targets of NSAIDS was strengthened by a recent report showing that stem cell elimination may be an effective chemoprevention of colon cancer by NSAIDS (34). Either way, the novel finding that these stem-like cells are modulated by chemopreventive agents is of great significance.
In conclusion, we demonstrate for the first time that female MIN mice appeared to have a more robust chemopreventive response than males. This was paralleled by the reduction in stem-like cells as assessed by DCAMKL1 immunostaining. This preliminary report provides the first compelling evidence to suggest that there is a gender-related difference that needs to be accounted for with chemoprevention and this may, potentially, be related to alteration in stem-like cell number. Future studies will address the translatability of these issues to human CRC prevention.
Acknowledgements
The study was supported by grants from National Institute of Health (NIH), Bethesda, MD USA [21-CA141112, UO1CA111257, RO1CA156186 and R21-CA140936].
Footnotes
This work was partially presented in an abstract form at the 111th Digestive Disease Week, May 2010, in New Orleans, USA.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352:2061–2068. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 3.Bresalier RS. Chemoprevention of colorectal neoplasia: advances and controversies (the COX-2 story) Curr Opin Gastroenterol. 2007;23:44–47. doi: 10.1097/MOG.0b013e328011c482. [DOI] [PubMed] [Google Scholar]
- 4.Guruswamy S, Rao CV. Multi-Target Approaches in Colon Cancer Chemoprevention Based on Systems Biology of Tumor Cell-Signaling. Gene Regul Syst Bio. 2008;2:163–176. doi: 10.4137/grsb.s486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, Scott EW, Huang EH. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boman BM, Fields JZ, Cavanaugh KL, Guetter A, Runquist OA. How dysregulated colonic crypt dynamics cause stem cell overpopulation and initiate colon cancer. Cancer Res. 2008;68:3304–3313. doi: 10.1158/0008-5472.CAN-07-2061. [DOI] [PubMed] [Google Scholar]
- 7.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 8.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 9.Sureban SM, May R, Ramalingam S, Subramaniam D, Natarajan G, Anant S, Houchen CW. Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology. 2009;137:649–659. doi: 10.1053/j.gastro.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ju J, Hong J, Zhou JN, et al. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)− epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–10631. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 11.May R, Sureban SM, Hoang N, et al. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27:2571–2579. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991;115:787–796. doi: 10.7326/0003-4819-115-10-787. [DOI] [PubMed] [Google Scholar]
- 13.Ladabaum U, Chopra CL, Huang G, Scheiman JM, Chernew ME, Fendrick AM. Aspirin as an adjunct to screening for prevention of sporadic colorectal cancer. A cost-effectiveness analysis. Ann Intern Med. 2001;135:769–781. doi: 10.7326/0003-4819-135-9-200111060-00007. [DOI] [PubMed] [Google Scholar]
- 14.Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2007;146:361–364. [PubMed] [Google Scholar]
- 15.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 16.Chan AT, Tranah GJ, Giovannucci EL, Hunter DJ, Fuchs CS. Genetic variants in the UGT1A6 enzyme, aspirin use, the risk of colorectal adenoma. J Natl Cancer Inst. 2005;97:457–460. doi: 10.1093/jnci/dji066. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Z, Luke BT, Izmirlian G, et al. Serum proteomic profiles suggest celecoxib-modulated targets and response predictors. Cancer Res. 2004;64:2904–2909. doi: 10.1158/0008-5472.can-03-3754. [DOI] [PubMed] [Google Scholar]
- 18.Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18. doi: 10.1111/j.1399-0004.2009.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 20.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 21.Laake I, Thune I, Selmer R, Tretli S, Slattery ML, Veierod MB. A prospective study of body mass index, weight change, and risk of cancer in the proximal and distal colon. Cancer Epidemiol Biomarkers Prev. 2010;19:1511–1522. doi: 10.1158/1055-9965.EPI-09-0813. [DOI] [PubMed] [Google Scholar]
- 22.Slatore CG, Au DH, Littman AJ, Satia JA, White E. Association of nonsteroidal anti-inflammatory drugs with lung cancer: results from a large cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:1203–1207. doi: 10.1158/1055-9965.EPI-08-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole BF, Logan RF, Halabi S, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101:256–266. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S, Martin C, Galanko J, et al. Use of nonsteroidal antiinflammatory drugs and distal large bowel cancer in whites and African Americans. Am J Epidemiol. 2008;168:1292–1300. doi: 10.1093/aje/kwn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira MA, Tao L, Wang W, Li Y, Umar A, Steele VE, Lubet RA. Modulation by celecoxib and difluoromethylornithine of the methylation of DNA and the estrogen receptor-alpha gene in rat colon tumors. Carcinogenesis. 2004;25:1917–1923. doi: 10.1093/carcin/bgh209. [DOI] [PubMed] [Google Scholar]
- 26.Shen R, Tao L, Xu Y, Chang S, Van Brocklyn J, Gao JX. Reversibility of aberrant global DNA and estrogen receptor-alpha gene methylation distinguishes colorectal precancer from cancer. Int J Clin Exp Pathol. 2009;2:21–33. [PMC free article] [PubMed] [Google Scholar]
- 27.Gu B, Watanabe K, Dai X. Epithelial stem cells: an epigenetic and Wnt-centric perspective. J Cell Biochem. 2010;110:1279–1287. doi: 10.1002/jcb.22650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermeulen L, Todaro M, de Sousa Mello F, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci U S A. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro-Alvarez N, Kondo E, Kawamoto H, et al. Isolation and propagation of a human CD133(−) colon tumor-derived cell line with tumorigenic and angiogenic properties. Cell Transplant. 2010;19:865–877. doi: 10.3727/096368910X508997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carothers AM, Davids JS, Damas BC, Bertagnolli MM. Persistent cyclooxygenase-2 inhibition downregulates NF-{kappa}B, resulting in chronic intestinal inflammation in the min/+ mouse model of colon tumorigenesis. Cancer Res. 2010;70:4433–4442. doi: 10.1158/0008-5472.CAN-09-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carothers AM, Moran AE, Cho NL, Redston M, Bertagnolli MM. Changes in antitumor response in C57BL/6J-Min/+ mice during long-term administration of a selective cyclooxygenase-2 inhibitor. Cancer Res. 2006;66:6432–6438. doi: 10.1158/0008-5472.CAN-06-0992. [DOI] [PubMed] [Google Scholar]
- 33.Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 2000;60:5040–5044. [PubMed] [Google Scholar]
- 34.Qiu W, Wang X, Leibowitz B, et al. Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proc Natl Acad Sci U S A. 2010;107:20027–20032. doi: 10.1073/pnas.1010430107. [DOI] [PMC free article] [PubMed] [Google Scholar]