Abstract
Rationale
Delta-opioid agonists enhance the antinociceptive efficacy of methadone and other mu-opioid agonists. However, relatively little is known about the degree to which delta agonists might enhance the abuse-related effects of mu agonists.
Objective
This study used a behavioral economic approach to examine effects of the delta agonist SNC80 [(+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxy-benzyl]-N,N-diethylbenzamide] on the reinforcing effects of methadone in a drug self-administration assay. Interactions between SNC80 and cocaine were also examined for comparison.
Methods
Rhesus monkeys (n=4), surgically implanted with indwelling intravenous catheters, were tested in two phases. In phase 1, drug self-administration dose-effect curves for methadone (0.0032–0.1 mg/kg/injection (inj)) and cocaine (0.0032–0.32 mg/kg/inj) alone were determined under a fixed-ratio 10 (FR 10) schedule of reinforcement. In phase 2, FR values were increased every 3 days (FR 1–FR 1800) during availability of methadone alone (0.032 mg/kg/inj) and in combination with varying proportions of SNC80 (0.1:1, 0.3:1, and 0.9:1 SNC80/methadone) or of cocaine alone (0.032 mg/kg/inj) and in combination with varying proportions of SNC80 (0.33:1, 1:1, and 3:1 SNC80/ cocaine). Demand curves related drug intake to FR price, and measures of reinforcement were derived.
Results
Methadone and cocaine alone each functioned as a reinforcer. SNC80 did not alter measures of reinforcement for either methadone or cocaine.
Conclusions
SNC80 at proportions previously shown to enhance methadone-induced antinociception did not enhance the abuse-related effects of methadone. These results support the proposition that delta agonists may selectively enhance mu agonist analgesic effects without enhancing mu agonist abuse liability.
Keywords: Self-administration, Methadone, Cocaine, Behavioral economics, SNC80, Monkey, Demand curve, Mu-opioid, Delta opioid
Introduction
Mu-opioid receptor agonists such as methadone are effective for the treatment of pain but have undesirable effects such as the potential for abuse that limit their clinical effectiveness and utility. One strategy in the development of new analgesics with improved therapeutic profiles has been combining mu agonists with other compounds targeting other biological receptor systems to selectively enhance analgesic effects and/or attenuate undesirable effects (Dietis et al. 2009). Toward this end, the delta-opioid system might be one possible biological target of interest. For example, we and others have shown that mixtures of delta and mu agonists often produce synergistic antinociceptive effects in rodents and non-human primates (Adams et al. 1993; Dykstra et al. 2002; Heyman et al. 1989; Negus et al. 2009; Stevenson et al. 2003; Stevenson et al. 2005). In contrast, interactions between delta and mu agonists are often additive, sub-additive, or antagonistic on endpoints related to undesirable effects of mu agonists (e.g., reduced bladder motility, sedation, and respiratory depression) or delta agonists (e.g., convulsions) (O’Neill et al. 1997; Sheldon et al. 1989; Stevenson et al. 2003; Su et al. 1998). The fact that delta agonists selectively enhance mu agonist antinociception suggests that mixtures of delta and mu agonists may function as safer analgesics than mu agonists alone. Abuse liability is another undesirable effect that could influence the conditions under which a drug or drug mixture could be made available for clinical use. We have previously shown that varying proportions of the high-efficacy delta agonist SNC80 [(+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N, N-diethylbenzamide] either attenuated or had no effect on self-administration of the mu agonist heroin, depending on the schedule contingencies (Stevenson et al. 2005). These data were interpreted to suggest that as with many other undesirable effects, delta agonists may decrease the reinforcing effects of mu agonists.
A behavioral economic approach may provide an especially sensitive tool to quantify the impact of delta agonists on the reinforcing effects of mu agonists (Hursh 1991; Hursh and Winger 1995; Negus et al. 2008). In this approach, “demand curves” are constructed to express the total consumption of a commodity as a function of that commodity’s price, with increases in price ultimately producing decreases in consumption. The shape of the demand curve can then be analyzed to derive quantitative measures of reinforcement. In the case of drug self-administration, this approach has been used to compare the reinforcing effects of different drugs or drug mixtures in rhesus monkeys (Ko et al. 2002; Negus et al. 2008; Wade-Galuska et al. 2007; Winger et al. 2006; Winger et al. 2002). For example, behavioral economic methods were used to show that the mu agonist fentanyl functioned as a more effective reinforcer than either the kappa opioid receptor agonist U69,593 or mixtures of U69,593+fentanyl (Negus et al. 2008). The present study used similar behavioral economic methods to evaluate effects of SNC80 on self-administration of the high-efficacy mu agonist methadone in rhesus monkeys. Methadone was selected as the mu agonist because (a) it is increasingly used to treat pain when other opioids are insufficiently effective or produce unacceptable adverse effects (Leppert 2009; Linderbeck 2008), and (b) our previous studies showed that SNC80 synergistically and robustly enhanced the antinociceptive effects of methadone (Stevenson et al. 2003). The primary goal of this study was to evaluate the degree to which SNC80 proportions that synergistically enhanced methadone antinociception might also enhance behavioral economic measures of methadone reinforcement.
Because previous studies of SNC80/methadone antino-ciceptive interactions were conducted in monkeys that were not opioid-dependent, the present study used cocaine as a maintenance drug to minimize exposure to methadone and the potential development of opioid dependence associated with methadone self-administration. Although this approach had the advantage of minimizing opioid dependence, SNC80 has been reported to share or enhance some abuse-related effects of cocaine, such as the discriminative stimulus effects of cocaine in rhesus monkeys (Negus et al. 1998) and the locomotor effects of cocaine in rats (Jutkiewicz et al. 2008). This raised the possibility that SNC80 might alter self-administration of a substitution test drug (i.e., methadone) by interacting with the cocaine self-administration history rather than with the substitution drug. To evaluate the impact of this potential confound, interactions between SNC80 and cocaine were also examined for comparison with interactions between SNC80 and methadone.
Materials and methods
Subjects
Four adult male rhesus monkeys (Macaca mulatta) were equipped with indwelling intravenous catheters using procedures similar to those described previously (Negus 2006). The externalized portion of the catheter was protected by a tether system consisting of a custom-fitted nylon vest connected to a flexible stainless steel cable and fluid swivel (Lomir Biomedical, Malone, NY, USA). The subjects were individually housed in stainless steel cages, and water was freely available. Monkeys were fed enough Lab Diet (Purina, Framingham, MA, USA) high-protein monkey biscuits after the behavioral session to maintain body weight, and the diet was supplemented with fresh fruit twice weekly. The temperature- and humidity-controlled colony room was maintained on a 12 h light/12 h dark cycle (lights on from 7 AM–7 PM). Animal maintenance and research were conducted in accordance with the guidelines provided by the National Institutes of Health Committee on Laboratory Animal Resources. The facility was licensed by the United States Department of Agriculture and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Protocols were approved by the Institutional Animal Care and Use Committee. Monkeys were checked daily by veterinary or technical staff and received daily environmental enrichment.
Apparatus and self-administration procedures
Each monkey was housed individually in a well-ventilated stainless steel chamber equipped with two syringe pumps (Model PHM-108, Med Associates) and a custom-designed operant conditioning panel mounted on the front wall as previously described (Stevenson et al. 2005). Self-administration sessions were conducted from 10 AM–12 NN 7 days per week. The green stimulus lights in the center response key were illuminated at the beginning of each session and were turned off at the end of the session and during time-out periods (see below). Responding was initially maintained by 0.032 mg/kg/injection (inj) cocaine under a fixed-ratio 10 (FR 10)/time out 60 s (TO 60″) schedule of reinforcement until the total number of injections per session varied by ≤20% for three consecutive days. We have reported previously that the relative reinforcing effects of mu opioid agonists may be influenced by the degree of opioid dependence (Negus 2006; Negus and Rice 2009). In this study, we wished to minimize dependence because previous studies of antinociception were conducted in non-dependent monkeys. Accordingly, cocaine injections were used as the reinforcing stimulus during training and maintenance of drug self-administration to minimize induction of opioid dependence associated with methadone self-administration. Saline was then substituted for cocaine until the number of injections per day decreased to fewer than 20. Subsequently, testing was conducted in two phases.
Phase 1
Dose-effect curves for methadone and cocaine were determined under the FR 10/TO 60″ schedule of reinforcement to determine relative potency of these two compounds. Specifically, saline, cocaine (0.00032–0.32 mg/kg/inj), or methadone (0.0032–0.1 mg/kg/inj) was made available for a single test session. Different doses of cocaine or methadone were made available by changing the drug concentration in the syringe, and injections were delivered in a constant volume of 0.1 ml over 0.95 s. Test sessions were separated by maintenance sessions, during which either 0.032 mg/kg/inj cocaine or saline was available. The sequence of maintenance sessions between test sessions was determined by self-administration performance, such that if a given test solution maintained more than 20 injections/session, then saline was substituted for at least 2 days and until responding declined to fewer than 20 injections/session. Conversely, if a given test solution maintained fewer than 20 injections/session, then 0.032 mg/ kg/inj cocaine was reinstated for at least 2 days and until self-administration increased to rates similar to previous performance during 0.032 mg/kg/inj cocaine availability. Subsequently, saline was substituted for at least 2 days and until responding declined to fewer than 20 injections/session. Thus, each test session was preceded by a period of self-administration at rates greater than 20 injections/session (maintained either by cocaine or by a test solution) and a period of saline substitution to extinguish self-administration to rates lower than 20 injections/session. Cocaine and methadone doses were tested in an irregular order across monkeys. For each drug, the lowest reinforcing dose was identified (i.e., the lowest dose to maintain a rate of self-administration significantly greater than that maintained by saline), and a dose of 0.5 log units higher than the lowest reinforcing dose was used for subsequent studies in phase 2. SNC80 alone was not tested because numerous studies have already demonstrated that SNC80 and other delta agonists do not maintain self-administration in rhesus monkeys (Negus et al. 1994; Negus 2004; Negus et al. 1998; Stevenson et al. 2005).
Phase 2
The reinforcing effects of methadone (0.032 mg/ kg/inj) or cocaine (0.032 mg/kg/inj) alone or in combination with different proportions of SNC80 were studied in phase 2. SNC80/methadone mixtures were identical to those that produced a proportion-dependent enhancement of methadone-induced thermal antinociception in Stevenson et al. (2003) and were 0.1:1, 0.3:1, and 0.9:1 SNC80/methadone. SNC80/cocaine mixtures were tested using proportions of 0.33:1, 1:1, and 3:1 SNC80/cocaine, and these proportions were based on the relative potency of SNC80 to enhance the discriminative stimulus effects of cocaine in rhesus monkeys (Negus et al. 1998). The highest proportion of 3:1 SNC80/ cocaine was studied briefly in only one subject, but the study was discontinued when the mixture produced a convulsion, and this mixture was not studied in other monkeys. Prior to each test, responding was maintained by 0.032 mg/kg/inj cocaine under an FR 10 schedule until rates of self-administration returned to baseline levels (± 20% self-administration rates in phase 1) and were stable for at least 2 days. Subsequently, the test solution was made available under an FR 1 schedule for at least 3 days and until the total number of injections per session varied by ≤20% for three consecutive days. Once responding was stable under the FR1 schedule, the FR value was increased every 3 days to FR 3, 10, 30, 100, 300, 1,000, and 1,800 or until the subject did not earn a reinforcer for two consecutive days at a given FR. At the conclusion of each test, access to the maintenance dose (0.032 mg/kg/inj) of cocaine was reinstated under an FR 10 schedule as described above until responding recovered to previous levels of cocaine self-administration. Subsequently, the next test drug or drug mixture was introduced. Methadone, cocaine, SNC80/ methadone mixtures, and SNC80/cocaine mixtures were tested in an irregular order between monkeys. All studies with one drug (cocaine or methadone) were tested in an individual subject before proceeding to studies with the other drug.
Data analysis
For phase 1 (dose-effect studies), dose-effect curves for cocaine and methadone self-administration were plotted to show the number of injections/session as a function of unit drug dose in milligrams/kilogram/injection (log scale). Rates of cocaine and methadone self-administration were compared to rates of saline-maintained responding by repeated-measures one-way analysis of variance (ANOVA) with unit drug dose as the main factor. Dunnett post-hoc tests were conducted to determine which unit drug doses maintained self-administration rates significantly different from those maintained by saline.
For phase 2 (FR studies), individual data from the last 2 days at each FR for each test solution were averaged, and these individual data were averaged to yield mean group data that were then plotted and analyzed in three ways. First, data were plotted as the number of injections/session as a function of FR (log scale). FR-effect curves for each drug solution were compared by repeated-measures two-way ANOVA, with drug solution and FR as the two main factors. A significant main effect was followed by the Bonferroni post-hoc method for multiple comparisons to compare self-administration rates at each FR. This preliminary analysis was then supplemented by two additional and complementary strategies for quantifying and comparing metrics of reinforcement (Hursh and Silberberg 2008; Negus et al. 2008). First, rates of self-administration for each test solution at each FR were normalized as the percent baseline number of injections/session delivered for that solution at an FR1. This approach controlled for different baseline rates of self-administration and permitted assessment of FR effects on baseline self-administration rates. Normalized rates of self-administration were then plotted as a function of log FR, and linear regression was used to determine the FR and 95% confidence limits at which self-administration decreased to 50% of baseline (the effective FR producing 50% baseline self-administration, abbreviated EFR50). All points between 20% and 80% baseline were included in the linear regression, as were the lowest FR associated with rates greater than 80% baseline and the highest FR associated with rates less than 20% baseline. Mean EFR50 values were compared using a repeated-measures one-way ANOVA with drug solution as the main factor. Second, data were also analyzed according to a behavioral economic demand curve approach where consumption (number of self-administered drug injections) was plotted as a function of price (FR requirement per injection), and the resulting curve was analyzed using the recently introduced exponential model of demand (Hursh and Silberberg 2008). This model is akin to previous adaptations of microeconomics concepts applied to the study of drug reinforcement (Hursh 1991; Hursh and Winger 1995; Negus et al. 2008; Wade-Galuska et al. 2007; Winger et al. 2002). Specifically, data were fit to the equation log Q=log Q0+k(e−α Q0C−1), where Q is the experimentally determined measure of consumption at any particular FR, Q0 is the predicted absolute consumption at price 0 and specifies the highest level of demand, k is the range of the exponential demand curve in log units shared across all individuals and conditions, α is the rate of change in consumption as a function of price and the primary measure of reinforcement, and C is a measure of cost and the primary independent variable, expressed here as the FR value. For drug self-administration, the Q0 parameter reflects pharmacological effects (e.g., dose) on drug intake, whereas α reflects reinforcement as the rate of change in consumption across the entire range of drug doses and FR values regardless of absolute consumption at any one dose or FR value. Because the α parameter is independent of consumption at any particular drug dose or FR value, it may provide a more comprehensive measure of reinforcement than approaches relying on a single point along the demand curve, such as EFR50 (described above) or the traditional behavioral economic metric Pmax (the point of maximum behavioral output). For the present study, drug injections per session at each FR value were entered into a custom-designed GraphPad 5 template embedded with the exponential model using the non-linear regression function (GraphPad Software, San Diego, CA, USA; template available upon request from PGR). Because log 0 cannot be calculated, data points from conditions that produced 0 consumption (i.e., no reinforcers delivered) were entered as 0.1. The k parameter was fixed at 4, and the primary dependent variables of Q0 and α were generated for each individual monkey under each condition. Insofar as α alone indicates sensitivity to price, a lower value suggests greater reinforcement. To make interpretation more intuitive, we used the inverse (1/α) for our analyses so that a larger value reflects greater reinforcement. Mean Q0 and 1/α values were then analyzed by repeated-measures one-way ANOVAs with drug solution as the main factor.
In addition to the analyses described above, the correlation between EFR50 and 1/α metrics of reinforcement was examined by linear regression using GraphPad 5. Statistical significance was set a priori at p<0.05 for all analyses.
Drugs (±)methadone HCl and cocaine HCl (National Institute on Drug Abuse, Bethesda, MD, USA) were dissolved in sterile water. SNC80 base (provided by Dr. Kenner C. Rice) was dissolved in 4% lactic acid in sterile water to a final concentration of 50 mg/ml, and dilutions were made with sterile water. All drug solutions were filter-sterilized using 0.22 μm Millipore filter and delivered intravenously. Doses were determined based on the salt or base forms listed above.
Results
Methadone and cocaine dose-effect curves
Figure 1 shows drug self-administration dose-effect curves for methadone and cocaine alone. The dose-effect curves for both methadone and cocaine displayed the prototypic inverted-U shape. For methadone, repeated-measures one-way ANOVA revealed a significant main effect of methadone dose (F(4,12)=3.4, p<0.05), and post-hoc analysis demonstrated that 0.01 mg/kg/inj methadone maintained significantly greater self-administration rates than saline (p<0.05). For cocaine, repeated-measures one-way ANOVA revealed a significant main effect of cocaine dose (F(5,10)=5.0, p<0.05). Post-hoc analysis revealed that 0.01 and 0.032 mg/kg/inj cocaine maintained significantly greater self-administration rates than saline (p<0.05). Based on these results, doses of 0.032 mg/kg/inj methadone and 0.032 mg/kg/inj cocaine were selected for phase 2 experiments involving the FR manipulations.
Fig. 1.
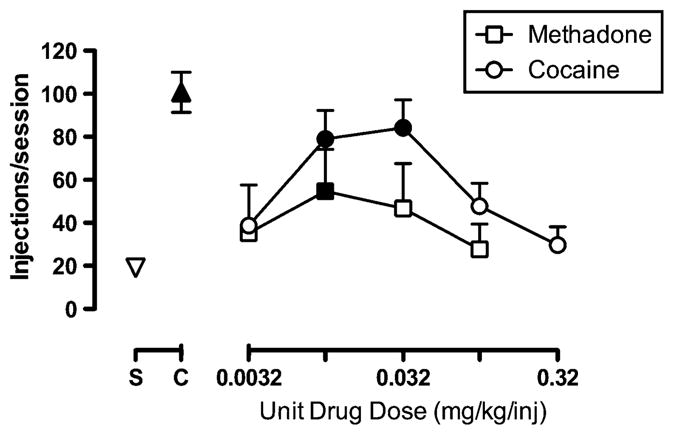
Self-administration of intravenous methadone (0.0032–0.1 mg/ kg/inj) or cocaine (0.0032–0.32 mg/kg/inj) under a fixed-ratio 10 schedule of reinforcement in rhesus monkeys (n=4). Abscissa: unit dose of methadone or cocaine in milligrams/kilogram/injection (log scale). Ordinate: number of injections earned in the 120-min session. Downward triangle above S represents the mean±SEM number of injections delivered during saline substitution sessions that preceded test sessions. Upward triangle above C represents mean±SEM number of injections delivered during maintenance sessions of 0.032 mg/kg/inj cocaine availability that preceded test sessions. Filled symbols represent drug doses that maintained significantly greater self-administration than saline (p<0.05)
FR manipulations for methadone alone and in combination with SNC80
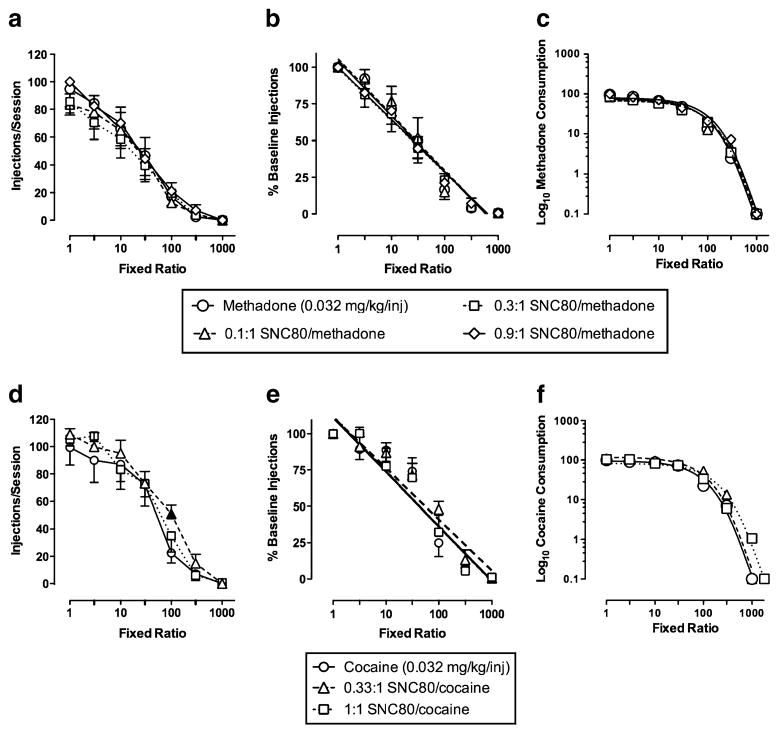
Figure 2 shows the effects of FR manipulations on self-administration maintained by methadone (0.032 mg/kg/inj) alone or in three mixtures with SNC80. Repeated-measures two-way ANOVA on data in the upper left of Fig. 2a revealed a main effect of fixed-ratio value (F (6,18)=44.5, p<0.05), but no significant main effect of SNC80 proportion and no significant interaction between fixed ratio and SNC80 proportion (p>0.05). The upper middle of Fig. 2b shows the same data as in Fig. 2a expressed as the percent self-administration rate at FR 1. Linear regression through these data was used to generate EFR50 values (Table 1) as a measure of reinforcement. The upper right of Fig. 2c shows the same data expressed as normalized demand curves. Non-linear regression through these curves using the exponential model of demand was used to generate the 1/α parameter (Table 1) as a measure of reinforcement. Repeated-measures one-way ANOVAs revealed no significant main effects of SNC80 on the EFR50 or 1/α measure of methadone reinforcement, or on predicted methadone consumption at zero price (Q0).
Fig. 2.
Effects of fixed-ratio manipulations on responding maintained by methadone (top panels) or cocaine (bottom panels) available alone or in mixtures with the delta-opioid agonist SNC80. Left panels (a and d) show the effects of varying the fixed ratio (log scale) on the numbers of injections per session. Center panels (b and e) show the same data but with rates of self-administration at each FR expressed as the percent self-administration rate at FR 1. Linear regression through these data was used to generate EFR50 values as a measure of reinforcement. Right panels (c and f) show the same data expressed as normalized demand curves. Non-linear regression through these curves using the exponential model of demand was used to generate 1/α values as a measure of reinforcement. All points represent the mean of four rhesus monkeys, and error bars show SEM. Filled symbols represent fixed ratios at which cocaine in some combination with SNC80 maintained significantly greater rates of self-administration than cocaine alone (p<0.05)
Table 1.
Mean EFR50, 1/α and Q0 values (± SEM) obtained for methadone (0.032 mg/kg/inj) available alone or in combination with various proportions of the delta-opioid agonist SNC80
| Self-administered solution | EFR50 | 1/α | Q0 |
|---|---|---|---|
| Methadone alone | 31 (±11) | 39,440 (±5,690) | 93 (±23) |
| 0.1:1 SNC80/methadone | 36 (±14) | 43,760 (±14,511) | 82 (±12) |
| 0.3:1 SNC80/methadone | 29 (±10) | 51,535 (±6,144) | 68 (±13) |
| 0.9:1 SNC80/methadone | 29 (±10) | 57,641 (±13,013) | 87 (±7) |
| ANOVAs |
F(3,9)=0.13 p>0.05 |
F(3,9)=1.02 p>0.05 |
F(3,9)=1.48 p>0.05 |
ANOVA results for effect of self-administered solution on EFR50, 1/α and Q0 values are shown in the bottom row
FR manipulations for cocaine alone and in combination with SNC80
Figure 2 also shows the effects of FR manipulations on self-administration maintained by cocaine (0.032 mg/kg/inj) alone or in two mixtures with SNC80. Repeated-measures two-way ANOVA of the data shown in the lower left of Fig. 2d revealed a significant main effect of SNC80 proportion (F(2,6)=6.4, p<0.05) and a main effect of fixed ratio (F(6,18)=54.7, p<0.05), but no significant interaction between SNC80 proportion and fixed ratio (p>0.05). Post-hoc analysis demonstrated that the 0.33:1 SNC80/cocaine mixture maintained significantly (p<0.05) greater rates of self-administration than cocaine alone at FR 100. The lower middle of Fig. 2e shows the same data as in Fig. 2d expressed as the percent self-administration rate at FR 1, and the lower right of Fig. 2f shows the same data expressed as normalized demand curves. Repeated-measures ANOVAs revealed no significant main effects of SNC80 on the EFR50 or 1/α measure of cocaine reinforcement, or on predicted cocaine consumption at zero price (Q0) (Table 2). Thus, the significant increase in self-administration observed at one FR value for one SNC80/cocaine proportion was not sufficient to significantly change these behavioral economic measures of cocaine reinforcement. Studies with a higher proportion of 3:1 SNC80/cocaine were terminated due to the emergence of convulsant effects.
Table 2.
Mean EFR50, 1/α and Q0 values (±SEM) obtained for cocaine (0.032 mg/kg/inj) available alone or in combination with various proportions of the delta-opioid agonist SNC80
| Self-administered solution | EFR50 | 1/α | Q0 |
|---|---|---|---|
| Cocaine alone | 59 (±16) | 66,182 (±16,663) | 112 (±16) |
| 0.33:1 SNC80/cocaine | 76 (±26) | 80,736 (±15,642) | 128 (±13) |
| 1:1 SNC80/cocaine | 59 (±22) | 81,792 (±29,588) | 106 (±10) |
| ANOVAs |
F(2,6)=0.40 p>0.05 |
F(2,6)=0.39 p>0.05 |
F(2,6)=4.77 p>0.05 |
ANOVA results for effect of self-administered solution on EFR50, 1/α and Q0 values are shown in the bottom row
Correlation between EFR50 and 1/α values
Figure 3 shows that the EFR50 and 1/α metrics of reinforcement were significantly correlated. Specifically, log EFR50 values are shown as a function of log 1/α values, and linear regression demonstrated that the slope of the line drawn through these points was significantly different from zero with an R2 of 0.68. The Pearson’s r value (95% confidence limits) was 0.83 (0.19–0.97).
Fig. 3.
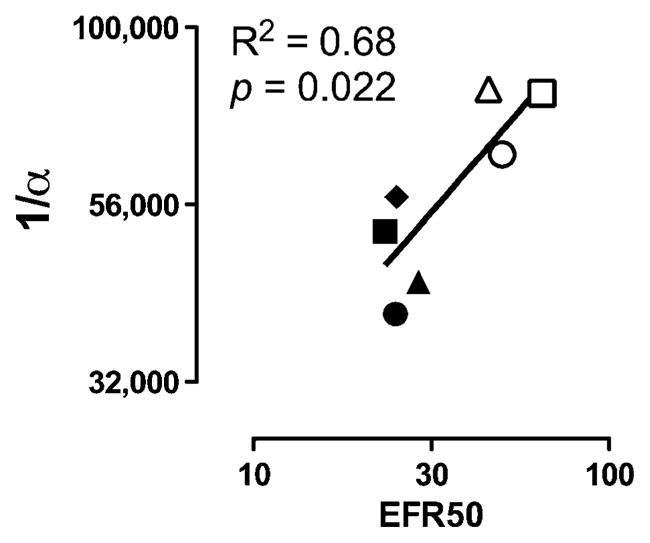
Correlation between EFR50 and 1/α metrics of reinforcement derived from curves shown in Fig. 2 b, c, e and f. Abscissa: log EFR50 values. Ordinate: log 1/α values. Each point represents the mean of four monkeys, and different symbols represent the different treatment conditions as shown in Fig. 2. Filled points represent methadone alone (filled circle) or in combinations with SNC80. Open points represent cocaine alone (open circle) or in combinations with SNC80. Linear regression was used to determine R2 and p values
Discussion
The main finding of this study was that the delta agonist SNC80 failed to alter the reinforcing effects of the mu agonist methadone at SNC80/methadone proportions previously shown to synergistically enhance methadone-induced antinociception (Stevenson et al. 2003). This agrees with a previous report that SNC80 enhanced antinociceptive but not reinforcing effects of heroin in rhesus monkeys (Stevenson et al. 2005) and extends this dissociation between antinociceptive and reinforcement-related delta/mu interactions to the frequently prescribed opioid analgesic methadone as assessed using behavioral economic measures. Overall, these findings support the proposition that delta agonists may be useful adjuncts that selectively enhance analgesic effects of mu opioids without enhancing abuse liability.
Preclinical expression and clinical implications of interactions between delta and mu opioid agonists
We and others have shown that delta and mu-opioid agonist combinations produce synergistic antinociception in both rodents and nonhuman primates (Adams et al. 1993; Banks et al. 2008; Dykstra et al. 2002; Heyman et al. 1989; Negus et al. 2009; Stevenson et al. 2003; Stevenson et al. 2005). Furthermore, synergistic delta–mu interactions appear to display some selectivity for antinociception, because additive, sub-additive, or antagonistic interactions have been observed for other endpoints such as suppression of food-maintained responding, bladder motility, and respiratory depression (Adams et al. 1993; Banks et al. 2010; Dykstra et al. 2002; Negus et al. 2009; O’Neill et al. 1997; Sheldon et al. 1989; Stevenson et al. 2003; Stevenson et al. 2005; Su et al. 1998). The present study extends these findings to behavioral economic measures of abuse liability and supports the general observation that delta and mu agonists do not synergistically enhance many of each other’s non-antinociceptive effects.
Further research will be required to explore the limits of this general observation, but the apparent selectivity of synergistic delta/mu interactions for antinociceptive endpoints suggests that delta agonists may enhance analgesia-related effects of mu opioids without enhancing potentially undesirable mu agonist effects related to sedation, respiratory depression, or abuse liability. Moreover, mu agonists attenuate delta agonist-induced convulsant effects (O’Neill et al. 1997). Overall, then, combined activation of delta and mu receptors may be more effective and/or safer for the treatment of pain than selective activation of mu or delta receptors alone.
The present study focused on potential delta/mu interactions on behavioral economic measures of drug reinforcement, and it was found that various proportions of SNC80 did not enhance the reinforcing effects of methadone. This finding is consistent with a previous report that SNC80 did not enhance heroin reinforcement (Stevenson et al. 2005), but several limitations of the present study should be noted. First, SNC80 proportions were evaluated in combination with only a single dose of methadone, and it is possible that SNC80 may have altered reinforcing effects of other methadone doses. One factor militating against this possibility is that the present study employed an experimental design that generated normalized demand curves for methadone±SNC80, and previous research with mu agonists and other classes of abused drugs have found that different doses of a given drug typically yield data that conform to a single underlying demand curve (e.g., Hursh and Winger 1995). Second, delta/mu interactions were examined with only the high-efficacy delta agonist SNC80 and the high-efficacy mu agonist methadone, and it is possible that other delta or mu agonists may have produced synergistic reinforcing effects. These high-efficacy agonists were used in part because they produced synergistic anti-nociception (Stevenson et al. 2003), and a primary goal of this study was to evaluate the degree to which synergistic delta/ mu antinociception might be accompanied by synergistic reinforcement. Delta/mu interactions involving lower-efficacy mu agonists may be of interest for future efforts to develop effective analgesics with low abuse liability, because SNC80 also synergistically enhanced antinociception produced by lower-efficacy mu agonists (e.g., nalbuphine; Stevenson et al. 2003). Conversely, interactions involving lower efficacy delta agonists may be of lesser interest, because agonists with lower efficacy at delta receptors than SNC80 produced little or no enhancement of mu agonist antinociception in monkeys (Negus et al. 2009). A final limitation of this study was that it examined delta/mu interactions in the absence of opioid dependence to permit comparison to previous studies of delta/ mu antinociceptive interactions in non-dependent monkeys and because opioids are often used to treat pain in non-dependent patients. However, opioid dependence can increase the reinforcing effects of mu agonists (Negus 2006), and future studies of delta/mu interactions under conditions of opioid dependence may be warranted insofar as patients receiving chronic opioids to treat chronic pain may develop opioid dependence.
Interactions between cocaine and SNC80
SNC80 alone did not support drug self-administration when substituted for cocaine in rhesus monkeys maintained on cocaine (Negus et al. 1998), and in the present study, SNC80 did not enhance behavioral economic measures of cocaine reinforcement in monkeys maintained on cocaine. We interpret these results to suggest that a cocaine self-administration history did not increase the reinforcing effects of SNC80 and was unlikely to confound evaluations of SNC80 effects on the reinforcing effects of substitution drugs (in this case, methadone). The failure of SNC80 to modulate cocaine reinforcement contrasts with previous findings that SNC80 may share and/or enhance other abuse-related effects of cocaine, including the discriminative stimulus effects of cocaine in rhesus monkeys (Negus et al. 1998) and the locomotor effects of cocaine in rats (Jutkiewicz et al. 2008). The emergence of convulsant activity in the one monkey tested with 3:1 SNC80/cocaine also suggests a positive interaction between the convulsant effects of SNC80 and cocaine (Danielsson et al. 2006; Matsuzaki 1978).
Behavioral economics as a strategy to examine reinforcing effects of drug combinations
Various strategies are available for evaluation of drug interactions on measures of reinforcement, and each approach has strengths and limitations. We have now used three approaches to assess reinforcing effects of delta/mu combinations. First, we examined self-administration of SNC80/heroin mixtures under a FR 30 schedule of reinforcement, and SNC80 produced a proportion-dependent decrease in rates of heroin self-administration. However, self-administration rates under FR schedules are determined not only by the reinforcing effects of the self-administered drug, but also by non-specific drug effects that often suppress responding (Woolverton and Johanson 1984; Woolverton and Nader 1990). Consequently, we could not definitively ascertain whether SNC80 decreased heroin reinforcement or enhanced heroin-induced suppression of response rates. As a second approach, concurrent-choice schedules generate a primary dependent measure (percent drug choice) that may be less sensitive to non-specific drug effects (Banks et al. 2008; Griffiths et al. 1975; Negus 2003). In a heroin versus food choice procedure, we found that varying proportions of SNC80 did not alter percent heroin choice, suggesting that SNC80 did not change heroin reinforcement (Stevenson et al. 2005). However, a limit of choice procedures is that experimental manipulations may alter the relative reinforcing effects of both reinforcers equally, resulting in no change in response allocation. The present study used a behavioral economic approach in which demand curves were generated from rates of drug self-administration at different FR values, and EFR50 and 1/α values were derived as metrics of reinforcement that were correlated with each other, relatively independent of non-specific drug effects, and free of the influence of alternative reinforcers (Hursh and Silberberg 2008; Negus et al. 2008). A limitation to this approach is the relatively long time required to test each drug or drug mixture dose; however, application of this approach also indicated no effect of SNC80 on mu agonist reinforcement. Taken together, these data provide convergent support for the conclusion that delta agonists do not enhance the reinforcing effects of mu agonists in rhesus monkeys.
Acknowledgments
We appreciate the technical assistance of Jennifer Gough. This research was supported by National Institutes of Health grants R01-DA011460 and T32-DA007027.
Contributor Information
Matthew L. Banks, Department of Pharmacology and Toxicology, Virginia Commonwealth University, 410 North 12th Street, PO Box 980613, Richmond, VA 23298, USA
Peter G. Roma, Institute for Behavior Resources, Baltimore, MD, USA. Department of Psychiatry and Behavioral Sciences, Johns Hopkins University, Baltimore, MD, USA
John E. Folk, Chemical Biology Research Branch, National Institute on Drug Abuse, National Institutes of Health, DHHS, Bethesda, MD, USA
Kenner C. Rice, Chemical Biology Research Branch, National Institute on Drug Abuse, National Institutes of Health, DHHS, Bethesda, MD, USA
S. Stevens Negus, Email: ssnegus@vcu.edu, Department of Pharmacology and Toxicology, Virginia Commonwealth University, 410 North 12th Street, PO Box 980613, Richmond, VA 23298, USA.
References
- Adams JU, Tallarida RJ, Geller EB, Adler MW. Isobolo-graphic superadditivity between delta and mu opioid agonists in the rat depends on the ratio of compounds, the mu agonist and the analgesic assay used. J Pharmacol Exp Ther. 1993;266:1261–1267. [PubMed] [Google Scholar]
- Banks ML, Gould RW, Czoty PW, Nader MA. Relationship between response rates and measures of reinforcing strength using a choice procedure in monkeys. Behav Pharmacol. 2008;19:365–369. doi: 10.1097/FBP.0b013e32830990bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Folk JE, Rice KC, Negus SS. Selective enhancement of fentanyl-induced antinociception by the delta agonist SNC162 but not by ketamine in rhesus monkeys: further evidence supportive of delta agonists as candidate adjuncts to mu opioid analgesics. Pharmacol Biochem Behav. 2010;97:205–212. doi: 10.1016/j.pbb.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson I, Gasior M, Stevenson GW, Folk JE, Rice KC, Negus SS. Electroencephalographic effects of the delta opioid agonist SNC80 in rhesus monkeys. Pharmacol Biochem Behav. 2006;85:428–434. doi: 10.1016/j.pbb.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietis N, Guerrini R, Calo G, Salvadori S, Rowbotham DJ, Lambert DG. Simultaneous targeting of multiple opioid receptors: a strategy to improve side-effect profile. Br J Anaesth. 2009;103:38–49. doi: 10.1093/bja/aep129. [DOI] [PubMed] [Google Scholar]
- Dykstra L, Granger A, Allen R, Zhang X, Rice K. Antinociceptive effects of the selective delta opioid agonist SNC80 alone and in combination with mu opioids in the squirrel monkey titration procedure. Psychopharmacology. 2002;163:420–429. doi: 10.1007/s00213-002-1100-8. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Wurster RM, Brady JV. Discrete-trial choice procedure: effects of naloxone and methadone on choice between food and heroin. Pharmacol Rev. 1975;27:357–365. [PubMed] [Google Scholar]
- Heyman JS, Jiang Q, Rothman RB, Mosberg HI, Porreca F. Modulation of mu-mediated antinociception by delta agonists: characterization with antagonists. Eur J Pharmacol. 1989;169:43–52. doi: 10.1016/0014-2999(89)90815-7. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration and drug abuse policy. J Exp Anal Behav. 1991;56:377–393. doi: 10.1901/jeab.1991.56-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. Normalized demand for drugs and other reinforcers. J Exp Anal Behav. 1995;64:373–384. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Baladi MG, Folk JE, Rice KC, Woods JH. The δ-opioid receptor agonist SNC80 (+)-4-[Œ±(R)-Œ±-[(2 S, 5R)-4-allyl-2, 5-dimethyl-1-piperazinyl]-(3-methoxybenzyl)-N, N-diethylbenzamide] synergistically enhances the locomotor-activating effects of some psychomotor stimulants, but not direct dopamine agonists, in rats. J Pharmacol Exp Ther. 2008;324:714–724. doi: 10.1124/jpet.107.123844. [DOI] [PubMed] [Google Scholar]
- Ko MC, Terner J, Hursh S, Woods JH, Winger G. Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther. 2002;301:698–704. doi: 10.1124/jpet.301.2.698. [DOI] [PubMed] [Google Scholar]
- Leppert W. The role of methadone in cancer pain treatment—a review. Int J Clin Pract. 2009;63:1095–1109. doi: 10.1111/j.1742-1241.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- Linderbeck LR. Update of the clinical issues regarding methadone (Dolophine) therapy in pain management. AACN Adv Crit Care. 2008;19:253–257. doi: 10.1097/01.AACN.0000330373.26519.58. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M. Alteration in pattern of EEG activities and convulsant effect cocaine following chronic administration in the rhesus monkey. Electroencephalogr Clin Neurophysiol. 1978;45:1–15. doi: 10.1016/0013-4694(78)90336-x. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with D-amphetamine and flupenthixol. Neuro-psychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS. Delta opioids and substance abuse. In: Chang KJ, Porreca F, Woods JH, editors. The delta receptor. Marcel Dekker; New York: 2004. pp. 401–431. [Google Scholar]
- Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs. food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2009;34:899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Butelman E, Chang K, DeCosta B, Winger G, Woods J. Behavioral effects of the systemically active delta opioid agonist BW373U86 in rhesus monkeys. J Pharmacol Exp Ther. 1994;270:1025–1034. [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther. 1998;286:362–375. [PubMed] [Google Scholar]
- Negus SS, Schrode K, Stevenson GW. Mu/kappa opioid interactions in rhesus monkeys: implications for analgesia and abuse liability. Exp Clin Psychopharmacol. 2008;16:386–399. doi: 10.1037/a0013088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Bear AE, Folk JE, Rice KC. Role of delta opioid efficacy as a determinant of mu/delta opioid interactions in rhesus monkeys. Eur J Pharmacol. 2009;602:92–100. doi: 10.1016/j.ejphar.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill SJ, Collins MA, Pettit HO, McNutt RW, Chang KJ. Antagonistic modulation between the delta opioid agonist BW373U86 and the mu opioid agonist fentanyl in mice. J Pharmacol Exp Ther. 1997;282:271–277. [PubMed] [Google Scholar]
- Sheldon RJ, Nunan L, Porreca F. Differential modulation by [D-Pen2, D-Pen5]enkephalin and dynorphin A-(1–17) of the inhibitory bladder motility effects of selected mu agonists in vivo. J Pharmacol Exp Ther. 1989;249:462–469. [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Linsenmayer DC, Rice KC, Negus SS. Opioid interactions in rhesus monkeys: Effects of δ+μ and δ+ κ agonists on schedule-controlled responding and thermal nocicep-tion. J Pharmacol Exp Ther. 2003;307:1054–1064. doi: 10.1124/jpet.103.056515. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Rice KC, Negus SS. Interactions between delta and mu opioid agonists in assays of schedule-controlled responding, thermal nociception, drug self-administration, and drug versus food choice in rhesus monkeys: studies with SNC80 [(+)-4-[(alpha±R)-alpha-((2 S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylben-zamide] and heroin. JJ Pharmacol Exp Ther. 2005;314:221–231. doi: 10.1124/jpet.104.082685. [DOI] [PubMed] [Google Scholar]
- Su YF, McNutt RW, Chang KJ. Delta-opioid ligands reverse alfentanil-induced respiratory depression but not antinociception. J Pharmacol Exp Ther. 1998;287:815–823. [PubMed] [Google Scholar]
- Wade-Galuska T, Winger G, Woods J. A behavioral economic analysis of cocaine and remifentanil self-administration in rhesus monkeys. Psychopharmacology. 2007;194:563–572. doi: 10.1007/s00213-007-0858-0. [DOI] [PubMed] [Google Scholar]
- Winger G, Hursh SR, Casey KL, Woods JH. Relative reinforcing strength of three N-methyl-D-aspartate antagonists with different onsets of action. J Pharmacol Exp Ther. 2002;301:690–697. doi: 10.1124/jpet.301.2.690. [DOI] [PubMed] [Google Scholar]
- Winger G, Galuska CM, Hursh SR, Woods JH. Relative reinforcing effects of cocaine, remifentanil, and their combination in rhesus monkeys. J Pharmacol Exp Ther. 2006;318:223–229. doi: 10.1124/jpet.105.100461. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Johanson CE. Preference in rhesus monkeys given a choice between cocaine and d, l-cathinone. J Exp Anal Behav. 1984;41:35–43. doi: 10.1901/jeab.1984.41-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Nader MA. Experimental evaluation of the reinforcing effects of drugs. In: Adler MW, Cowans A, editors. Modern methods in pharmacology: testing and evaluation of drugs of abuse. Wiley-Liss, Inc; New York: 1990. pp. 165–192. [Google Scholar]