Abstract
Extra-adrenal myelolipoma is a relatively rare entity, with fewer than 50 cases reported in literature. We present a case of a 79 year-old female who presented for evaluation of hip fracture following trauma, where a lobulated presacral mass with mixed fat/soft tissue attenuation was incidentally seen on initial bone algorithm pelvic CT. Subsequent MRI showed signal characteristics of a lesion with mixed fat and soft tissue composition. The lesion demonstrated stability on follow-up imaging. An elective surgical resection was performed which yielded a grossly fatty mass. The diagnosis of presacral myelolipoma was confirmed on microscopic examination. Following description of our case, we conduct a literature review of the imaging characteristics, diagnosis, and treatment of presacral myelolipoma.
Keywords: Myelolipoma, extra adrenal myelolipoma, presacral myelolipoma, presacral MRI, presacral CT, lipomatous tumor, MR imaging
CASE REPORT
A 79-year-old female with known atrial fibrillation, hypercholesterolemia, well controlled hypertension, osteopenia, and temporal arteritis controlled by chronic corticosteroids initially presented for evaluation of possible right hip fracture following trauma.
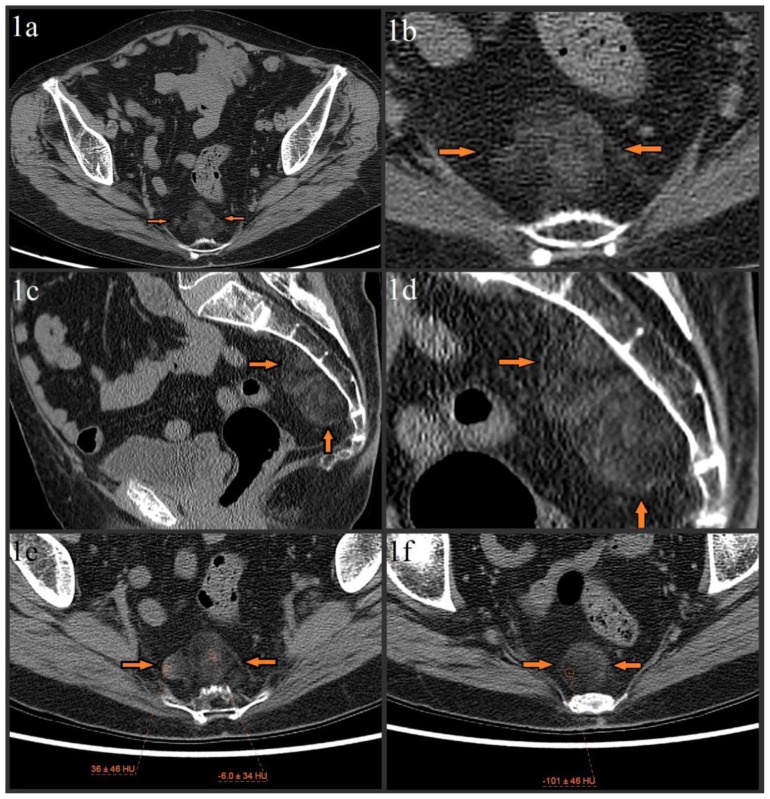
Initial pelvic/hip CT evaluation with bone algorithm showed mild degenerative changes of the bilateral hips and no acute hip fracture (not shown). No destructive osseous lesions were noted. A 5.8 × 2.9 × 4.8 cm lobulated, heterogeneous presacral mass with mixed fat and soft tissue attenuation was incidentally noted (figures 1a–1f). MR imaging was recommended for further characterization.
Figure 1.
79 year old female with presacral myelolipoma. The CT was performed on a GE® 64-slice CT scanner. All images are from the patient’s initial noncontrast bone algorithm pelvic CT with standard soft tissue windows (width = 400, center = 50). 2mm slices were acquired, using 120 kVp and variable mAs, which ranged from 614 mAs to 633 mAs for the provided axial images.
Figures 1a–1f: Axial (figure 1a, magnified in figure 1b) and sagittal (figure 1c, magnified in figure 1d) images show incidental 5.8 × 2.9 × 4.8 cm lobulated, heterogeneous presacral mass without evidence of erosion/invasion of the anterior sacrum (orange arrows). The mass spans from S3 to S5 and is composed of fat and soft tissue elements. Precise measurement of the soft tissue elements’ attenuation is difficult due to significant admixture, but attempts show a range from about −10 HU to about 20 HU, with a single area of higher (mid 30 HUs) attenuation in the lesion’s right lateral margin (image 1e). The fat elements have attenuation values of about −100 HU (image 1f).
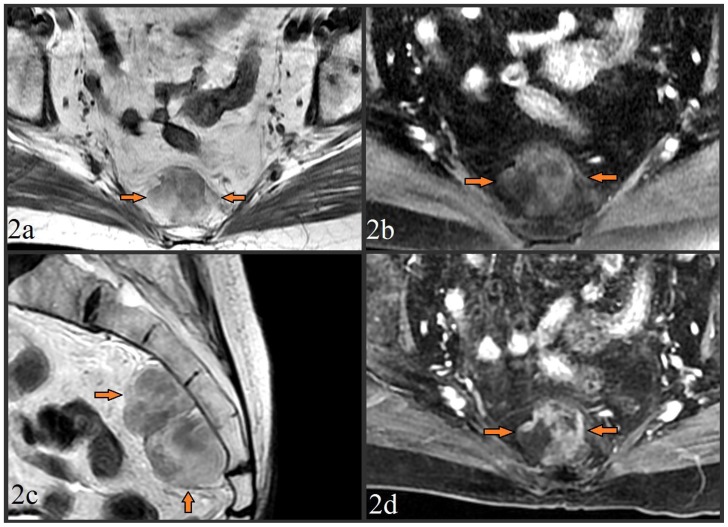
Subsequent pelvic MR showed a 6.4 × 3.1 × 5.7 cm mass posterior to the rectosigmoid colon which abutted the anterior aspects of the S3–S5 vertebrae. T1-weighted fast spin-echo (FSE) imaging demonstrated the lesion to be predominately iso-intense compared to surrounding fat (figure 2a), with “drop out” of signal intensity in these fatty areas on the corresponding fat-suppressed FSE T1-weighted sequence (figure 2b). No invasion of the sacral cortex or lower sacral nerve roots was seen (figure 2c). The fatty regions were again noted to be admixed with areas of soft tissue composition, which enhanced on post-gadolinium T1-weighted fat-suppressed liver acquisition and volume acquisition (LAVA) images (figure 2d). A differential diagnosis of extra-adrenal presacral myelolipoma versus low grade liposarcoma and immature teratoma was suggested.
Figure 2.
MR images from a 79 year old female with presacral myelolipoma. All images were acquired on a GE® 1.5 Tesla MR scanner.
Figure 2a. Pre-contrast T1-weighted fast spin echo (FSE) axial image (TR=500.0, TE=9.0, TI=0.0, FA=90.0) at the level of the midsacrum demonstrated a 6.4 × 3.1 × 5.7 cm lobulated presacral mass with mixed fat/soft tissue signal (arrows). No bony invasion was seen.
Figure 2b. Pre-contrast fat-suppressed T1-weighted FSE axial image (TR=516.7, TE=7.7, TI=0.0, FA=90.0) taken at the same level, showed loss of signal intensity in the areas which were previously iso-intense to fat, providing further confirmation of a significant fat component of the mass.
Figure 2c. Pre-contrast T2-weighted FSE image (TR=5800.0, TE=119.8, TI=0.0, FA=90.0) in the sagittal plane to provide craniocaudad visualization of the 6.4 × 3.1 × 5.7 cm mass which spanned from S3 to S5. The mass was again noted to be lobulated, of mixed fat/soft tissue constituency, and without bony invasion.
Figure 2d. Post-contrast (15cc of gadopentetate dimeglumine) T1-weighted fat-suppressed liver acquisition with volume acquisition (LAVA) sequence axial image (TR=4.4, TE=2.1, TI=7.0, FA=12.0) taken at the same level of images 2b/2c showed enhancement of the non-fatty soft tissue elements, and redemonstrated loss of signal in the mass’ fatty elements.
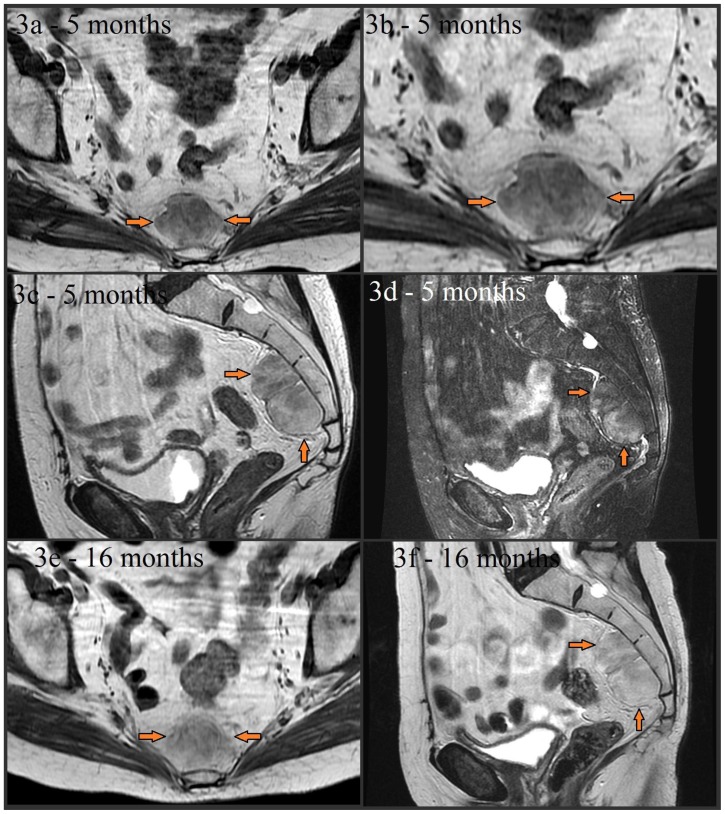
Follow up MRI at 5 and 16 months was performed, which failed to demonstrate any significant interval growth or change in signal characteristics (figures 3a–3f). Preoperative biopsy was not performed, as the diagnostic possibility of liposarcoma raised concern for potential biopsy tract malignant seeding. Although the mass remained stable in size, the patient’s anxiety about possible malignancy and the overall size of the mass prompted the patient to pursue elective surgical resection.
Figure 3.
Noncontrast MR images from a 79 year old (80 year old at the time of images 3e and 3f) female with presacral myelolipoma. All images were acquired on a GE® 1.5 Tesla MR scanner.
Follow-up axial noncontrast T1-weighted FSE (image 3a and magnified in 3b, TR=400.0, TE=8.5, TI=0.0, FA=90.0), noncontrast sagittal T2-weighted (image 3c, TR=3166.7, TE=122.1, TI=0.0, FA=90.0), and noncontrast sagittal T2-weighted fat-suppressed (image 3d, TR=3450.0, TE=122.1, TI=0.0, FA=90.0) images taken about 5 months later showed stability of the presacral mass (arrows) without significant interval change in size, appearance, or signal characteristics. The mass is again noted to be lobulated, well-circumscribed, and have mixed soft tissue and fat composition (with signal dropout on fat-suppressed image 3d). A lack of sacral invasion is again seen. Noncontrast T1-weighted FSE (image 3e, TR=433.3, TE=7.5, TI=0.0, FA=90.0) and noncontrast sagittal T2-weighted (image 3f, TR=5450.0, TE=119.9, TI=0.0, FA=90.0) images obtained 16 months later also showed continued overall stability.
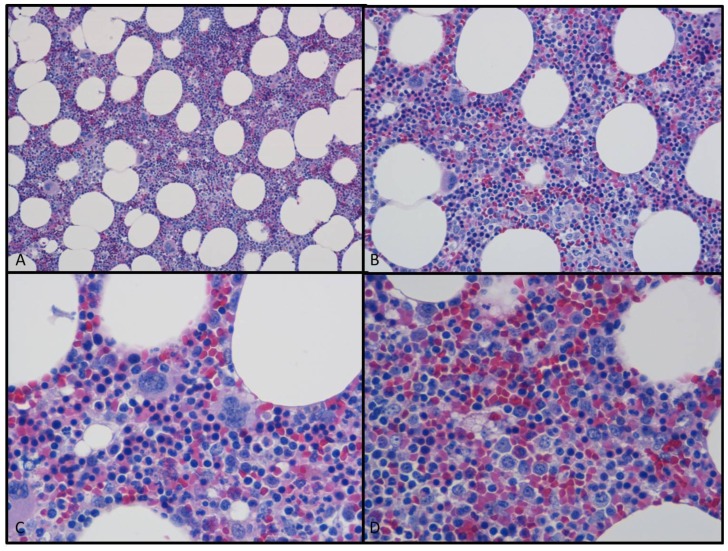
Following placement of an epidural catheter for analgesia and administration of general anesthesia, the patient’s abdomen was entered by a lower midline incision. Small bowel was mobilized out of the pelvis and packed in the upper abdomen. The peritoneum overlying the base of the rectal mesentery was incised in order to gain access to the presacral mass. The mass was noted to be somewhat adherent to the sacrum, requiring dissection for satisfactory separation. The gross specimen measured approximately 7.0 × 3.0 × 5.9 cm and was described as a fatty mass on gross pathology. No bony erosion or infiltration was noted at surgery. Microscopic examination (figures 4a–4d) showed mature adipose tissue with areas of extramedullary hematopoiesis and focal myxoid/reactive changes. These collective findings yielded a pathologic diagnosis of presacral myelolipoma.
Figure 4.
Photomicrograph from the obtained surgical specimen using H&E (hematoxylin and eosin) staining. Images 4a (10× magnification) and 4b (20× magnification) showed mature adipose tissue with prominent cellular stroma. Images 4c and 4d are higher magnification (both 40x) views which showed that the stroma consisted of all three hematopoietic cell lineages; myeloid, erythroid, and megakaryocytic forming cell lines.
The patient recovered well from the surgery without complication, and was discharged home on postoperative day 2. There has been no complication or tumor recurrence since the procedure.
DISCUSSION
Myelolipomas are benign lesions which contain mature adipose cells and combinations of myeloid and erythroid elements [1]. They almost always occur in the adrenal glands, with less than 50 cases of extra-adrenal myelolipomas having been reported in literature. The presacral region is the most frequent extra-adrenal location. Other reported locations include the pelvic retroperitoneum, musculofascial tissue, mediastinum, kidney, stomach, and liver [1–7]. The etiology of myelolipomas is unknown, although some theories suggest metaplasia of uncommitted adrenal cortical mesenchymal cells or migration of hematopoietic tissue to the adrenal gland during intrauterine development. Another potential explanation which may have particular relevance to extra-adrenal myelolipoma involves reactivation of peritoneal connective tissue hematopoiesis (which is normally seen in embryonic life), a phenomenon which has been observed in certain septic and infectious conditions, and in rats after administration of ACTH and methyltestosterone [2,4–5,8–11].
Extra-adrenal myelolipomas are typically well-circumscribed round or oval masses. They are variable in size, and have been reported to measure up to 26 cm in diameter [5]. They are often incidentally found in older patients with a female to male predominance of 2:1 [5–6]. There has been some association of presacral myelolipoma with Cushing syndrome, Addison disease, adrenal hyperplasia, and chronic exogenous steroid use (which was noted in our case, as the patient was taking chronic prednisone for suppression of temporal arteritis) [6]. Presacral myelolipomas are typically asymptomatic. However, continued growth can lead to symptoms from hemorrhage or mass effect on adjacent structures (namely the bladder, sacral nerve plexus, rectum, and ureters) [1,3,5–6]. Malignant degeneration has not been reported [5]. Small areas of hemorrhage within the mass can lead to calcification, and like our case, the masses can adhere to the sacrum without frank bone invasion.
The imaging characteristics of extra-adrenal myelolipomas are similar to their adrenal counterparts, having an appearance that varies with the composition of the tumor. Ultrasonography will show a hyperechoic mass if the tumor is predominately fatty, or mostly hypoechoic echotexture if the tumor is primarily composed of erythroid/myeloid cells [5,12]. Small areas of hemorrhage within the mass can eventually calcify and obscure internal echotexture [12–13].
Similarly, myelolipoma appearance on CT also depends on the composition of the lesion. An encapsulated mass (again, typically in the adrenal) with macroscopic fatty tissue intermixed with areas of soft tissue can strongly suggest the diagnosis of myelolipoma [5,12]. Although adrenal adenomas can be lipid-rich, their density is not usually less than −20 HU, while myelolipomas usually (but not always) have some areas with attenuations less than −30 HU [12–13]. The amount of fat in myelolipomas is quite variable, and some areas can have higher attenuation values if the fat is diffusely mixed with hematopoietic tissue [13]. The hematopoietic soft tissue elements may enhance after injection of intravenous contrast [5,12].
On MR imaging, the fatty components of a myelolipoma yield high signal intensity on T1-weighted images, which demonstrate fat suppression when applied. Hematopoietic elements will have lower signal intensity on T1-weighted images and intermediate signal intensity on T2-weighted images. Intra-tumoral hemorrhage may also be seen, and its resultant appearance and intensity characteristics will vary depending on the age of the blood [5]. As our images show, the liver acquisition with volume acquisition (LAVA) sequence can aid in the distinction of hematopoietic and fat elements, as the unique fat suppression technique generates enhanced contrast of soft tissue from fat compared to a more typical fat suppression method. In-phase and out-of-phase gradient recalled echo (GRE) sequences may also be useful, as areas of a lesion with intracellular fat will lose signal on out-of-phase images, while areas of macroscopic fat (more typical of myelolipoma) will maintain signal intensity [12]. As in our case, the hematopoietic soft tissue elements may enhance after administration of intravenous gadolinium contrast [5,12].
Differentiating presacral myelolipoma from other fat containing retroperitoneal tumors (namely liposarcoma, teratoma, and extramedullary hematopoiesis) may be difficult depending on the amount of visible fat and clinical history, as there is overlap in their imaging and microscopic appearances. Thus, histologic correlation is often required. Although neurogenic tumors (ganglioneuroma, ganglioneuroblastoma, schwannoma, neurofibroma, etc.) are the second most common type of presacral tumors, they are usually easily distinguished from potential myelolipoma due to the heterogeneous, macroscopic fat-containing appearance of myelolipoma which is not typical for neurogenic tumors [14,15].
Well-differentiated liposarcoma is the most common fat-containing retroperitoneal tumor [16,17]. The malignant nature of the tumor causes it to exhibit more infiltrative growth without the well-defined borders and circumscription that myelolipoma tends to exhibit. Additionally, on biopsy, liposarcomas contain lipoblasts and cellular atypia as opposed to the mature adipose, myeloid, and erythroid cells seen in myelolipoma [7,17].
On imaging, teratomas also may contain areas of fat, soft tissue, and calcium (calcium may be seen in myelolipoma following hemorrhage), rendering their inclusion in the differential diagnosis logical. However, microscopic examination may also show other mesenchymal tissue elements, none of which were seen in our case [7].
Extramedullary hematopoietic tumors may resemble myelolipoma microscopically, as microscopic fat may be present within rests of erythroid and myeloid hyperplasia. Like extra-adrenal myelolipoma, extramedullary hematopoietic tumors may be uncommonly found in the presacral space (they are most commonly seen in the mediastinum, and less commonly in the spinal epidural space, falx cerebri, or perirenal space) [2,4,7]. However, their imaging characteristics are quite different than myelolipoma, as they are usually multifocal, poorly circumscribed, and with insufficient macroscopic fat to allow detection by gross or radiologic means. Their clinical settings are also different, as extramedullary hematopoietic tumors are usually seen in young to middle aged men, and in association with myeloproliferative disorders and chronic hemolytic anemias, as opposed to the 2:1 older female preponderance and asymptomatic/incidental nature of extra-adrenal myelolipoma [2,4,7].
The role of preoperative biopsy for any tumor which is presacral in location is debated. Traditionally, many surgeons consider presacral biopsy a contraindication in any lesion which is resectable due to the risks of seeding the biopsy needle tract with potentially malignant cells and infecting the lesion (particularly for transperitoneal, transretroperitoneal, transrectal, and transvaginal approaches) [15]. Others suggest that preoperative biopsy should be considered, as the pathologic results have the ability to strongly impact management, particularly for lesions which are large or invade adjacent structures [15].
There are three common approaches for surgical resection of presacral tumors: the anterior (transabdominal) approach, the posterior (perineal) approach, and the combined abdominoperineal approach. In general, the anterior approach is used for lesions without sacral invasion and with an inferior margin above the level of S4 [15,18]. The posterior approach is usually used for small, benign lesions which are below the level of S3. This approach can be further subdivided into transrectal, transsacral, transsphincteric, transanorectal, and transsacrococcygeal techniques. For presacral tumors which span above and below the level of S3 (such as our patient’s myelolipoma), a combined abdominoperineal approach from a low midline incision can be used (very similar to the technique used in our case) [15,18].
In summary, an older woman presenting with an asymptomatic, circumscribed presacral mass with mixed fat and soft tissue imaging characteristics should prompt the radiologist to consider the diagnosis of extra-adrenal myelolipoma. Differentiation of myelolipoma from the other considerations discussed above will likely require the collective consideration of clinical history with multi-modality imaging appearance. While percutaneous biopsy may be considered, surgical excision is rarely indicated. Despite this rare indication, some patients (such as ours) may elect for surgical removal.
TEACHING POINT
Myelolipomas typically appear as lobulated, well-circumscribed lesions with varying ultrasound, CT, and MR imaging characteristics according to the differing prevalence of fat and erythroid/myeloid elements. Myelolipomas can rarely be extra-adrenal, with the presacral location being the most common.
Table 1.
Differential diagnosis table for presacral myelolipoma
| Diagnosis | US | CT | MRI |
|---|---|---|---|
| Presacral myelolipoma | Similar to adrenal myelolipoma and varies according to composition; hyperechoic mass if predominately fatty, or mostly hypoechoic if tumor is primarily composed of erythroid/myeloid cells. | Similar to adrenal myelolipoma and varies according to composition; there will be a well-encapsulated, lobular lesion with mixed areas of low attenuation (frequently less than −30 HU) and soft tissue density (with possible soft tissue density enhancement after injection of intravenous contrast). | Similar to adrenal myelolipoma and varies according to composition; there will be a well-encapsulated, lobular lesion with fatty components yielding high signal intensity on T1-weighted images, which demonstrate fat suppression when applied. Hematopoietic elements have lower signal intensity on T1-weighted images and intermediate signal intensity on T2-weighted images. Hematopoietic soft tissue elements may enhance after administration of intravenous gadolinium contrast |
| Liposarcoma | Can also have mixed echogenicity, however tends to have more infiltrative growth with less well- defined borders/circumscription than presacral myelolipoma | Can also have mixed fat/soft tissue attenuation, however tends to have more infiltrative growth with less well-defined borders/circumscription than presacral myelolipoma. Can also present with metastasis. | Can also have mixed fat/soft tissue signal intensities, however tends to have more infiltrative growth with less well-defined borders/circumscription than presacral myelolipoma. Can also present with metastasis. |
| Teratoma | Potentially very difficult to distinguish from presacral myelolipoma, as teratomas also may have marked heterogeneous echogenicity from fat and soft tissue composition. Acoustic shadowing from calcium may also be seen (which can also be seen in myelolipoma following hemorrhage). | Like presacral myelolipoma, teratomas also may contain areas of fat and soft tissue attenuation. While calcium is not usually seen in presacral myelolipoma, its presence does not provide definitive differentiation, as calcium may be seen in myelolipoma following hemorrhage. | Like presacral myelolipoma, teratomas may also appear as well-circumscribed, lobular masses with mixed fat, soft tissue, and calcium signal characteristics. |
| Extramedullary hematopoiesis | Infrequently detectable and usually multifocal and poorly circumscribed. | Infrequently detectable by radiologic means secondary to insufficient macroscopic fat. Also, usually multifocal and poorly circumscribed. | Infrequently detectable secondary to insufficient macroscopic fat. Also, usually multifocal and poorly circumscribed. |
Table 2.
Summary table for presacral myelolipoma
| Etiology | This is not entirely certain for myelolipomas in adrenal or extra-adrenal locations. Some theories involve metaplasia of uncommitted adrenal cortical mesenchymal cells or migration of hematopoietic tissue to the adrenal gland during intrauterine development. Another hypothesis (which may have particular relevance to extra-adrenal myelolipoma) involves reactivation of peritoneal connective tissue hematopoiesis (a normal phenomenon of embryonic life). |
| Incidence | Extra-adrenal myelolipoma is relatively rare, with fewer than 50 reported cases in the literature. Of these extra-adrenal locations, the presacral region is the most common location. |
| Gender ratio | 2:1 female to male predominance |
| Age predilection | More frequent in the older (50+) population |
| Risk factors | Cushing syndrome, Addison disease, adrenal hyperplasia, chronic exogenous steroid use |
| Treatment | Varies on case by case basis, but usually none. Surgical resection may be indicated when mass effect causes symptoms |
| Prognosis | Excellent |
| Findings on imaging |
Ultrasound- Similar to adrenal myelolipoma and varies according to composition. The lobulated presacral mass will often have heterogeneous echogenicity with mixed hyperechoic fatty and hypoechoic hematopoietic areas. If the lesion is predominately fatty, then it will be more uniformly hyperechoic. If the lesion is predominately composed of hematopoietic cells, then the mass will be more uniformly hypoechoic. CT- Similar to adrenal myelolipoma and varies according to composition. A well-encapsulated, lobular presacral mass with mixed areas of fat/low attenuation (frequently less than −30 HU) and soft tissue density will be seen. The soft tissue elements sometimes enhance after intravenous contrast administration. MRI- Similar to adrenal myelolipoma and varies according to composition. The presacral mass will usually appear well-encapsulated, lobular, and feature fatty areas which yield high signal intensity on T1-weighted images with significant loss of signal when fat suppression is applied. Hematopoietic elements have lower signal intensity on T1-weighted images and intermediate signal intensity on T2-weighted images. Hematopoietic soft tissue elements may enhance after administration of intravenous gadolinium contrast. When using in-phase and out-of-phase gradient recalled echo (GRE) imaging, areas of the mass with intracellular fat will lose signal on out-of-phase images, while areas of macroscopic fat will maintain signal intensity. |
ABBREVIATIONS
- ACTH
adrenocorticotropic hormone
- CT
computed tomography
- FA
flip angle
- FSE
fast spin echo
- GRE
gradient recalled echo
- H&E
hematoxylin and eosin
- HU
Hounsfield units
- LAVA
liver acquisition with volume acquisition
- MRI
magnetic resonance imaging
- MR
magnetic resonance
- S3
third sacral vertebra
- S4
fourth sacral vertebra
- S5
fifth sacral vertebra
- TE
echo time
- TI
time for inversion
- TR
repetition time
- T
Tesla
- US
ultrasound
REFERENCES
- 1.Sutker B, Balthazar EJ, Fazzini E. Presacral Myelolipoma: CT findings. J Comput Assist Tomogr. 1985;9:1128–1130. doi: 10.1097/00004728-198511000-00028. [DOI] [PubMed] [Google Scholar]
- 2.Chen KTK, Felix EL, Flam MS. Extra adrenal myelolipoma. Am J Clin Pathol. 1982 Sep;78(3):386–389. doi: 10.1093/ajcp/78.3.386. [DOI] [PubMed] [Google Scholar]
- 3.Prahlow IA, Loggie BW, Cappellari JO, Scharling ES, Teot LA, Iskandar SS. Extra-adrenal myelolipoma: report of two cases. South Med J. 1995;88(6):639–643. doi: 10.1097/00007611-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Grignon DI, Shkrum MJ, Smout MS. Extra-adrenal myelolipoma. Arch Pathol Lab Med. 1989 Jan;113(1):52–54. [PubMed] [Google Scholar]
- 5.Kammen BF, Elder DE, Fraker DL, Siegelman ES. Extra adrenal myelolipoma: MR imaging findings. AJR. 1998;171:721–723. doi: 10.2214/ajr.171.3.9725304. [DOI] [PubMed] [Google Scholar]
- 6.Singla AK, Kechejian G, Lopez MJ. Giant presacral myelolipoma. Am Surg. 2003 Apr;69:334–338. [PubMed] [Google Scholar]
- 7.Dann PH, Krinsky GA, Israel GM. Case 135: Presacral Myelolipoma. Radiology. 2008 Jul;248:314–316. doi: 10.1148/radiol.2481050321. [DOI] [PubMed] [Google Scholar]
- 8.Amin MD, Tickoo SK, Schultz D. Myelolipoma of the renal sinus. An unusual site for rare extra-adrenal lesion. Arch Pathol Lab Med. 1999 Jul;123(7):631–634. doi: 10.5858/1999-123-0631-MOTRS. [DOI] [PubMed] [Google Scholar]
- 9.Talwalkar SS, Shaheen SP. Extra-adrenal myelolipoma in the renal hilum: a case report and review of the literature. Arch Pathol Lab Med. 2006;130(7):1049–1052. doi: 10.5858/2006-130-1049-EMITRH. [DOI] [PubMed] [Google Scholar]
- 10.Fowler MR, Williams RB, Alba JM, Byrd CR. Extra-adrenal myelolipomas compared with extramedullary hematopoietic tumors: a case of presacral myelolipoma. Am J Surg Pathol. 1982;6:363–374. doi: 10.1097/00000478-198206000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Selye H, Stone H. Hormonally induced transformation of adrenal gland into myeloid tissue. Am J Pathol. 1950;26:211–233. [PMC free article] [PubMed] [Google Scholar]
- 12.Cyran KM, Kenney PJ, Memel DS, Yacoub I. Adrenal myelolipoma. AJR. 1996;166:395–400. doi: 10.2214/ajr.166.2.8553954. [DOI] [PubMed] [Google Scholar]
- 13.Kenney PJ, Wagner BJ, Rao P, Heffess CS. Myelolipoma: CT and pathologic features. Radiology. 1998 Jul;208(1):87–95. doi: 10.1148/radiology.208.1.9646797. [DOI] [PubMed] [Google Scholar]
- 14.Hobson KG, Ghaemmaghami V, Roe JP, Goodnight JE, Khatri VP. Tumors of the retrorectal space. Dis Colon Rectum. 2005;48(10):1964–1974. doi: 10.1007/s10350-005-0122-9. [DOI] [PubMed] [Google Scholar]
- 15.Hassan I, Wietfeldt ED. Presacral Tumors: Diagnosis and Management. Clin Colon Rectal Sur. 2009 May;22(2):84–93. doi: 10.1055/s-0029-1223839. PMCID: PMC2780241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim T, Murakami T, Oi H, et al. CT and MR imaging of abdominal liposarcoma. AJR. 1996;166:829–833. doi: 10.2214/ajr.166.4.8610559. [DOI] [PubMed] [Google Scholar]
- 17.Liang EY, Cooper JE, Lam WW, Chung SC, Allen PW, Metreweli C. Case report: myolipoma or liposarcoma - a mistaken identity in the retroperitoneum. Clin Radiol. 1996;51:295–297. doi: 10.1016/s0009-9260(96)80350-3. [DOI] [PubMed] [Google Scholar]
- 18.Dozois EJ, Jacofsky DJ, Dozois RR. Presacral Tumors. In: Beck DE, Wolff BG, Fleshman JW, editors. The ASCRS Textbook of Colon and Rectal Surgery. New York: Springer; 2007. pp. 501–514. [Google Scholar]